Specimen Management
1. State four critical parameters that should be monitored in the laboratory from specimen collection to set up and describe the effects each may have on the quality of the laboratory results (e.g., false negatives or positives, inadequate specimen type, incorrect sample).
2. Identify the proper or improper labeling of a specimen, and determine adequacy of a specimen given a patient scenario.
3. Define and differentiate backup broth, nutritive media, and differential and selective media.
4. Describe the oxygenation states (atmospheric conditions) associated with anaerobic, facultative anaerobic, capnophilic, aerobic, and microaerophilic organisms. Provide an example for each.
• Cultivation (growth), identification, and antimicrobial susceptibility testing of microorganisms
• Direct detection of infecting organisms by microscopy
• Direct detection of specific products of infecting organisms using chemical, immunologic, or molecular techniques
• Detection of antibodies produced by the patient in response to an infecting organism (serology)
This chapter presents an overview of issues involved in infectious disease diagnostic testing. Many of these issues are covered in detail in separate chapters.
General Concepts for Specimen Collection and Handling
Appropriate Collection Techniques
• Selection of appropriate anatomic site and specimen
• Collection instructions including type of swab or transport medium
• Transportation instructions including time and temperature
• Labeling instructions including patient demographic information (minimum of two patient identifiers)
• Special instructions such as patient preparation
Instructions should be written so that specimens collected by the patient (e.g., urine, sputum, or stool) are handled properly. Most urine or stool collection kits contain instructions in several languages, but nothing substitutes for a concise set of verbal instructions. Similarly, when distributing kits for sputum collection, the microbiologist should be able to explain to the patient the difference between spitting in a cup (saliva) and producing good lower respiratory secretions from a deep cough (sputum). General collection information is shown in Table 5-1. An in-depth discussion of each type of specimen is found in Part VII.
TABLE 5-1
Collection, Transport, Storage, and Processing of Specimens Commonly Submitted to a Microbiology Laboratory*
| Specimen | Container | Patient Preparation | Special Instructions | Transportation to Laboratory | Storage before Processing | Primary Plating Media | Direct Examination | Comments |
| Abscess (also Lesion, Wound, Pustule, Ulcer) | ||||||||
| Superficial | Aerobic swab moistened with Stuart’s or Amie’s medium | Wipe area with sterile saline or 70% alcohol | Swab along leading edge of wound | < 2 hrs | 24 hrs/RT | BA, CA, Mac, CNA optional | Gram | Add CNA if smear suggests mixed gram- positive and gram-negative flora |
| Deep | Anaerobic transporter | Wipe area with sterile saline or 70% alcohol | Aspirate material from wall or excise tissue | < 2 hrs | 24 hrs/RT | BA, CA, Mac, CNA Anaerobic BBA, LKV, BBE |
Gram | Wash any granules and “emulsify” in saline |
| Blood or Bone Marrow Aspirate | ||||||||
| Blood culture media set (aerobic and anaerobic bottle) or Vacutainer tube with SPS | Disinfect venipuncture site with 70% alcohol and disinfectant such as Betadine | Draw blood at time of febrile episode; draw two sets from right and left arms; do not draw more than three sets in a 24-hr period; draw ≥20 ml/set (adults) or 1-20 ml/set (pediatric) depending on patient’s weight | Within 2 hrs/RT | Must be incubated at 37° C on receipt in laboratory | Blood culture bottles may be used. BA, CA BBA-anaerobic | Direct gram Stain from positive blood culture bottles | Other considerations: brucellosis, tularemia, cell wall–deficient bacteria, leptospirosis, or AFB | |
| Body Fluids | ||||||||
| Amniotic, abdominal, ascites (peritoneal), bile, joint (synovial), pericardial, pleural | Sterile, screw-cap tube or anaerobic transporter or direct inoculation into blood culture bottles | Disinfect skin before aspirating specimen | Needle aspiration | < 15 min | Plate as soon as received Blood culture bottles incubate at 37° C on receipt in laboratory |
May use an aerobic and anaerobic blood culture bottle set for body fluids BA, CA, thio CNA, Mac (Peritoneal) BBA, BBE, LKV anaerobic |
Gram (vaginal fluid is recommended) | May need to concentrate by centrifugation or filtration —stain and culture sediment |
| Bone | ||||||||
| Sterile, screw-cap container | Disinfect skin before surgical procedure | Take sample from affected area for biopsy | Immediately/RT | Plate as soon as received | BA, CA, Mac, thio | Gram | May need to homogenize | |
| Cerebrospinal Fluid | ||||||||
| Sterile, screw-cap tube | Disinfect skin before aspirating specimen | Consider rapid testing (e.g., Gram stain; cryptococcal antigen) | < 15 min | < 24 hrs Routine Incubate at 37° C except for viruses, which can be held at 4° C for up to 3 days | BA, CA (Routine) BA, CA, thio (shunt) |
Gram—best sensitivity by cytocentrifugation (may also want to do AO if cytocentrifuge not available) | Add thio for CSF collected from shunt | |
| Ear | ||||||||
| Inner | Sterile, screw-cap tube or anaerobic transporter | Clean ear canal with mild soap solution before myringotomy (puncture of the ear drum) | Aspirate material behind drum with syringe if ear drum intact; use swab to collect material from ruptured ear drum | < 2 hrs | 24 hrs/RT | BA, CA, Mac (add thio if prior antimicrobial therapy) BBA-(anaerobic) |
Gram | Add anaerobic culture plates for tympanocentesis specimens |
| Outer | Aerobic swab moistened with Stuart’s or Amie’s medium | Wipe away crust with sterile saline | Firmly rotate swab in outer canal | < 2 hrs/RT | 24 hrs/RT | BA, CA, Mac | Gram | |
| Eye | ||||||||
| Conjunctiva | Aerobic swab moistened with Stuart’s or Amie’s medium | Sample both eyes; use swab premoistened with sterile saline | < 2 hrs/RT | 24 hrs/RT | BA, CA, Mac | Gram, AO, histologic stains (e.g., Giemsa) | Other considerations: Chlamydia trachomatis, viruses, and fungi | |
| Aqueous/vitreous fluid | Sterile, screw cap tube | < 15 min/RT | Set up immediately on receipt | BA, Mac, 7H10, Ana | Gram/AO | |||
| Corneal scrapings | Bedside inoculation of BA, CA, SDA, 7H10, thio | Clinician should instill local anesthetic before collection | < 15 min/RT | Must be incubated at 28° C (SDA) or 37° C (everything else) on receipt in laboratory | BA, CA, SDA, 7H10, Ana, thio | Gram/AO The use of 10-mm frosted ring slides assists with location of specimen due to the size of the specimen |
Other considerations: Acanthamoeba spp., herpes simplex virus and other viruses, Chlamydia trachomatis, and fungi | |
| Foreign Bodies | ||||||||
| IUD | Sterile, screw-cap container | Disinfect skin before removal | < 15 min/RT | Plate as soon as received | Thio | |||
| IV catheters, pins, | Sterile, screw-cap container | Disinfect skin before removal | Do not culture Foley catheters; IV catheters are cultured quantitatively by rolling the segment back and forth across agar with sterile forceps four times; ≥15 colonies are associated with clinical significance | < 15 min/RT | Plate as soon as received if possible store < 2 hrs 4° C | BA, Thio prosthetic valves | ||
| GI Tract | ||||||||
| Gastric aspirate | Sterile, screw-cap tube | Collect in early AM before patient eats or gets out of bed | Most gastric aspirates are on infants or for AFB | < 15 min/RT | Must be neutralized with sodium bicarbonate within 1 hr of collection | BA, CA, Mac, HE, CNA, EB | Gram/AO | Other considerations: AFB |
| Gastric biopsy | Sterile, screw-cap tube (normal saline < 2 hrs transport medium recomended) | Rapid urease test or culture for Helicobacter pylori | < 1 hr/RT | 24 hrs/4° C | Skirrow’s, BA, BBA | H&E stain optional: Immunostaining | Other considerations: urea breath test Antigen test (H. pylori ) |
|
| Rectal swab | Swab placed in enteric transport medium | Insert swab ~ 2.5 cm past anal sphincter; feces should be visible on swab | Within 24 hrs/RT | < 48 hrs/RT or store 4° C | BA, Mac, XLD HE, Campy, EB | Methylene blue for fecal leukocytes | Other considerations: Vibrio, Yersinia enterocolitica, Escherichia coli O157:H7 | |
| Stool culture | Clean, leak-proof container; transfer feces to enteric transport medium (Cary-Blair) if transport will exceed 1 hr | Routine culture should include Salmonella, Shigella, and Campylobacter; specify Vibrio, Aeromonas, Plesiomonas, Yersinia, Escherichia coli O157:H7, if needed Follow-up may include Shiga toxin assay as recommened by CDC |
Within 24 hrs/RT Unpreserved < 1 hr/RT |
72 hrs/4° C | BA, Mac, XLD, HE, Campy, EB, optional: Mac-S; Chromogenic agar | Methylene blue for fecal leukocytes Optional: Shiga toxin testing |
See considerations in previous rectal swabs Do not perform routine stool cultures for patients whose length of stay in the hospital exceeds 3 days and whose admitting diagnosis was not diarrhea; these patients should be tested for Clostridium difficile |
|
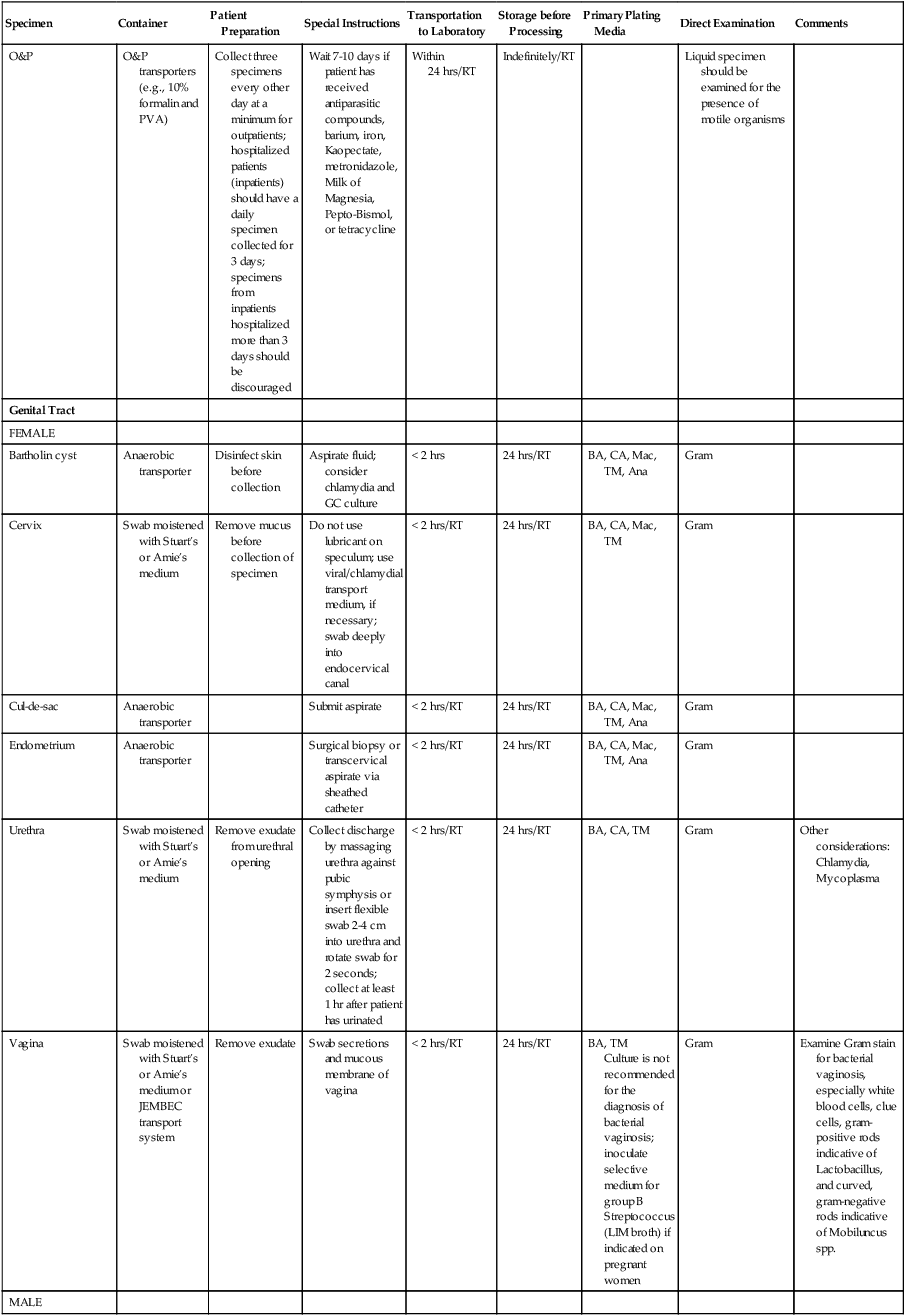
| O&P | O&P transporters (e.g., 10% formalin and PVA) | Collect three specimens every other day at a minimum for outpatients; hospitalized patients (inpatients) should have a daily specimen collected for 3 days; specimens from inpatients hospitalized more than 3 days should be discouraged | Wait 7-10 days if patient has received antiparasitic compounds, barium, iron, Kaopectate, metronidazole, Milk of Magnesia, Pepto-Bismol, or tetracycline | Within 24 hrs/RT | Indefinitely/RT | Liquid specimen should be examined for the presence of motile organisms | ||
| Genital Tract | ||||||||
| FEMALE | ||||||||
| Bartholin cyst | Anaerobic transporter | Disinfect skin before collection | Aspirate fluid; consider chlamydia and GC culture | < 2 hrs | 24 hrs/RT | BA, CA, Mac, TM, Ana | Gram | |
| Cervix | Swab moistened with Stuart’s or Amie’s medium | Remove mucus before collection of specimen | Do not use lubricant on speculum; use viral/chlamydial transport medium, if necessary; swab deeply into endocervical canal | < 2 hrs/RT | 24 hrs/RT | BA, CA, Mac, TM | Gram | |
| Cul-de-sac | Anaerobic transporter | Submit aspirate | < 2 hrs/RT | 24 hrs/RT | BA, CA, Mac, TM, Ana | Gram | ||
| Endometrium | Anaerobic transporter | Surgical biopsy or transcervical aspirate via sheathed catheter | < 2 hrs/RT | 24 hrs/RT | BA, CA, Mac, TM, Ana | Gram | ||
| Urethra | Swab moistened with Stuart’s or Amie’s medium | Remove exudate from urethral opening | Collect discharge by massaging urethra against pubic symphysis or insert flexible swab 2-4 cm into urethra and rotate swab for 2 seconds; collect at least 1 hr after patient has urinated | < 2 hrs/RT | 24 hrs/RT | BA, CA, TM | Gram | Other considerations: Chlamydia, Mycoplasma |
| Vagina | Swab moistened with Stuart’s or Amie’s medium or JEMBEC transport system | Remove exudate | Swab secretions and mucous membrane of vagina | < 2 hrs/RT | 24 hrs/RT | BA, TM Culture is not recommended for the diagnosis of bacterial vaginosis; inoculate selective medium for group B Streptococcus (LIM broth) if indicated on pregnant women |
Gram | Examine Gram stain for bacterial vaginosis, especially white blood cells, clue cells, gram-positive rods indicative of Lactobacillus, and curved, gram-negative rods indicative of Mobiluncus spp. |
| MALE | ||||||||
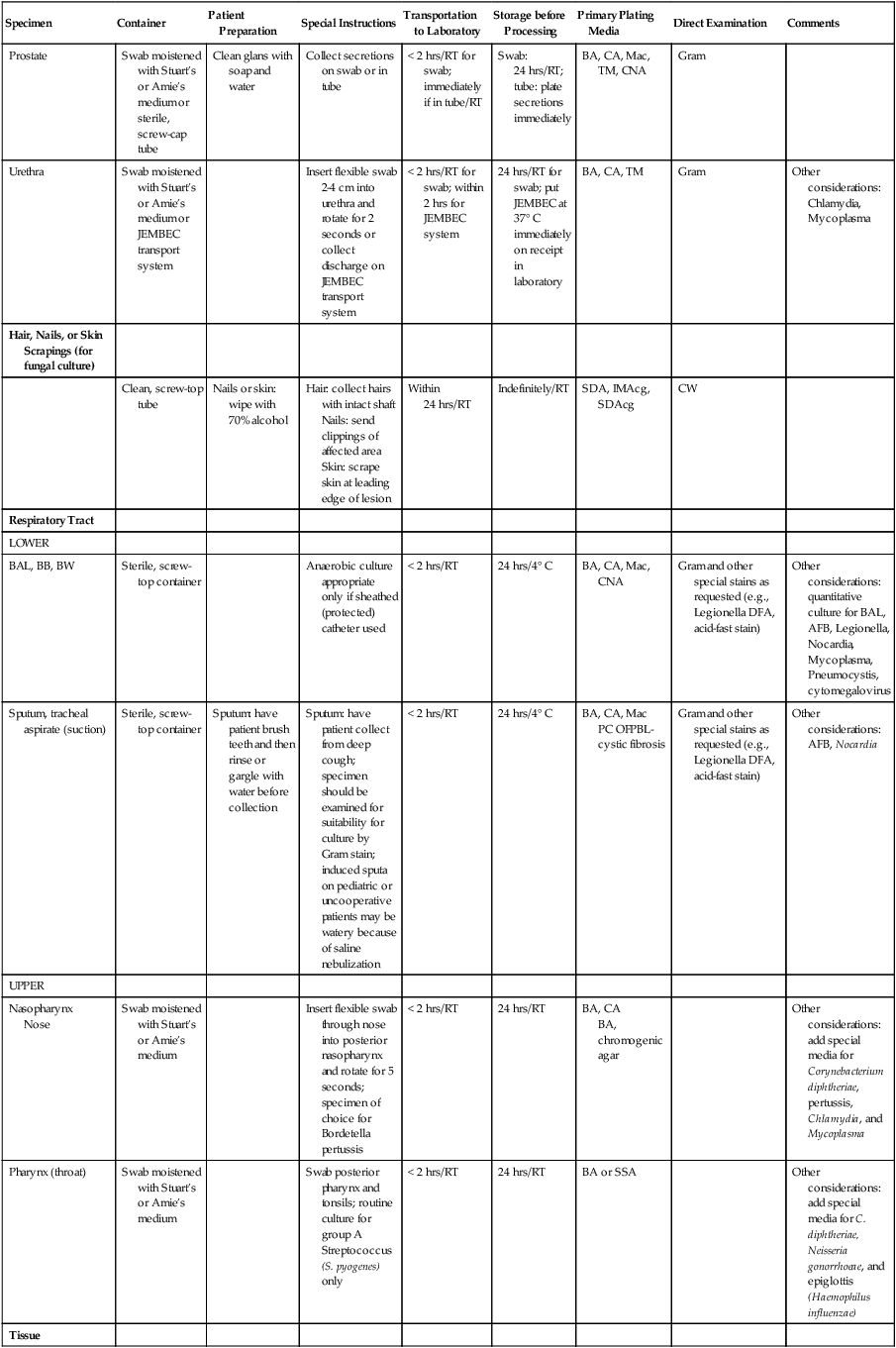
| Prostate | Swab moistened with Stuart’s or Amie’s medium or sterile, screw-cap tube | Clean glans with soap and water | Collect secretions on swab or in tube | < 2 hrs/RT for swab; immediately if in tube/RT | Swab: 24 hrs/RT; tube: plate secretions immediately | BA, CA, Mac, TM, CNA | Gram | |
| Urethra | Swab moistened with Stuart’s or Amie’s medium or JEMBEC transport system | Insert flexible swab 2-4 cm into urethra and rotate for 2 seconds or collect discharge on JEMBEC transport system | < 2 hrs/RT for swab; within 2 hrs for JEMBEC system | 24 hrs/RT for swab; put JEMBEC at 37° C immediately on receipt in laboratory | BA, CA, TM | Gram | Other considerations: Chlamydia, Mycoplasma | |
| Hair, Nails, or Skin Scrapings (for fungal culture) | ||||||||
| Clean, screw-top tube | Nails or skin: wipe with 70% alcohol | Hair: collect hairs with intact shaft Nails: send clippings of affected area Skin: scrape skin at leading edge of lesion |
Within 24 hrs/RT | Indefinitely/RT | SDA, IMAcg, SDAcg | CW | ||
| Respiratory Tract | ||||||||
| LOWER | ||||||||
| BAL, BB, BW | Sterile, screw-top container | Anaerobic culture appropriate only if sheathed (protected) catheter used | < 2 hrs/RT | 24 hrs/4° C | BA, CA, Mac, CNA | Gram and other special stains as requested (e.g., Legionella DFA, acid-fast stain) | Other considerations: quantitative culture for BAL, AFB, Legionella, Nocardia, Mycoplasma, Pneumocystis, cytomegalovirus | |
| Sputum, tracheal aspirate (suction) | Sterile, screw-top container | Sputum: have patient brush teeth and then rinse or gargle with water before collection | Sputum: have patient collect from deep cough; specimen should be examined for suitability for culture by Gram stain; induced sputa on pediatric or uncooperative patients may be watery because of saline nebulization | < 2 hrs/RT | 24 hrs/4° C | BA, CA, Mac PC OFPBL-cystic fibrosis |
Gram and other special stains as requested (e.g., Legionella DFA, acid-fast stain) | Other considerations: AFB, Nocardia |
| UPPER | ||||||||
| Nasopharynx Nose |
Swab moistened with Stuart’s or Amie’s medium | Insert flexible swab through nose into posterior nasopharynx and rotate for 5 seconds; specimen of choice for Bordetella pertussis | < 2 hrs/RT | 24 hrs/RT | BA, CA BA, chromogenic agar |
Other considerations: add special media for Corynebacterium diphtheriae, pertussis, Chlamydia, and Mycoplasma | ||
| Pharynx (throat) | Swab moistened with Stuart’s or Amie’s medium | Swab posterior pharynx and tonsils; routine culture for group A Streptococcus (S. pyogenes) only | < 2 hrs/RT | 24 hrs/RT | BA or SSA | Other considerations: add special media for C. diphtheriae, Neisseria gonorrhoeae, and epiglottis (Haemophilus influenzae) | ||
| Tissue | ||||||||
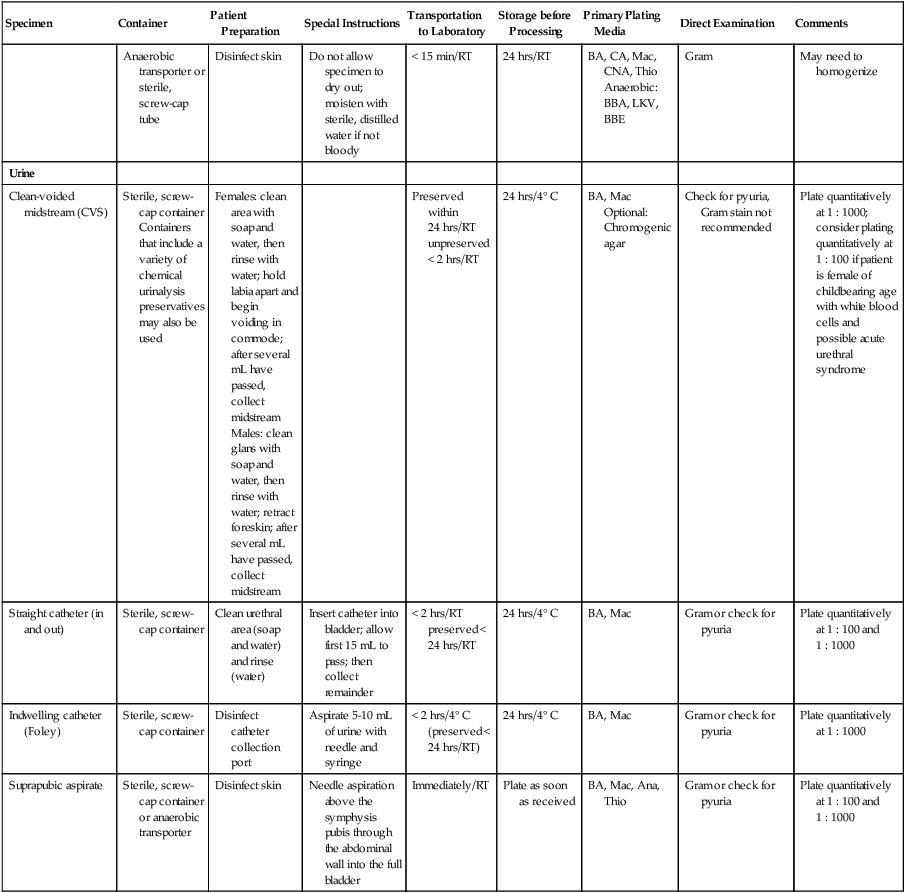
| Anaerobic transporter or sterile, screw-cap tube | Disinfect skin | Do not allow specimen to dry out; moisten with sterile, distilled water if not bloody | < 15 min/RT | 24 hrs/RT | BA, CA, Mac, CNA, Thio Anaerobic: BBA, LKV, BBE |
Gram | May need to homogenize | |
| Urine | ||||||||
| Clean-voided midstream (CVS) | Sterile, screw-cap container Containers that include a variety of chemical urinalysis preservatives may also be used |
Females: clean area with soap and water, then rinse with water; hold labia apart and begin voiding in commode; after several mL have passed, collect midstream Males: clean glans with soap and water, then rinse with water; retract foreskin; after several mL have passed, collect midstream |
Preserved within 24 hrs/RT unpreserved < 2 hrs/RT | 24 hrs/4° C | BA, Mac Optional: Chromogenic agar |
Check for pyuria, Gram stain not recommended | Plate quantitatively at 1 : 1000; consider plating quantitatively at 1 : 100 if patient is female of childbearing age with white blood cells and possible acute urethral syndrome | |
| Straight catheter (in and out) | Sterile, screw-cap container | Clean urethral area (soap and water) and rinse (water) | Insert catheter into bladder; allow first 15 mL to pass; then collect remainder | < 2 hrs/RT preserved < 24 hrs/RT |
24 hrs/4° C | BA, Mac | Gram or check for pyuria | Plate quantitatively at 1 : 100 and 1 : 1000 |
| Indwelling catheter (Foley) | Sterile, screw-cap container | Disinfect catheter collection port | Aspirate 5-10 mL of urine with needle and syringe | < 2 hrs/4° C (preserved < 24 hrs/RT) | 24 hrs/4° C | BA, Mac | Gram or check for pyuria | Plate quantitatively at 1 : 1000 |
| Suprapubic aspirate | Sterile, screw-cap container or anaerobic transporter | Disinfect skin | Needle aspiration above the symphysis pubis through the abdominal wall into the full bladder | Immediately/RT | Plate as soon as received | BA, Mac, Ana, Thio | Gram or check for pyuria | Plate quantitatively at 1 : 100 and 1 : 1000 |
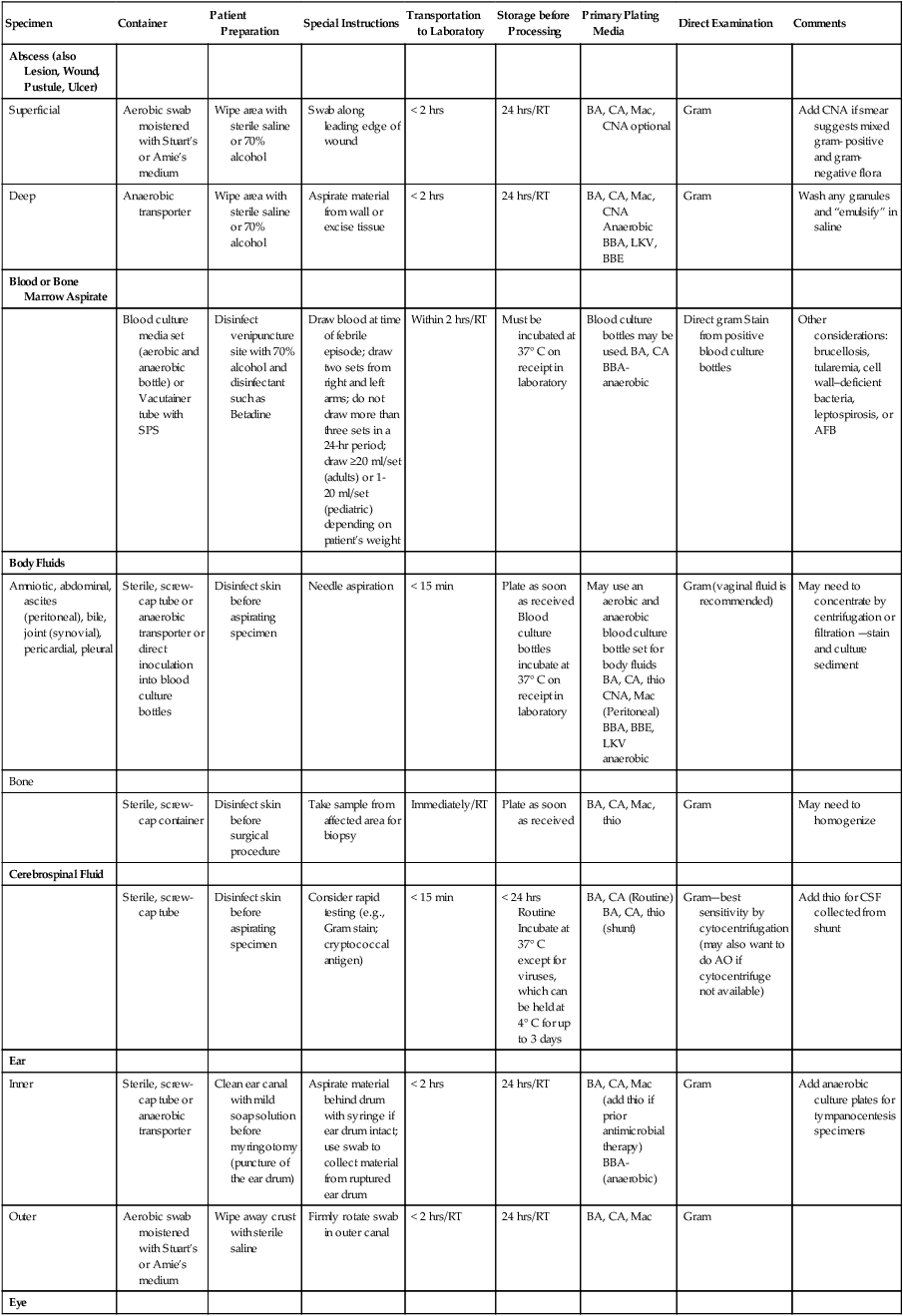
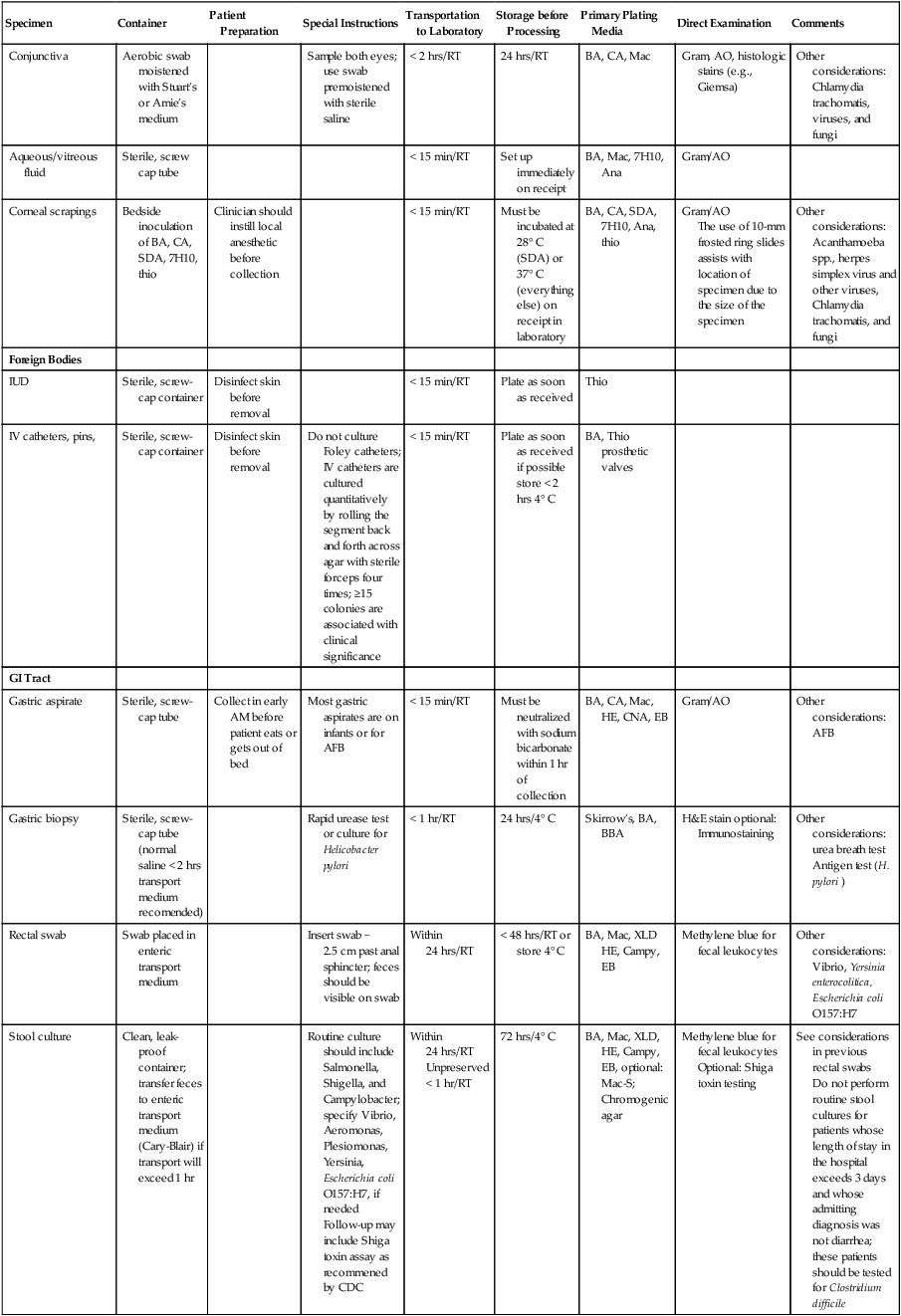
7H10, Middlebrook 7H10 agar; AFB, acid-fast bacilli; AM, morning; Ana, anaerobic agars as appropriate (see Chapter 41); AO, acridine orange stain; BA, blood agar; BAL, bronchial alveolar lavage; BB, bronchial brush; BBA, brucella blood agar; BBE, Bacteroides bile esculin agar; BW, bronchial wash; CA, chocolate agar; Campy, selective Campylobacter agar; CNA, Columbia agar with colistin and nalidixic acid; CW, calcofluor white stain; DFA, direct fluorescent antibody stain; EB, enrichment broth; GC, Neisseria gonorrhoeae; transport using JEMBEC system with modified Thayer-Martin; GI, gastrointestinal; Gram, Gram stain; HBT, human blood-bilayer Tween agar; HE, Hektoen enteric agar; hrs, hours; IMAcg, inhibitory mold agar with chloramphenicol and gentamicin; IUD, intrauterine device; LKV, laked blood agar with kanamycin and vancomycin; Mac, MacConkey agar; Mac-S, MacConkey-sorbitol; mL, milliliters; OFPBL, oxdative-fermentative polymixin B-bacitracin-lactose-agar; O&P, ova and parasite examination; PC, Pseudomonas cepacia agar; PVA, polyvinyl alcohol; RT, room temperature; SDA, Sabouraud dextrose agar; SDAcg, Sabouraud; dextrose agar with cycloheximide and gentamicin; SPS, sodium polyanethol sulfonate; SSA, group A streptococcus selective agar; thio, thioglycollate broth; TM, Thayer-Martin agar; XLD, xylose lysine deoxycholate agar.
*Specimens for viruses, chlamydia, and mycoplasma are usually submitted in appropriate transport media at 4° C to stabilize respective microorganisms.
Specimen Transport
Ideally, specimens should be transported to the laboratory within 2 hours of collection. All specimen containers should be leak-proof, and the specimens should be transported within sealable, leak-proof, plastic bags with a separate section for paperwork; resealable bags or bags with a permanent seal are common for this purpose. Bags should be marked with a biohazard label (Figure 5-1). Many microorganisms are susceptible to environmental conditions such as the presence of oxygen (anaerobic bacteria), changes in temperature (Neisseria meningitidis), or changes in pH (Shigella). Thus, use of special preservatives or holding media for transportation of specimens delayed for more than 2 hours is important to ensure organism viability (survival).
Specimen Preservation
Specimen Storage
If specimens cannot be processed as soon as they are received, they must be stored (see Table 5-1). Several storage methods are used (refrigerator temperature [4° C], ambient [room] temperature [22° C], body temperature [37° C], and freezer temperature [either –20° or –70° C]), depending on the type of transport media (if applicable) and the etiologic (infectious) agents suspected. Specimens should never be stored in the refrigerator and should remain at room temperature. Urine, stool, viral specimens, sputa, swabs, and foreign devices such as catheters should be stored at 4° C. Serum for serologic studies may be frozen for up to 1 week at –20° C, and tissues or specimens for long-term storage should be frozen at –70° C.
Rejection of Unacceptable Specimens
• The information on the label does not match the information on the requisition or the specimen is not labeled at all (patient’s name or source of specimen is different).
• The specimen has been transported at the improper temperature.
• The specimen has not been transported in the proper medium (e.g., specimens for anaerobic bacteria submitted in aerobic transports).
• The quantity of specimen is insufficient for testing (the specimen is considered quantity not sufficient [QNS]).
• The specimen transport time exceeds 2 hours postcollection or the specimen is not preserved.
• The specimen was received in a fixative (formalin), which, in essence, kills any microorganism present.
• The specimen has been received for anaerobic culture from a site known to have anaerobes as part of the normal flora (vagina, mouth).
• Processing the specimen would produce information of questionable medical value (e.g., Foley catheter tip).
Direct Microscopic Examination
The most common stain in bacteriology is the Gram stain, which helps the clinician to visualize rods, cocci, white blood cells, red blood cells, or squamous epithelial cells present in the sample. The most common direct fungal stains are KOH (potassium hydroxide), PAS (periodic-acid Schiff), GMS (Grocott’s methenamine silver stain), and calcofluor white. The most common direct acid-fast stains are AR (auramine rhodamine), ZN (Ziehl-Neelsen), and Kinyoun. Chapter 6 describes the use of microscopy in clinical diagnosis in more detail.
Selection of Culture Media
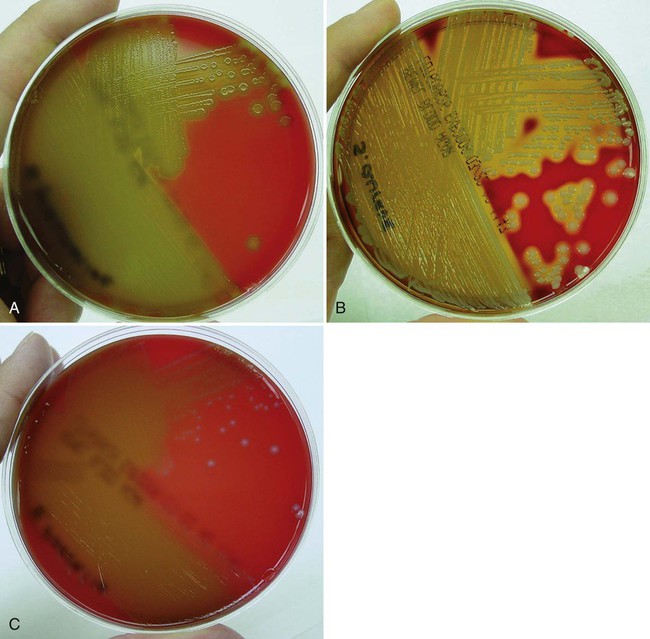
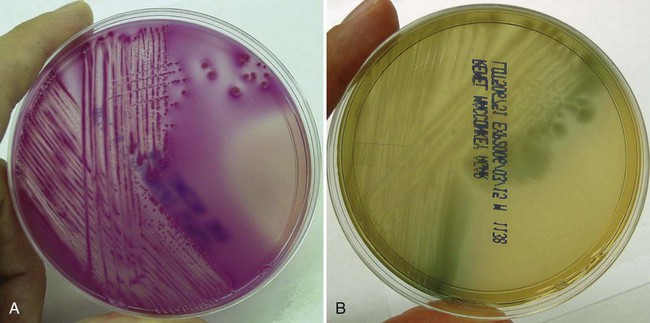
Primary culture media are divided into several categories. The first are nutritive media, such as blood or chocolate agars. Nutritive media support the growth of a wide range of microorganisms and are considered nonselective because, theoretically, the growth of most organisms is supported. Nutritive media can also be differential, in that microorganisms can be distinguished on the basis of certain growth characteristics evident on the medium. Blood agar is considered both a nutritive and differential medium because it differentiates organisms based on whether they are alpha (α)-, beta (β)-, or gamma (γ)- hemolytic (Figure 5-2). Selective media support the growth of one group of organisms, but not another, by adding antimicrobials, dyes, or alcohol to a particular medium. MacConkey agar, for example, contains the dye crystal violet, which inhibits gram-positive organisms. Columbia agar with colistin and nalidixic acid (CNA) is a selective medium for gram-positive organisms because the antimicrobials colistin and nalidixic acid inhibit gram-negative organisms. Selective media can also be differential media if, in addition to their inhibitory activity, they differentiate between groups of organisms. MacConkey agar, for example, differentiates between lactose-fermenting and nonfermenting gram-negative rods by the color of the colonial growth (pink or clear, respectively); this is shown in Figure 5-3. In some cases (sterile body fluids, tissues, or deep abscesses in a patient on antimicrobial therapy), backup broth (also called supplemental or enrichment broth) medium is inoculated, along with primary solid (agar) media, so small numbers of organisms present may be detected; this allows detection of anaerobes in aerobic cultures and organisms that may be damaged by either previous or concurrent antimicrobial therapy. Thioglycollate (thio) broth, brain-heart infusion broth (BHIB), and tryptic soy broth (TSB) are common backup broths.
Routine primary plating media and direct examinations for specimens commonly submitted to the microbiology laboratory are shown in Table 5-1. Chapter 7 on bacterial cultivation reemphasizes the strategies described here for selection and use of bacterial media.
Inoculation on Solid Media
Specimens can be inoculated (plated) onto solid media either quantitatively by a dilution procedure or by means of a quantitative loop, or semiquantitatively using an ordinary inoculating loop. Urine cultures and tissues from burn victims are plated quantitatively; everything else is usually plated semiquantitatively. Plates inoculated for quantitation are usually streaked with a 1 : 100 or 1 : 1000 loop. Plates inoculated for semiquantitation are usually streaked out in four quadrants. Detailed methods for streaking solid media are provided in Chapter 7, Figure 7-9. Semiquantitation is referred to as streaking for isolation, because the microorganisms present in the specimen are successively diluted out as each quadrant is streaked until finally each morphotype is present as a single colony. Numbers of organisms present can subsequently be graded as 4+ (many, heavy growth) if growth is out to the fourth quadrant, 3+ (moderate growth) if growth is out to the third quadrant, 2+ (few or light growth) if growth is in the second quadrant, and 1+ (rare) if growth is in the first quadrant. This tells the clinician the relative numbers of different organisms present in the specimen; such semiquantitative information is usually sufficient for the physician to be able to treat the patient.
Specimen Workup
Communication of Laboratory Findings
Laboratory newsletters should be used to provide physicians with material such as details of new procedures, nomenclature changes, and changes in usual antimicrobial susceptibility patterns of frequently isolated organisms. This last information, discussed in more detail in Chapter 12 is very useful to clinicians when selecting empiric therapy. Empiric therapy is based on the physician determining the most likely organism causing a patient’s clinical symptoms and then selecting an antimicrobial that, in the past, has worked against that organism in a particular hospital or geographic area. Empiric therapy is used to start patients on treatment before the results of the patient’s culture are known and may be critical to the patient’s well-being in cases of life-threatening illnesses.
Critical (Panic) Values
• Positive spinal fluid Gram stain or culture
• Streptococcus pyogenes (group A Streptococcus) in a surgical wound
• Gram stain suggestive of gas gangrene (large boxcar-shaped gram-positive rods)
• Blood smear positive for malaria
• Positive cryptococcal antigen test
• Detection of a select agent (e.g., Brucella) or other significant pathogen (e.g., Legionella, vancomycin-resistant S. aureus, or other antibiotic-resistant organisms as outlined by the facility and infection control policies).