Chapter 103 Spinal Intradural Vascular Malformations
Spinal vascular malformations are a family of lesions involving abnormalities of the arteries or veins surrounding the spinal column, spinal cord, and nerve roots. They are relatively rare1–3 and may manifest as a hemorrhage, myelopathy, radiculopathy, or back pain.4–7 These lesions can be divided into two broad categories: those that are intradural and those whose abnormal arterial connections are extradural. Extradural lesions are the most common and account for approximately 80% of spinal vascular malformations and are considered in another chapter. This chapter discusses the intradural lesions and includes glomus arteriovenous malformations (AVMs), juvenile AVMs, and intradural direct arteriovenous fistulas (AVFs). Cavernous malformations of the spinal cord and intradural spinal aneurysms are also discussed. In addition to detailing the symptoms, diagnosis, treatment, natural history, and outcomes of these lesions, the demographics and symptoms of these lesions are contrasted with those of the extradural lesions.
History and Nomenclature
The early classification of spinal AVMs occurred prior to the advent of spinal angiography. Patients with signs and symptoms of myelopathy underwent surgery, and the lesions were characterized based on their pathologic appearance.8–17
With the advent of spinal angiography in the 1960s, a more refined nomenclature developed based on the pattern of arterial input and venous drainage.18–20 This resulted in the creation of the terms type I, type II, and type III AVMs still in common use today.2,21,22
A type I lesion is a dural AVF whose single or, occasionally, multiple arteriovenous connections lie within the dura of the nerve root sheath and result in a dilated, arterialized coronal venous plexus.23,24 Prior to angiography, this dilated vein was erroneously felt to be the site of pathology rather than the arteriovenous connection that is the true source of pathology. Surgical treatment consisted of stripping the veins, often with poor results.25 With the recognition of the fistulous component of this lesion, surgical therapy has been directed at ligation of the abnormal arteriovenous connection and has led to significantly better outcomes.
Type II lesions, or glomus AVMs, are analogous to intracranial AVMs and consist of a tightly packed nidus of vessels over a short segment of the spinal cord. These lesions tend to manifest at an earlier age than the type I lesions and tend to occur at the cervicothoracic junction rather than at the thoracolumbar junction.1,26 Like type I lesions they may be amenable to surgical excision. Type III lesions, or juvenile AVMs, arise in single or multiple adjacent somites and therefore are frequently both extradural and intradural and may involve soft tissue and bone in addition to the spinal cord and dura.27,28 In the cord they form a diffuse nidus with normal spinal cord existing between loops of abnormal vessels. The embryologic term metameric was historically used in connection with these lesions because it connotes involvement of tissue derived from the entire somite.15 Surgical cure of these metameric or type III lesions (juvenile AVMs) is difficult and often requires a multidisciplinary approach.
Shortly after the introduction of the type I to III classification an additional type IV lesion was proposed by Heros et al.13 This lesion is a direct connection between an intradural artery and a vein in the subarachnoid space without a definable nidus. The lesions are frequently ventral and involve the anterior spinal artery. Surgical cure is possible when the lesions are small. Embolization may be a helpful adjuvant and may be palliative for the larger lesions.
The understanding and classification of spinal vascular malformations continue to evolve. Recently, a type V spinal AVM was proposed based on the observation that some type III AVMs are outside the spinal cord and dura and are therefore not truly metameric.29 Their epidural location drastically changes the potential for treatment. Spetzler et al.,30 in addition to their other nomenclature contributions, have proposed that juvenile AVMs of the conus medullaris be considered a separate category of spinal AVM because complex juvenile lesions at the level of the conus may have a more favorable prognosis with surgical resection.
With the advent of MRI, cavernous malformations of the spinal cord have been identified with increasing frequency.1,7–9,16,31–53 Like their intracranial counterparts, they are sinusoidal venous channels that appear with stair-step neurologic decline from repeated hemorrhage. Some controversy exists over the indication for surgical resection of these lesions. Intradural spinal aneurysms are also being diagnosed with increasing frequency and may be traumatic, flow related from AVM feeding vessels, or rarely congenital such as an aneurysm of a posterior inferior cerebellar artery that has a spinal origin. These lesions appear with subarachnoid hemorrhage and may require direct surgical repair or endovascular vessel sacrifice.
Embryology and Vascular Anatomy
The fetal spinal vascular network develops in four stages.54 The first, or primitive segmental, stage, occurs between weeks 2 and 3 of gestation. During this stage, 31 pairs of segmental vessels originate from paired dorsal aortas and grow toward the neural tube along the developing nerve roots. The segmental vessels divide into ventral and dorsal branches and form capillary networks on the ventrolateral surface of the neural tube. These networks ultimately develop into paired primitive ventral arterial tracts, the precursors of the anterior spinal artery. The anterior spinal artery develops when these paired ventral arterial tracts fuse during the third stage of development.
The transitional stage is the third embryologic stage of spinal vascular development and occurs between the sixth week and fourth month of fetal growth. The major development in this stage is the formation of the adult pattern of vascular supply. The primitive ventral longitudinal arterial tracts fuse, and the number of segmental arteries supplying the spinal cord is reduced.55 By 10 weeks’ gestational age, adult patterns of superficial spinal cord vessels are present. The last stage, called the terminal stage, occurs after 4 months of development and is the phase of maturation and increased tortuosity of the major spinal cord vessels.
The most likely stage of embryologic development at which spinal vascular malformations can arise is the second stage (3–6 weeks). Maldevelopment in this stage leads to persistence of thin-walled tortuous vessels that exhibit primitive capillary interconnections, arteriovenous shunts, and poorly developed elastic and medial layers that closely resemble intracranial angiomas.11 The concept that intradural vascular malformations are congenital and are the result of fetal vascular maldevelopment is supported by the fact that 20% of patients with intradural AVMs have other associated congenital vascular anomalies (Table 103-1). Furthermore, these malformations are present in younger patients and are distributed throughout the entire spinal axis. This favors a common dysembryogenic basis of intradural spinal and other vascular malformations.
TABLE 103-1 Congenital Vascular Anomalies Associated with Intradural Spinal Arteriovenous Malformations
| Congenital Anomaly | Reference |
|---|---|
| Brain arteriovenous malformation | Brion et al.10; Bruni et al.33; Di Chiro and Wener18; Hebold17; Jellinger et al.91 |
| Cerebral aneurysm | Aminoff and Logue4; Djindjian6; Djindjian et al.71; Hebold17 |
| Vascular agenesis | Hebold17 |
| Rendu-Osler-Weber syndrome | Doppman et al.21; Hebold17 |
| Klippel-Trénaunay-Weber syndrome | Cogen and Stein68; Hebold17; Heros et al.78 |
| Soft tissue hemangiomas | Djindjian et al.58 |
| Hemangioblastomas | Hall et al.14 |
Glomus Arteriovenous Malformations
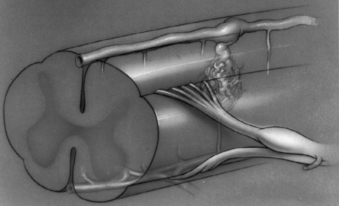
Glomus, or type II, spinal AVMs are high-flow malformations in which a tightly packed malformation nidus is located within a short segment of the pia or the spinal cord parenchyma. They may occur anywhere along the longitudinal axis of the spinal cord, although some reports indicate a higher incidence of glomus AVMs in the cervicothoracic junction.1,26 The feeding arteries of glomus AVMs usually arise from distinct medullary arteries and also supply the spinal cord.56 The malformations are frequently found in the ventral aspect of the spinal cord and derive their blood supply from medullary branches of the anterior spinal artery. Venous drainage is through the coronal venous plexus, and, unlike dural (type I) AVMs, the venous drainage usually occurs in both a rostral and a caudal direction57 (Fig. 103-1). With dural lesions, caudal venous drainage is extremely rare.
The clinical symptoms of glomus-type intramedullary AVMs is usually apoplectic in nature because of sudden hemorrhage from the malformation. These AVMs usually become symptomatic before adulthood and frequently appear with subarachnoid hemorrhage (SAH). Neurologic symptoms often involve the upper extremities, if the nidus is in or near the cervical portion of the spinal cord. By comparison, upper extremity involvement is exceedingly rare in the more commonly observed dural (type I) AVMs. There is no gender predilection for intradural AVMs, whereas at least 85% of spinal dural AVMs occur in males. SAH or intramedullary hemorrhage occurs in 50% of patients with intradural vascular malformations and is attributed to the frequently associated presence of arterial or venous aneurysms.1,58,59 Of 54 patients with confirmed intradural spinal AVMs who were studied at the National Institute of Health, 30 patients (52%) had experienced SAH and 24 patients (44%) had aneurysms associated with either the draining or feeding vasculature.59 SAH occurs as the initial symptom most commonly in glomus-type malformations, whereas weakness is most common in any other spinal vascular malformation.
At the time of diagnosis, most patients with intradural AVMs have some motor and sensory deficit. Spastic paraparesis and pain and temperature sensory deficits are the most frequent neurologic findings during onset.59 A bruit heard over the affected dermatome may also be present. With intradural AVMs, specific neurologic symptoms reflect the location of the nidus along the longitudinal axis of the spinal cord. Strenuous activity or postural changes rarely exacerbate the symptoms of intradural glomus-type AVMs, although this is a common finding with dural type I lesions or juvenile (type III) spinal malformations.
Adequate and appropriate radiologic investigation is paramount for confirming the diagnosis of an intradural vascular malformation. Plain spine radiographs may be useful to rule out other pathology and have been found by some to be abnormal in patients with high-flow intradural AVMs. Rosenblum et al.59 found that 15% of patients with intradural AVMs had widened interpeduncular distances on plain spine radiographs. No increase in spinal canal dimension was observed with type I dural lesions.
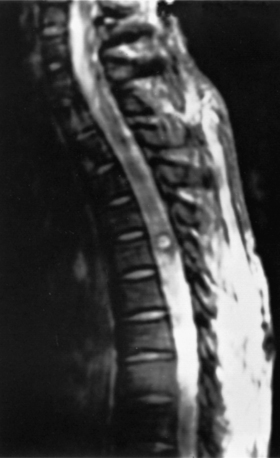
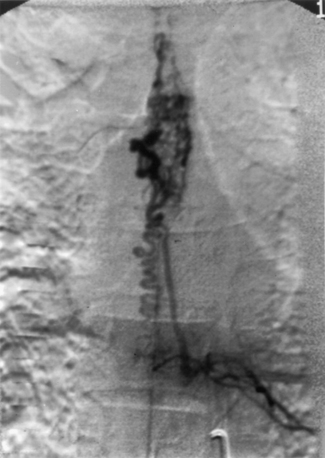
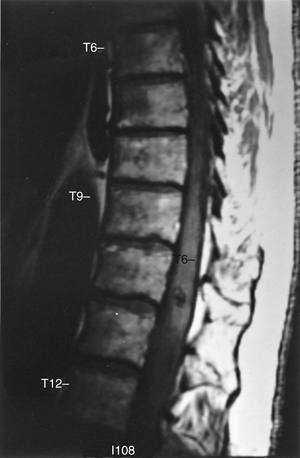
Although total spine myelography was the radiologic test of choice for many years, most patients now undergo MRI as a screening test instead of myelography (Fig. 103-2). Although the sensitivity of myelography and CT for detecting a spinal AVM is high, the anatomic information is nonspecific.60 Selective spinal angiography with high-resolution digital imaging remains the diagnostic test of choice to provide the most precise anatomic information (Fig. 103-3).
The indications for surgical resection of glomus, or type II, spinal AVMs are difficult to generalize because of the rarity of these lesions, the variability of symptoms, and their poorly understood natural history. Several authors have reported excellent results with surgical resection of glomus AVMs, although extrapolation of these results, obtained by highly specialized microvascular surgeons, to a general neurosurgical practice would be grossly misleading.61 Rosenblum59 reported a series of 43 patients who underwent surgery to resect intradural spinal malformations. In 22 patients (51%) the neurologic status was unchanged, 14 patients (33%) improved, and 6 patients (14%) were neurologically worse after surgery. In this series, residual malformation was detected in one third of the patients who underwent postoperative angiography.59 This finding is extremely important if the natural history of intramedullary spinal AVMs parallels that of cranial AVMs, because partial resection offers no benefit but poses a tremendous risk. A more recent series reported that 6 of 15 (40%) patients improved, 8 of 15 (53%) patients were stable, and 1 (7%) patient became worse after resection of a type II lesion.62
Favorable features for surgical resection of a glomus AVM include the radiographic appearance of a compact nidus located in an accessible portion of the spinal cord and an informed patient who understands the risks and the potential benefits of the procedure.63 The operative procedure requires precise localization of the nidus and adequate exposure to minimize spinal cord manipulation. Use of the operative microscope and microsurgical technique is mandatory to minimize trauma to the spinal cord. Dissection is initially directed at the feeding vessels entering at the periphery of the lesion. The plane of dissection is deepened until the arterial supply has been eliminated and only venous drainage remains. The venous drainage is divided, and the nidus delivered out of the spinal cord. The presence of an intramedullary clot is helpful for defining the periphery of the nidus, although SAH hampers visualization. It is best not to attempt surgery in the period immediately after an acute spinal SAH; it should be delayed.
The embolization of type II spinal AVMs through endovascular techniques can be a useful adjunct to surgical resection. Preoperative embolization may reduce the blood flow and the number of vessels supplying the malformation and thus decreases the technical difficulty of surgical resection. The risk of embolization of glomus AVMs is the inadvertent occlusion of radiculomedullary afferent vessels supplying vital regions of the adjacent spinal cord parenchyma and potential worsening of the patient’s neurologic status.64 Embolization has been advocated as a sole treatment for these lesions in some cases65 and has been shown to favorably alter the hemodynamics of the lesions.66 The high tendency toward revascularization and the need for frequent repeat procedures are significant limitations of this strategy. Newer embolic agents such as the nonadhesive liquid agent Onyx may reduce the revascularization rate.67
Juvenile Arteriovenous Malformations
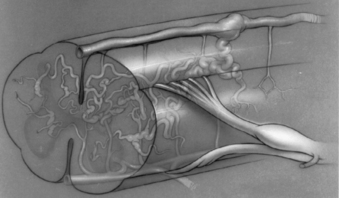
Juvenile, or type III, spinal AVMs are extremely rare, formidable lesions that constitute approximately 7% of all spinal AVMs.68,69 These AVMs can be distinguished from glomus, or type II, malformations by several characteristics. Juvenile spinal AVMs are exceedingly rare, large, high-flow intramedullary AVMs. They usually have multiple feeding vessels over several spinal segments and often extend into and involve the epidural space, vertebrae, paravertebral musculature, and soft tissues27,28 (see Fig. 103-3). Juvenile malformations may have integumentary representation with cutaneous extension of the AVM within the somites corresponding to the spinal level of involvement.70 They are frequently located in the cervical or upper thoracic region and involve several spinal segments. The entire transverse area of the spinal cord is usually involved with malformation, and functional neural tissue is present within the interstices of the lesion (Fig. 103-4).
Subarachnoid, or intramedullary, hemorrhage is an uncommon occurrence in juvenile type III spinal AVMs.59 The typical symptom is one of progressive neurologic deterioration that occurs during early adulthood or adolescence. Postural changes, Valsalva maneuver, and pregnancy have been reported to exacerbate the clinical symptoms.4,46,71–73 There may be an overlying bruit if significant soft tissue involvement is present.68
Due to the extensive involvement of the spinal cord, type III spinal AVMs can only rarely be removed with acceptable morbidity.18,19,69,74 Touho et al.75 and Spetzler et al.73 have reported cases of successful removal of juvenile spinal AVMs in patients who had a definable nidus within the spinal cord. Malis69,74 described his experience in the treatment of spinal AVMs. He reported a series of 43 patients in which 3 underwent attempted surgical resection of type III malformations. In this series one patient died, one had no improvement of severe neurologic deficits, and one patient was left paraplegic after attempted surgical resection.69,74 Ommaya et al.22 and Djindjian6 have reported some success with arterial embolization as the sole treatment regimen. Hall et al.14 reported delayed recanalization of spinal AVMs that had previously undergone complete angiographic embolization as the only treatment. Similarly, Bao and Ling76 reported that 17 of 22 (75%) patients with a type III lesion, treated with embolization, required repeat embolization because of recanalization or clinical recurrence. Cyanoacrylate, a more permanent liquid embolic agent, may provide higher cure rates but is difficult to control.76
Intradural Arteriovenous Fistulas
True intradural or perimedullary AVFs are very rare, with only a few cases reported over the past decade.77–80 Heros et al.78 suggested classifying true intradural fistulas separately from previously described spinal AVMs and proposed referring to them as type IV spinal AVMs. Others have subsequently reported similar cases that could be classified as type IV spinal AVMs.4,80 These lesions are direct AVFs that involve the normal arterial supply of the spinal cord and drain into the coronal venous plexus that often becomes dilated and aneurysmal. Of those cases reported in the United States, 10 of 14 patients had AVFs involving the anterior spinal artery, whereas 4 of the patients reported on by Tomlinson et al.78,80 had posterior spinal artery supply of the fistula.
Three types of intradural extramedullary fistulas have been described based on radiographic appearance and intraoperative observations. The first type may be a simple connection between an elongated but normal-caliber, anterior spinal artery and the coronal venous plexus as described by Aminoff et al.77 The second type consists of a dilated anterior spinal artery with a fistulous connection to a dilated aneurysmal venous system, similar to the case reported by Heros et al.78 The third type consists of a large fistula from multiple arterial pedicles with rapid blood flow and a massively dilated system of draining veins.13,81,82
The etiology of intradural direct AVFs remains unclear, although a congenital etiology is a likely possibility. In the patients reported by Aminoff et al.77 and Heros et al.,78 the symptoms and history were inconsistent with fistulas of an acquired nature. Wakai et al.82 have also reported a similar case in a 9-year-old child in whom an unusual intradural AVF was treated. This patient suffered two distinct SAHs separated by approximately 1 year. The patient underwent three negative cerebral arteriograms before an MRI was obtained that demonstrated a spinal intradural AVF. The typical symptoms are usually related to progressive neurologic deterioration rather than hemorrhage, although SAH can occur and may be a repetitive occurrence if the fistula remains undiagnosed and untreated. In patients with these rare lesions, symptoms of progressive myelopathy may be due to compressive mass effect from venous aneurysms, arterial vascular steal phenomenon, venous hypertension, or subacute intraparenchymal hemorrhage.
The diagnosis is usually confirmed with selective spinal angiography. Treatment is contingent on the precise localization and anatomic delineation of the fistula. Surgical interruption of the AVF is the definitive treatment when feasible. Because of the frequent involvement of the anterior spinal artery and the commonly ventral location of the fistulas, complex ventral spinal approaches are often necessary to adequately expose the fistula for resection.78,83 When the arterial feeders are separated from the venous outflow, complete removal of the dilated venous structures is hazardous and usually unnecessary.
If the fistula is of giant proportions and the risks of surgical intervention are deemed too great, endovascular occlusion techniques may be considered. Hall et al.14 reported six patients who were followed after complete embolization of spinal AVMs. Three patients underwent endovascular treatment of type I spinal AVFs, whereas three others underwent embolization of glomus malformations as the sole treatment modality. All but one patient developed recanalization of the malformation, and four of the six patients had symptomatic recurrence.14 Definitive treatment of spinal intradural direct AVFs by endovascular techniques is feasible, but the long-term efficacy remains to be demonstrated. Furthermore, endovascular occlusion of feeding arteries in too proximal a location may result in delayed recruitment of collateral arterial channels, whereas extremely distal occlusion may compromise an already tenuous venous outflow and precipitate neurologic deterioration.13,79 Endovascular treatment as the sole modality is perhaps most useful with giant AVFs and least appropriate for small fistulas of the first type.
Cavernous Angiomas
Spinal cavernous malformations are rare and represent 5% to 12% of all spinal vascular malformations.40 Most spinal cavernous malformations arise within the vertebral bodies, although many reports of intradural and intramedullary cavernous malformations exist.1,7–9,16,31–53 Cavernous angiomas occur throughout the nervous system. They are vascular malformations, pathologically composed of closely opposed, blood-filled spaces lined by a single layer of epithelium. The vessel walls of the malformation vary from thin capillary-sized vessels to thick, hyalinized vessels densely packed with collagen. Typically, there are no elastic fibers and no smooth muscle within the walls,46 and the vessels of the cavernous malformations are arranged in a sinusoidal network without intervening neural tissue. The neural parenchyma surrounding these malformations is often gliotic and hemosiderin laden.45,49 According to McCormick and Nofzinger,45 spinal cavernous malformations are pathologically indistinguishable from cerebral cavernous malformations.
The clinical symptoms of patients with intradural spinal cavernous malformations usually include progressive paraparesis and sensory loss, along with pain. Symptoms may exist or progress over many years. In the pre-MRI era, diagnosis was difficult, and cavernous angioma was often confused with multiple sclerosis.44,84 Symptoms appear most commonly during the fourth decade, although as many as 10% may occur in children.85 Females account for 70% of patients diagnosed with spinal cavernous malformations.34,46 These lesions may occur anywhere along the neuroaxis and occur with equal frequency in the cervical and thoracic cord. The average size at diagnosis is 17 mm and is similar to cranial cavernous malformations; no correlation between size and the incidence of hemorrhage has been shown.86 Familial cavernous malformations account for 50% of all cases of CNS cavernous angiomas, and in these cases, genetic transmission is believed to be autosomal dominant.16,87 Spontaneous development of new cavernomas has been documented in rare patients followed for existing lesions,50 and patients with cavernous malformations of the spine have an increased risk of multiple neuroaxis cavernous malformations.88
Cavernous malformations produce symptoms through repetitive hemorrhages. Typically, hemorrhage is associated with small amounts of bleeding into the surrounding neural parenchyma. In rare cases of intradural extramedullary cavernomas, SAH has been reported.31,33,38,48,51 In a literature review by Canavero et al.,34 the risk of hemorrhage was estimated to be 1.6% per person-year of exposure. This is roughly two times the estimated annual risk of hemorrhage with cranial cavernous malformations. Pregnancy appears to statistically increase the risk of hemorrhage, as does a cervical location of the cavernoma.34,52
Before the availability of MRI, cavernous malformations of the spinal cord were difficult to visualize radiographically. Myelography is uniformly unreliable and may only reveal subtle widening of the spinal cord. CT may also demonstrate pathologic spinal cord widening or the presence of acute hemorrhage, calcifications, or a syrinx cavity. Angiography is uniformly negative and carries unnecessary risk because the diagnosis can be confirmed with MRI. MRI is the diagnostic procedure of choice for intradural cavernous malformations and is virtually 100% reliable. The appearance of spinal cavernous malformations on MRI is similar to that of cerebral lesions. There is typically mixed signal intensity on both T1- and T2-weighted images that variably enhance with gadolinium (Fig. 103-5). Regions of acute hemorrhage, edema, or hemosiderin deposition may be observed immediately surrounding these lesions. Hemosiderin deposition produces a ringlike region of decreased signal intensity on both T1- and T2-weighted images.37
Optimal treatment of a spinal cavernous malformation remains unclear. In the review of 57 patients by Canavero et al.,34 the single most important factor relating to outcome was neurologic status at onset of symptoms. When neurologic status was poor, patients typically did poorly with surgical treatment. In their review, the age of the patient, site of the lesion, duration of the condition, and extent of removal had no significant effect on outcome. Because neurologic improvement is common after a bleeding episode, the reported improvement with surgical resection may be coincidental.
The goal of microsurgical removal of cavernous malformations is to prevent further hemorrhage and subsequent neurologic deterioration. Intraoperative ultrasonography is useful for precise intraoperative localization and for limiting the length of the myelotomy.42 After exposure of the involved spinal cord, slight staining of the dorsal surface is often observed. A myelotomy is made over the region of staining, and microsurgical technique under high magnification is used to dissect the gliotic plane surrounding the lesion. Bleeding on the periphery of the malformation is usually due to low-flow, low-pressure vessels that are easily controlled with bipolar electrocoagulation. The resected lesions resemble small “berries” of vascular tissue similar to cerebral cavernous malformations. Preoperative treatment of patients with high-dose steroids and the use of intraoperative somatosensory evoked potentials may be useful to minimize the inherent morbidity of intramedullary spinal cord surgery.
Although some surgeons have reported good results with radiosurgery for occult vascular malformations of the brain, others have reported poor results and have pathologically confirmed posttreatment radiation necrosis when brainstem lesions have been treated with stereotactic radiosurgery.89,90 Because of the lack of evidence of therapeutic benefit and the risk of radionecrosis, microsurgical resection, if clinically warranted, appears to be the only therapeutic treatment option. There is no apparent indication for radiosurgery in the treatment of spinal cavernous malformations.
Cogen P., Stein B.M. Spinal cord arteriovenous malformations with significant intramedullary components. J Neurosurg. 1983;59:471.
Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. J Neurosurg. 1986;64:134.
Krayenbuhl H., Yasargil M.G., McClintock H.G. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg. 1969;30:427.
Oldfield E.H., Doppman J.L. Spinal arteriovenous malformations. Clin Neurosurg. 1986;34:161.
Spetzler R.F., Detwiler P.W., Riina H.A., et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(Suppl 2):145.
1. Criscuolo G.R., Long D.M. Vascular anomalies of the spinal cord. In: Frymoyer P., Dueker K., Hadler N., et al, editors. The adult spine: principles and practice. Philadelphia: JB Lippincott; 1991:679.
2. Doppman J.L., DiChiro G., Dwyer A.J., et al. Magnetic resonance imaging of spinal arteriovenous malformations. J Neurosurg. 1987;66:830.
3. Symon L., Kuyama H., Kendall B. Dural arteriovenous malformations of the spine: clinical features and surgical results in 55 cases. J Neurosurg. 1984;60:238.
4. Aminoff M.J., Logue V. Clinical features of spinal vascular malformations. Brain. 1974;97:197.
5. Aminoff M.J., Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211.
6. Djindjian R. Embolization of angiomas of the spinal cord. Surg Neurol. 1975;4:411.
7. Logue V. Angiomas of the spinal cord: review of the pathogenesis, clinical features and the results of surgery. J Neurol Neurosurg Psychiatry. 1979;42:1.
8. Bergstrand A., Hook O., Lidvall H. Vascular malformations of the spinal cord. Acta Neurol Scand. 1964;40:169.
9. Bicknell J.M., Carlow T.J., Kornfeld M. Familial cavernous angiomas. Arch Neurol. 1978;35:746.
10. Brion S., Netzky M.G., Zimmermann H.M. Vascular malformations of the spinal cord. Arch Neurol Psychiatry. 1952;68:339.
11. Cushing H., Bailey P. Tumors arising from the blood vessels of the brain: angiomatous malformations and hemangioblastomas. Springfield, IL: Charles C. Thomas; 1928.
12. Gaupp J. Casustische Beitrage zur pathologischen Anatomie des Rückenmarks und seiner Haute. Beitr pathol Anat. 1987;2:510.
13. Gueguen B., Merland J.J., Riche M.C., et al. Vascular malformations of the spinal cord: intrathecal perimedullary arteriovenous fistulas fed by medullary arteries. Neurology. 1987;37:969.
14. Hall W.A., Oldfield E.H., Doppman J.L. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg. 1989;70:714.
15. Hamilton M.G., Anson J.A., Spetzler R.F. Arteriovenous and other vascular malformations of the spine. In: Menezes A.H., Sonntag V.K.H., editors. Principles of spinal surgery. New York: McGraw-Hill, 1996.
16. Hayman L.A., Evans R.A., Ferrell R.E. Familiar cavernous angiomas: natural history and genetic study over a 5-year period. Am J Med Genet. 1982;11:147.
17. Hebold O. Aneurysmen der kleinsten Rückenmarksgefässe. Arch Psychiatr Nervenkr. 1885;16:813.
18. DiChiro G., Wener L. Angiography of the spinal cord: a review of contemporary techniques and applications. Neurosurgery. 1973;39:1.
19. DiChiro G., Doppman J.L., Ommaya A.K. Selective arteriography of arteriovenous aneurysms of the spinal cord. Radiology. 1967;88:1065.
20. DiChiro G., Doppman J.L., Ommaya A.K. Radiology of spinal arteriovenous malformations. Prog Neurol Surg. 1971;4:329.
21. Doppman J.L., DiChiro G., Oldfield E.H. Origin of spinal arteriovenous malformation and normal cord vasculature from a common segmental artery: angiographic and therapeutic considerations. Radiology. 1985;154:687.
22. Ommaya A.K., DiChiro G., Doppman J. Ligation of arterial supply in the treatment of spinal cord arteriovenous malformations. J Neurosurg. 1969;30:69.
23. Doppman J.L., DiChiro G., Dwyer A.J., et al. Selective arteriography of the spinal cord. St. Louis: Warren H. Green; 1969.
24. Kendall B.E., Logue V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 1977;13:181.
25. Krayenbuhl H., Yasargil M.G., McClintock H.G. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg. 1969;30:427.
26. Ommaya A.K. Spinal arteriovenous malformations. Williams R.H., Rengachary S.S., editors. Neurosurgery. New York: McGraw-Hill. 1985;vol 2:1495.
27. Kaplan P., Hollenberg R.D., Fraser F.C. Spinal arteriovenous malformation with hereditary cutaneous hemangiomas. Am J Dis Child. 1976;130:1329.
28. Merry G.S., Appleton D.B. Spinal arterial malformation in a child with hereditary hemorrhagic telangiectasia. J Neurosurg. 1976;44:613.
29. Morgan H., Morrill K. Vascular lesions of the spine. In: Batjer H.H., Loftus C.M., editors. Textbook of neurological surgery. Philadelphia: Lippincott-Williams & Wilkins; 2003:1847.
30. Spetzler R.F., Detwiler P.W., Riina H.A., et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(Suppl 2):145.
31. Acciarri N., Padovani R., Pozzati E., et al. Spinal cavernous angioma: a rare cause of subarachnoid hemorrhage. Surg Neurol. 1992;37:453.
32. Anson J.A., Spetzler R.F. Surgical resection of intramedullary spinal cord cavernous malformations. J Neurosurg. 1993;78:446.
33. Bruni P., Massari A., Greco R., et al. Subarachnoid hemorrhage from cavernous angioma of the cauda equina: case report. Surg Neurol. 1994;41:226.
34. Canavero S., Pagni C.A., Duca S., et al. Spinal intramedullary cavernous angiomas: a literature metaanalysis. Surg Neurol. 1994;41:381.
35. Cosgrove G.R., Bertrand G., Fontaine S., et al. Cavernous angiomas of the spinal cord. J Neurosurg. 1988;68:31.
36. DiChiro G. Combined retino-cerebellar angiomatosis and deep cervical angiomas: case report. J Neurosurg. 1957;14:685.
37. Fontaine S., Melanson D., Cosgrove R., et al. Cavernous hemangiomas of the spinal cord: MR imaging. Radiology. 1988;166:839.
38. Heimberger K., Schnaberth G., Koos W., et al. Spinal cavernous hemangioma (intradural-extramedullary) underlying repeated subarachnoid hemorrhage. J Neurol. 1982;226:289.
39. Houdart R., Djindjian R., Hurth M. Angiomas of the spinal cord: clinical study. Mechanism of spinal cord involvement. Therapeutic possibilities. Apropos of 33 cases. Rev Neurol. 1968;118:97.
40. Jellinger K. Pathology of spinal vascular malformations and vascular tumors. In: Pia H.W., Djindjian R., editors. Spinal angiomas: advances in diagnosis and therapy. Berlin: Springer-Verlag; 1978:18.
41. Lindboe C.F., Nordal J.H. Multiple neurilemmomas of the cauda equina, cavernous hemangioma of the spinal cord, and degeneration of the lateral corticospinal tracts in a man with a clinical diagnosis of multiple sclerosis. Clin Neuropathol. 1985;4:260.
42. Lunardi P., Acqui M., Ferrante L., et al. The role of intraoperative ultrasound imaging in the surgical removal of intramedullary cavernous angiomas. Neurosurgery. 1994;34:520.
43. McCormick P.C. Spinal vascular malformations. Semin Neurol. 1993;13:349.
44. McCormick P.C., Michelsen W.S., Post K.D., et al. Cavernous malformations of the spinal cord. Neurosurgery. 1988;2:459.
45. McCormick W.F., Nofzinger J.D. “Cryptic” vascular malformations of the central nervous system. J Neurosurg. 1966;24:865.
46. Ogilvy C.S., Louis D.N., Ojemann R.G. Intramedullary cavernous angiomas of the spinal cord: clinical presentation, pathological features, and surgical management. Neurosurgery. 1992;31:219.
47. Ojemann R.G., Crowell R.M., Ogilvy C.S. Management of cranial and spinal cavernous angiomas. Clin Neurosurg. 1993;40:98.
48. Pagni C.A., Canavero S., Forni M. Report of a cavernoma of the cauda equina and review of the literature. Surg Neurol. 1990;33:124.
49. Russell D.S., Rubinstein L.J. Pathology of tumors of the nervous system, ed 5. London: E. Arnold; 1989.
50. Scott R.M., Barnes P., Kupsky W., et al. Cavernous angiomas of the central nervous system in children. J Neurosurg. 1992;76:38.
51. Veda S., Saito A., Inomori S., et al. Cavernous angioma of the cauda equina producing subarachnoid hemorrhage: case report. J Neurosurg. 1987;66:134.
52. Villani R.M., Arienta C., Caroli M. Cavernous angioma of the central nervous system. J Neurosurg Sci. 1989;33:229.
53. Wyburn-Mason R. The vascular abnormalities and tumors of the spinal cord and the membranes. London: Henry Kimpton; 1943.
54. Turnbull I.M. Bloody supply of the spinal cord. Vinken P.J., Bruyn G.W., editors. Handbook of clinical neurology. Amsterdam: North Holland. 1972;vol 12:478.
55. Torr J.B. The embryological development of the anterior spinal artery in man. J Anat. 1957;91:587.
56. Doppman J.L. The nidus concept of spinal cord arteriovenous malformations: a surgical recommendation based upon angiographic observations. Br J Radiol. 1971;44:758.
57. Rosenblum B.R., Oldfield E.H., Doppman J.L. Pathogenesis of spinal arteriovenous malformations. Surg Forum. 1987;37:489.
58. Djindjian R., Hurth M., Houdart R. Angiomas medullaires, dysplasies vasculaires segmentaires ou generalisees et chacomatoses. Rev Neurol. 1971;124:121.
59. Rosenblum B.R., Oldfield E.H., Doppman J.L., et al. Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVMs in 81 patients. J Neurosurg. 1987;67:795.
60. Oldfield E.H., Doppman J.L. Spinal arteriovenous malformations. Clin Neurosurg. 1986;34:161.
61. Morgan M.K. Outcome from treatment for spinal arteriovenous malformation. Neurosurg Clin North Am. 1999;10(1):113.
62. Connolly E.S.Jr., Zubay G.P., McCormick P.C., et al. The posterior approach to a series of glomus (type II) intramedullary spinal cord arteriovenous malformations. Neurosurgery. 1998;42(4):774.
63. Ohata K., Takami T., El-Naggar A., et al. Posterior approach for cervical intramedullary arteriovenous malformation with diffuse-type nidus: report of three cases. J Neurosurg. 1999;91(Suppl 1):105.
64. Anson J.A., Spetzler R.F. Interventional neuroradiology for spinal pathology. Clin Neurosurg. 1992;39:388.
65. Biondi A., Merland J.J., Reizine D., et al. Embolization with particles in thoracic intramedullary arteriovenous malformations: long-term angiographic and clinical results. Radiology. 1990;177(3):651.
66. Touho H., Karasawa J., Ohnishi H., et al. Hemodynamic evaluation of spinal arteriovenous malformations before and after embolization: preliminary report. Neurol Med Chir (Tokyo). 1995;35(7):445.
67. Molyneux A.J., Coley S.C. Embolization of spinal cord arteriovenous malformation with an ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx liquid embolic system): report of two cases. J Neurosurg. 2000;93(Suppl 2):304.
68. Cogen P., Stein B.M. Spinal cord arteriovenous malformations with significant intramedullary components. J Neurosurg. 1983;59:471.
69. Malis L.I. Arteriovenous malformations of the spinal cord. Youmans J.R., editor. Neurological surgery. Philadelphia: WB Saunders. 1982;ed 2:1850.
70. Krayenbuhl H., Benini A., Bollinger A., et al. Ein Fall von Klippel-Trenaunay-Weber Syndrom mit arteriovenoser Fistel im Bereich der Brustwirbelsäule und Rückenmarkkompression. Neurochirurgic. 1970;13:228.
71. Djindjian M., Djindjian R., Rey A., et al. Intradural extramedullary spinal arteriovenous malformations fed by the anterior spinal artery. Surg Neurol. 1977;8:85.
72. Kulkarni M.V., Burks D.D., Price A.C., et al. Diagnosis of spinal arteriovenous malformation in a pregnant patient by MR imaging. J Comput Assist Tomogr. 1985;9:171.
73. Spetzler R.F., Zabramski J.M., Flom R.A. Management of juvenile spinal AVMs by embolization and operative excision. J Neurosurg. 1989;70:629.
74. Malis L.I. Microsurgery for spinal and arteriovenous malformations. Clin Neurosurg. 1979;26:543.
75. Touho H., Karasawa J., Shishido H., et al. Successful excision of a juvenile-type spinal arteriovenous malformation following intraoperative embolization. J Neurosurg. 1991;75:647.
76. Bao Y., Ling F. Classification and therapeutic modalities of spinal vascular malformations in 80 patients. Neurosurgery. 1997;40(1):75.
77. Aminoff M.J., Gutin P.H., Norman D. Unusual type of spinal arteriovenous malformation. Neurosurgery. 1988;22:589.
78. Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. J Neurosurg. 1986;64:134.
79. Riche M.C., Melke J.P., Merland J.J. Embolization of spinal cord vascular malformations via the anterior spinal artery. Am J Neuroradiol. 1983;4:378.
80. Tomlinson F.H., Rufenacht D.A., Sundt T.M., et al. Arteriovenous fistulas of the brain and the spinal cord. J Neurosurg. 1993;7:16.
81. Riche M.C., Scialfa G., Gueguen B., et al. Giant extramedullary arteriovenous fistula supplied by the anterior spinal artery: treatment by detachable balloons. Am J Neuroradiol. 1983;4:391.
82. Wakai S., Inoh S., Iwanaga H., et al. Successful surgical obliteration of a huge intradural arteriovenous fistula of the spinal cord in a child. Childs Nerv Syst. 1992;8:347.
83. Williams F.C., Zabramsic J.M., Spetzler R.F., et al. Anterolateral transthoracic transvertebral resection of an intramedullary spinal arteriovenous malformation. J Neurosurg. 1991;71:1004.
84. Pia H.W. Diagnosis and treatment of spinal angiomas. Acta Neurochir. 1973;28:1.
85. Deutsch H., Shrivistava R., Epstein F., et al. Pediatric intramedullary spinal cavernous malformation. Spine. 2001;26(18):E427.
86. Robinson J.R., Awad I.A., Little J.R. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709.
87. Rigamonti D., Hadley M.N., Drayer B.P., et al. Cerebral cavernous malformations: incidence and familiar occurrence. N Engl J Med. 1988;319:343.
88. Vishteh A.G., Zabramski J.M., Spetzler R.F. Patients with spinal cord cavernous malformations are at an increased risk for multiple neuroaxis cavernous malformations. Neurosurgery. 1999;45(1):30.
89. Kondziolka D., Lunsford D., Coffey R.J., et al. Stereotactic radiosurgery of angiographically occult vascular malformations: indications and preliminary experience. Neurosurgery. 1990;27:892.
90. Weil S., Tew J.M., Seiner L. Comparison of radiosurgery and microsurgery for treatment of cavernous malformation of the brain stem. J Neurosurg. 1990;72:336A.
91. Jellinger K., Minauf M., Garzuly F., et al. Angiodysgenetische nekrotisierende Myelopathie. Arch Psychiat Nervenkr. 1968;21:377.