Chapter 104 Spinal Dural Vascular Malformations
The most common type of spinal cord arteriovenous malformation (AVM) is the spinal-dural arteriovenous fistula (SDAVF), also known as a type I spinal AVM. First described by Gaupp1 in 1888 as “hemorrhoids of the pia mater,” spinal-dural AVMs have recently become better recognized and understood with the advent of modern superselective neuroangiography. As a distinct subtype of spinal AVMs, these lesions require specific treatments that differ from those for intradural or intraparenchymal vascular malformations. At present, these AVMs are best treated surgically, although endovascular techniques may play an increasing role in the future.
Spinal Vascular Anatomy
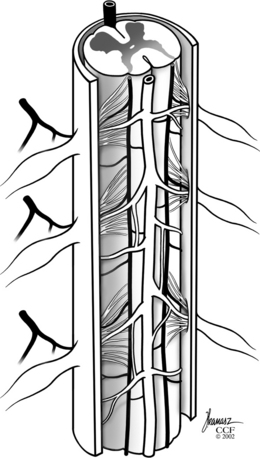
A comprehensive knowledge of the vascular anatomy of the spinal cord is necessary to understand the pathologic and clinical aspects of SDAVFs and their differentiation from other spinal AVMs. The spinal cord receives its blood supply from three separate longitudinal vessels: one anterior spinal artery and two posterior spinal arteries (Fig. 104-1).
The venous drainage of the spinal cord is via small radial veins that run from the center to the periphery of the cord and into the coronal venous plexus that ascends and descends along its dorsal surface. These surface veins converge to form medullary veins that exit at the root sleeve. The coronal veins along the dorsal spinal cord surface become dilated and tortuous in patients with SDAVFs, often forming a convoluted vascular mass along the dorsal aspect of the spinal cord.
Classification
Although this chapter addresses only SDAVFs, the classification system for spinal AVMs should be understood to appreciate the differences between these lesions and other types of AVMs. Recognizing and properly categorizing spinal AVFs, particularly distinguishing between dural and intramedullary lesions, is important for treatment decisions. Historically, spinal-dural AVMs were first referred to as angioma racemosum venosum by Wyburn-Mason2 in his 1943 monograph. This was later shortened to just angioma racemosum by Bergstrand et al.3 and Krayenbuhl et al.4 Malis5 later referred to them as long dorsal AVMs. Currently, dural AVF or type I spinal AVM is the most appropriate term.6,7
Type II spinal AVMs, also known as glomus AVMs, represent intramedullary AVMs with a true compact nidus.2,5 Type III spinal AVMs are also known as juvenile AVMs and are much less common. They are larger, more extensive lesions that often involve intramedullary, extramedullary, and extradural spaces over more than one spinal level.5,8 Last, type IV AVMs are intradural extramedullary AVFs that were first described by Djindjian et al.9 and later classified as type IV lesions by Heros et al.10 Unlike type I dural AVFs that arise from dural branches, these lesions are fed from the anterior spinal artery or, less commonly, from the posterior spinal artery. They flow directly into an enlarged venous outflow tract, lie outside the spinal cord and its pia mater, and vary in size and flow.11
Pathophysiology
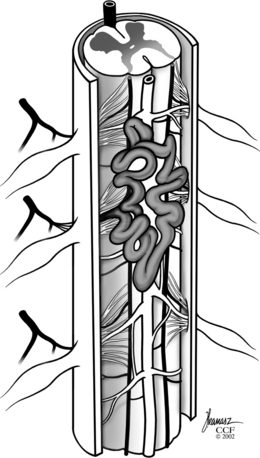
It is easiest to think of SDAVFs as consisting of two relevant compartments: a vascular malformation (AVM) nidus located in the spinal dura and the medullary vein and coronal venous plexus draining the AVM. Usually, a single radiculomedullary artery enters the dural root dorsolaterally at the dural root sleeve. This artery supplies an AVM that is typically embedded within the dura mater around the proximal nerve root sleeve and/or adjacent spinal dura (Fig. 104-2). The venous outflow of the AVM is then via retrograde flow through a medullary vein that has anastomosis with the coronal venous plexus. This medullary vein and coronal venous plexus is obvious on the superselective spinal angiogram. This medullary vein and coronal venous plexus are normal but dilated from the flow through the AVM lying in the dural wall.
Although most SDAVFs have a single arterial feeder, some may have two arterial feeders that enter at separate levels.5,12 The additional feeders appear to travel within the dura mater to the fistula nidus located in the wall of the dura, where they converge and communicate with the intradural efferent medullary vein. No valves are present within the radial veins or coronal plexus and, therefore, the increased pressure is transmitted to the spinal cord parenchyma. It is critical to recognize the additional feeding branches when these are present, because failure to obliterate all inflow channels can lead to recurrence of the AVF.13
Clinical Characteristics
Most patients with type I dural AVFs are between the ages of 40 and 70, with few showing symptoms before age 30. Over 80% of patients are male, and no familial tendency has been identified.14,15 This differs from types II and III spinal AVMs, which typically appear in patients younger than age 40 and have less male predominance. This age discrepancy suggests that type I lesions may be acquired rather than congenital.
The typical pattern of symptoms and clinical course was first described by Aminoff and Logue,16,17 and this description has been supported by other more recent reports.13–1518 The most common symptom associated with dural AVFs is pain, which may be local, radicular, or nonspecific. Most patients also experience leg weakness and sensory changes by the time of diagnosis.14,15 Spastic paraparesis, along with loss of pain and temperature sensation, is the most common neurologic pattern. Most patients have a distinct sensory level corresponding to the level of the vascular nidus. Disturbances of bladder, bowel, and sexual function are less common initially but become more frequent over time.
Most patients experience a gradual onset of symptoms and a slowly progressive clinical deterioration.16,17 Only 10% to 15% of patients experience an acute onset of symptoms, in contrast to patients with types II and III AVMs that lead to an acute onset of symptoms in more than 50% of patients. The progressive neurologic deterioration occurring with these lesions was first documented by Aminoff and Logue.17 At 6 months after onset of symptoms, only 56% of patients had unrestricted activity, and 19% were severely disabled. At 3 years after onset, only 9% had no restrictions, and 50% were severely disabled.
Because of the infrequency and gradual course of SDAVFs, symptoms are often present long before the diagnosis of SDAVF is made. In the series of 55 patients studied by Symon et al., only 33% were diagnosed within 1 year of symptom onset, and 66% were not diagnosed for more than 3 years.15 In fact, given the large amount of edema found on T2-weighted MRI, many patients will have undergone spinal cord biopsy in search of a tumor prior to proper diagnosis. On rare occasions, onset of symptoms can be acute, caused by thrombosis within the draining medullary veins. This produces a catastrophic, acute necrotizing myelitis that is often referred to as Foix-Alajouanine syndrome.19 Subarachnoid hemorrhage (SAH) is extremely uncommon with SDAVMs.14,15 In contrast, other types of spinal AVMs, particularly type II lesions, have a significant incidence of SAH.
Exercise and certain postures can exacerbate symptoms in patients with dural AVMs.14–16 Because almost all dural AVMs have rostrally directed venous outflow, the greater venous hydrostatic pressure in the upright position may explain why symptoms worsen with standing.20 Types II and III AVMs, which have both rostral and caudal venous drainage, do not produce symptoms that change with position. Worsening symptoms have also been associated with physical activity, probably because of increased draining venous pressure during systemic hypertension.21
Radiologic Evaluation
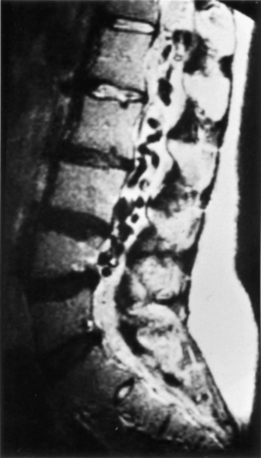
Since the first diagnosis of spinal AVM was made by myelography in 1927,20 most patients have undergone myelography as part of their radiologic evaluation. Although the typical findings of tortuous channels outlined by intrathecal contrast are almost pathognomonic for spinal AVM, in recent years myelography has largely been replaced by MRI as the initial imaging study.22 Irregular, serpentine flow void signals suggest vessels can often be seen along the dorsal surface of the spinal cord (Fig. 104-3). MRI can also differentiate type I from type II and type III lesions, and it is the test of choice for visualizing spinal cord cavernous malformations. Moreover, T2-weighted MRI images often suggest extensive edema of the cord.23,24
The definitive radiologic study for SDAVFs is selective spinal angiography. Aortography may demonstrate the general location of the AVM; however, this large-volume contrast injection may limit the extent of the superselective injections available because of contrast load reasons. Generally, bilateral selective injections of radiculomedullary branches are performed in both anteroposterior and lateral views to demonstrate the precise location, extension, hemodynamic characteristics, and venous drainage of the lesion. Multiple levels above and below the nidus must be studied to identify any additional feeding vessels. It is also essential to visualize the anterior spinal artery above and below the AVM to determine whether it has a supply in common with the AVM. Although this is a rare configuration with dural AVFs, it is a critical factor in planning treatment. Most dural AVFs are located along the dorsal aspect of the spinal cord, although 15% of patients may have dilated veins ventral to the spinal cord, and almost all of these lesions are found in the midthoracic to lower thoracic or thoracolumbar region.5,14,15 This distribution differs from that of other types of spinal AVMs, which occur throughout the length of the spinal cord. If there is high clinical and radiographic suspicion of the presence of an SDAVF, selective angiography is not complete until all possible vessels that may contribute to the spinal vasculature have been imaged. This includes vertebral, external carotid, and sacral arteries. Occasionally, before this can be accomplished, the maximum volume of contrast that the patient’s kidneys can safely tolerate is reached. Therefore, scheduling the spinal angiogram over 2 days’ time enables completion of the examination.
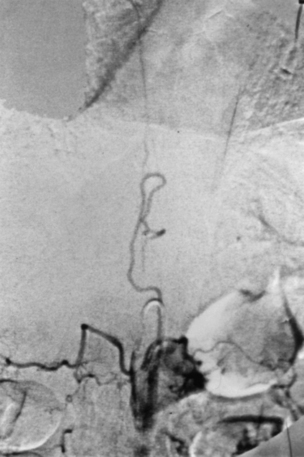
Characteristically, the radiculomedullary feeding vessel is observed to disperse into a cluster of small abnormal vessels within or adjacent to the dura inside the neural foramen (Figs. 104-4 and 104-5). The transition from artery to vein, representing the AVF itself, is usually observed at the medial margin of this cluster. When additional feeders are present, they usually run within the dura to the level of the fistula. Flow is then seen progressively throughout the dilated dorsal venous plexus that typically extends for three to five spinal segments, but occasionally, dilated veins are seen extending over the full length of the cervical, thoracic, and lumbar regions. The blood flow is slow through the intradural veins, and 16 to 20 seconds is often required for contrast to clear.13 Associated arterial or venous aneurysms are extremely uncommon with dural AVFs, in contrast to intramedullary lesions.
Venous drainage from dural AVFs is typically in a rostral direction,14 unlike that from intramedullary AVFs, which drain both rostrally and caudally. It has been suggested that this rostral drainage of dural AVFs, along with their occurrence in the lower spine, is additional evidence for an acquired etiology of dural AVFs.14 The pattern of venous drainage through anatomically normal, but dilated, venous channels, despite increased hydrostatic pressure, supports theories that a diminished, rather than an increased, venous outflow may be associated with the formation of dural AVFs. Congenital malformations would be expected to occur along the entire spinal axis, as seen with intramedullary AVMs.
Endovascular Treatment
Embolization of SDAVFs has been reported, most commonly with cyanoacrylate “glue” or with polyvinyl alcohol (PVA) particles.18,22,25–27 Because the spinal arteries do not participate in the dural fistula, these lesions are potentially well suited for endovascular treatment, with minimal risk to the normal spinal cord.
The goal of endovascular treatment is the same as that for surgery, namely, to interrupt the fistula itself, including the distal feeding vessel and, most importantly, the proximal efferent intradural arterialized vein.28 Because most patients improve after obliteration of the fistula, thus making excision of the venous plexus unnecessary, several authors27,29,30 have recommended embolization as the initial treatment of choice.
The most important factor that determines the feasibility of embolizing a dural AVF is the normal supply to the spinal cord. An anterior spinal artery supplied by the same arterial feeder as the AVF is a relative contraindication to embolization.26,31 Inability to selectively catheterize the radiculomedullary artery because of its size or configuration is another contraindication. The second most important factor that determines the feasibility of embolization is the durability of the embolic agents. Previous reports concerning the use of PVA suggest a high rate of recurrence within only a few months of treatment.1,26 Experience with the use of PVA would indicate that this material is not a permanent embolic agent. Cyanoacrylate glues such as n-butyl cyanoacrylate (NBCA) are likely to be more permanent.
In addition to treatment failure, the complications of endovascular treatment include direct clinical or neurologic deterioration. Neurologic deterioration after embolization is usually due to inadvertent occlusion of feeding arteries to the normal spinal cord because of an unrecognized connection, improper placement or dislodgement of the catheter, improper particle size, or failure to discontinue embolization when the fistula is occluded.26,31,32 With dural AVFs, the greatest risk of deterioration is from occlusion of the venous drainage at a site considerably distal from the fistula.33 This would occlude normal venous drainage of the spinal cord. Distal occlusion can aggravate venous hypertension, impede normal blood flow through the spinal cord, and potentially cause enlargement or rupture of the AVF.
Several large series of patients, in which surgical and endovascular treatments were compared, showed comparable clinical results with the two approaches.22,26,27 Failures included a number of patients in whom the dural AVF could not be successfully obliterated initially, patients in whom interruption of the fistula had failed at a later stage, and one patient who became paraplegic after the cyanoacrylate embolus migrated into the distal veins.22,26,27,34
Late recanalization after initial obliteration with PVA particles is a well-recognized phenomenon.18,22,25,35 Of 17 patients with these lesions reported from the Mayo clinic, 14 underwent embolization with PVA particles or microfibrillary collagen.18 Although initial obliteration of the AVM was accomplished in all but one patient, delayed follow-up angiography demonstrated recanalization in 13 of the 15 patients studied, with the average time for recanalization being only 5 months. Similar results have been reported from the National Institutes of Health in two of three patients25 and by Djindjian et al.,35 who found recanalization in 10 of 12 patients. PVA is not an adequate embolization material for the sole treatment of these lesions and should be used only for preoperative embolization of lesions that will then be treated surgically. Recanalization is less common after embolization with cyanoacrylate glue.34 However, the distal extent of embolization with glue is more difficult to regulate because of its polymerization characteristics, making complications from normal venous obstruction more likely. In addition, microcatheters have been glued in place with the use of cyanoacrylates. Because of the greater risk of complications with glue and the high rate of recanalization with particulate embolization, direct surgical treatment is generally considered preferable in suitable patients.
As newer liquid embolic agents, such as Onyx, with better material properties become available, the role of endovascular treatment of these lesions holds significant promise36,37 for future therapy.
Surgical Treatment
Although Krause38 performed the first surgical exposure of a spinal AVM in 1910, the first successful surgical treatment of an SDAVF, at the T9 sensory level, was reported by Elsberg39 in 1916 in a patient with paraparesis. He ligated and excised a large “vein” that traversed the dura adjacent to the T8 nerve root, and the patient made a complete neurologic recovery. A number of subsequent reports, however, described poor results,3,40,41 and it was not until the advent of modern neuroangiography, which allowed preoperative evaluation of these lesions, that therapy improved.4,42
For many years the standard surgical treatment for dural AVFs included stripping the enlarged venous plexus from the dorsal spinal cord.5,42,43 It subsequently become clear, however, that this extensive resection is unnecessary and, also, potentially dangerous. Obliteration of the AVF alone is sufficient to eliminate the AVM in the dural sleeve. Surgery is much safer without resection of the dorsal veins, because manipulation of the spinal cord is minimized, risk to normal vessels is diminished, and the operation is shortened. In addition to complicating the surgery, resection of the dorsal vessels may injure the spinal cord by interrupting its normal venous drainage because the radial veins have no anastomotic system within the spinal cord parenchyma.13
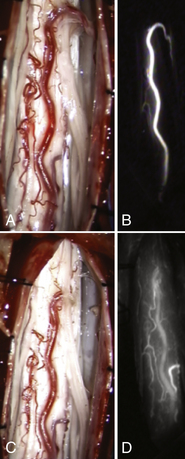
An emerging adjunctive method for delineating intraoperative vascular anatomy is indocyanine green (ICG) angiography. Employing this method, the operating field is illuminated by near-infrared excitation light and ICG is injected intravenously. The intravenous fluorescence is imaged instantly with a video camera integrated into the microscope, allowing differentiation between arterial, capillary, and venous phases (see Figs. 104-5A and B). Recently, its use has been reported in the surgical treatment of cerebral arteriovenous malformations,44 the resection of tumors encasing the extracranial vertebral artery,45 and during surgical obliteration of SDAVF.46–48
After identification, the offending vessel is carefully dissected free from surrounding tissues and the arachnoid. This should be done with sharp dissection with microscissors. It is best to avoid blunt dissection, which can tear small vessels from the radial spinal cord veins and cause bleeding and impaired venous drainage. After the intradural arterialized vein is freed, it is coagulated with bipolar cautery and divided. Alternatively, a temporary aneurysm clip can be applied to the vessel, and observation of the coronal venous plexus for a color change to a more purplish hue may provide the surgeon with reassurance prior to definitive occlusion. At this point, the inner dural surface should be carefully inspected and coagulated. Care should be taken to identify and interrupt any other feeding vessels running in or under the dura from adjacent levels. When the nidus and efferent vein have been obliterated, the large dorsal veins should have decreased turgor and flow. The surgeon should allow several minutes for direct inspection because the venous plexus can remain arterialized for 5 to 10 minutes as a result of the sluggish venous outflow. Microvascular Doppler imaging can be of assistance if insonation is performed before and after venous interruption. ICG angiography can be obtained a second time to verify the eradication of AV shunting at this time (see Figs. 104-5C and D). If the veins do not become blue and soft after 5 to 10 minutes, additional feeders should be sought and interrupted. There is no need to resect or strip the dorsal venous plexus from the dorsal surface of the spinal cord. Attempting this only causes bleeding and interferes with normal venous drainage of the spinal cord and may lead to venous infarction.
All patients undergo postoperative spinal angiography, usually the day after surgery, which includes selective bilateral angiography of the spinal level involved and of two levels above and below the lesion. If residual flow through the AVF is present, reoperation is performed. With this basic approach, good surgical results were achieved in 24 patients with SDAVFs.12 Of the 24 patients, 17 improved, 6 remained unchanged, and 1 worsened slightly.
Similarly, good results after obliteration of the dural AVF alone have been reported by others (Table 104-1). One of the largest series was reported by Symon et al.,15 who operated on 50 of 55 patients with dural AVFs. Through a limited laminectomy, this group identified the communication between the AVM and the dorsal venous plexus. If the nidus was accessible on the dura, it was coagulated or excised. If the nidus was separated from the coronal plexus by several levels, it was left undisturbed, and the intradural arterialized vein was interrupted. Improvement after surgery was related mainly to preoperative disability, with 65% of patients with severe preoperative disabilities and 80% of moderately disabled patients showing improvement. The authors15 stressed that attempts to resect the coronal venous plexus are unnecessary and potentially damaging. Although previously considered a factor, spinal cord compression by these enlarged veins is improbable. Furthermore, because obliteration of the fistula causes collapse of the veins, resection to “decompress” the spinal cord is not a reasonable indication.
Table 104-1 Reported Results of Surgical Series of Fistula Obliteration for Spinal Dural Arteriovenous Fistulas
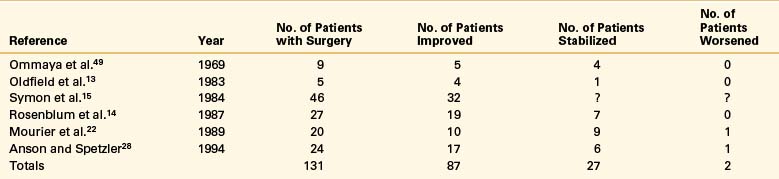
Rosenblum et al.14 reported surgical results in 27 patients with spinal-dural AVMs and 54 patients with intradural AVMs. After surgical obliteration of the AVF, 72% of patients improved and 28% stabilized, in comparison with surgical results in 43 patients treated for intramedullary AVMs, in which 33% improved, 51% remained unchanged, and 14% worsened. Outcome after surgery did not correlate with the presence or degree of preoperative sensory loss or with the rate of neurologic deterioration. There was a direct correlation, however, between preoperative and postoperative motor function.
Similarly, good results were described by Oldfield et al.13 in five patients treated by coagulating and excising the cluster of abnormal vessels at the nidus and by dividing the intradural arterialized vein. In all patients, neurologic function improved progressively within days of surgery.
Summary
Although SDAVFs are rare lesions, it is important to recognize and treat them appropriately. If left untreated, they almost invariably cause progressive neurologic deterioration, with paraparesis, sensory symptoms, and urinary disturbances, as well as pain. Unlike intradural spinal AVMs, these lesions usually appear in men older than age 40, have a gradual onset of symptoms that is often affected by activity, and usually localize to the lower half of the spinal column. Spinal cord dysfunction is produced by venous hypertension and not by compression or vascular steal as once thought.
Aminoff M.J., Logue V. Clinical features of spinal vascular malformation. Brain. 1974;97:197-210.
Aminoff M.J., Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211-218.
Djindjian M., Djindjian R., Rey A., et al. Intradural extramedullary spinal arteriovenous malformations fed by the anterior spinal artery. Surg Neurol. 1977;8:85-93.
Djindjian R., Merland J.J., Djindjian M., et al. Embolization in the treatment of medullary arteriovenous malformations in 38 cases (In French). Neuroradiology. 1978;16:428-429.
Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64:134-139.
Hettige S., Walsh D. Indocyanine green video-angiography as an aid to surgical treatment of spinal dural arteriovenous fistulae. Acta Neurochir (Wien). 2010;152:533-536.
Masaryk T.J., Ross J.S., Modic M.T., et al. Radiculomeningeal vascular malformations of the spine: MR imaging. Radiology. 1987;164:845-849.
Oldfield E.H., Di Chiro G., Quindlen E.A., et al. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg. 1983;59:1019-1030.
Warakaulle D.R., Aviv R.I., Niemann D. Embolisation of spinal dural arteriovenous fistulae with Onyx. Neuroradiology. 2003;45(2):110-112.
1. Gaupp J. Hämorrhoiden der Pia Mater Spinalis im Gebiet des Lendenmarks. Beitr Pathol. 1888;2:516-518.
2. Wyburn-Mason R. The vascular abnormalities and tumors of the spinal cord and its membranes. London: Henry Kimpton; 1943.
3. Bergstrand A., Hook O., Lidvall H. Vascular malformations of the spinal cord. Acta Neurol Scand. 1964;40:169-183.
4. Krayenbuhl H., Yasargil M.G., McClintock H.G. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg. 1969;30:427-435.
5. Malis L.I. Arteriovenous malformations of the spinal cord. In: Youmans J.R., editor. Neurological surgery: a comprehensive reference guide to the diagnosis and management of neurosurgical problems. ed 2. Philadelphia: WB Saunders; 1982:1850-1874.
6. Anson J.A., Spetzler R.F. Classification of spinal arteriovenous malformations and implications for treatment. BNI Quarterly. 1992;8:2-8.
7. Kendall B.E., Logue V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 1977;13:181-189.
8. Spetzler R.F., Zabramski J.M., Flom R.A. Management of juvenile spinal AVMs by embolization and operative excision: case report. J Neurosurg. 1989;70:628-632.
9. Djindjian M., Djindjian R., Rey A., et al. Intradural extramedullary spinal arteriovenous malformations fed by the anterior spinal artery. Surg Neurol. 1977;8:85-93.
10. Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64:134-139.
11. Barrow D.L., Colohan A.R., Dawson R. Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). J Neurosurg. 1994;81:221-229.
12. Anson J.A., Spetzler R.F. Spinal dural arteriovenous malformations. In: Barrow W., Awad I., editors. Dural arteriovenous malformations. Park Ridge, IL: American Association of Neurologic Surgeons; 1993:175-191.
13. Oldfield E.H., Di Chiro G., Quindlen E.A., et al. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg. 1983;59:1019-1030.
14. Rosenblum B., Oldfield E.H., Doppman J.L., et al. Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVMs in 81 patients. J Neurosurg. 1987;67:795-802.
15. Symon L., Kuyama H., Kendall B. Dural arteriovenous malformations of the spine: clinical features and surgical results in 55 cases. J Neurosurg. 1984;60:238-247.
16. Aminoff M.J., Logue V. Clinical features of spinal vascular malformation. Brain. 1974;97:197-210.
17. Aminoff M.J., Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211-218.
18. Morgan M.K., Marsh W.R. Management of spinal dural arteriovenous malformations. J Neurosurg. 1989;70:832-836.
19. Foix C.H., Alajouanine T.H. La myelite necrotique subaigue. Rev Neurol (Paris). 1926;46:1-42.
20. Perthes G. Ueber das rankenangiom der weichen haute des gehirns und ruckenmarks. Deutsche Ztschr Chir. 1927;203:93-103.
21. Hassler W., Thron A., Grote E.H. Hemodynamics of spinal dural arteriovenous fistulas: an intraoperative study. J Neurosurg. 1989;70:360-370.
22. Mourier K.L., Gelbert F., Rey A., et al. Spinal dural arteriovenous malformations with perimedullary drainage: indications and results of surgery in 30 cases. Acta Neurochir (Wien). 1989;100:136-141.
23. Doppman J.L., DiChiro G., Dwyer A.J., et al. Magnetic resonance imaging of spinal arteriovenous malformations. J Neurosurg. 1987;66:830-834.
24. Masaryk T.J., Ross J.S., Modic M.T., et al. Radiculomeningeal vascular malformations of the spine: MR imaging. Radiology. 1987;164:845-849.
25. Hall W.A., Oldfield E.H., Doppman J.L. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg. 1989;70:714-720.
26. Merland J.J., Reizine D. Embolization techniques in the spinal cord. In: Dondelinger R.F., Rossi P., Kurdziel J.C., et al, editors. Interventional radiology. New York: Thieme; 1990:433-442.
27. Merland J.J., Reizine D. Treatment of arteriovenous spinal cord malformations. Semin Interv Radiol. 1987;4:281-290.
28. Anson J.A., Spetzler R.F. Interventional neuroradiology for spinal pathology. Clin Neurosurg. 1992;39:388-417.
29. Choi I.S., Berenstein A. Surgical neuroangiography of the spine and spinal cord. Radiol Clin North Am. 1988;26:1131-1141.
30. Mourier K.L., Gelbert F., Reizine D., et al. Phase contrast magnetic resonance of the spinal cord: preliminary results in spinal cord arteriovenous malformations. Acta Neurochir (Wien). 1993;123:57-63.
31. Berenstein A., Kricheff I.I. Catheter and material selection for transarterial embolization: technical considerations. Part II: materials. Radiology. 1979;132:631-639.
32. Riche M.C., Melki J.P., Merland J.J. Embolization of spinal cord vascular malformations via the anterior spinal artery. Am J Neuroradiol. 1983;4:378-381.
33. Djindjian R. Embolization of angiomas of the spinal cord. Surg Neurol. 1975;4:411-420.
34. Lundqvist C., Berthelsen B., Sullivan M., et al. Spinal arteriovenous malformations: neurological aspects and results of embolization. Acta Neurol Scand. 1990;82:51-58.
35. Djindjian R., Merland J.J., Djindjian M., et al. Embolization in the treatment of medullary arteriovenous malformations in 38 cases (in French). Neuroradiology. 1978;16:428-429.
36. Nogueira R.G., Dabus G., Rabinov J.D., et al. Onyx embolization for the treatment of spinal dural arteriovenous fistulae: initial experience with long-term follow-up. Technical case report. Neurosurgery. 2009;64(1):E197-E198.
37. Warakaulle D.R., Aviv R.I., Niemann D., et al. Embolisation of spinal dural arteriovenous fistulae with Onyx. Neuroradiology. 2003;45(2):110-112.
38. Krause F. Chirurgie des Gehirns and Ruckenmarks nach eigenen Erfahrungen, II: Band,. Berlin: Urban and Schwarzenberg; 1911. 775–776
39. Elsberg C.A. Diagnosis and Treatment of Surgical Diseases of the Spinal Cord and Its Membranes,. Philadelphia: WB Saunders; 1916. 194–204
40. Newman M.J.D. Racemose angioma of the spinal cord. QJ Med. 1959;28:97-108.
41. Svien H.J., Baker H.L.Jr. Roentgenographic and surgical aspects of vascular anomalies of the spinal cord. Surg Gynecol Obstet. 1961;112:729-735.
42. Shephard R.H. Some new concepts in intradural spinal angioma. Riv Patol Nerv Ment. 1965;86:276-283.
43. Yasargil M.G., DeLong W.B., Guarnaschelli J.J. Complete microsurgical excision of cervical extramedullary and intramedullary vascular malformations. Surg Neurol. 1975;4:211-214.
44. Killory B.D., Nakaji P., Gonzales L.F., et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009;65(3):456-462.
45. Bruneau M., Sauvageau E., Nakaji P., et al. Preliminary personal experiences with the application of near-infrared indocyanine green videoangiography in extracranial vertebral artery surgery. Neurosurgery. 2010;66(2):305-311.
46. Colby G.P., Coon A.L., Sciubba D.M., et al. Intraoperative indocyanine green angiography for obliteration of a spinal dural arteriovenous fistula. J Neurosurg Spine. 2009;11(6):705-709.
47. Raabe A., Beck J., Gerlach R., et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52(1):132-139. discussion 139
48. Hettige S., Walsh D. Indocyanine green video-angiography as an aid to surgical treatment of spinal dural arteriovenous fistulae. Acta Neurochir (Wien). 2010;152:533-536.
49. Ommaya A.K., Di Chiro G., Doppman J. Ligation of arterial supply in the treatment of spinal cord arteriovenous malformations. J Neurosurg. 1969;30:679-692.