Somatic Growth and Maturation
Determinants of Normal Growth
The body grows primarily through proliferation of cells by mitosis.1,2 In contrast, increased cell size generally plays a greater role in organ growth as development approaches completion. Growth factors and other environmental signals are necessary for the entry of a quiescent cell into the cell cycle, and they affect cell division by modulating passage through the first phase of the mitotic cell cycle (G1).3,4 The first subphase of G1 requires “competence factors,” such as fibroblast growth factor, which induces cells to become competent to synthesize DNA. Cells then require essential amino acids to progress to a critical point in the cycle at which “progression factors” can induce completion of G1. Progression factors are exemplified by insulin-like growth factors (IGFs), insulin, thyroxine, and hydrocortisone. Growth factors modulate the internal regulatory pathways governed by cyclins and cyclin-dependent kinases (CDKs), which are proto-oncogenes, and CDK inhibitors (CDKI), which are tumor suppressors. Specifically, growth factors lead to accumulation of the D-type cyclins, which sense and mediate growth factor stimulation in G1. The binding of cyclin D with CDK 4/6 results in phosphorylation of “pocket” proteins (pRb, p107, and p130) and release of inhibitory control of these proteins on the E2F family transcription factors, which then drive expression of various effectors of DNA synthesis.5 It is important to note that free E2F initiates a positive feedback loop by increasing cyclin E transcription and protein stabilization, because binding of cyclin E with CDK2 also phosphorylates pocket proteins and causes further release of E2F. The balance between cyclin, CDK, and CDKI activity therefore determines the start of DNA synthesis (S phase of the cell cycle). From this point onward, cell-cycle processes depend entirely on intracellularly triggered controls involving cyclins. After completing DNA synthesis, the cell finishes doubling its entire contents (G2 phase) and then undergoes the M phase of the cycle, during which cell division is completed.
The cell cycle to a great extent is also regulated by nuclear factor-κB (NF-κB).6 This transcription factor is held inactive in the cytoplasm when bound to its inhibitory partner Iκβ. When Iκβ undergoes regulated serine phosphorylation, it is polyubiquinated, which targets it for degradation within the proteosome. This releases NF-κB to move to the nucleus, where it promotes cell-cycle progression and inhibits programmed cell death (apoptosis).
Cell senescence is the response of the cell to conditions such as telomere attrition, DNA damage, and oncogene activation—processes that appear to be interrelated. During each mitotic cycle, a portion of the terminal end (the telomere) of each chromosome is lost; this eventually shortens the chromosome to the point where cell proliferation becomes impossible and the cell dies.7 The enzyme telomerase supports the synthesis of telomeric DNA, which maintains telomere length and proliferative potential. Telomerase is present in somatic cells of the fetus, permitting continued growth. With maturation of the fetus, however, telomerase levels begin to fall and decline progressively with aging, thus limiting mortality.
Somatic Growth
Prenatal and postnatal requirements for growth differ in several respects. Embryonic growth is determined primarily by genetic programming of local sequential inductions.8 Coordination of cell differentiation and morphogenesis requires a class of developmental genes that belong to the homeobox family.9–11 Homeobox genes encode transcription factors that bind DNA, thereby controlling gene expression, cell differentiation, and organ development. Abnormalities of several homeobox genes are known to cause organ malformation and to affect linear growth. Fetal growth depends heavily on the delivery of nutrients, metabolic substrates, and oxygen, as well as IGFs, from the mother. The placenta regulates many of these and contributes to the fetal nutritional and hormonal milieu.12–14 Placental size is a determinant of fetal growth, and placental growth itself is influenced by genomic factors,14,15 growth factors, maternal weight and nutrition (perhaps via leptin),16 parity, parental size,17 and uterine blood flow. Placental regulation of fetal blood flow is, in turn, a determinant of growth; discordance in the size of monozygous twins has been attributed to the unequal distribution of blood flow that results from placental arteriovenous anastomoses.18
Both placental growth and function involve hormones. Specific deletion of placental IGF-2, which is an imprinted gene expressed from the paternal allele, sequentially reduces placental growth, decreases nutrient delivery, and restricts fetal growth.14 The placenta also influences fetal growth through its elaboration of hormones. For example, human placental lactogen seems to influence regulation of fetal IGF-1,19 although the role of the placental variant of GH in regulating fetal IGF-1 and growth remains unclear.20,21 Umbilical cord leptin appears to be an index of fetal nutrition in humans and correlates with birth size independent of IGF-1.22,23
Familial and environmental variables that predict birth size independent of gestational age include parental heights, sibling birth weight, maternal weight, parity, glycemic status and history of smoking, altitude, gender, and uterine constraints, such as the number of fetuses carried.17,24–30
Some of the hormonal requirements for fetal growth differ from those that regulate postnatal growth. For example, prenatal growth is less dependent on GH and thyroxine. Individuals with congenital GH deficiency or resistance often have normal birth length despite low IGF-1 levels, although in large population studies, average birth length is reduced by 1 standard deviation (SD).12,31,32 Similarly, newborns with congenital hypothyroidism typically have normal birth size, although their bone maturation lags during the last trimester.33
In contrast, the IGF system affects prenatal and postnatal growth, although specific influences and regulatory components differ according to stage of development. Immunoreactive IGF is present in most fetal tissues. Rodent models that lack IGF-1, IGF-2, or the IGF-1 receptor have reduced birth weights, suggesting a role for both IGF-1 and IGF-2 in prenatal growth.34–37 IGF-1 and IGF-2 act through the IGF-1, insulin, and IGF-2 receptors, and IGF-2 is approximately equal in importance to IGF-1 for fetal growth, each contributing about 40%.37,38 IGF-2 abundance in early pregnancy promotes fetal growth and viability near term, primarily through effects of IGF-2 on placental growth and differentiation.39 IGF-1, in contrast, is important for fetal but not placental growth, and its effects are mediated by decreased release of vasoconstrictors, thus optimizing placental blood flow and nutrient delivery to the fetus. Six IGF binding proteins (IGFBPs) regulate the amount of free IGF available and are regulated in turn by IGFBP proteases. High levels of IGFBPs have been associated with fetal growth inhibition, likely from sequestration of fetally derived IGF-1.40–43 IGFBP-3 also prolongs the half-life of IGFs in circulation, and levels are reduced in the cord serum of small for gestational age (SGA) fetuses.44
Pregnancy-associated plasma protein A (PAPP-A), which cleaves IGFBP-4, increases free IGF availability,45 and low first-trimester PAPP-A levels in pregnant women are associated with lower birth weight.46
In contrast to fetal growth, postnatal growth is less dependent on IGF-2 than on IGF-1 levels. Whereas animals lacking IGF-2 that survive may have relatively normal postnatal growth, those with IGF-1 deficiency remain stunted. During fetal life, IGF-1 is relatively independent of GH and is regulated greatly by nutritional status (particularly glucose availability and consequent fetal insulin secretion) and placental lactogen, whereas postnatal levels are dependent on both GH and nutritional status.8,12,19,31 Although the serum IGF level is even lower prenatally than in infancy, it rises during gestation and correlates with size at birth.12 IGF-2 blood levels are higher than those of IGF-1 in utero, in contrast to postnatal life. Human correlates, substantiating the role of IGFs in prenatal growth, are the identification of a patient with homozygous partial deletion of the gene encoding IGF-1 and identification of patients with IGF-1 receptor mutations with severe intrauterine growth retardation, as well as postnatal growth failure.47
In addition to IGFs, insulin influences fetal growth. Infants of diabetic mothers and children with Beckwith-Wiedemann syndrome (with hyperinsulinism) have excessive fetal growth, and those with pancreatic agenesis have poor fetal growth. Mutations in the insulin receptor and in IRS-1, a downstream molecule in the signaling pathways of both IGF-1 and insulin receptors, are also associated with suboptimal fetal growth and insulin resistance.48
Sex hormones may play a subtle role in normal fetal growth: Plasma levels of testosterone, estradiol, and dehydroepiandrosterone are at or above pubertal levels by mid-gestation; estrogen promotes fetal bone development49; and androgen action seems to account for the greater birth weight of boys compared with girls.8,31
An understanding of the regulation of fetal growth has assumed particular importance because of potential links between prenatal growth and later disease. Strong experimental evidence in animal models indicates that an adverse fetal environment, as reflected in birth size, can lead to a poor health outcome in adults.50 Diverse causes of poor fetal growth (including maternal undernutrition or glucocorticoid exposure) can have similar deleterious effects on postnatal health (including hypertension, cardiovascular disease, and glucose intolerance). Considerable human data support such a “fetal origins of adult disease” model.36 The risks for insulin resistance, diabetes, and visceral adiposity are particularly high in SGA infants with rapid postnatal “catch-up growth.”51–53 Although the underlying mechanisms are not fully understood, evidence points to the development of a “thrifty” phenotype, with fetal metabolism adapting to undernutrition through epigenetic modifications in key genes (including the glucocorticoid receptors, peroxisome-proliferator–activated receptor [PPAR-α], Pdx1, and Glut4), which persists into adulthood.54–56 Another proposed mechanism invokes a defect in placental inactivation of maternal cortisol due to decreased 11β-hydroxysteroid dehydrogenase activity, leading to elevated fetal cortisol and reprogramming of the hypothalamic-pituitary-adrenal axis for hyperresponsiveness to stress.57 Dehydroepiandrosterone (DHEAS) and androstenedione levels have been reported to be higher in SGA children,58 and early pubarche and polycystic ovary syndrome (PCOS) may be important sequelae of SGA.59 In addition, a reduction in the total number of nephrons as a consequence of intrauterine growth retardation with compensatory effects influencing the renin-angiotensin system may predispose these individuals to subsequent hypertension.60
Postnatal Growth
Familial and environmental determinants of early postnatal growth include gestational age at delivery, birth size, parental heights, socioeconomic status, and breastfeeding.17
Genetic determinants exist for both postnatal and prenatal growth.61 Genes on the Y chromosome seem to enhance stature commencing in antenatal life,61,62 and the X chromosome carries genetic determinants, including the SHOX gene (see Skeletal Dysplasias below), which promotes linear growth and regulates body proportions.61,63 The epigenetic process of genomic imprinting, like X-inactivation, is due to methylation of genes to silence them,15 and clusters of autosomal “imprinted” genes also regulate growth. Although the exact nature of most imprinted genes is unknown, the genes for IGF-2 and its receptor on chromosome 15 are imprinted. The IGF-2 gene is silenced in eggs and thus is maternally imprinted, whereas the IGF-2 receptor is paternally imprinted. Other genes that regulate height include those implicated in Noonan syndrome64 and other genetic causes of short stature (described later). In addition, genome-wide analysis has allowed the identification of single nucleotide polymorphisms in regions that include candidate and novel genes and implicate pathways (let-7 targets, chromatin remodeling proteins, Hedgehog signaling) that may regulate height.65,66
The axial and appendicular skeletons account for the vast majority of postnatal linear growth. These bones are formed by endochondral ossification, which commences with chondrocytes of the epiphyses laying down an orderly cartilage template, which osteoblasts then convert to bone.67–69 The cranium and some of the clavicle are formed by direct intramembranous ossification. The cycle of bone cell remodeling for structural purposes is linked closely to the overall metabolic needs for calcium and phosphorus homeostasis, primarily through the actions of parathormone and calciferol. Chondrocyte proliferation is inhibited by parathyroid hormone–related protein (PTHrP) and fibroblast growth factor (FGF) paracrine signaling mediated through the PTH receptor and FGF receptor 3 (FGFR3). This effect is opposed by Indian hedgehog signaling, which operates in a negative feedback loop with PTHrP. The natriuretic factor system, particularly involving the C type, similarly appears to play a local role in endochondral ossification, in this case as positive regulators.70,71
Nutrition and metabolism must be adequate for normal growth. Adult height has been used as a marker for the nutritional status of populations during childhood and has been shown to be related to cognitive function.72,73 Calories seem particularly critical for cell multiplication. Two percent to 13% of normal energy consumption goes into promoting growth.1,74 Protein intake is particularly important for normal growth in cellular size. It must be adequate with respect to both amount and provision of essential amino acids or their ketoanalogues.75–77 Essential fatty acids are necessary for normal growth in lower animals, but this may not hold true for primates.78 Vitamins A and D are important for normal growth.1,79 Trace metals, such as zinc and copper, are probably essential for normal growth and sexual maturation80–83 because of their role as cofactors for enzyme function. The pH must be maintained at optimal levels to conserve mineral homeostasis.84
The general level of activity seems to promote overall body growth, just as normal muscular activity is necessary for limb growth. The mechanism is unclear; it may be related to neural trophic factors or to blood flow. The efficiency of nitrogen accretion and growth is decreased in inactive rats.85
A complex interplay of extracellular peptides with intracellular transcription factors and signaling systems governs the development of the hypothalamic and the pituitary Growth hormone (GH) secretory system.86,87 Pituitary secretion of GH is normally under the immediate control of hypothalamic hormones: somatostatin, which inhibits its release, and growth hormone-releasing hormone (GHRH). GHRH stimulates GH synthesis and secretion. Defects in GHRH synthesis or action stunt growth in mouse models, and humans with a defect in the GHRH receptor similarly show growth failure,88 attesting to the fundamental importance of GHRH in GH secretion and growth.
The balance between GHRH and somatostatin is determined by a complex flux of input from higher cerebral centers, which mediate nutritional, metabolic, and endocrine signals (Fig. 18-1).89–97 Diverse neurotransmitters are involved; they include acetylcholine, galanin, and neuropeptide Y. Dopamine is inhibitory to GH release in the newborn period.98
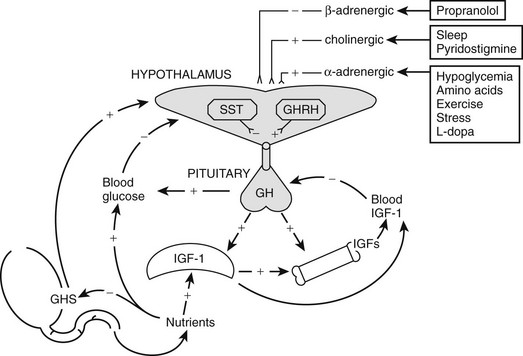
FIGURE 18-1 Growth hormone (GH) regulatory axis: Major factors regulating GH release. GH is secreted after the integration of diverse hypothalamic stimuli. It stimulates insulin-like growth factor-1 (IGF-1) production by the liver, bone, and other tissues, as well as gluconeogenesis. GH release from the pituitary gland is under tonic inhibition by hypothalamic somatostatin, and GH-releasing hormone (GHRH) stimulates GH release when somatostatin (SST) tone wanes owing to fluctuations in input from higher neural centers. Ghrelin is an endogenous GH secretagogue (GHS) and orexogenic peptide mainly secreted by the stomach in response to fasting. Its major indirect effect on GH is to antagonize SST release at the level of the hypothalamus. Small amounts of GHS are formed in the hypothalamus, however, and these weakly stimulate GHRH release directly; neonatally, GHS pituitary expression is high. SST tone is inhibited by cholinergic, dopaminergic, and α-adrenergic neuronal inputs to the hypothalamus and is stimulated by β-adrenergic ones. Negative feedback effects are exerted primarily by the long-loop actions of blood glucose and IGF-1, but also by short-loop signals between the various signal peptides of the axis. IGF-1 and blood glucose also exert negative feedback effects on GH release. Pharmacologic stimuli to GH release are shown in boxes. Solid lines indicate major regulatory pathways, dotted lines minor ones. +, Stimulator of GH, GHS, or IGF-1 release; −, inhibitor of GH, GHS, or IGF-1 release.
Endocrine input includes the endogenous GH secretagogue (GHS) ghrelin, an orexigenic peptide originating in the stomach and hypothalamus. Ghrelin stimulates GH release, primarily through promoting GHRH release, but also by acting directly on the pituitary, through specific receptors (GHSR). In addition, there is negative feedback on GH secretion by circulating IGF-1, which is primarily of hepatic origin, and glucose levels.99,100
After secretion, GH is approximately 50% bound to GH-binding protein (GHBP). GHBP rises through childhood.101 It is the extracellular domain of the GH receptor (GHR); its underlying alternate splicing may be differentially regulated from the intact GHR.102 GHR is a member of the cytokine family. One molecule of GH binds to two GHR molecules, indicating receptor dimerization, which is critical for GH action. (This leads to activation of a receptor-associated Janus tyrosine kinase [JAK-2] and, in turn, transduction through a number of pathways, including the MAPK [mitogen-activated protein kinase] and STAT [signal transducers and activators of transcription] pathways.103) These paths result in activation of genes (including IGF-1) that mediate GH’s biological effects. Abnormalities of the GHR and its signaling system result in GH insensitivity and growth failure (see discussion to follow).
GH appears to stimulate growth through a combination of direct effects and effects mediated by IGFs.104 GH stimulates the production of endocrine IGF-1 and its major binding protein (IGFBP-3). It also directly induces the clonal expansion and differentiation of target stem cells (such as prechondrocytes), and these differentiating cells (chondrocytes) then respond to GH by forming IGF-1 and IGF-1 receptors, which makes them responsive to the growth-promoting effect of both endocrine IGF-1 and IGFs secreted locally (autocrine and paracrine IGFs).105,106
IGF-1, produced by the liver and other tissues, is a critical regulator of postnatal growth and represents a major mechanism by which GH promotes growth. Circulating IGF-binding proteins (IGFBPs) sequester IGFs, whereas at the cell surface they can promote IGF action and exert novel actions.107 Defects in IGF-1 synthesis or action lead to growth failure in humans and laboratory animals. The effects of IGF-1 may depend on the tissue of origin. Local production of IGF-1 in peripheral tissues appears to mediate GH-induced somatic growth, whereas circulating IGF-1, which originates primarily in the liver, may not be essential for growth, but provides negative feedback for the GH axis.106 The free (unbound) IGF-1 is thought to be the biologically active fraction of circulating IGF-1, but the validity of current assays for this moiety is in question.108,109 IGF-1 production is regulated primarily by GH when nutrition is normal. IGF-2 is produced by cells independently of GH and seems to be normally important only for local growth regulation.110 IGF-2 levels seem to be modulated locally by the activity of a metabolizing receptor complex consisting of the IGF-2 receptor and glypican-3.111
There is more to the regulation of plasma IGF-1 concentrations and bioactivity than GH. Hepatic IGF-1, the major source of blood IGF-1, is fundamentally under broad regulation by nutrition. Undernutrition decreases plasma IGF-1 levels despite normal or elevated GH concentrations.112 Overnutrition (i.e., obesity) has the opposite effect.89 Studies in rats suggest that insulin plays a role in mediating nutritional effects on hepatic IGF-1 formation through its stimulation of amino acid uptake.113,114 The increased plasma-free IGF-1 concentration in obese patients has been attributed to the suppressive effect of their insulin excess on IGFBP-1.115 Thyroid hormone and cortisol are necessary for hepatic IGF-1 production, and prolactin has a slight effect on it.116
Factors other than GH and nutrition—including age—determine IGF production, and these are poorly understood. Plasma IGF-1, IGFBP-3 levels,117 and somatomedin activity118 rise slowly during the prepubertal years with no change in GH production (Fig. 18-2).119 As a result, IGF-1 levels in normal children younger than 5 years of age overlap with those of GH-deficient children, making use of these tests in diagnosing GH deficiency difficult in young children. During puberty, IGF-1 levels rise further, and since IGFBP-3 levels rise to a lesser degree, free-plasma IGF-1 rises even more markedly.117 The pubertal increase in IGF-1 is mediated by sex hormone stimulation of GH secretion,120–122 although a separate direct effect on IGF-1 has been suggested.123 IGF-1 levels during adolescence, therefore, correlate more with pubertal development and bone age than with chronologic age.
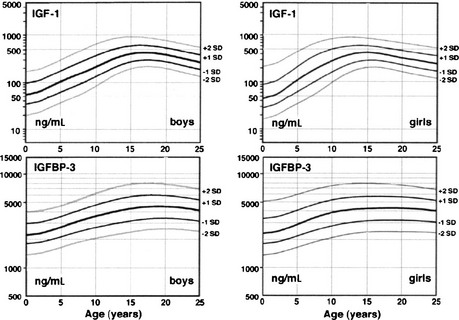
FIGURE 18-2 Plasma insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) normal ranges from infancy to adulthood. Increases after 10 years of age are related to pubertal stage rather than to age. IGF-1 values are given in terms of the World Health Organization reference preparation 87/518, which is of low (44%) purity with respect to authentic recombinant human IGF-1, so the values shown are in excess of the true IGF-1 concentration.41 (Data from Diagnostic Systems Laboratories, Inc., 1997, Webster, TX.)
The relationship of plasma IGF-1 levels to normal linear growth is not a simple one. Plasma IGF-1 levels do not correlate with growth rate in childhood except during the pubertal growth spurt, when levels peak about a year after peak height velocity.124 IGFBPs in plasma determine the unbound concentration of IGF-1, transport the IGFs to target cells, and influence the interaction of IGFs with their receptors; a tissue IGFBP-protease system modulates IGF-1 bioavailability to target cells.105,107,125,126 IGFBPs also appear to be bioactive molecules that have IGF-independent functions.107,126 IGF bioactivity may be influenced by circulating somatomedin inhibitory activity, which is attributable to both glucocorticoids and incompletely characterized peptides.127,128 The cytokines interleukin-6 and tumor necrosis factor-α have direct inhibitory effects on chondrocytes.129
Growth may be normal with subnormal GH production in the poorly understood “growth without growth hormone syndrome.”130 Most often, this syndrome has been identified after surgical treatment for large hypothalamic and pituitary tumors, but the syndrome occasionally has been recognized in benign forms of hypopituitarism.131 IGF-1 levels may be low, but bioactivity normal. Most such patients are obese, so insulin excess or sensitivity has been suspected to be the underlying growth factor. Individual variation in local aromatase activity and thus availability of estrogen has also been suggested.132 Hyperprolactinemia is seldom found.
Thyroid hormone is necessary for postnatal bone growth because of both indirect effects on the GH-IGF axis and direct effects on bone growth.133 Thyroid hormone is required for normal GH secretion in response to GHRH and for normal GH action as indexed by GHBP, IGF, and IGFBP levels. Hypothyroidism (and, to a lesser degree, mutations of the thyroid receptor-β) produces short stature and delays bone maturation.
Glucocorticoids in above-normal amounts are inhibitors of linear growth.128,134 The mechanism is both indirect and direct. Glucocorticoid excess inhibits spontaneous GH secretion by stimulating somatostatin tone. The bioactivity of plasma IGF-1 falls during glucocorticoid therapy; this may reflect an increase in IGF-binding protein.135,136 Glucocorticoids themselves directly hinder cartilage growth,137 perhaps in part by inhibiting GH and IGF-1 induction of their respective receptors.138
Increased secretion of sex hormones clearly initiates the pubertal growth spurt. The growth-promoting actions of sex hormones require adequate GH; GH-deficient children will not undergo a normal pubertal growth spurt unless GH is replaced. About half of the contribution of sex hormones to the pubertal growth spurt is due to their stimulation of the GH-IGF axis, which appears to be mediated primarily by estrogen.91,120,121,139 The remaining effects of sex steroids on growth are direct or are mediated by a direct effect on IGF.123,140–142
Estrogen and androgen both stimulate bone growth, bone turnover, and epiphyseal growth.123,143,144 Androgen appears to stimulate and estrogen to inhibit periosteal bone formation, while estrogen promotes greater cortical thickening by inhibiting endosteal bone resorption. Estrogen is particularly effective in reducing bone turnover, however, and estrogens are responsible for epiphyseal closure.145 To some extent, these effects may be exerted prenatally, since maternal estrogen can have permanent effects on fetal bone development.146 Differences between these actions of sex hormones account for women’s bones being shorter and narrower than men’s.
Early pubertal amounts of estradiol (about 0.25 mg/month) stimulate growth in girls, in contrast to inhibition of growth by high doses of estrogen.147 Peak growth velocity of boys occurs at a testosterone production rate of about 50 to 100 mg/month.148 Whether other sex steroids play an independent role in growth is unknown; it has been reported that dehydroepiandrosterone sulfate promotes calcification of cartilage, and subandrogenic doses of androstenedione promote growth.49
Patterns of Normal Somatic Growth
Standards for intrauterine growth are shown in Fig. 18-3.149 Race, altitude, and gender cause subtle differences from these norms.150
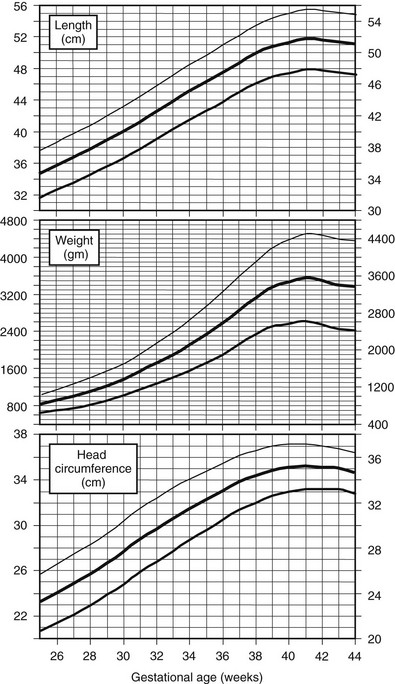
FIGURE 18-3 Intrauterine growth charts. Data represent birth weights according to gestational age of live-born Caucasian infants at sea level. Infants with major congenital malformations were excluded. (Data from Usher R, McLean F: Intrauterine growth of live-born Caucasian infants at sea level: Standards obtained from measurements in seven dimensions of infants born between 25 and 44 weeks of gestation. J Pediatr 74[6]:901–910, 1969.)
Healthy infants born prematurely have weights appropriate for gestational age and continue to grow at the same rate that they would have grown in utero.151 When corrected for postconceptional age, length and weight follow postnatal standards. Consequently, the lengths of children born prematurely remain slightly less through infancy than those of children born at term, but the differences become negligible over time. Very premature infants, however, require intensive care to survive and uniformly lose weight during the first weeks of life; it takes several years for the great majority to catch up to the weight and length of term infants, and females achieve greater catch-up growth than males.152,153 In contrast to premature infants, 10% to 15% of those born small for gestational age prove to have persistent short stature beyond 4 years of age154 (see discussion that follows).
Postnatal Growth
1. Linear growth is assessed as supine length until 2 to 3 years of age (using a firm box with inflexible head board and movable foot board) and, thereafter, as erect height measured with the use of calibrated stadiometers. Stature then is plotted on growth charts. Traditionally, these linear growth standards have been derived from cross-sectional data as shown in Fig. 18-4.155 Because differences in the timing of puberty can influence normal growth rates, longitudinal growth charts are useful in sequential assessment of individual children.156,157 Height SD in relation to the mean for age and gender can be determined from the Centers for Disease Control and Prevention website.155 Syndrome-specific growth charts have been developed for Turner’s syndrome, Down syndrome, and achondroplasia.158–160 There is a “secular trend” toward increasing height of populations with time associated with improvements in nutrition and health,161 although the most recent U.S. data suggest little change in the past 25 years.
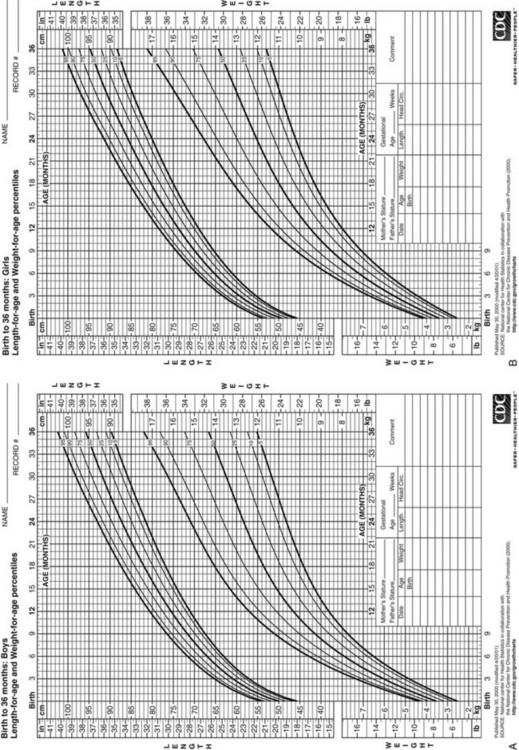
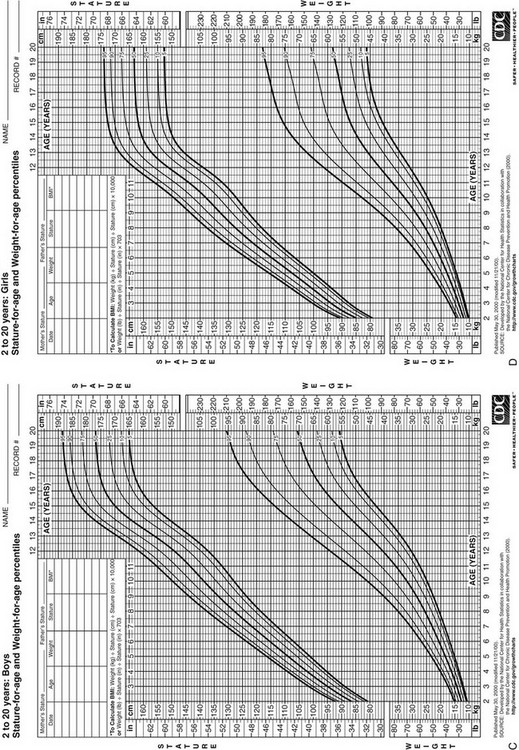
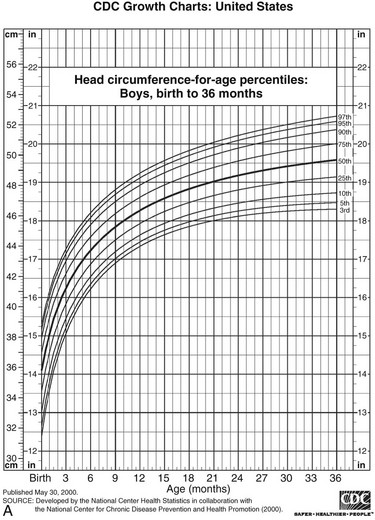
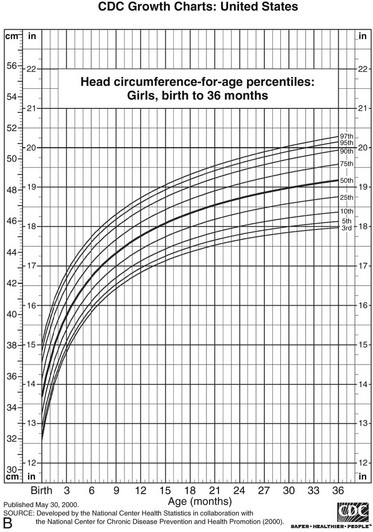
FIGURE 18-4 Postnatal growth standards. Current standards for height and weight of normal children in the United States. Figures A and B are infant growth charts for boys and girls, respectively. Figures C and D are for older boys and girls, respectively. (From Centers for Disease Control and Prevention. CDC growth charts: United States, National Center for Health Statistics, Atlanta, GA.)
2. Weight and body mass index (BMI; weight [kg]/height [cm]2) are measured and plotted on appropriate growth charts.155 Weight is a labile parameter relative to height, being sensitive to acute illness and changes in nutrition, activity, and muscle mass. Whether weight is appropriate can be estimated by comparing a child’s percentile position for weight with respect to height age or by calculating the BMI. BMI is the recommended parameter for assessing whether children over 2 years old are overweight (BMI 85th to 94th percentile for age) or obese (BMI at or above the 95th percentile for age).
3. Growth velocity is assessed from sequential height measurements and can be plotted on growth velocity charts (Fig. 18-5).156,157 A minimum interval of 6 months is needed for meaningful assessments of growth velocity. Growth occurs in three phases—infantile, childhood, and pubertal—each of which has distinct characteristics.162 Linear growth velocity is most rapid in infancy, averaging 15 cm/year in the first 2 years of life. Two thirds of infants cross percentile channels on the linear growth curves.163 The growth of infants seems to result from an initial steep vector, which is generated by the GH- and thyroxine-independent cell proliferation that uniquely drives intrauterine growth, superimposed on a shallow vector, which is dependent on the endocrine factors that determine subsequent growth during childhood.
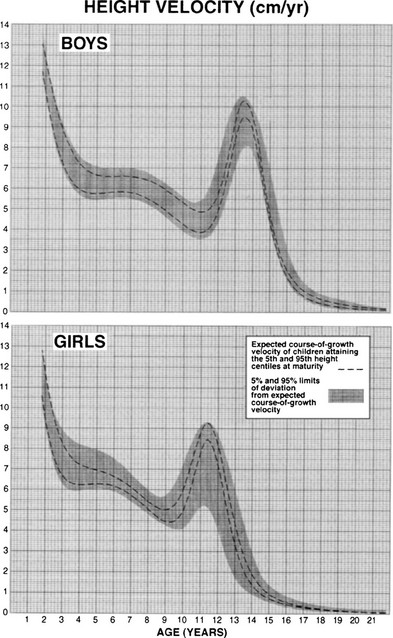
FIGURE 18-5 Longitudinal height velocity standards derived from the Fels, Berkely, and Denver growth studies.141 (Courtesy R.D. Bock.)
4. Growth patterns: From the end of infancy until puberty begins, growth normally proceeds along a channel that closely corresponds to a given height-attained percentile on cross-sectional growth standards. A child normally establishes this channel by 2 to 3 years of age,163 although, on rare occasions, a gradual drift by as many as 40 percentile positions in height attained may occur over a period of several years in normal children.164 The velocity of growth (cm per year) actually decelerates slightly during this period (see Fig. 18-5) and averages about 6 cm per year in midchildhood.157 However, normal children cross height-velocity percentiles to maintain their height channel (Fig. 18-6).156,165,166 A growth velocity that is consistently along the third percentile will lead to a subnormal height.
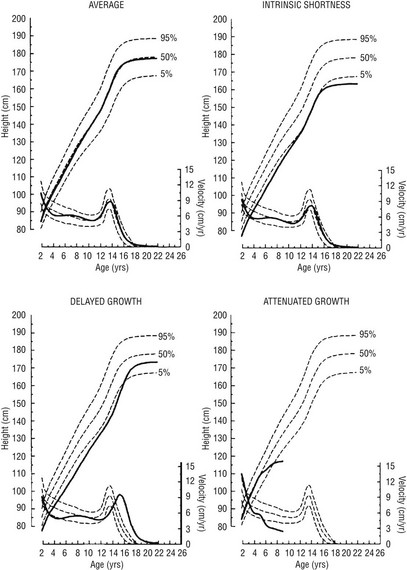
FIGURE 18-6 Linear growth curves in children with various types of growth patterns. Note that three prepubertal children of similar short stature at 9 years of age have different prognoses for growth. The growth curve of an average-size child is shown for comparison. On each chart, the upper scale shows the height attained, and the lower scale shows the height velocity. Normal percentiles are from the National Center for Health Statistics. Growth curves were generated by the TRI-FOUR program of Bock et al. (Bock RD, du Toit SHC, Thissen D: A.U.X.A.L: Auxological Analysis of Longitudinal Data, Scientific Software, 1993, Chicago, IL. Courtesy of R.D. Bock.)
The growth channel seems to be genetically determined. Children grow as if to reach a genetically predetermined height. This target height, which represents the child’s genetic potential, can be approximated by calculating the midparental height (the average of the parents’ heights) and adding 6.5 cm for boys or subtracting 6.5 cm for girls (to adjust for the average differences between men and women). Alternative functions have been proposed for children with short parents. However, all such predictions are accurate only within a range of 7 to 10 cm.167
Deflections from this channel are firmly resisted, as if growth is being developmentally canalized.168 The mechanisms by which the growth channel is maintained are unknown. They may involve recognition of cell density, which is a determinant of the cell population in culture systems.169 In the course of a year, healthy children maintain their percentile position with respect to height attained by means of short-term fluctuations in growth velocity, termed stasis and saltation.170 These oscillations may be marked, growth sometimes seeming nil over 3-month periods, and are a potential source of error in growth diagnosis. GH variability has been reported to increase during periods of short-term growth.171 Variations tend to be seasonal, a “blooming” trend most often occurring in the spring.
During puberty, children may again cross height-attained percentiles because the pubertal growth spurts of individuals occur out of phase. The magnitude of this pubertal growth spurt is apparent only from growth-velocity standards based on age of menarche or longitudinal data. Peak growth velocity occurs approximately 1 year before menarche172 in girls, and at a bone age of approximately 12 years in girls and 13 years in boys.173 Girls on average achieve only 7 cm further growth after menarche.174 During the course of sexual maturation, the epiphyseal cartilage plates become progressively obliterated, and growth ceases when the process is complete. Only about 1 cm of growth occurs after fusion is complete in the femur and tibia.
The causes of the decrease in growth velocity from the fetal to the neonatal and subsequent early childhood years are not known but may be a consequence of differential expression of IGF-1 versus IGF-2, their receptors, and the various IGF binding proteins in the growth plate with increasing age.175 In addition, fibroblast growth factors may play a role.176
Some of the greater ultimate height of boys than of girls results from their later puberty and consequent longer period of prepubertal growth166; boys additionally have a slightly greater peak linear growth velocity than girls.156 Early maturers have more brisk pubertal growth than late maturers; however, they also stop growing earlier.156 This tendency occurs at comparable levels of bone maturation (Table 18-1).177 The growth patterns of nonwhite American children differ from those of whites in some particulars.178 Immigrant children go into a phase of catch-up growth in an optimum nutritional environment.179
Table 18-1
Percentage of Adult Height Achieved at Successive Bone Ages, Variation in Height Prediction From Bone Age, and Variation in Bone Age in Relation to Chronologic Age
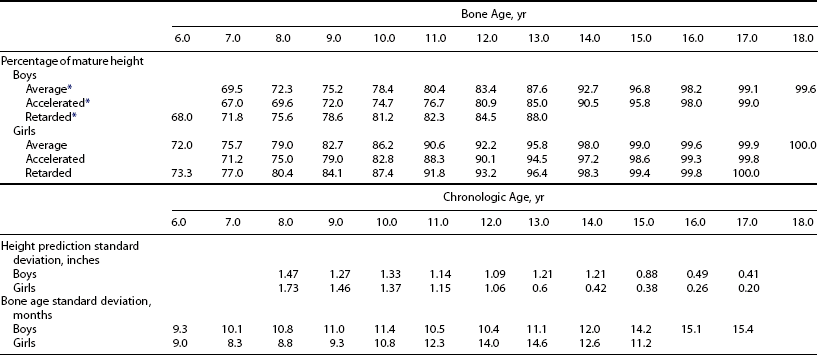
*With respect to whether bone age is within 1 year of chronologic age.
From Gruelich WW, Pyle SI: Radiographic Atlas of Skeletal Development of the Hand and Wrist. Palo Alto, CA, Stanford University Press, 1959.
5. Body proportions (arm span and upper-to-lower segment ratio) change in concert with growth. The limbs are relatively short in infancy. By about 11 years of age, adult proportions are reached (Figs. 18-7 and 18-8).180,181 Occasional marked changes in segmental proportions appear during puberty.182 Many growth disorders are characterized by abnormal body proportions (see later discussion).
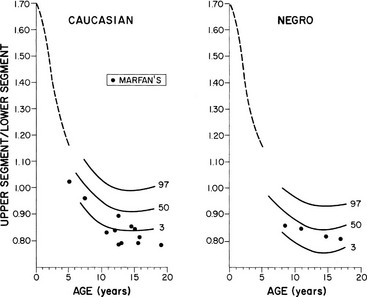
FIGURE 18-7 Normal standards for the ratio of the upper segment to the lower segment of the body. The lower segment represents the measurement from the top of the symphysis pubis to the heel; the upper segment is computed by subtracting the lower segment from height. The dotted line shows the average for young children in 1932. (Percentile and Marfan data from McKusick VA: Heritable Disorders of Connective Tissue, ed 4. St Louis, Mosby, 1972.)
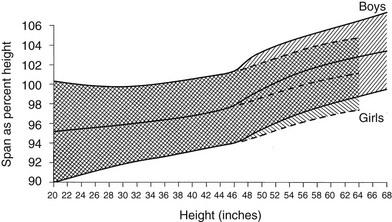
FIGURE 18-8 Standards for arm span as a percentage of height. The shaded area represents the normal range, smoothed. (Data from Engelbach W: Endocrine Medicine, vol 1. Springfield, IL, Charles C Thomas, 1932, p 261.)
6. Head circumference increases most rapidly during early infancy (Fig. 18-9). It is related to both skeletal and brain growth, and about half the variation is familial.183–185
Catch-Up and Catch-Down Growth and Compensatory Growth
Catch-up growth occurs upon relief from any disorder that has caused a deviation from a child’s genetic growth channel and restores the child to his or her original channel.168,186–188 In classic (“type 1”) catch-up growth, the rate of growth is supranormal and exceeds that expected for the age at which growth had been arrested. During adolescence, it may resemble the pubertal growth spurt. This type of catch-up growth has been further subclassifed.189 A different kind of catch-up growth (“type 2”) occurs following adequate therapy for sexual precocity.190 In this situation, restoration of height potential occurs because restitutional linear growth proceeds without bone maturation advancement, that is, height age catches up to bone age (Table 18-2). This is particularly true for sexual precocity that occurs very early; however, suppression of pubertal progression around the lower limit of normal age for pubertal onset may not be associated with similar benefits for height potential. Complete compensation for growth failure can occur upon correction of the underlying disorder if diagnosed early. Catch-up may be incomplete, however, if the growth disorder is of many years’ duration and extends into the age at which puberty normally occurs.
Table 18-2
Definitions of Growth Parameters
| Parameter | Definition |
| Bone age | Age for which bone maturation is average |
| Chronologic age | Calendar age |
| Height age | Age for which height is average |
| Weight age | Age for which weight is average |
Growth plate physiology plays an important role in mediating “catch-up” and “catch-down” growth. The growth plate goes through a programmed pattern of senescence through childhood and adolescence, dependent on factors intrinsic to the growth plate.191,192 Information regarding previous growth history appears to be retained in the memory of the growth plate, likely in resting zone “stem cell–like” chondrocytes, and influences future structural and functional changes in the growth plate, with effects on “catch-up” and “catch-down” growth.193
Endocrine deficiencies causing short stature have important effects on the growth plate. Following induced hypothyroidism and hypercortisolism in experimental models, growth plate chondrocyte proliferation slows down, with a slower depletion of resting zone chondrocytes, leading to preservation of proliferative capacity and slowing of senescence. With normalization of hormone levels, growth plates grow more rapidly, resulting in “catch-up” growth.194,195 However, akin to humans, catch-up growth after correction of hypothyroidism remains incomplete, with adult height being less than in euthyroid controls. Estrogen administration accelerates growth plate senescence, and fusion occurs with exhaustion of proliferative potential.195 The duration of estrogen exposure to induce epiphyseal fusion depends on age. A younger child requires more years of estrogen exposure than an older child, likely because a longer duration of estrogen exposure is required to exhaust the larger reserve capacity of growth plate chodrocytes in a younger child. Finally, although GH deficiency leads to marked growth deficits, provided sufficient time is available, GH replacement causes sustained “catch-up” growth sufficient to achieve target height.196
In contrast to situations of hormone replacement for endocrine deficiencies, “catch-up” growth in other situations of short stature is not as optimal. In SGA infants, an early increase in growth rate has been associated with an increase in IGF-1 levels after intrauterine constraints are eliminated, because high GH levels from intrauterine undernutrition and associated GH insensitivity take time to normalize.51 However, “catch-up” does not occur in as many as 10% to 15%, and only about half of very low birth weight, SGA infants demonstrate complete “catch-up.” About a third remain shorter than target height, with the initial “catch-up” being followed by “catch-down” growth.197 In addition, following GH therapy in short children born SGA, cessation of GH therapy before epiphyseal fusion leads to a reduction in growth velocity SD score (SDS) and height SDS over a 5-year period, with maximum growth deceleration occurring during the first year after GH therapy is stopped. Another form of “catch-down” growth occurs in children born in families with a history of short stature, in whom length SDS decreases over the first 2 years of life to the familial range, and the extent of loss of length SDS is greater in appropriate for gestational age (AGA) than SGA babies.
Compensatory growth is the term used for the local organ regeneration that occurs after the mass of an organ has been reduced, as by removal or destruction of a portion of that organ.168 Examples include the compensatory growth that occurs after partial hepatectomy or loss of a kidney. Local IGF-1 and IGF-2 are involved in this type of growth.110
Skeletal Maturation: Bone Age In Prediction of Adult Height and Pubertal Milestones
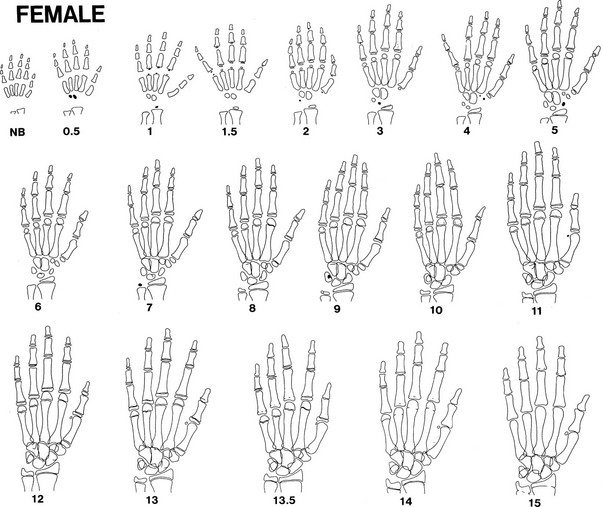
Bone growth is accompanied by a predictable pattern of bone maturation. After epiphyseal ossification centers first appear, they undergo modeling in shape and then fuse with the shaft. Bone maturation is assessed as bone age (BA, skeletal age), based on x-rays of the left wrist (see Table 18-2). Figs. 18-10 and 18-11 schematically show the Gruelich and Pyle BA standards.198,199 The normal range for BA is indicated in Table 18-1. The evaluation is most reliable if the maturation of each center is assessed for calculation of the average,200 to circumvent normal variations in the epiphyseal ossification pattern.201 Other atlas methods are available for assessing bone maturation.202 Skeletal development of young black children is about 0.67 SD advanced over whites of comparable economic status.203 Other ethnic differences exist that are, to an unknown extent, nutritional.202 Bone age is influenced by thyroid hormone, growth hormone, sex steroids, and unknown factors. In both boys and girls, estrogen is responsible for ultimate fusion of the epiphyses.
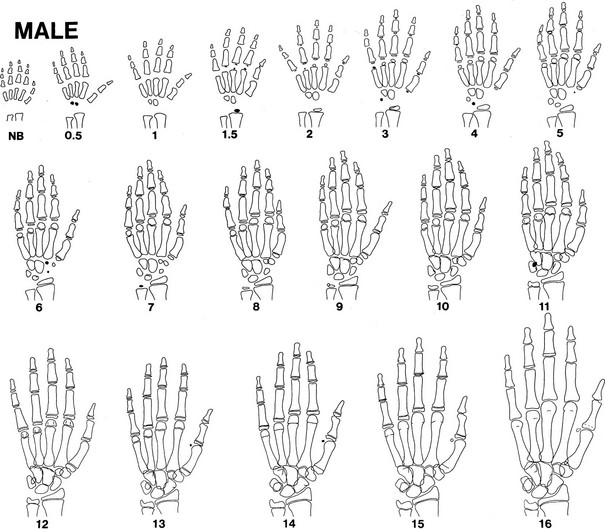
FIGURE 18-10 Progression of ossification of the hand and wrist in boys. Tracings are modified from the standards of Gruelich and Pyle,198 according to the manner of Wilkins.199 Newly apparent ossification centers are shown in black. Late perfusion is depicted as a single line at the junction of the epiphysis and the shaft. Bony projections, which appear as a double contour within the outlines of a center, are not illustrated after their appearance has matured.
BA is a better predictor of pubertal milestones than is chronologic age. It is as if bone and neuroendocrine maturation have common genetic, nutritional, and endocrine determinants.204 A BA of 11 to 12 years corresponds better to the onset of puberty in girls and boys, respectively, than do these chronologic ages. Peak height-velocity phase differences are 25% less when plotted against BA instead of chronologic age.165 In girls, menarche has been demonstrated to occur at a mean skeletal age of approximately 13 years.198,205
Bone age can be used to predict ultimate height potential, since the degree of bone maturation is inversely proportional to the amount of epiphyseal cartilage growth remaining. It follows that if a child’s BA and height age (HA; see Table 18-2) are equal, he or she has the potential to reach an average adult height. The fraction of final height achieved at each BA is known (see Table 18-1). Therefore, predicted adult height can be calculated by dividing a child’s current height by this fraction (method of Bayley and Pinneau).177 The error inherent in this method is less than 1.5 inches in normal children (see Table 18-1). However, spontaneous shifts by as much as 5 inches in predicted height may occur in 3% of the population for reasons that are unclear.206 The error is not reduced by serial readings.200 Because height prediction methods were developed based on normal children, the error is greater in children who are very short207 or have abnormalities such as bone dysplasias.
To reduce the error in height prediction, elaborate tables have been devised that take into consideration not only a child’s BA and height but also the height and weight of the genetic target.208 Genetic influences on height predicted from bone age can be roughly accounted for by adding one-third the amount that the midparental height differs from the average.174
Three methods for assessing height predictions based, in part, on bone age have been developed.177,208,209 All are based on data from normal children. The Bayley-Pinneau method can be applied simply to young children with abnormal bone ages, so it is used most frequently in children with growth disorders; however, its accuracy has not been verified in many of these.