Chapter 145 Spinal Infections
Vertebral Osteomyelitis and Spinal Epidural Abscess
Epidemiology of Spinal Infection
During recent decades, there has been a steady rise in the incidence of VO and SEA, traditionally felt to be relatively infrequent infections of the vertebral column. Although older series reported rates of VO and SEA of 0.2 to 2 per 10,000 hospitalizations, researchers in more recent series in both Europe and the United States have reported a 10- to 15-fold increase in the incidence of VO and SEA at large tertiary care centers.1–6
Additionally, though older series reported a biphasic distribution in the age-related incidence of VO and SEA, with incidence peaking in the first and fifth to sixth decades of life, newer series have shown a rising incidence of these infections in adult patients with impaired host defenses, frequent bacteremia, or both.7–10 In particular, in recent population-based reports, patients with AIDS and intravenous drug users have been shown to have exceedingly high relative rates of VO or SEA.11–13 Patients with diabetes, liver cirrhosis, renal disease on dialysis, and patients on chronic immunosuppression have all shown a proclivity toward the development of spinal infections.14 The widespread use of MRI technology, coupled with the greater concentration of these patients at tertiary care centers, may account for at least a portion of the greater relative incidence of these spinal infections documented in recent years.
Risk Factors for Vertebral Osteomyelitis and Spinal Epidural Abscess
Several risk factors have been associated with osteomyelitis and SEAs. Many of these risk factors are associated with an immunocompromised state and appear to lead to the development of the infection. Diabetes, for example, lowers the immune response and is associated with spinal infections. Studies have shown diabetes to be associated with SEAs in 18% to 54% of cases3 and 31% of VO cases.15 IV drug abuse has also been associated with the development of spinal infections. This may be secondary to the sharing of unsterile needles, leading to systemic infections and endocarditis, which then predispose individuals to spinal infection. There also seems to be an association between alcoholism and spinal infections. This association may have multifactorial causes, including diet and hygiene and increased predisposition to spinal trauma.16 Other associated risk factors that decrease immune system function include malignancy and long-term steroid use.
Since one of the major mechanisms of the development of VO and SEA include hematogenous dissemination of an infection from a remote source, infections and bacteria originating from other parts of the body serve as risk factors for spinal infections. Therefore, spinal infections have been associated with other infections, including bacterial endocarditis, urinary tract infections, skin abscesses, pneumonia, dental infections, and sepsis. In several studies, skin and soft-tissue infections were found to be the most common cause of SEAs via hematogenous spread.11,14 In VO, however, the most common source of infection seems to be from the urinary tract.17 Hematogenous seeding of spinal epidural infections account for approximately 50% of cases.18,19
In addition to hematogenous dissemination, SEAs may develop by direct extension from an area of VO. In such cases, the abscess tends to be located in the ventral epidural space. Several studies have shown this association of SEA with VO, especially in ventrally located abscesses. Infections extending to the spinal epidural space can also directly spread from a psoas muscle abscess. This route of spread is responsible for approximately 10% to 30% of cases.19
Spinal infections may also develop from direct seeding of bacteria via an invasive procedure. Open spinal surgery, including laminectomies, discectomies, and operations involving spinal instrumentation, as well as less invasive procedures, such as lumbar punctures and epidural injections, have been shown to be risk factors for the development of spinal infections. Interestingly, the rate of infection due to less invasive procedures seems to be larger than that due to open spinal surgeries. Researchers in recent studies have found the risk of developing a SEA after epidural catheterization to be from 0.04% to 0.07%.19 Moreover, leaving catheters and drains in place at the time of surgery seems to increase the risk of acquiring a spinal infection.18,19 Trauma to the spine has also been associated with the development of SEA and VO. Trauma may predispose patients to spinal infections because of disruption of anatomic barriers.18
Clinical Presentation
The classic triad of symptoms of SEA is fever, back pain, and neurologic dysfunction. Investigators in some studies, however, have reported that this triad is present in only 10% to 15% of patients at the time of initial presentation.18,19 Important features of the clinical presentation of SEA are that the symptoms progress through several stages and that this progression can occur rapidly. A classic paper by Heusner1 described the stages of clinical SEA presentation:
The course of time through which these stages progress is highly variable. A patient may present with symptoms that have been ongoing from days to weeks.19 An abscess caused by hematogenous spreading tends to have a clinical presentation that progresses more rapidly through these stages as compared to an abscess formed secondarily to local extension. Once neurologic dysfunction is present, however, the average time to complete paralysis is 24 hours and often can occur in even less time. Thus, the onset of neurologic dysfunction should prompt the need for emergent surgical decompression to prevent progression to paralysis.
Localized pain and tenderness over the involved area of the spine is found very commonly as the initial presentation of SEA (71% in one large meta-analysis).11 Oftentimes pain is described as sharp and stabbing19 This pain reflects the level of the spine involved. The pain may occur in a dermatomal distribution in the lower extremities, for example, or a patient may experience pain in the chest wall or abdomen which may mimic other conditions such as pancreatitis.19 Neurologic dysfunction secondary to a SEA may include motor weakness, including sphincter dysfunction and sensory loss. Motor deficits were shown to be present in 54% of cases in one study.20 A large meta-analysis showed paralysis affects approximately 34% of patients with SEA.11
Fever is also a common manifestation of SEA, with an incidence between 66% and 75%.16 Symptoms and signs of meningitis may also be present, including headaches and nuchal rigidity. This may represent a parameningeal reaction. In patients with a chronic infection course, symptoms such as unintended weight loss and malaise can occur. Sometimes the clinical presentation of SEA may be dominated by that of sepsis, making the detection of a SEA more obscure. In children, the symptoms are more likely to be nonspecific, and the child may simply complain of not feeling well.18,21
The clinical manifestations of VO are similar to those of SEA in that localized pain exacerbated by motion and tenderness almost always occurs and is very frequently the initial symptom. Often-times the pain may be associated with paraspinal muscle spasm. In one review, the authors reported a 92% incidence of back pain in VO.17 Radicular pain in VO has been reported with incidences of up to 25-33%.15 Neurologic deficits can occur with VO and are due to either associated SEA or spinal instability. Investigators in one study reported neurologic impairment in 28% of patients with VO.22 Neurologic involvement seems to be more common in cases of VO affecting the cervical or thoracic spine as compared to the lumbar spine.23 Fever can also be present in VO; however, this presentation is not as consistent a finding as back pain and tenderness.
Laboratory Findings
Laboratory findings associated with VO and SEA reflect the underlying inflammatory nature of the disease and are not specific. The erythrocyte sedimentation rate (ESR) is the most consistently elevated laboratory value in spinal infection, with the ESR typically being greater than 40. In one meta-analysis, the average ESR was 77.2,8 ESR has been shown to be greater than 20 in 95% of cases in some studies.3,19 The ESR is also helpful in monitoring the disease process during therapy, with declining values of ESR to be expected with appropriate therapy. The C-reactive protein (CRP), an acute phase reactant, has also been found to be elevated in patients with spinal infections.2,8 Obtaining CRP levels may be useful because CRP levels have been shown to rise more quickly during inflammation and to return to normal more quickly than ESR levels.24 In addition, one study showed CRP values obtained over 2 weeks after surgery for SEAs can provide prognostic information regarding outcomes.20
The peripheral white blood cell count may be elevated in patients with spinal infections, but many times may be normal. An elevation of the peripheral white blood cell count may be more indicative of a systemic infectious process. The average peripheral white blood cell count in one large meta-analysis of SEAs was 15,700/μL.11
In patients with SEA, a cerebrospinal fluid (CSF) sample may show an elevated white blood cell count and protein level, which are indicative of a parameningeal process. In addition, the CSF values may be reflective of concomitant encephalitis or meningitis, which may present along with a spinal infection in a significant proportion of cases.2 However, a lumbar puncture is often inadvisable in the setting of a SEA because of the risk of seeding bacteria into the thecal sac, especially in abscesses in the lumbar region that are located in the dorsal epidural space.
Blood cultures are often positive in the setting of a spinal infection. The organism grown on blood culture may be the same as the causative organism in a SEA or VO, especially if the spinal infection was caused by a hematogenous seeding. Blood cultures have been reported to be positive in approximately 50% of cases in some studies.15 Positive blood cultures can be useful for guiding antibiotic therapy in the setting in which direct cultures from the spinal infection cannot be obtained. Because blood cultures may become negative if obtained after the administration of antibiotics, it is important to obtain blood cultures prior to the initiation of antibiotics if possible.
Radiographic Studies
Narrowing of the intervertebral disc space can be seen in plain films as early as 2 weeks from the onset of symptoms but usually appear at week 6 or 8.15,26–28 As the infection progresses, there is an increase in vertebral body destruction followed, in some cases, by subsequent spinal deformity. Lytic lesions, demineralization, and scalloping of the end plates may occur.25 As the infection resolves and healing takes place, new bone formation and subsequent fusion can occur, but this may not be evident for as long as 1 year.
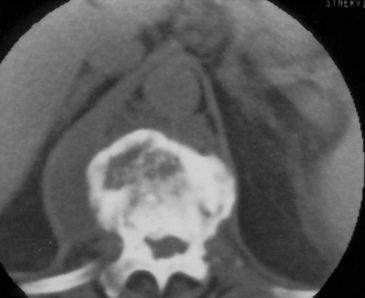
For the above-described reasons, computed tomography (CT) has become an important diagnostic tool in the diagnosis of spinal infections, especially for VO. Among its benefits, CT provides excellent detail of the bony anatomy, and it clearly demonstrates the lytic lesions as well as gas within the disc space or soft tissues (Fig. 145-1). Although identification of SEA has been reported using CT alone, it is generally thought that plain CT is not particularly sensitive for visualizing SEA.29 Indirect findings such as intraspinal gas have been described in the presence of SEA.25
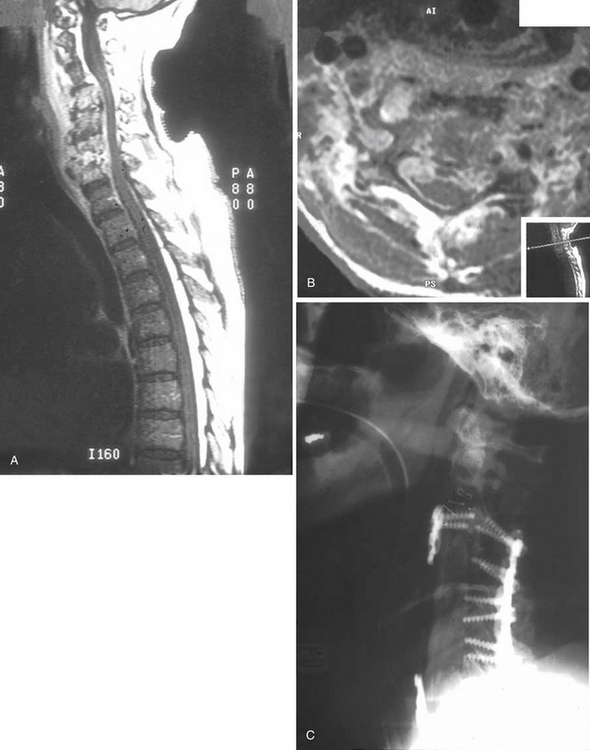
CT is the most valuable tool when performing image-guided percutaneous biopsies of the vertebral body and paravertebral tissues to obtain culture material, and CT is also effective in performing aspiration of paravertebral abscesses.30 Image-guided CT biopsy has been shown to be safe and effective in obtaining diagnostic material at all levels of the spine; however, this technique yields a lower diagnostic rate than the previously reported biopsy of neoplastic vertebral lesions, especially if performed in patients who have undergone previous antibiotic treatment.31 Therefore, it is imperative to perform the biopsy prior to administration of antibiotics to maximize the yield of a positive culture.
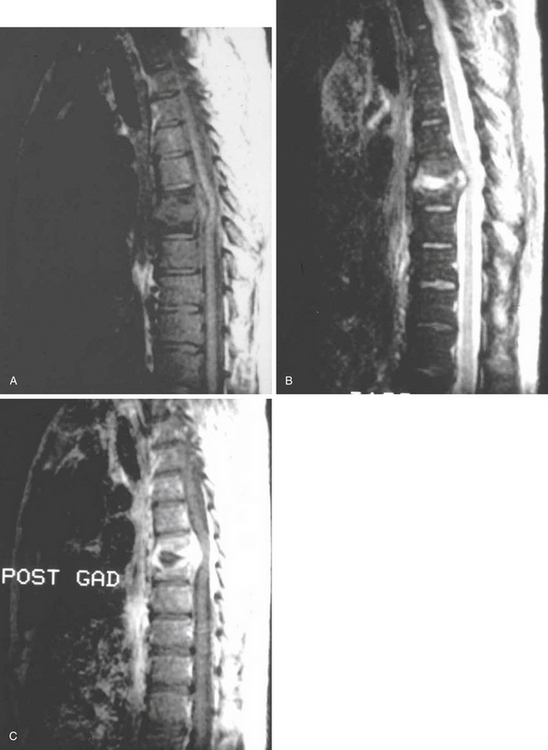
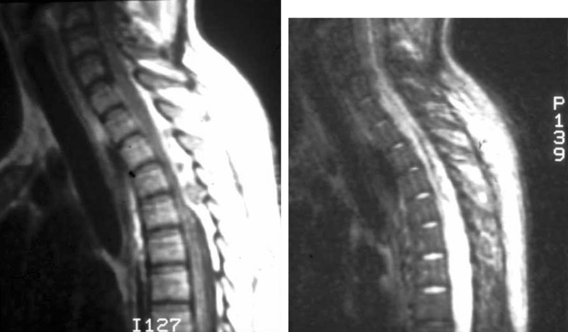
MRI has lately become the single most important imaging modality in the evaluation of infectious and inflammatory diseases of the spine.32–35 The advantages of MRI over other imaging modalities include the ability to perform multiplanar imaging and to directly visualize the paraspinal soft tissues and spinal cord as well as it being a noninvasive procedure. Additionally, MRI abnormalities seen in patients with VO are apparent long before the findings can be detected on plain radiographs. Classic MRI findings of VO described by Modic and colleagues32,33 typically include the following. (1) The clinician can usually see confluent low signals from the vertebral bodies and intervertebral disc space on T1-weighted (T1W1) images, that is, decreased signal intensity in the affected vertebral body with loss of delineation of the end plates from the intervertebral disc. (2) The practitioner can see increased signal intensity from the involved vertebral body and disc space on T2W1 images (Fig. 145-2).
MRI is also the imaging modality of choice for SEA and is helpful in differentiating other conditions, such as spinal myelitis and spinal cord infarction. The following are typical findings. (1) T1W1 imaging is beneficial in detecting a hypo- or isointense epidural mass. (2) T2W1 imaging is useful for visualizing a hyperintense epidural mass, which often enhances with gadolinium. There are usually three patterns of enhancement: (1) dense homogeneous, (2) nonhomogeneous with scattered areas of no uptake, and (3) thin peripheral enhancement.36 On the other hand, an unenhanced MRI scan can cause clinicians to miss some SEAs.37
MRI is very helpful in differentiating an infectious from a neoplastic process. In most of the cases, the paravertebral soft-tissue swelling that accompanies osteomyelitis completely surrounds the spine ventrally. However, neoplastic processes usually have minimal to no soft-tissue reaction, and bony changes produced by tumors may often involve the posterior elements, whereas osteomyelitis seldom compromises the posterior elements unless the patient has had a prior surgical procedure.38 It may also be possible to differentiate pus from granulation tissue with the use of MRI.37 In such cases, more discrete areas of increased signal intensity on T2W1 images are believed to show liquid pus, and administration of gadolinium results in enhancement of chronic granulation tissue, though there is usually little to no enhancement of the abscess cavity itself.
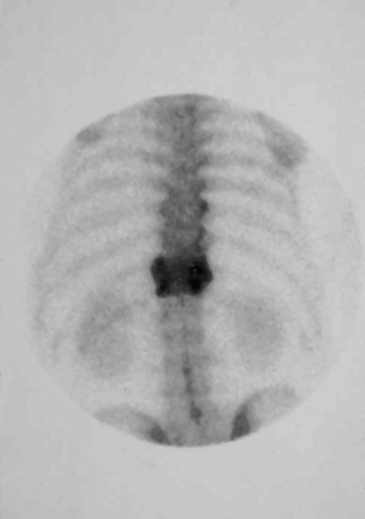
Other diagnostic studies include radionuclide imaging with technetium, gallium-67, and indium-111, which may provide valuable adjunct to diagnosis, particularly for VO, especially before changes on plain radiographs become evident. In cases of VO, technetium scans generally show a diffuse uptake of tracer in the region of infection (Fig. 145-3). Technetium scans have fairly high sensitivity of approximately 90% but specificity of only 78% and accuracy of 86%.32 The probability of a positive study is increased with the duration of symptoms and, in long-standing cases, approaches 100%; however, false-positive increased uptake may be due to degenerative changes, recent surgery, or fracture. False-negative scans also occur, particularly in children and elderly patients.
Management and Treatment
Antibiotic Therapy
Empiric antibiotic therapy should include coverage of those organisms most likely to cause VO and SEA, namely, Gram-positive organisms such as Staphylococcus, as well as Gram-negative organisms such as E. coli and anaerobes.17 Such coverage may include vancomycin, a third-generation cephalosporin, and metronidazole given at bactericidal levels. Given that staphylococci and streptococcal species account for the vast majority of causative agents in VO and SEA, the addition of an aminoglycoside, fluoroquinolone, or rifampin may be considered for their potential synergistic effect, especially in cases of extensive bony involvement, where vancomycin and cephalosporins may have poor penetration into devascularized bone or the disc.26
While there have been no prospective, randomized studies comparing the duration of various antiobiotic therapy regimens for the treatment of VO and SEA, most practitioners treat patients for at least 4 to 6 weeks with intravenous antiobiotic therapy with the goals of sterilizing infected vertebral levels, preventing neurologic decline, and preventing the formation of painful spinal deformities.3 In patients with prominent disease over multiple spinal levels or in immunocompromised patients, therapy extended to 8 weeks has been associated with more effective prevention of relapse of infection.10 Diminished pain, malaise, fever, and reduced ESR levels over the course of antibiotic therapy correlate well with radiographic evidence of resolution of the disease.2,8
Role of Immobilization
Immobilization of the spine in patients with VO has traditionally been employed to achieve one of two goals: to reduce pain and to prevent postinfection spinal deformity. Given that the risk of postinfection spinal deformity is greatest in patients in whom the disease process causes more than a 50% reduction in vertebral body height or in which there is involvement of two or more adjacent vertebral bodies, patients presenting with these pathologies are most often immobilized. Although immobilization has played an important role in the treatment of VO patients with spinal instability, its importance in VO patients without spinal instability has remained less clear. Some authors have employed immobilization for all VO patients until pain is minimal or absent, with the type of orthotic used being dependent upon the region of the spine affected.38
Surgery for Vertebral Osteomyelitis
Many authors believe that uncomplicated cases of VO, especially at early stages of the disease, will respond well to conservative treatment and can be effectively managed with antibiotics and immobilization with good outcomes and minimum morbidity.39 Usually, surgical intervention is reserved for more complicated and advanced cases.39
Major indications for surgery include the following:
• The presence of neurologic deficit due to spinal cord compression
• To obtain a tissue sample for a bacteriologic diagnosis, especially in those cases where closed biopsy is unsafe or when no source of infection has been identified after multiple attempts of adequately performed closed biopsies, negative blood cultures, and where no other source of infection is identified
• In the case of a significant abscess causing either neurologic symptoms or persistent fever and systemic signs of sepsis
• In cases refractory to adequate nonoperative management
• In cases of spinal instability, significant vertebral body destruction and/or spinal deformity27,39
• In cases of spinal instability, significant vertebral body destruction and/or spinal deformity27,39
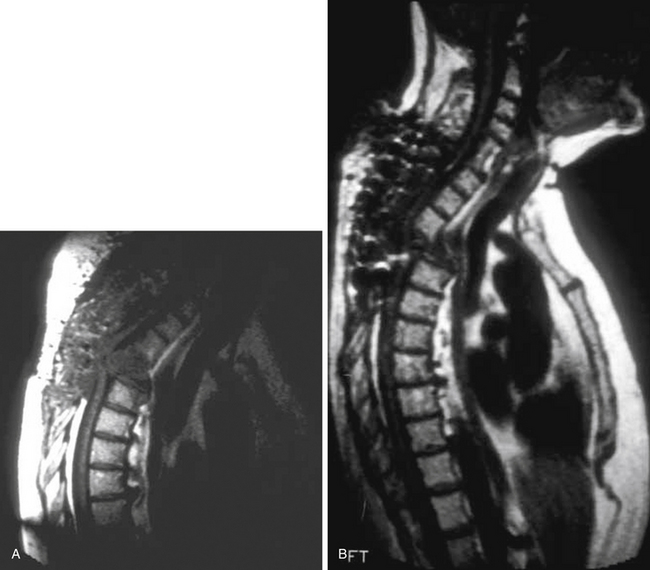
Spinal deformity and instability usually occur in 2% to 28% of patients with VO. Spinal deformity in most cases begins with a loss of anterior column height and progresses to kyphosis. Bony involvement usually includes the anterior and middle columns; posterior column involvement is unusual in the absence of previous surgery. In very advanced cases, severe deformity and even complete dislocation may occur. Therefore, posterior decompressive procedures are usually contraindicated in this condition, unless they are combined with circumferential reconstruction of the spine.28 Indeed, in such instances, an unstabilized laminectomy almost always exacerbates the problem (Fig. 145-4). The goal in such cases is to restore the integrity of the anterior column with a weight-bearing construct.28
In most surgical cases, the involved vertebrae have little viable structure to support the reconstructive strut; therefore, multilevel corpectomies are required to expose end plates that are strong enough to support the construct.28 Anterior column reconstruction can be performed using either an autologous iliac graft26,28 or titanium cages packed with cancellous iliac autograft or allograft.26 If there is absence or loss of posterior element integrity, posterior instrumentation should be added to the anterior construct.26 In cases with posterior element integrity, the cages can be stabilized with a rigid anterior device.
Over the past several years, a large number of authors have reported the successful use of instrumentation in the presence of infection as long as all obviously infected material has been removed39,40 (Fig. 145-5). Instrumentation is usually used in those cases (1) where the infectious process has destroyed bone and the spine has become unstable under physiologic loading and/or (2) when the surgical procedure itself has rendered the spine unstable. In most cases, the recurrence of the infectious process is not related to the implants themselves, but to the compromised general health of the patient.39
Surgery for Spinal Epidural Abscess
• Most importantly, the ability to decompress the spinal cord, thereby preventing further neurologic deterioration
• The removal of devitalized and infected tissue
• The ability to make a bacteriologic diagnosis on the basis of intraoperative cultures taken from the epidural space (This is extremely important in terms of choosing the appropriate antibiotic regimen.)
The type of surgical procedure chosen varies, depending upon different independent situations. As a general rule, the procedure level should be based on the site of maximal compression. For spontaneous abscesses that are located dorsally and not associated with VO, a laminectomy over the involved levels generally provides adequate decompression, and, if treated with appropriate antibiotic therapy, simple laminectomies are usually not followed by instability41 (Fig. 145-6). All purulent material should be removed by using both suction and irrigation. Patients with more chronic abscesses may have a significant amount of granulation tissue that is often adherent to the dura, but it can be carefully dissected in most cases. Once all purulent material has been removed, most practitioners prefer primary closure without a drain to prevent a possible source of infection.
In most cases, abscesses do not extend over more than three or four segments of the spine; therefore, laminectomy can easily be performed over the entire area of involvement. Some patients occasionally develop a SEA that extends over many segments and, in rare situations, involves the entire spinal axis. For these cases, Schulz and colleagues42 have described a minimally invasive technique for decompression of abscesses that extend over a long distance. This technique involves performing a one-segment or two-segment laminectomy at the most rostral and caudal levels of the abscess and then advancing a Fogarty 5-French catheter (Edwards Lifesciences, Irvine, CA) through the epidural space from the caudal laminectomy to the rostral one. The balloon is then inflated with 1 to 1.5 mL of saline and slowly and gently withdrawn. The procedure is repeated until all purulent material has been adequately removed.
CT-guided percutaneous aspiration has also been used as a minimally invasive technique for drainage of SEA.43,44 It is effective only for posterior abscesses that primarily contain pus and is contraindicated for ventral abscesses. The major reported complication associated with this procedure is dural penetration with subsequent contamination of the CSF and development of meningitis.
SEA has usually been considered a surgical disease. Lately, however, there has been an increasing tendency toward more conservative nonoperative treatment.3,11,45 This approach is based mainly on early identification of the pathologic organism and subsequent specific antibiotic therapy. Usually, nonoperative therapy is reserved for the following patients:
• Patients for whom the surgical morbidity is unacceptable high
• Patients who are neurologically intact or have minimal neurologic findings
• Patients with extensive spinal involvement
• Patients who have been paraplegic for more than 48 hours
• Patients with lumbar abscesses and no neurosurgical deficit
Outcomes
The mortality associated with SEAs has declined by about 50% since the 1950s, and current mortality rates range from 15% to 20%.2,5,8,20,27 This decrease is generally attributed to the ability of clinicians to diagnose the condition earlier and to improved antibiotics and surgical capabilities. One study, however, showed that 45% of patients with SEA will have some long-term neurologic dysfunction, and 22% will have severe weakness or paralysis.46 The prognosis for patients with greater neurologic compromise is significantly worse than that of those who do not. Patients who are treated before the onset of neurologic deficit have a significantly better prognosis than those patients whose neurologic deficit has manifested, with the duration of symptoms prior to treatment correlating to the severity of the outcome. Additionally, the time to recovery of neurologic function may be longer than the duration of the symptoms prior to intervention, and, in some cases, patients may not recover fully despite prompt intervention.19 A study by Khanna et al. showed that age and degree of thecal sac compression are independently associated with poor outcomes.46 They proposed a grading scheme to predict outcomes in patients with SEAs, with the criteria of the grading system being age, degree of thecal sac compression, and duration of symptoms.46 A poor outcome was found in 0% of patients classified as grade 0 compared with 87.5% in patients evaluated as grade III.46 The rapidity with which neurologic dysfunction progresses to paralysis highlights the importance of prompt treatment to prevent significant disability or mortality.
The mortality associated with VO appears to be lower than that of SEA. Reviewers who evaluated 309 cases found the mortality rate to be approximately 5% and the rate of permanent neurologic deficit to be 7%.17 Investigators in other studies have quoted mortality rates as high as 11%.47 The outcomes after VO seem to be worse in diabetic patients. Additionally, a longer duration of symptoms before VO diagnosis and neurologic involvement is associated with poorer outcomes.15
Baker A.S., Ojemann R.G., Swartz M.N., Richardson E.P.Jr. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
Broner F.A., Garland D.E., Zigler J.E. Spinal infections in the immunocompromised host. Orthop Clin North Am. 1996;27:37-46.
Chen W., Jiang L., Dai L. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16:1307-1316.
Fischer E.G., Greene C.S.Jr., Winston K.R. Spinal epidural abscess in children. Neurosurgery. 1981;9:257-260.
Hanley E.N., Phillips E.D. Profiles of patients who get spine infections and the type of infections that have a predilection for the spine. Semin Spine Surg. 1990;2:257-267.
Heusner A.P. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845-854.
Hlavin M.L., Kaminski H.J., Ross J.S., Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27:177-184.
Khanna R.K., Malik G.M., Rock J.P., Rosenblum M.L. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery. 1996;39:958-964.
Leys D., Lesoin F., Viaud C., et al. Decreased morbidity from acute bacterial spinal epidural abscesses using computed tomography and nonsurgical treatment in selected patients. Ann Neurol. 1985;17:350-355.
Lu C.H., Chang W.N., Lui C.C., et al. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg. 2002;104:306-310.
McGahan J.P., Dublin A.B. Evaluation of spinal infections by plain radiographs, computed tomography, intrathecal metrizamide, and CT-guided biopsy. Diagn Imaging Clin Med. 1985;54:11-20.
McHenry M., Easley K., Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342-1350.
Osenbach R.K., Hitchon P.W., Menezes A.H. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33:266-275.
Rea G.L., McGregor J.M., Miller C.A., Miner M.E. Surgical treatment of the spontaneous epidural abscess. Surg Neurol. 1992;37:274-279.
Reihsaus E., Waldbaur H., Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175-205.
Rezai A., Woo H., Errico T., Cooper P. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44:1018-1026.
Rigamonti D., Liem L., Sampath P., et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189-197.
Rothman S.L. The diagnosis of infections of the spine by modern imaging techniques. Orthop Clin North Am. 1996;27:15-31.
Sapico F.L., Montgomerie J.Z. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1:754-776.
Schultz K.D.Jr., Comey C.H., Haid R.W.Jr. Pyogenic spinal epidural abscess: a minimally invasive technique for multisegmental decompression. J Spinal Disord. 2001;14:546-549.
Soehle M., Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51:79-87.
Tang H., Lin H., Liu Y., Li C. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76-81.
Waldvogel F.A., Medoff G., Swartz N.M. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med. 1970;282:316-322.
1. Heusner A.P. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845-854.
2. Baker A.S., Ojemann R.G., Swartz M.N., Richardson E.P.Jr. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
3. Rigamonti D., Liem L., Sampath P., et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189-197.
4. Espersen F., Frimodt-Møller N., Thamdrup Rosdahl V., et al. Changing pattern of bone and joint infections due to Staphylococcus aureus: study of cases of bacteremia in Denmark, 1959-1988. Rev Infect Dis. 1991;13:347-358.
5. Hlavin M.L., Kaminski H.J., Ross J.S., Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27:177-184.
6. Jones N.S., Anderson D.J., Stiles P.J. Osteomyelitis in a general hospital: a five-year study showing an increase in subacute osteomyelitis. J Bone Joint Surg Br.. 1987;69:779-783.
7. Digby J.M., Kersley J.B. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br.. 1979;61:47-55.
8. Waldvogel F.A., Medoff G., Swartz N.M. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med. 1970;282:316-322.
9. Broner F.A., Garland D.E., Zigler J.E. Spinal infections in the immunocompromised host. Orthop Clin North Am.. 1996;27:37-46.
10. Jensen A.G., Espersen F., Skinhøj P., Frimodt-Møller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158:509-517.
11. Reihsaus E., Waldbaur H., Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev.. 2000;23:175-205.
12. Adler M.W. ABC of AIDS: development of an epidemic. BMJ. 2001;322:1226-1229.
13. Centers for Disease Control and Prevention (CDC). Trends in injection drug use among persons entering addiction treatment––New Jersey, 1992-1999. JAMA. 2001;285:2706-2707.
14. Hanley E.N., Phillips E.D. Profiles of patients who get spine infections and the type of infections that have a predilection for the spine. Semin Spine Surg. 1990;2:257-267.
15. McHenry M.C., Easley K.A., Locker G.A. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342-1350.
16. Tang H.-J., Lin H.-J., Liu Y.-C., Li C- M. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76-81.
17. Sapico F.L., Montgomerie J.Z. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1:754-776.
18. Grewal S., Hocking G., Wildsmith J.A. Epidural abscesses. Br J Anaesth. 2006;96:292-302.
19. Sendi P., Bregenzer T., Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008;101:1-12.
20. Soehle M., Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51:79-87.
21. Fischer E.G., Greene C.S.Jr., Winston K.R. Spinal epidural abscess in children. Neurosurgery. 1981;9:257-260.
22. Nolla J., Ariza J., Gómez-Vaquero C., et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Sem Arthritis Rheum. 2002;31:271-278.
23. Nather A., David V., Hee H.T., Thambiah J. Pyogenic vertebral osteomyelitis: a review of 14 cases. J Orthop Surg (Hong Kong). 2005;13:240-244.
24. Kushner I.. C-reactive protein and the acute-phase response, Handbook of Neurosurgery, 6th ed New York, NY, Thieme, 2006 , Hosp Pract (Off Ed). 1990;25:13,16,21-28.25. Greenberg MS. Spinal epidural abscess.240-242
26. Rezai A.R., Woo H.H., Errico T.J., Cooper P.R. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44:1018-1026.
27. Cahill D.W., Abshire B.B.. Pyogenic vertebral osteomyelitis, Batjer H.H., Loftus C.M., editors. Textbook of Neurological Surgery: principles and Practice, Vol 4. Philadelphia, PA: Lippincott Williams & Wilkins, 2002. 3249–3247
28. Leys D., Lesoin F., Viaud C., et al. Decreased morbidity from acute bacterial spinal epidural abscesses using computed tomography and nonsurgical treatment in selected patients. Ann Neurol. 1985;17:350-355.
29. Smith A.S., Blaser S.I. MR of infectious and inflammatory diseases of the spine. Crit Rev Diagn Imaging. 1991;32:165-189.
30. McGahan J.P., Dublin A.B. Evaluation of spinal infections by plain radiographs, computed tomography, intrathecal metrizamide, and CT-guided biopsy. Diagn Imaging Clin Med. 1985;54:11-20.
31. de Lucas E.M., González Mandly A., Gutiérrez A., et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. 2009;28:315-320.
32. Bertino R.E., Porter B.A., Stimac G.K., Tepper S.J. Imaging spinal osteomyelitis and epidural abscess with short TI inversion recovery (STIR). AJNR Am J Neuroradiol. 1988;9:563-564.
33. Modic M.T., Feiglin D.H., Piraino D.W., et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
34. Angtuaco E.J., McConnell J.R., Chadduck W.M., Flanigan S. MR imaging of spinal epidural sepsis. AJR Am J Roentgenol. 1987;149:1249-1253.
35. Rothman S.L. The diagnosis of infections of the spine by modern imaging techniques. Orthop Clin North Am.. 1996;27:15-31.
36. Post M.J., Sze G., Quencer R.M., et al. Gadolinium-enhanced MR in spinal infection. J Comput Assist Tomogr. 1990;14:721-729.
37. Sandhu F.S., Dillon W.P. Spinal epidural abscess: evaluation with contrast enhanced MR imaging. AJNR Am J Neuroradiol. 1991;158:1087-1093.
38. Osenbach R.K., Hitchon P.W., Menezes A.H. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33:266-275.
39. Chen W.-H., Jiang L.-S., Dai L- Y. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16:1307-1316.
40. Liljenqvist U., Lerner T., Bullmann V., et al. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12:606-612.
41. Rea G.L., McGregor J.M., Miller C.A., Miner M.E. Surgical treatment of the spontaneous epidural abscess. Surg Neurol. 1992;37:274-279.
42. Schultz K.D.Jr., Comey C.H., Haid R.W.Jr. Pyogenic spinal epidural abscess: a minimally invasive technique for multisegmental decompression. J Spinal Disord. 2001;14:546-549.
43. Tabo E., Ohkuma Y., Kimura S., et al. Successful percutaneous drainage of epidural abscess with epidural needle and catheter. Anesthesiology. 1994;80:1393-1395.
44. Lu C.H., Chang W.N., Lui C.C., et al. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg. 2002;104:306-310.
45. Tang H.J., Lin H.J., Liu Y.C., Li C.M. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76-81.
46. Khanna R.K., Malik G.M., Rock J.P., Rosenblum M.L. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery. 1996;39:958-964.
47. Pigrau C., Almirante B., Flores X., et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118:1287.