Sphingomonas paucimobilis and Similar Organisms
1. Identify cultivation methods and colonial characteristics for Sphingomonas paucimobilis and similar organisms.
2. State the initial clues that alert the CL to the presence of this group of organisms for clinical laboratorians.
3. Select identification approaches for this group of organisms.
4. Identify susceptibility testing methods appropriate for this group of organisms.
Epidemiology, Spectrum of Disease, and Antimicrobial Therapy
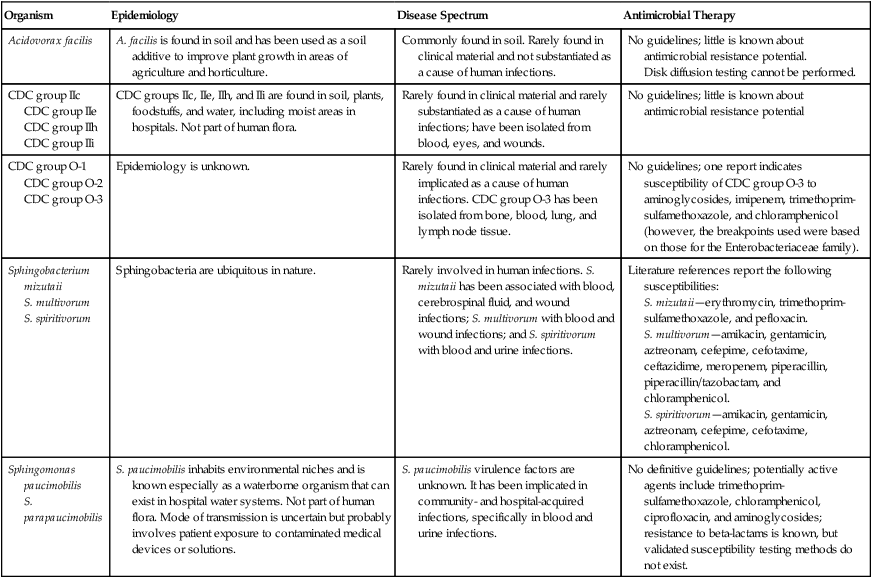
As demonstrated in Table 27-1, these organisms are rarely or only occasionally isolated from human materials and have limited roles as agents of infection. Because they are infrequently encountered in the clinical setting, little information is available on their epidemiology, ability to cause human infections, and potential for antimicrobial resistance. For example, even though O-1 and O-2 organisms have been submitted to the Centers for Disease Control and Prevention (CDC) after being isolated from clinical materials such as blood, cerebrospinal fluid (CSF), wounds, and pleural fluid, their natural habitat is unknown. The genus Sphingobacterium is ubiquitous in nature, and Sphingomonas spp. are known for their waterborne nature. Some of these groups are present in hospital settings, such as hospital water supplies. When the organisms discussed in this chapter are encountered in clinical specimens, their clinical significance and potential as contaminants should be considered; human infections have been documented, so care must be taken to determine whether these organisms are infectious agents or contaminants.
TABLE 27-1
Epidemiology, Spectrum of Disease, and Antimicrobial Therapy
| Organism | Epidemiology | Disease Spectrum | Antimicrobial Therapy |
| Acidovorax facilis | A. facilis is found in soil and has been used as a soil additive to improve plant growth in areas of agriculture and horticulture. | Commonly found in soil. Rarely found in clinical material and not substantiated as a cause of human infections. | No guidelines; little is known about antimicrobial resistance potential. Disk diffusion testing cannot be performed. |
| CDC group IIc CDC group IIe CDC group IIh CDC group IIi |
CDC groups IIc, IIe, IIh, and IIi are found in soil, plants, foodstuffs, and water, including moist areas in hospitals. Not part of human flora. | Rarely found in clinical material and rarely substantiated as a cause of human infections; have been isolated from blood, eyes, and wounds. | No guidelines; little is known about antimicrobial resistance potential |
| CDC group O-1 CDC group O-2 CDC group O-3 |
Epidemiology is unknown. | Rarely found in clinical material and rarely implicated as a cause of human infections. CDC group O-3 has been isolated from bone, blood, lung, and lymph node tissue. | No guidelines; one report indicates susceptibility of CDC group O-3 to aminoglycosides, imipenem, trimethoprim-sulfamethoxazole, and chloramphenicol (however, the breakpoints used were based on those for the Enterobacteriaceae family). |
| Sphingobacterium mizutaii S. multivorum S. spiritivorum |
Sphingobacteria are ubiquitous in nature. | Rarely involved in human infections. S. mizutaii has been associated with blood, cerebrospinal fluid, and wound infections; S. multivorum with blood and wound infections; and S. spiritivorum with blood and urine infections. | Literature references report the following susceptibilities: S. mizutaii—erythromycin, trimethoprim-sulfamethoxazole, and pefloxacin. S. multivorum—amikacin, gentamicin, aztreonam, cefepime, cefotaxime, ceftazidime, meropenem, piperacillin, piperacillin/tazobactam, and chloramphenicol. S. spiritivorum—amikacin, gentamicin, aztreonam, cefepime, cefotaxime, chloramphenicol. |
| Sphingomonas paucimobilis S. parapaucimobilis |
S. paucimobilis inhabits environmental niches and is known especially as a waterborne organism that can exist in hospital water systems. Not part of human flora. Mode of transmission is uncertain but probably involves patient exposure to contaminated medical devices or solutions. | S. paucimobilis virulence factors are unknown. It has been implicated in community- and hospital-acquired infections, specifically in blood and urine infections. | No definitive guidelines; potentially active agents include trimethoprim-sulfamethoxazole, chloramphenicol, ciprofloxacin, and aminoglycosides; resistance to beta-lactams is known, but validated susceptibility testing methods do not exist. |
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Cultivation
Media of Choice
Colonial Appearance
Table 27-2 describes the colonial appearance and distinguishing characteristics (e.g., pigment) of each organism on 5% sheep blood agar. When these organisms do grow on MacConkey agar, they appear as lactose nonfermenters.
TABLE 27-2
Colonial Appearance and Characteristics
| Organism | Medium | Appearance |
| Acidovorax facilis | BA | No distinctive appearance |
| CDC group IIc | BA | No distinctive appearance but colonies sticky |
| CDC group IIe | BA | No distinctive appearance |
| CDC group IIh | BA | No distinctive appearance |
| CDC group IIi | BA | No distinctive appearance |
| CDC group O-1, O-2, O-3 | BA | Yellow pigment present in O-1 and O-2 but not in O-3 |
| Sphingobacterium spp. | BA | Yellow pigment present in S. mizutaii |
| Sphingomonas paucimobilis S. parapaucimobilis |
BA | Small, circular, smooth, convex; bright yellow growth pigment |
BA, 5% sheep blood agar.
Approach to Identification
The ability of many commercial identification systems to identify accurately the organisms discussed in this chapter may be limited or uncertain. Tables 27-3 through 27-6 show some biochemical tests that are helpful for presumptive differentiation among the various organisms in this group.
TABLE 27-3
Key Biochemical and Physiologic Characteristics
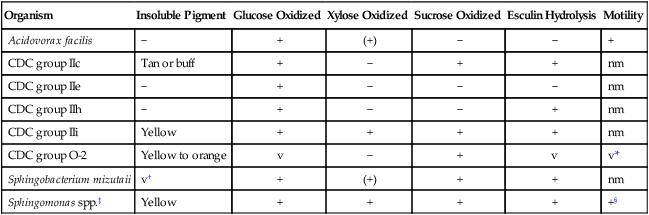
| Organism | Insoluble Pigment | Glucose Oxidized | Xylose Oxidized | Sucrose Oxidized | Esculin Hydrolysis | Motility |
| Acidovorax facilis | − | + | (+) | − | − | + |
| CDC group IIc | Tan or buff | + | − | + | + | nm |
| CDC group IIe | − | + | − | − | − | nm |
| CDC group IIh | − | + | − | − | + | nm |
| CDC group IIi | Yellow | + | + | + | + | nm |
| CDC group O-2 | Yellow to orange | v | − | + | v | v* |
| Sphingobacterium mizutaii | v† | + | (+) | + | + | nm |
| Sphingomonas spp.‡ | Yellow | + | + | + | + | +§ |
nm, Nonmotile; v, variable; +, >90% strains positive; −, >90% strains negative; (+), delayed.
*Only 20% are motile; motility is only apparent upon wet mount or flagellar staining.
†Yellow pigment production may be enhanced by incubation at room temperature.
‡Includes S. paucimobilis and S. parapaucimobilis.
§Usually nonmotile in motility medium, but motility is present on wet mount.
Data compiled from Daneshvar MI, Hill B, Hollis DG et al: CDC group O-3: phenotypic characteristics, fatty acid composition, isoprenoid quinone content, and in vitro antimicrobic susceptibilities of an unusual gram-negative bacterium isolated from clinical specimens, J Clin Microbiol 36:1674, 1998; Hollis DG, Moss CW, Daneshvar MI, Wallace-Shewmaker PL: CDC group IIc phenotypic characteristics, fatty acid composition, and isoprenoid quinone content, J Clin Microbiol 34:2322, 1996; and Weyant RS, Moss CW, Weaver RE et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1996, Williams & Wilkins.
TABLE 27-4
Specific Biochemical Characteristics for Differentiation of CDC groups IIc, IIe, and IIh*
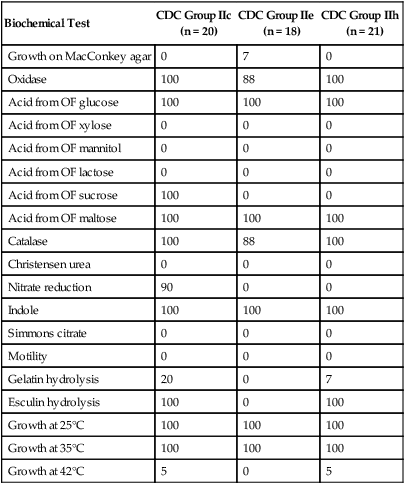
| Biochemical Test | CDC Group IIc (n = 20) |
CDC Group IIe (n = 18) |
CDC Group IIh (n = 21) |
| Growth on MacConkey agar | 0 | 7 | 0 |
| Oxidase | 100 | 88 | 100 |
| Acid from OF glucose | 100 | 100 | 100 |
| Acid from OF xylose | 0 | 0 | 0 |
| Acid from OF mannitol | 0 | 0 | 0 |
| Acid from OF lactose | 0 | 0 | 0 |
| Acid from OF sucrose | 100 | 0 | 0 |
| Acid from OF maltose | 100 | 100 | 100 |
| Catalase | 100 | 88 | 100 |
| Christensen urea | 0 | 0 | 0 |
| Nitrate reduction | 90 | 0 | 0 |
| Indole | 100 | 100 | 100 |
| Simmons citrate | 0 | 0 | 0 |
| Motility | 0 | 0 | 0 |
| Gelatin hydrolysis | 20 | 0 | 7 |
| Esculin hydrolysis | 100 | 0 | 100 |
| Growth at 25°C | 100 | 100 | 100 |
| Growth at 35°C | 100 | 100 | 100 |
| Growth at 42°C | 5 | 0 | 5 |
*Results indicate percent positive after 48 hours.
Data compiled from Hollis DG, Moss CW, Daneshvar MI, Wallace-Shewmaker PL: CDC group IIc phenotypic characteristics, fatty acid composition, and isoprenoid quinone content, J Clin Microbiol 34:2322, 1996.
TABLE 27-5
Specific Biochemical Characteristics for Differentiation of the Sphingobacterium spp.
| Biochemical Test | S. multivorum | S. spiritivorum | S. mizutaii |
| Oxidation of ethanol | Negative | Positive | Negative |
| Oxidation of mannitol | Negative | Positive | Negative |
| Oxidation of rhamnose | Negative | Positive | Positive |
| Christensen urease | Positive | Positive | Negative* |
| DNase | Negative† | Positive | ND‡ |
| Susceptibility to polymyxin B | Resistant | Resistant | Resistant |
| Indole | Negative | Negative | Negative |
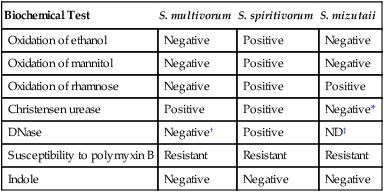
‡Not determined. Some microbiology texts classify S. mizutaii as DNase positive, and some major literature references identify it as DNase negative.
Data compiled from Freney J, Hansen W, Ploton C et al: Septicemia caused by Sphingobacterium multivorum. J Clin Microbiol 25:1126, 1987.
TABLE 27-6
Specific Biochemical Characteristics for Differentiation of the Sphingomonas spp.
| Biochemical Test | S. paucimobilis | S. parapaucimobilis |
| Oxidation of glucose | Positive | Positive |
| Oxidation of xylose | Positive | Positive |
| Oxidation of maltose | Positive | Positive |
| Esculin hydrolysis | Positive | Positive |
| Motility | Positive* | Positive |
| Indole | Negative | Negative |
| Susceptibility to polymyxin B | Susceptible | Variable |
| Hydrogen sulfide (H2S) (lead acetate paper suspended over KIA) | Negative | Positive |
| Citrate | Negative | Positive |
| DNase | Positive | Negative |
H2S, Hydrogen sulfide; KIA, Kligler iron agar.
Data compiled from Winn WC, Allen SD, Janda WM et al: Koneman’s color atlas and textbook of diagnostic microbiology, ed 6, Philadelphia, 2006, Lippincott Williams & Wilkins.
Comments Regarding Specific Organisms
Acidovorax facilis.
A. facilis is a straight to slightly curved, gram-negative rod that occurs singly or in short chains. It is aerobic and has a single polar flagellum, which makes it motile. On nutrient agar, it forms unpigmented colonies, and 30°C is its optimum temperature. A. facilis is oxidase positive and urease variable (some grow on Christensen urea agar but lack urease activity). Key characteristics are shown in Table 27-3. A. facilis is commonly found in soil, and no evidence of pathogenicity in healthy humans has been identified. A role for A. facilis as an opportunistic pathogen has not been proven or rejected.
CDC groups IIc, IIe, IIh, IIi, O-1, O-2, and O-3.
CDC groups IIc, IIe, IIh, IIi, O-1, and O-2 are short, straight rods that may appear as “II-forms” (i.e., bacteria with thickened ends and thin centers). The phenotypic characteristics of CDC group IIc are most similar to those of CDC groups IIe and IIh, the major difference being that CDC group IIc produces acid from sucrose, hydrolyzes esculin, and usually reduces nitrate. Strains of CDC groups IIe and IIh are similar to Empedobacter brevis (see Chapter 26) in that they oxidize glucose and maltose and produce indole. CDC group IIi resembles S. multivorum but produces indole. S. parapaucimobilis resembles CDC group O-1 in that both are motile, esculin positive, and positive for hydrogen sulfide (H2S) in lead acetate; however, S. parapaucimobilis oxidizes more carbohydrates (CDC O-1 is weakly positive in OF glucose and negative in OF xylose, maltose, and mannitol).
CDC O-3 bacteria, which are predominantly curved rods, do not produce yellow pigment. They are motile by a single polar flagellum. They grow well on a Campylobacter-selective medium and may be misidentified as a Campylobacter sp. CDC group O-3 are aerobic, glucose-oxidizing organisms that utilize xylose, sucrose, and maltose. They do not grow on MacConkey agar. They are oxidase positive, hydrolyze esculin, and are negative for urease, indole, nitrate, and gelatin. Key characteristics of the CDC groups are shown in Tables 27-3 and 27-4. CDC O-3 has been reported as susceptible to aminoglycosides, imipenem, chloramphenicol, and trimethoprim-sulfamethoxazole and resistant to beta-lactam antimicrobials.
Sphingobacterium mizutaii.
S. mizutaii exhibits II-forms. It can produce a yellow pigment, and it does not grow on MacConkey agar. Although aflagellate and therefore frequently classified as nonmotile, it can be motile by gliding movement. It is able to grow in the presence of 40% bile; it also is oxidase positive, catalase positive, esculin positive, indole negative, and urease negative (although a report exists that 20% are positive for Christensen urease). Key characteristics are shown in Tables 27-3 and 27-5. Reported infections in humans have included septicemia (blood culture), meningitis (CSF specimen), and cellulitis (wound source). This bacterium has been reported to be susceptible to erythromycin, trimethoprim-sulfamethoxazole, and pefloxacin.
Sphingobacterium multivorum.
S. multivorum is yellow pigmented, oxidase positive, and esculin positive. It is OF glucose positive; it does not produce acid from mannitol, ethanol, or rhamnose; and it is Christensen urease positive. This bacterium grows on blood agar plate (BAP), Mueller-Hinton agar, Burkholderia cepacia–selective agar (BCSA), and MacConkey agar. Key characteristics are shown in Table 27-5. These organisms are ubiquitous in nature and rarely associated with serious infection; however, cases of septicemia and peritonitis have been reported. This bacterium is nonmotile and resistant to polymyxin B, characteristics that distinguish it from S. paucimobilis. Susceptibility to amikacin, gentamicin, aztreonam, cefepime, cefotaxime, ceftazidime, meropenem, piperacillin, and chloramphenicol has been reported in a small study of eight isolates.
Sphingobacterium spiritivorum.
S. spiritivorum is yellow pigmented and positive for oxidase and esculin. It does not grow on MacConkey agar but does grow on BAP, Mueller-Hinton agar, and BCSA. It produces acid in OF glucose and in mannitol, ethanol, and rhamnose. Like S. multivorum, this bacterium is ubiquitous in nature but rarely pathogenic for humans. It can be distinguished from S. paucimobilis by the fact that it is nonmotile and resistant to polymyxin B. Key characteristics are shown in Table 27-5. S. spiritivorum has been isolated environmentally from hospitals, most commonly from blood and urine. Susceptibility testing by Kirby-Bauer (KB) disk diffusion on 13 isolates showed susceptibility to amikacin, gentamicin, aztreonam, cefepime, cefotaxime, and chloramphenicol.
Sphingomonas paucimobilis.
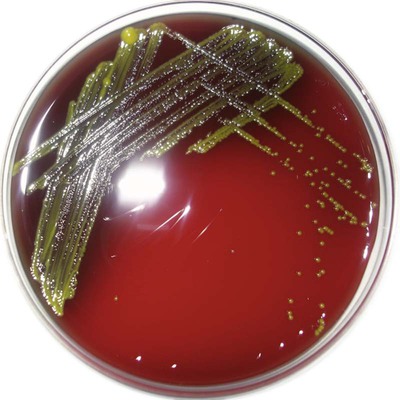
S. paucimobilis is a medium-size, straight, gram-negative rod with a single polar flagellum; growth requires at least 48 hours’ incubation on sheep blood agar (Figure 27-1). Optimal growth occurs at 30°C in 5% CO2 or ambient air; it does grow at 37°C but not at 42°C. It grows as a deep yellow colony on tryptic soy and blood agars. It is obligately aerobic, grows in broth (e.g., brain-heart infusion, thioglycollate, blood culture media), and does not grow on MacConkey agar (90% do not grow; 10% grow as lactose nonfermenters). S. paucimobilis oxidatively utilizes glucose, xylose, and sucrose. Biochemical test results of interest include the following: esculin hydrolysis positive; motile by wet mount or in motility medium when incubated at 18° to 22°C (nonmotile when incubated at 37°C); oxidase positive (90% to 94% positive); catalase positive; urease negative; and indole negative. S. paucimobilis is susceptible to polymyxin B, a trait that distinguishes it from Sphingobacterium spp. Key characteristics are shown in Table 27-6.
Sphingomonas parapaucimobilis.
S. parapaucimobilis is similar to S. paucimobilis in many ways. It is a medium-size, straight, gram-negative rod that grows with a deep yellow pigment. It is obligately aerobic, motile, and does not grow on MacConkey agar. S. parapaucimobilis can be distinguished from S. paucimobilis by several characteristics. S. parapaucimobilis is H2S positive, as indicated by blackening of lead acetate paper suspended over Kligler iron agar (KIA); it is Simmons citrate positive (S. paucimobilis is negative); and it is negative for extracellular DNAse (S. paucimobilis is positive). Like S. paucimobilis, S. parapaucimobilis is acid in OF glucose, OF xylose, and OF maltose but negative in OF mannitol. It has been distinguished from Sphingobacterium spp. by its susceptibility to polymyxin B; however, S. parapaucimobilis is sometimes variable to polymyxin B. Key characteristics are shown in Table 27-6. Antimicrobial susceptibility testing indicates that S. parapaucimobilis displays variable resistance but is usually susceptible to tetracycline, chloramphenicol, sulfamethoxazole, aminoglycosides, third-generation cephalosporins, and fluoroquinolone. S. parapaucimobilis has been associated with human infections; specifically, it has been isolated from sputum, urine, and the vagina.