45 Spinal Cord Myelopathies
Acute Myelopathies
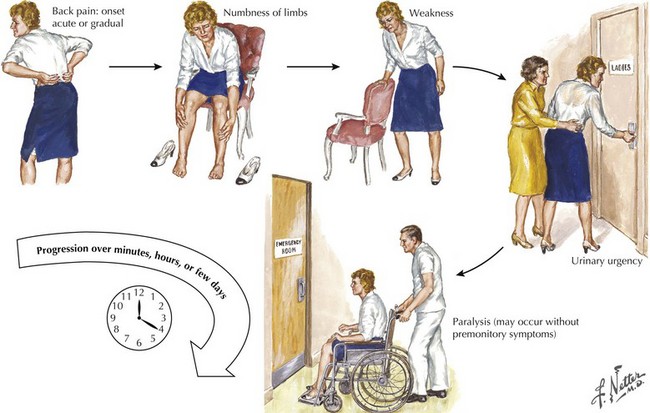
The patient presenting with an acute myelopathy provides one of the most challenging emergencies that a neurologic physician will confront. These clinical settings require expeditious and thorough evaluation including careful history, detailed neurologic examination, and immediate definitive neuroradiologic studies. In most instances, a magnetic resonance image (MRI) is the perfect study. The primary exception is the patient with a pacemaker or other medical apparatus that is not compatible with a magnet. Expeditious therapeutic intervention can sometimes reverse/stabilize a potentially disastrous clinical outcome (Fig. 45-1). Three common causes of an acute myelopathy are spinal fracture/dislocations (Chapter 60), acute transverse myelitis in young adults, and metastatic tumors in late middle- and senior-aged individuals (Chapter 53).
Acute Extradural Spinal Lesions
Trauma
Central Herniated Disc
Clinical Vignette
Comment: Initially, this patient was thought to have a basically untreatable brainstem stroke, that is, a lesion in an entirely different part of the neuraxis. A more careful subsequent clinical examination, lying the patient on his side and examining for a specific sensory level, was the key to diagnosis, as patients with brainstem stroke rarely have total loss of sensory function in a spinal cord–level distribution (see Figs. 44-11 and 44-12).
Acute spinal cord injuries, with subsequent paraplegia or quadriplegia, are among the most dreaded sequelae to a serious bodily injury (Box 45-1). There is a tragic propensity for traumatic myelopathies to occur among vigorous and healthy young persons. Automobile and motorcycle accidents as well as sports injuries are the most common etiologies. Gunshot wounds, whether resulting from war, accident, or assault, are another source of traumatic spinal cord injury.
Box 45-1 Acute Extradural Extramedullary Myelopathies
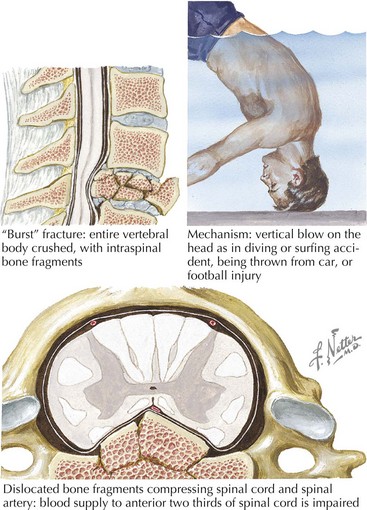
Cervical spinal fracture dislocation with resultant ligamentous tear, allowing bony fragments to directly tear or transect the spinal cord, is the common denominator in this setting (Fig. 45-2). Sometimes, there is concomitant compromise of the spinal arteries causing an associated spinal cord infarction, hematomyelia, or both. Typically, in the acute setting, spinal shock results with complete paralysis, loss of sensation, areflexia distal to the trauma site, and loss of bladder and bowel function.
Occasionally, these traumatic spinal lesions are amenable to immediate surgical correction or external traction (Chapter 60). Prognosis is always guarded. Once emergent management is completed, these patients are cared for at specialized spinal rehabilitation centers. Treatment of autonomic and sphincter dysfunction has greatly improved the long-term survival for many patients. Spinal cord repair, leading to functional recovery, is one of the greatest challenges for 21st-century neuroscientists.
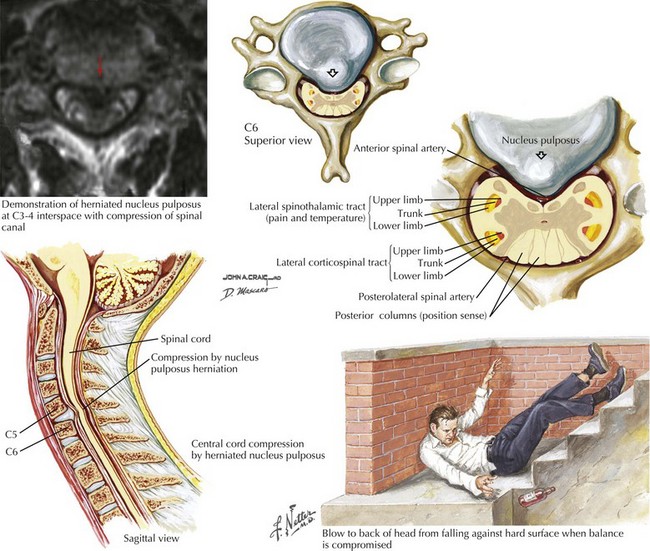
As these lesions are eminently treatable with neurosurgical intervention, consideration of these less common lesions is vital in patients who have sudden unexplained falls leading to immediate paralysis. Anatomically, the extruded disc compressed the anterior spinal cord between two vertebral bodies. Occasionally, these lesions present subacutely or chronically. Because associated bony pain or tenderness may be present, these lesions often mimic metastatic or primary tumors. However, if the patient does not have a history of malignancy, a benign mechanism, such as a central disc, must be sought (Figs. 45-3 and 45-4). Rarely, a dural arteriovenous malformation (AVM) or spinal epidural hematoma (SEH) mimics this clinical picture.
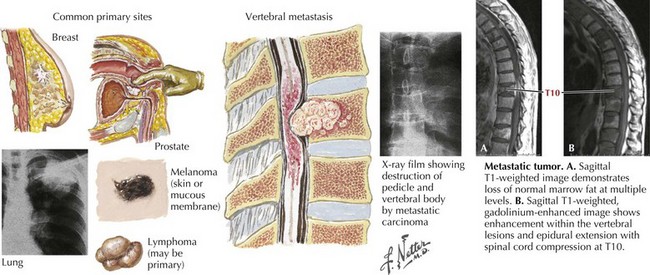
Metastatic Malignancies
One of the most common etiologies for a nontraumatic acute or subacute extradural myelopathy occurs with various forms of metastatic carcinoma or lymphoma (Fig. 45-5). These patients initially develop spinal pain secondary to bony metastases. Within a matter of days, they begin to note symptoms of spinal cord compromise, usually with a spastic gait or bladder. Once these symptoms appear, the progression to paraplegia may be very rapid. On occasion, the spinal metastasis may be the presenting sign of a previously undiagnosed lung cancer. Emergency MRI is indicated. Treatment of metastatic extradural tumors includes radiation and sometimes surgery, chemotherapy, or both. In contrast to metastatic disease, primary intrinsic spinal cord tumors are usually either intradural extramedullary or of intramedullary origin.
Epidural Abscess
Clinical Vignette
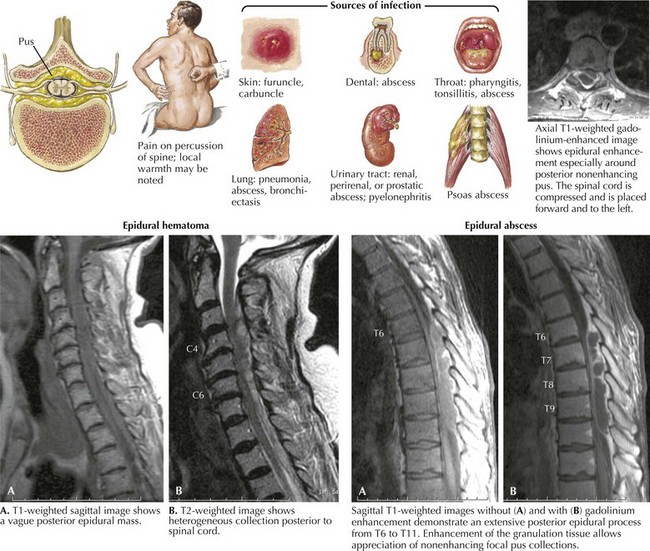
Epidural spinal abscess is a rare clinical process occurring in 2–20 cases per 100,000 hospital admissions. Its incidence appears to be increasing; these are usually seen in middle-aged adults, more often men. Despite its rarity, the potential for an epidural abscess to cause permanent paraplegia makes it one of the most urgent spinal cord emergencies (Fig. 45-6). Even though back pain is such a ubiquitous and usually benign process, it is very important for any physician to consider the potential diagnosis of an epidural abscess in every patient presenting with acute and increasing back pain, especially when febrile. The epidural space in the posterior thoracic cord is the primary site for an epidural spinal abscess to develop. These may extend to the cervical cord and rarely into the lumbar spine. Experimental studies suggest that the mass effect of the abscess, leading to cord compression, is the important clinicopathologic mechanism.
Staphylococcus aureus is the predominant etiologic microorganism leading to epidural spinal abscesses. Usually a distant septic focus provides bacterial seeding via the bloodstream, for example, skin furuncles, dental abscesses, simple pharyngitis, or a recently infected traumatic site (see Fig. 45-6). Often there is a concomitant history of diabetes mellitus, alcoholism, drug abuse, or recent spinal or extraspinal trauma. Less commonly, epidural spinal abscesses develop subsequent to vertebral osteomyelitis, pulmonary or urinary infection, sepsis, or extremely rarely bacterial endocarditis. Invasive procedures, including epidural anesthesia, spinal surgery, vascular access lines, and paravertebral injections, also provide potential mechanisms for bacterial seeding. Corticosteroid therapy may contribute to immune suppression and the possibility of secondary nosocomial infections.
Acute Intradural Intramedullary Spinal Lesions
Myelitis Secondary to Multiple Sclerosis
Clinical Vignette
Cervical spine MRI demonstrated an area of increased signal with ill-defined enhancement located posteriorly and on the left side of the cord (Fig. 45-7A). Brain MRI with and without gadolinium demonstrated a few periventricular lesions and other abnormalities oriented perpendicularly along vessels (Dawson fingers) within the corpus callosum. Visual evoked responses (VERs) were prolonged for her right eye. Her cerebrospinal fluid (CSF) was significant for 10 white blood cells (99% lymphocytes, 1% monocytes), and the presence of oligoclonal bands that was not identified in her serum.
Comment: This vignette presents a classic clinical picture of early multiple sclerosis (MS). The patient has an incomplete demyelinating myelitis consistent with classic hemicord syndrome, affecting ipsilateral motor and proprioceptive function and crossed sensory fibers. This is known as the Brown–Sequard syndrome. However, she also had involvement of her right arm, implying extension into the right anterior horn. The presence of the subclinical brain MRI abnormalities, as well as the prolonged VERs, point to multiplicity of demyelinating lesions in time and space consistent with the diagnosis of MS (Table 45-1).
| Inflammatory Transverse myelitis |
Anterior cord syndrome
Central cord syndrome
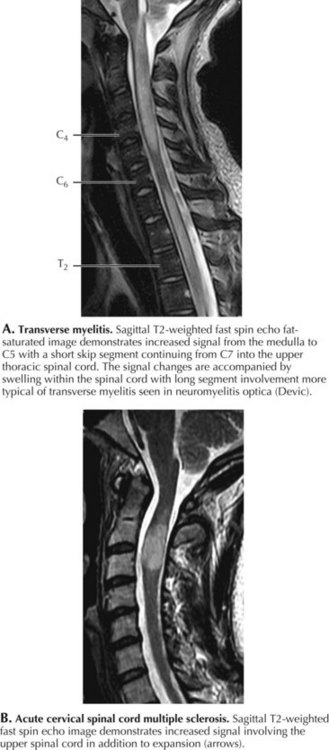
Transverse Myelitis
Clinical Vignette
Very often, there is no underlying pathology identified with many transverse myelitis (TM) cases. Acute transverse myelitis most likely represents another type of autoimmune CNS disorder. A nonspecific “viral infection” may be the inciting mechanism or sometimes a bacterial infection, and rarely this may occur subsequent to immunization. Sometimes, a transverse myelitis is preceded by or associated with optic neuritis. This is known as neuromyelitis optica (NMO), an unusual autoimmune demyelinating disorder associated with a very specific serum autoantibody referred to as NMO-IgG (Fig. 45-7). This antibody binds to the CNS-dominant water channel, aquaporin 4; this is a structure that is normally present on astrocytes. The diagnostic criteria for neuromyelitis optica include optic neuritis, acute myelitis, and two of the following three: brain MRI not characteristic for multiple sclerosis, contiguous spinal cord MRI lesion extending over three or more vertebral segments, and NMO-IgG positive status.
MRI with gadolinium is the procedure of choice to exclude compressive lesions, especially when history and exam suggest a specific level of spinal cord dysfunction (see Fig. 45-7). Brain lesions consistent with MS are demonstrated with MRI in approximately half of all TM patients. Transverse myelitis appears with T2 signal hyperintensity on MRI. The area of signal abnormality may be focal or extensive in cross section and length. Gadolinium enhancement is frequent. Cord swelling is present to variable degrees. Large cross-sectional area, multisegment length, cord expansion, and peripheral enhancement are most consistent with a diagnosis of TM. In contrast, MS lesions tend to be smaller, usually involving only 1–2 segments, and are often multifocal. Total cross-sectional area and multisegment length are uncommon in MS, although cord expansion and enhancement are frequent with larger acute inflammatory MS lesions. Gadolinium-enhanced brain MRI, and optical coherence tomography (OCT), even more than visual evoked potentials (Chapter 46), help determine the presence of multifocal demyelinating disease. Associated cerebral white matter lesions and oligoclonal bands in CSF increase the later probability of developing unequivocal MS. Oligoclonal bands, however, are nonspecific for multiple sclerosis and not always present in individuals with multiple sclerosis.
Spinal Cord Infarction/Ischemic Myelopathy
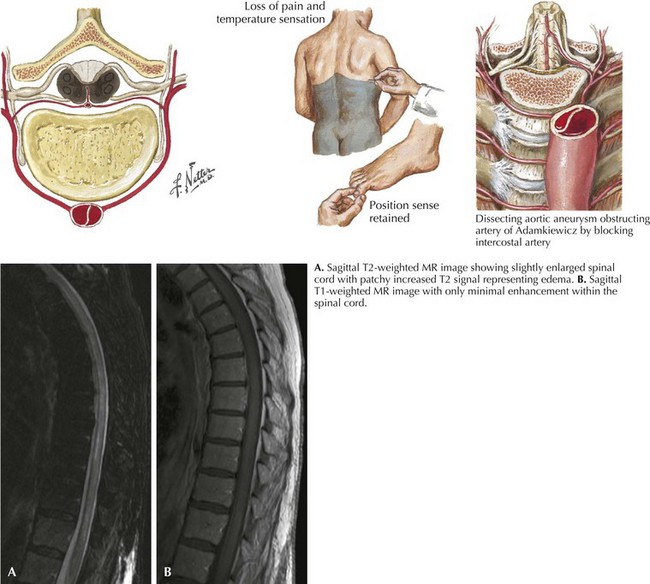
Anterior Spinal Artery Syndrome
Clinical Vignette
The clinical picture of paralysis secondary to loss of corticospinal tract function and sensory change from impaired spinothalamic function, as well as infarction of the anterior horn cells, but with very preserved posterior column function, is classic for an anterior spinal artery distribution spinal cord infarction (Fig. 45-8). Onset of anterior spinal artery syndrome, although usually sudden, may occasionally be gradual over hours or days.
Chronic Myelopathies
Extradural Myelopathies
Cervical Spondylosis
Clinical Vignette
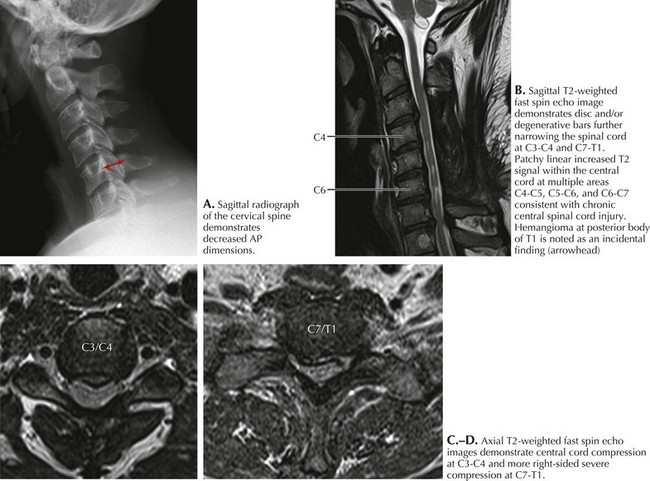
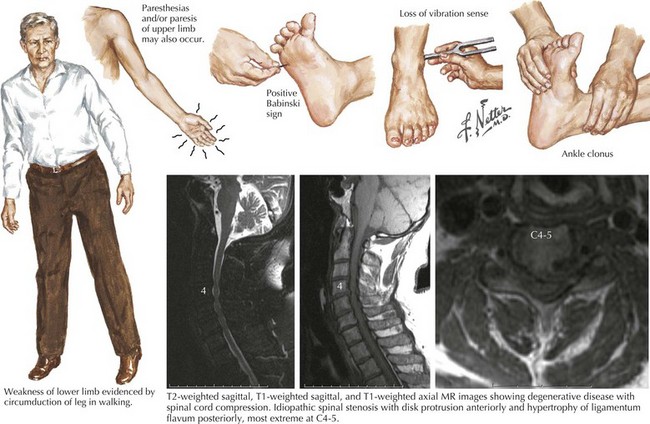
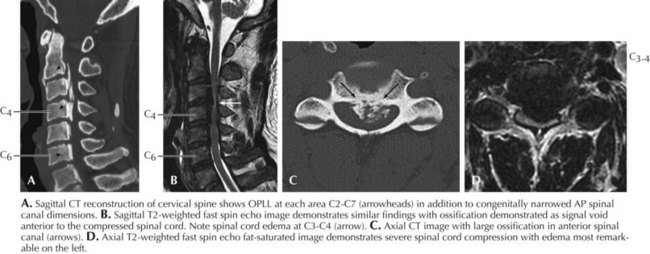
Spondylosis, a normal aging process, is the most common cause of a cervical myelopathy (Figs. 45-9 and 45-10; Box 45-2). This results from disc degeneration followed by reactive osteophyte formation, fibrocartilaginous bars, spondylotic transverse bars, articular facet hypertrophy, and thickening of the ligamentum flavum causing spinal canal narrowing. Subsequently, gradual spinal cord compression may occur; it is particularly likely in patients having congenitally narrowed spinal canals. In its simplest form, a chronically herniated central nucleus pulposus in patients with congenital stenosis can produce a cervical myelopathy. Although many senior individuals have radiographic signs of cervical spondylosis, most are asymptomatic. When a myelopathy develops, clinical findings can present acutely, subacutely, or over many years. Sometimes both a cervical myelopathy and adjacent radiculopathy may occur in the same spondylotic patient.
Clinical Vignette
His examination demonstrated bilateral ankle clonus, and a left Babinski sign. The patient likened this feeling of clonus to his skiing experience. Cervical spinal cord MRI demonstrated disc extrusion primarily at the C5–C6 level along with abnormal intramedullary enhancement compatible with a severe spondylotic cervical myelopathy (see Figs. 45-9 and 45-10). In this instance, the patient’s vivid description of a most unusual complaint (“mogul clonus”) led to careful investigation and eventual surgery. Six months postoperatively, “skiing the bumps” no longer produced clonus. Most importantly, his risk of permanent spinal cord injury was greatly lessened if he were to subsequently sustain a severe fall.
Spinal Cord Arteriovenous Malformations
Pathophysiology and Etiology
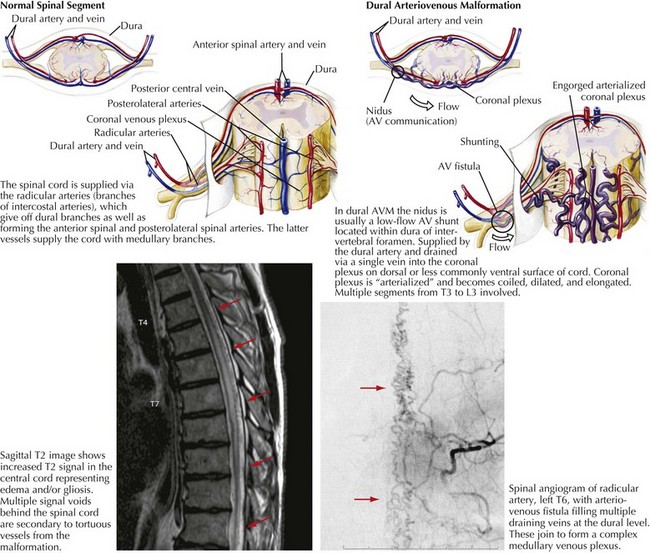
Dural AVMs primarily occur in the mid to lower thoracic and lumbar spine. Many dural AVMs are AV fistulas that essentially have a single hole in an artery connected to a vein (Fig. 45-11). Radicular or dural branches from the segmental arteries supply the AVM, usually within the dura of an intervertebral foramen. The AVM nidus is typically a low-flow shunt drained by a single vein that joins the coronal plexus on the dorsal cord surface. The coronal plexus is arterialized by the AVM fistula and becomes dilated, coiled, and elongated. Blood flow through this vessel is slower than normal and thus leads to venous congestion with increased venous pressure that is transmitted intramedullarly, reducing the arteriovenous pressure gradient within the cord. Cord perfusion decreases, leading to prolonged ischemia/hypoxia and a progressive myelopathy. The effects of hypoxia that are initially reversible later lead to an irreversible degenerative cord necrosis. The corticospinal tracts, lateral white matter, and dorsal columns are especially vulnerable.
Intradural Extramedullary Spinal Cord Lesions
Meningioma
Clinical Vignette
Spinal cord meningiomas comprise at least 25% of primary spinal cord tumors although they are less common than intracranial meningiomas (Box 45-3). Most are intradural. They can be located ventrally, dorsally or laterally to the cord.
Pathophysiology and Etiology
Spinal cord meningiomas have the same histologic classification as intracranial meningiomas (Chapter 51) and can become densely calcific. Meningothelial (syncytial), psammomatous, transitional, and fibroblastic meningiomas are seen; meningotheliomatous meningiomas are the most frequent.
Diagnosis
Intradural extramedullary spinal tumors include meningiomas, schwannomas, and neurofibromas. Ependymomas can affect the filum terminale or any part of the spinal cord. Other spinal cord tumor categories include extradural and intradural intramedullary lesions (Table 45-2). Additional diagnoses in the differential are MS, spinal dural AVMs, vitamin B12 deficiency, central disc herniation, and syringomyelia.
| Type | Examples |
|---|---|
| Congenital or acquired |
MRI provides an excellent means to diagnose intradural extramedullary lesions (Fig. 45-12). Meningiomas enhance homogeneously with gadolinium. Axial MRI helps to determine if the tumor is circumferential. When an initial MRI at the thoracic level is normal, further study needs to be performed to the foramen magnum. This is because what initially appears to be sensory localizing signs at the low thoracic level may actually be found due to a lesion situated higher in the canal. CT/myelogram is also a valuable diagnostic tool that can demonstrate a partial or complete block and also assess for tumor calcification, but is primarily used when MRI cannot be performed. Plain spine films may demonstrate erosion of a pedicle or articular process by the meningioma and intraspinal calcification.
Intradural Intramedullary Spinal Cord Lesions
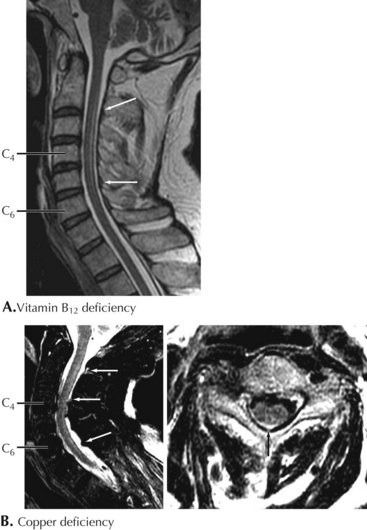
Vitamin B12 Deficiency
Clinical Presentation
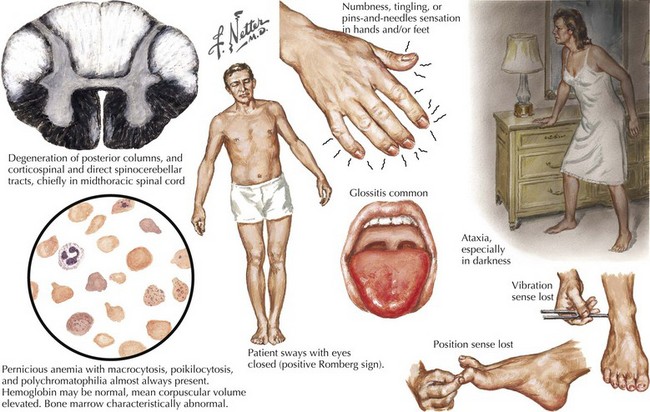
Although most patients with PA do not have signs of neurologic compromise, some individuals occasionally present with what appears to be a primary neurologic syndrome and minimal or no hematologic abnormalities. Distal paresthesia, particularly in the hands, and gait ataxia are the typical presenting symptoms in patients with primary neurologic involvement (Fig. 45-13). Loss of vibratory sensation, abnormal joint position sense, sensory ataxia, and positive Romberg signs correlate with posterior column damage. Later appearance of lower extremity weakness and spasticity reflect the corticospinal tract damage. Muscle stretch reflexes can be increased or decreased depending on whether the myelopathy or the peripheral neuropathy components predominate. Memory loss, confusion, paranoia, irritability, and hallucinations rarely develop.
Copper Deficiency Myelopathy
AIDS-Associated Vacuolar Myelopathy
HTLV-I Myelopathy
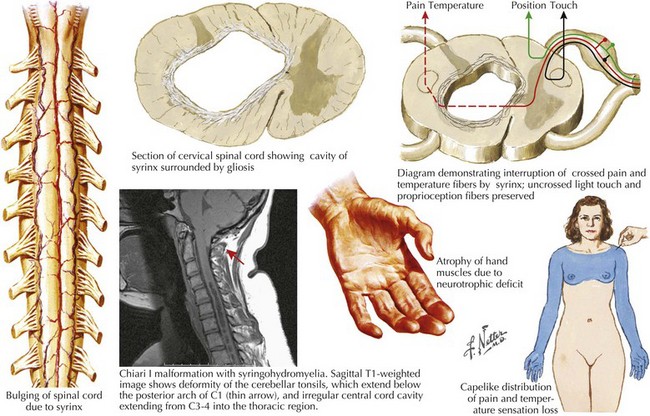
Syringomyelia
Pathophysiology and Etiology
The syrinx is in essence an enlarging tubular cavity within the central spinal canal. It is thought to arise from a diverticulum directly communicating with the central canal. The syrinx maximally affects the cervical cord, but has the potential to sometimes extend rostrally into the brainstem (syringobulbia) and distally as far as the lower thoracic cord. Typically these cystic structures enlarge transversely within the cord, gradually putting isolated pressure on both the decussating pain and temperature fibers as they cross through the central gray matter as well as the anterior horn gray matter (Fig. 45-14). Eventually the enlarging size of the syrinx also affects the corticospinal tracts in the lateral funiculus, and if large enough even the posterior columns, thus occasionally modifying proprioceptive sensation.
Diagnosis
MRI is the diagnostic modality of choice. CT/myelography is also useful when MRI is contraindicated.
Hereditary Spastic Paraplegia
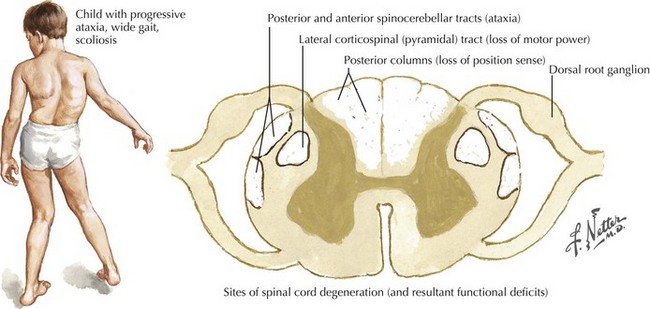
Friedreich Ataxia
Pathophysiology and Etiology
The spinal cord atrophies secondary to a fiber loss in the corticospinal and spinocerebellar tracts. Neuronal loss also occurs in Clarke’s column, the dorsal root ganglia, and especially the dentate nuclei (Fig. 45-15). Compensatory gliosis follows axonal degeneration and demyelination. The heart is affected, with myocardial muscle fibers replaced by myophages and fibroblasts.
Anderson O. Myelitis. Current Opinions in Neurology. 2000;13(3):1311-1316.
Bhigjee AI, Madurai S, Bill PLA, et al. Spectrum of myelopathies in HIV seropositive South African patients. Neurology. 2001;57:348-351. In South Africa, the majority of HIV-seropositive patients presenting with a myelopathy have a treatable cause. Vacuolar myelopathy is rare. These features must be borne in mind when managing these patients
Bohlman HH. Cervical spondylosis and myelopathy. Instr Course Lect. 1995;44:81-97.
Chan KF, Boey ML. Transverse myelopathy in SLE: clinical features and functional outcomes. Lupus. 1996;5:294-299.
Clarke MJ, Patrick TA, White JB, et al. Spinal extradural arteriovenous malformations with parenchymal drainage: venous drainage variability and implications in clinical manifestations. Neurosurg Focus. 2009 Jan;26(1):E5. Although nontraumatic spinal arteriovenous malformations and fistulas restricted to the epidural space are rare, they can lead to significant neurologic morbidity. Careful diagnostic imaging is essential to their detection and the delineation of the pathologic anatomy. Aggressive endovascular and open operative treatment can provide arrest and reversal of neurologic deficits
de Seze J, Stojkovic T, Breteau G, et al. Acute myelopathies—clinical, laboratory and outcome profiles in 79 cases. Brain. 2001;124:1509-1521.
Ferguson RJL, Caplan LR. Cervical spondylitic myelopathy. Neurologic Clinics. 1985;3(2):373-382.
Fink JK. The hereditary spastic paraplegias: nine genes and counting. Archives of Neurology. 2003;60(8):1045-1049.
Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien). 2004 Feb;146(2):103-110.
Harris MK, Hadi Maghzi A, Etemadifar M, et al. Acute demyelinating disorders of the central nervous system. Curr Treat Options Neurol. 2009 Jan;11(1):55-63. This paper summarizes clinical manifestations of these disorders, as well as treatment strategies. Currently, immunosuppression with corticosteroids for the acute events is the mainstay of treatment
Kerr DA, Ayetey H. Immunopathogenesis of acute transverse myelitis. Current Opinion in Neurology. 2002;15:339-347.
Kovacs B, Lafferty TL, Brent LH DeHoratius RJ. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Annals of Rheum Dis. 2000;59:120-124.
Kumar N, McEvoy K, Ahlskog JE. Myelopathy due to copper deficiency following gastrointestinal surgery. Arch Neurol. 2003;60:1782-1785. This paper is not only the first report on copper myelopathy but also the seminal one to date
Kumar N. Spinal cord, root, and plexus disorders. Continuum. June 2008;14:1-271. This is a brilliant overview of the subject
Lestini WF, Wiesel SW. The pathogenesis of cervical spondylosis. Clinical Orthopedics and Related Research. 1989;239:69-93.
Levin MC, Jacobson S. HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP): a chronic progressive neurologic disease associated with immunologically mediated damage to the central nervous system. Journal of Neuro-Virology. 1997;3:126-140.
McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005 Sep;4(9):543-555.
Makinson A, Morales RJ, Basset D, et al. Diagnostic approaches to imported schistosomal myeloradiculopathy in travelers. Neurology. 2008;71:66-68.
Narvid J, Hetts SW, Larsen D, et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008 Jan;62(1):159-166. discussion 166-7. Sixty-three patients presenting symptoms were consistent with progressive myelopathy, and included lower extremity weakness (33 patients, 52%), parasthesias (19 patients, 30%), back pain (15 patients, 24%), and urinary symptoms (four patients, 6%). Thirty-nine patients underwent an initial endovascular embolization, with 27 requiring only this first procedure for complete obliteration. On the other hand, 24 patients underwent an initial surgical procedure, with 20 of them treated successfully with a single operation
Novy J, Carruzzo A, Maeder P, et al. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006 Aug;63(8):1113-1120. An excellent review based on 14 years’ experience with 27 patients
Pandolfo M. Friedreich ataxia. Arch Neurol. 2008 Oct;65(10):1296-1303.
Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2008 Nov 18. Epub ahead of print
Thiele RH, Hage ZA, Surdell DL, et al. Spontaneous spinal epidural hematoma of unknown etiology: case report and literature review. Neurocrit Care. 2008;9(2):242-246.