Chapter 70 Spinal injuries
There are few injuries that have a more devastating impact on both the patient and their family than spinal cord injuries (SCI). The physical, psychological and functional sequelae of permanent disability are immense. In addition, the economic cost to the individual and to society, with the loss of productivity and costs of hospitalisation, rehabilitation and ongoing care, are enormous.
AETIOLOGY
The incidence of SCI in developed countries is 12–53 new cases per million population per year (excluding deaths before reaching hospital).1 There is some variation in the incidence and causes in different countries. About 80% of SCI are male, usually in the 15–35 years age group.
SCI are due mainly to motor car, motor bike and bicycle accidents (50%), falls (15–20%) and sporting injuries (10–25%).1 Alcohol ingestion is frequently an associated factor. Work-related injuries account for 10–25%, and physical violence for 10–20% of SCI, especially from gunshot injury in the USA. Sporting and recreational injuries appear to be increasing (but with fewer now due to diving), and there is an increasing incidence of SCI in the elderly, especially from falls.2 Ischaemic SCI is occasionally due to aortic injury or cross clamping. Pre-existing spinal pathology predisposes to SCI, including osteoarthritis, spinal canal stenosis, ankylosing spondylitis, rheumatoid arthritis and congenital abnormalities.
Fifty-five per cent of SCI are cervical (most at the C4–6 levels), with thoracic, thoracolumbar and lumbosacral injuries each being 15% of SCI. Forty-five per cent of SCI are complete and 55% incomplete. Between 20 and 60% of patients with SCI have significant associated injuries such as head and chest injuries.1
PATHOGENESIS
SPINAL INJURY
CERVICAL SPINE
Injury to the cervical spine has been classified several ways3–5 relating to the mechanism of injury using the two-column concept. Differences appear to be due to the fact that compression of the anterior column is associated with distraction of the posterior column, and vice-versa. Injuries may be grouped according to the predominant mechanism of injury (Table 70.1) with these mechanisms having characteristic radiological patterns.
SPINAL CORD INJURY
Trauma to the spinal cord results in immediate primary and delayed secondary injury processes.
SECONDARY INJURY
An understanding of secondary injury mechanisms has come from experimental SCI in animals.1,7 Local hypoperfusion and ischaemia begin at the site of injury, extending progressively over hours from the site of injury in both directions. There is loss of spinal cord autoregulation, complicated by arterial hypotension with high SCI. Apart from ischaemia, other mechanisms may contribute to the secondary injury. These include the release of free radicals, eicosanoids, calcium, proteases, phospholipases and excitotoxic neurotransmitters (e.g. glutamate).
Petechial haemorrhages begin in the grey matter, progress over hours and may result in significant haemorrhage into the cord. There is oedema, cellular chromatolysis and vacuolation, and ultimately neuronal necrosis. Apoptosis, especially of oligodendrocytes, also occurs.1 In the white matter vasogenic oedema, axonal degeneration and demyelination follow. Infiltration of polymorphs occurs in the haemorrhagic areas. Late coagulative necrosis and cavitation subsequently take place.
CLINICAL PRESENTATION
Spinal and spinal cord injuries should be suspected after severe trauma or head injuries, if there are motor or sensory symptoms or signs, or the patient reports neck or back pain.
NEUROLOGICAL ASSESSMENT
TERMINOLOGY
The terminology for SCI has now been standardised.8
Table 70.3 American Spinal Injury Association (ASIA) impairment scale
INCOMPLETE SCI
Incomplete SCI tend to occur in characteristic syndromes.10
UNCONSCIOUS OR UNCOOPERATIVE PATIENTS
The neurological examination may be difficult if the patient is unconscious or uncooperative. In this case a number of signs may be helpful in drawing attention to a SCI as in Table 70.4. Confirmation of SCI may be established by MRI or somatosensory-evoked potentials (SSEP).
Table 70.4 Signs of SCI in unconscious or uncooperative patients
|
Paradoxical pattern of breathing with indrawing of upper chest on inspiration (in the absence of upper respiratory tract obstruction or chest injury)
|
ASSOCIATED INJURIES
CERVICAL SPINE INJURY/TETRAPLEGIA
Overall with major trauma the incidence of cervical spine injury (CSI) is 1–3%.11 However, 2–10% of patients with head injuries have a CSI. The more severe the head injury, the more likely is CSI to be present. Patients with head injuries should be assumed to have a CSI until proved otherwise. Of patients with a cervical SCI, 25% have some degree of head injury, with 2–3% having a severe injury.1,12
THORACOLUMBAR SPINE INJURY/PARAPLEGIA
Chest injuries are often present and are difficult to assess with the inability to take an erect chest X-ray, and the presence of a mediastinal haematoma associated with the vertebral injury. Contrast-enhanced helical CT of the chest may be used to assess other chest injuries, and to exclude an aortic injury,13 although aortography may be preferred for this purpose.
Abdominal injuries are difficult to diagnose if there is a SCI. However, they should be suspected if there is excessive hypotension, referred shoulder pain, or free gas or fluid seen on X-ray of the spine or chest. Abdominal CT, diagnostic peritoneal lavage or ultrasound may be used to delineate these injuries.14
IMAGING
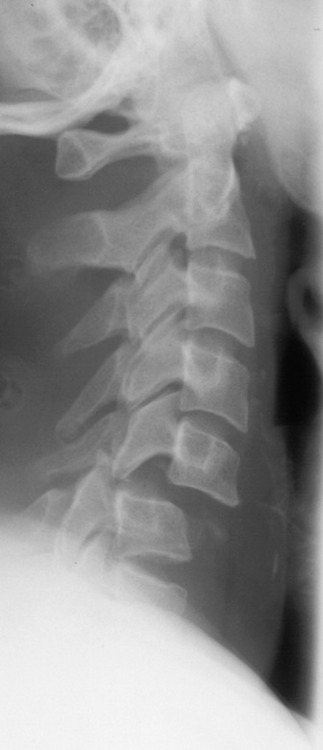
PLAIN X-RAYS (Figure 70.1)
Plain X-rays remain the primary screening method for suspected spinal injuries in symptomatic patients, except in unconscious or multitrauma patients. However, the absence of a detectable abnormality does not exclude a spinal injury. Lesions may not be seen if the views are inadequate or of poor technical quality, or the observer is inexperienced. Even without these limitations, plain X-rays of the neck frequently miss fractures and subluxations,15,16 and a high index of suspicion should be maintained. Adequate views of the entire cervical spine from the base of the skull down to and including the C7–T1 junction should always be obtained. Abnormalities of the prevertebral soft tissues are often present and indicative of subtle injuries.4
With the cervical spine the three-view trauma series is standard in most hospitals (Table 70.5). This consists of lateral, anteroposterior (AP) and odontoid (open mouth) views. A five-view series with supine oblique views has been recommended as improving the diagnostic yield,17 but other studies show no difference in the detection rate. Lateral and AP views are standard screening views for the thoracolumbar spine.
Table 70.5 Basic examination of plain X-rays of cervical spine
| Must include occipital condyles to C7–T1 junction | |
| Lateral is the most important view and will show most injuries | |
| Check for: | |
COMPUTED TOMOGRAPHY (CT) SCANNING
The sensitivity of CT scanning is much greater than plain X-rays in the detection of spinal injuries,15 especially with the newer multidetector CT machines. CT scanning now has an essential role in the detection and evaluation of spinal injuries, as well as the exclusion of injuries with spinal clearance.18
Indications for CT scanning have been regarded as:
However, CT scanning should now be undertaken in all suspected or proven spinal injuries. If patients are unconscious or have suffered severe or multiple trauma, CT scanning should be undertaken as the first line imaging, without screening plain X-rays, for the evaluation of cervical and thoracolumbar injuries.2,19 Sagittal and coronal reconstructions should be routinely obtained. CT scanning of the spine should be coordinated with CT of other regions if needed. CT myelography has now been replaced by MRI, but may be indicated if MRI is not available or is contraindicated.
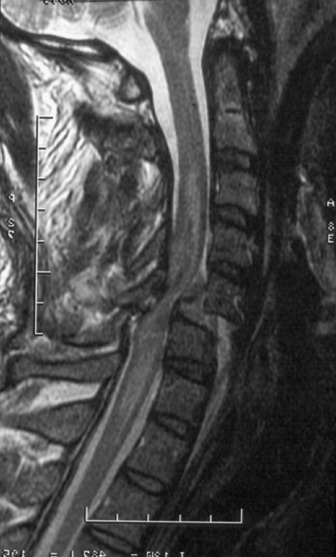
MAGNETIC RESONANCE IMAGING (MRI) (Figure 70.2)
MRI allows visualisation of soft tissues including ligaments, intervertebral discs and the spinal cord itself where it may show the site, extent and nature of the SCI.4 However, it involves a prolonged study with physical isolation of the patient in a difficult environment. Nevertheless, it provides important information in evaluation of the neurological deficit, assessment of prognosis and in the planning of surgical management of SCI. MRI is urgent if there is an unexplained neurological deficit, discordance between the skeletal and neurological levels of injury or worsening neurological status.
CLEARANCE OF THE CERVICAL SPINE
Although familiarity with common injuries is desirable, in view of the severe consequences of a missed spinal injury, clearance of the spine must only be undertaken by a suitably experienced specialist after adequate imaging. Neurological deterioration with missed spinal injuries still occurs, usually as a result of insufficient imaging.20
The Canadian C-spine rule21,22 now provides guidelines for the need for imaging in alert, stable trauma patients in an emergency department setting, but this would not frequently apply to patients in the intensive care unit (ICU). Clearance of the cervical spine in unconscious ICU patients remains controversial with no standardised approach.23 The best approach would seem to be fine-cut helical multidetector CT scanning with reconstructions,18 reserving the further use of MRI for selected cases such as those with a high-velocity mechanism of injury or high injury severity score, rather than using flexion/extension imaging to detect unstable ligamentous injuries.16,24,25
Early clearance of CSI is desirable to allow early removal of the cervical collar with its associated problems of pressure sores, difficult access for airway management and insertion of central venous lines, and increased intracranial pressure with head injuries.26
MANAGEMENT
PREHOSPITAL MANAGEMENT
IMMOBILISATION
If a CSI is suspected, the patient may have to be extricated from the scene of the accident. Manual stabilisation of the head in a neutral position is used with four people lifting and one controlling the head and neck. The neck is immobilised in a rigid, hard collar that should be applied without neck movement. The patient is placed supine on a rigid spine board with straps and occipital padding.26 Sandbags should be placed on either side of the head, and the head secured with the application of broad tape across the forehead. With a suspected thoracolumbar spinal injury (TLSI) the patient is again lifted as a single unit or log rolled as necessary.
EMERGENCY MANAGEMENT
The initial management of spinal injuries follows the usual trauma triage principles with a primary survey, resuscitation and a careful secondary survey. The measures previously mentioned for establishing an adequate airway, and for providing adequate ventilation and circulation should be continued to minimise any further spinal cord insult.
After stabilisation, definitive X-rays and CT are undertaken whilst maintaining spinal immobilisation. A cervical collar does not provide rigid immobilisation and manual stabilisation is still necessary with patient transfers.27 The prevention of hypothermia is a continuing issue in both the emergency and radiology departments. Morphine analgesia is likely to be required for pain at the spinal fracture site and/or other injury.
With SCI, a nasogastric tube should be inserted to prevent aspiration of gastric content and bowel distension from the associated ileus. A urinary catheter should also be inserted to monitor the urine output and prevent overdistension of the bladder. Attention to the skin, taking care to prevent pressure areas, is important. If high-dose corticosteroids are to be used they should be started within 8 hours from the time of SCI (see pharmacological treatment). If stable, early transport to a definitive care facility such as a multidisciplinary SCI referral centre is recommended. This approach has been shown to result in fewer complications and a reduced hospital length of stay.2 Initially, patients (especially tetraplegics) should be admitted to the ICU for the detection and management of respiratory and cardiovascular problems.28
HOSPITAL/ICU MANAGEMENT
RESPIRATORY
Respiratory dysfunction
Respiratory dysfunction is a major cause of morbidity after SCI, especially in tetraplegics.29,30
Tetraplegia
The diaphragm is innervated by the C3–5 segments. With a C5 SCI the diaphragm is intact but about 50% still need short-term mechanical ventilation (MV). With a C4 SCI there is partial loss of diaphragmatic innervation, and nearly all need short-term MV. With a C3 SCI most of the diaphragmatic innervation is lost, and all need initial MV, with about 50% requiring permanent MV.29 Scalene muscle weakness and intercostal muscle paralysis lead to a paradoxical pattern of breathing, and abdominal muscle paralysis produces an inability to cough.
The vital capacity (VC) is markedly reduced and is usually 1.0–1.5 l on presentation. In C5–6 complete SCI the VC is decreased to 31% of predicted during the first week, and with C4 lesions to 24%.31 It often undergoes a further decrease due to atelectasis, and sometimes from ascent of the neurological level. However, there is usually a significant increase in VC by 3–5 weeks after injury, reaching values of 44–51% of predicted by 3 months.31 This is thought to be due to stiffening of the ligaments and joints of the rib cage, and the return of intercostal muscle tone preventing indrawing of the chest wall by the diaphragm. The VC and tidal volume are dependent on posture and surprisingly are greatest when supine due to the more cephalad position of the diaphragm.30,32 Hypoxaemia is very common in the early phase.
Respiratory complications
Endotracheal intubation with CSI
The options are awake fibreoptic nasotracheal intubation or orotracheal intubation under general anaesthesia. No particular approach has been shown to be superior, and either is acceptable depending on individual circumstances. Factors determining the choice include the urgency of the situation, the skill and training of medical staff, the availability of equipment, the conscious state of the patient and the associated injuries.11
Awake nasotracheal intubation with topical anaesthesia allows continuous neurological assessment, but should only be attempted with an alert cooperative patient. It is contraindicated if urgent intubation is required, or if there is coagulopathy, nasal obstruction or basal skull fracture. Blind intubation often requires multiple attempts, and has a significant complication and failure rate. Fibreoptic intubation is a more acceptable technique for those with appropriate training, although desaturation during the procedure is common.
Orotracheal intubation under anaesthesia is a safe technique, despite the greater potential for neck movement, and there is no evidence to suggest that neurological outcome is worse.11,33 Preparation should be made for a rapid sequence induction and a difficult intubation. The anterior part of the collar is removed to allow adequate mouth opening, manual stabilisation of the head is performed without traction, and cricoid pressure is applied. Intravenous atropine is given, followed by the induction agent and muscle relaxant. Suxamethonium should be avoided between 10 days and 7 months after SCI to prevent severe hyperkalaemia.34 Rocuronium is then the preferred relaxant.
Weaning from MV
With high tetraplegics weaning can be very prolonged, difficult and is frequently complicated by recurrent atelectasis. Weaning is usually started after resolution of any pulmonary pathology, in the supine position and having a VC of at least 10 ml/kg. No method of weaning has been shown to be superior. Weaning is most conveniently begun by conversion to pressure support ventilation, ideally with flow triggering. Incremental reduction in the pressure support level is carried out as tolerated, before conversion to a T-piece or tracheostomy shield. Alternatively, T-piece trials may be used from the outset, but need much more psychological support to prevent patient anxiety and loss of confidence. Nearly all C4 or lower tetraplegics, and about 50% of C3 tetraplegics can be weaned from MV given time.29 When weaned, patients may be discharged from the ICU, but tracheostomy decannulation should be delayed until it is clear that the patient is coping off MV, atelectasis does not recur, sputum production is minimal and aspiration is not occurring.
C1–3 tetraplegics who cannot be weaned are usually managed with permanent MV via a home ventilator. Some of these patients may be suitable for phrenic nerve pacing.35 Other tetraplegics with very limited ventilatory reserve are likely to require nocturnal ventilatory support.
CARDIOVASCULAR
Cardiovascular management
Monitoring should include ECG, arterial blood pressure (BP), subclavian or femoral central venous pressure (CVP) and urine output. Some plasma volume expansion is necessary to correct a degree of relative hypovolaemia. Previously mean arterial pressures (MAP) of 60–70 mmHg have been tolerated if the urine output was adequate (> 0.5 ml/kg per hour). However, animal evidence strongly suggests that hypotension after SCI contributes to secondary ischaemic injury of the cord. Clinical studies of BP elevation suggest improved neurological outcomes without adverse effects.36 Consensus guidelines now recommend prompt correction of hypotension, and maintenance of MAP at 85–90 mmHg with vasopressors for the first 7 days after SCI.37
Cardiovascular complications
Venous thromboembolism
Venous thromboembolism has a 40% incidence without prophylaxis, with pulmonary embolism accounting for up to 15% of deaths in the first year after SCI. Consensus guidelines38 for prevention recommend intermittent pneumatic compression or electrical calf muscle stimulation, plus enoxaparin 30 mg twice daily or adjusted dose unfractionated heparin (to a high normal activated partial thromboplastin time) starting within 72 hours and continued for 8–12 weeks depending on risk factors. If high risk and anticoagulation fails or is contraindicated, an inferior vena caval filter should be inserted.
Bradycardia, asystole
Due to disruption of sympathetic pathways and unopposed vagal activity these problems are extremely common after high SCI. With severe cervical SCI the incidence of persistent bradycardia was 100% and transient marked bradycardia (heart rate < 45 per min) 71%, with cardiac arrest occurring in 16% of patients. These arrhythmias peaked in the first week and resolved 2–6 weeks after injury.39 Tracheal suction in the presence of hypoxia appears to be a cause of cardiac arrest preventable with atropine.
Autonomic hyperreflexia
This is not a problem in the early phase. It begins within 6 months of SCI after recovery from spinal shock, and is usually only significant with SCI above the T6 level.40 In response to an afferent stimulus below the neurological level there is excessive paroxysmal autonomic reflex activity. The stimulus is often bladder or bowel distension, but may be cutaneous stimulation or surgery. The massive sympathetic efferent response causes intense vasoconstriction that produces severe hypertension, with the risk of seizures and cerebral haemorrhage. Baroreceptor-mediated bradycardia may occur with reflex vasodilatation above the neurological level in paraplegics. Treatment consists of preventing or removing the stimulus, placing the patient in an upright posture and, if necessary, short-acting i.v. antihypertensive drugs such as nitroprusside.
NEUROLOGICAL
Drug treatment strategies are aimed to reduce secondary injury mechanisms, and to allow recovery of damaged neurones. Encouraging results have been achieved with animal experiments but only minimal improvement in human SCI using corticosteroids. In the NASCIS 2 trial,41 methylprednisolone – if started within 8 hours of SCI (30 mg/kg bolus followed by 5.4 mg/kg per hour for 23 hours) – resulted in mildly improved motor and sensory scores at 6 months and 1 year. A further trial, NASCIS 3,42 showed some improvement in motor score and functional outcome if methylprednisolone started within 3–8 hours of SCI was given for 48 hours rather than 24 hours. However, this was associated with more sepsis and pneumonia. The administration of methylprednisolone has been widely, but not universally, accepted. Criticism of these studies has followed from reanalysis of the NASCIS data43 and systematic review of all published studies.44 An attempt to set guidelines45 concluded that methylprednisolone for 24 or 48 hours was only an option for treatment of acute SCI, and that it should only be undertaken with the knowledge that evidence suggesting harmful side-effects was more consistent than suggestion of clinical benefit. No additional evidence has been forthcoming and the controversy remains.46,47
In human studies, GM-1 ganglioside, tirilazad, naloxone and nimodipine have shown no benefit.
SKELETAL
Conservative measures for CSI include closed reduction, traction in head tongs or a halo, collars or the application of a halo-thoracic brace. For TLSI postural realignment may be used. However, some of these techniques require prolonged periods of immobilisation in bed. Mechanical turning beds facilitate this approach. Some believe that most spinal injuries, and especially TLSI, should be treated without surgery.27
Surgical techniques utilise open reduction and internal fixation to provide sufficient stability to allow early mobilisation. External supports such as a collar or brace may be added. Surgery is now utilised more frequently with the aim of achieving early stability and pain control, earlier mobilisation and rehabilitation, less muscle wasting, fewer hospital complications and earlier time for hospital discharge. If surgery is undertaken it is probably best performed at the earliest opportunity that other injuries and complications allow. In a multicentre study in North America, surgery was performed on 65% of acute SCI with little agreement as to timing.48 Although claims have been made for fewer complications with earlier mobilisation and hospital discharge, there is no clear evidence in favour of surgical management.
With regard to the SCI itself, animal studies have consistently shown improved neurological outcomes with early surgical decompression of the spinal cord.49 A recent systematic review of human studies concluded that early (< 24 hours) decompression produced better outcomes than delayed decompression or conservative treatment, especially for incomplete SCI with neurological deterioration.50 However, there is no conclusive evidence of benefit of early decompression of the spinal cord in clinical practice, and this aspect also remains controversial.49
GASTROINTESTINAL
A range of GI problems are associated with SCI. Paralytic ileus and acute gastric dilatation are frequent problems in the first few days. A nasogastric tube should be inserted immediately to protect against aspiration and respiratory impairment from abdominal distension. It may then be used for early enteral nutrition once these problems have resolved. Stress ulceration with GI bleeding has an incidence of 3–5%.41 H2 antagonists or proton pump inhibitors should be used for prevention. Acute acalculous cholecystitis, pancreatitis and the superior mesenteric artery syndrome are uncommon. Constipation and faecal impaction readily develop after a number of days. A preventative bowel regimen consisting of faecal softeners, suppositories and laxatives should be started early.
URINARY
After SCI there is paralysis of the bladder detrusor and urinary retention with the risk of bladder overdistension. A urethral catheter should be inserted early and placed on continuous drainage. Bladder training techniques with intermittent catheterisation are delayed until the patientis stable and has been discharged from the ICU. Bacterial colonisation of the urine is accepted while catheterised.
METABOLIC
Hyponatraemia has been reported to be common after complete and severe incomplete SCI.51 Immobility after SCI leads to calcium mobilisation from bone, hypercalciuria and occasionally hypercalcaemia. Atrophy of denervated muscle, muscle wasting, weight loss and negative nitrogen balance are marked. Enteral nutrition should meet caloric needs (preferably estimated by indirect calorimetry) but nitrogen loss has to be accepted.52
PAIN
Pain is common after SCI. Pain may result from vertebral fractures or the gut. Neuropathic pain may occur at, or below the level of SCI. Intravenous opioids, ketamine, lidocaine and gabapentin are effective for neuropathic pain.53
OUTCOME
NEUROLOGICAL RECOVERY
The prognosis for neurological recovery is best judged by neurological examination 72 hours post SCI. Most complete tetraplegics regain one motor level and do not recover functional lower limb movement. Incomplete SCI have much more recovery, and more than 50% of incomplete tetraplegics become ambulatory.54,55 Significant spinal cord haemorrhage seen on MRI indicates a very low probability of motor recovery, but if < 4 mm the prognosis is better.56
SURVIVAL
Hospital survival after SCI is now more than 90%, with increasing life expectancy in the longer term. Long-term survival studies have shown a higher mortality risk with a higher neurological level, complete SCI, older age and earlier year of injury.57 Most deaths are now due to respiratory and heart disease, with a decreasing proportion due to urinary complications.
1 Sekhon LH, Fehlings MG. Epidemiology, demographics and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2-S12.
2 Fisher CG, Noonan VK, Dvorak MF. Changing face of spine trauma care in North America. Spine. 2006;31:S2-S8.
3 Holdsworth F. Fractures, dislocations and fracture-dislocations of the spine. J Bone Jt Surg. 1970;52A:1534-1551.
4 Harris JH, Mirvis SE. The Radiology of Acute Cervical Spine Trauma, 3rd edn., Baltimore: Williams and Wilkins; 1996:213-244.
5 Allen BL, Ferguson RL, Lehmann TR, et al. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine. 1982;7:1-27.
6 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
7 Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
8 Maynard FM, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35:266-274.
9 Waters RL, Adkins RH, Yakura JS. Definition of complete spinal cord injury. Paraplegia. 1991;29:573-581.
10 Tator CH. Classification of spinal cord injury based on neurological presentation. In: Narayan RK, Wilberger JE, Povlishock JT, editors. Neurotrauma. New York: McGraw-Hill; 1996:1059-1073.
11 Crosby ET. Airway management in adults after cervical spine trauma. Anesthesiology. 2006;104:1293-1318.
12 Holly LT, Kelly DF, Counelis GJ, et al. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors and injury characteristics. J Neurosurg. 2002;96(3 Suppl):285-291.
13 Lang-Lazdunski L, Pons F, Jancovici R. Update on the emergency management of chest trauma. Curr Opin Crit Care. 1999;5:488-499.
14 Schuster-Bruce M, Nolan J. Priorities in the management of blunt abdominal trauma. Curr Opin Crit Care. 1999;5:500-510.
15 Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34:32-39.
16 Cooper DJ, Ackland HM. Clearing the cervical spine in unconscious head injured patients – the evidence. Crit Care Resusc. 2005;7:181-184.
17 Turetsky DB, Vines FS, Clayman DA, et al. Technique and use of supine oblique views in acute cervical spine trauma. Ann Emerg Med. 1993;22:685-689.
18 Brown CV, Antevil JL, Sise MJ, et al. Spiral computed tomography for the diagnosis of cervical, thoracic and lumbar spine fractures: its time has come. J Trauma. 2005;58:890-895.
19 Tins BJ, Cassar-Pullicino VN. Imaging of acute cervical spinal injuries: review and outlook. Clin Radiol. 2004;59:865-880.
20 Levi AD, Hurlbert RJ, Anderson P, et al. Neurological deterioration secondary to unrecognized spinal instability following trauma – a multicentre study. Spine. 2006;31:451-458.
21 Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286:1841-1848.
22 Stiell IG, Clement CM, McKnight RD, et al. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Eng J Med. 2003;349:2510-2518.
23 Lien D, Jacques T, Powell K. Cervical spine clearance in Australian intensive care units. Crit Care Resusc. 2003;5:91-96.
24 Ackland HM, Cooper DJ, Malham GM, et al. Magnetic resonance imaging for clearing the cervical spine in unconscious intensive care trauma patients. J Trauma. 2006;60:668-673.
25 Hogan GJ, Mirvis SE, Shanmuganathan K, et al. Exclusion of unstable cervical spine injury in obtunded patients with blunt trauma: is MR imaging needed when multi-detector row CT findings are normal? Radiology. 2005;237:106-113.
26 Hadley MN. Cervical spine immobilization before admission to hospital. Neurosurgery. 2002;50(Suppl):S7-S15.
27 Rechtine GR. Nonoperative management and treatment of spinal injuries. Spine. 2006;31:S22-S27.
28 Hadley MN. Management of acute spinal cord injuries in an intensive care unit or other monitored setting. Neurosurgery. 2002;50(Suppl):S51-S57.
29 Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. Chest. 1990;97:1446-1452.
30 Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803-814.
31 Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124:41-44.
32 Baydur A, Adkins RH, Milic-Emili J. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001;90:405-411.
33 Shatney CH, Brunner RD, Nguyen TQ. The safety of orotracheal intubation in patients with unstable cervical spine fracture or high spinal cord injury. Am J Surg. 1995;170:676-680.
34 Yentis SM. Suxamethonium and hyperkalaemia. Anaesth Intensive Care. 1990;18:92-101.
35 DiMarco AF. Restoration of respiratory muscle function following spinal cord injury. Review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147:273-287.
36 Vale FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239-246.
37 Hadley MN. Blood pressure management after acute spinal cord injury. Neurosurgery. 2002;50(Suppl):S58-S62.
38 Hadley MN. Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosurgery. 2002;50(Suppl):S73-S80.
39 Lehmann KG, Lane JG, Piepmeier JM, et al. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol. 1987;10:46-52.
40 Colachis SC. Autonomic hyperreflexia with spinal cord injury. J Am Paraplegia Soc. 1992;15:171-186.
41 Bracken MB, Shepard MJ, Collins WF, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. N Eng J Med. 1990;322:1405-1411.
42 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. JAMA. 1997;277:1597-1604.
43 Hurlbert JR. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(1 Suppl):1-7.
44 Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury – a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
45 Hadley MN. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50(Suppl):S63-S72.
46 Baptiste DC, Fehlings MG. Pharmacological approaches to repair of the injured spinal cord. J Neurotrauma. 2006;23:318-334.
47 Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-343.
48 Tator CH, Fehlings MG, Thorpe K, et al. Current use and timing of spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J Neurosurg. 1999;91(1 Suppl):12-18.
49 Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28-S35.
50 La Rosa G, Conti A, Cardali S, et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using ameta-analytical approach. Spinal Cord. 2004;42:503-512.
51 Peruzzi WT, Shapiro BA, Meyer PR, et al. Hyponatremia in acute spinal cord injury. Crit Care Med. 1994;22:252-258.
52 Hadley MN. Nutritional support after spinal cord injury. Neurosurgery. 2002;50(Suppl):S81-S84.
53 Acute spinal cord injury. Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute Pain Management: Scientific Evidence, 2nd edn. 2005, 148-150.
54 Kirshblum SC, O’Connor KC. Levels of spinal cord injury and predictors of neurological recovery. Phys Med Rehabil Clin N Am. 2000;11:1-27.
55 Burns AS, Ditunno JF. Establishing prognosis and maximizing functional outcomes after spinal cord injury. Spine. 2001;26:S137-S145.
56 Boldin C, Raith J, Fankhauser F, et al. Predicting neurological recovery in cervical spinal cord injury with postoperative MR imaging. Spine. 2006;31:554-559.
57 Frankel HL, Coll JR, Charlifue SW, et al. Long term survival in spinal cord injury: a fifty year study. Spinal Cord. 1998;36:266-274.