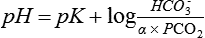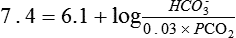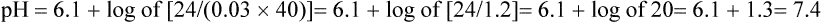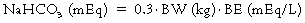Sodium bicarbonate
The carbonic acid/bicarbonate buffering system
Because CO2 is not sufficiently soluble in blood, a number of mechanisms have had to evolve over time to facilitate the transport of CO2 from the periphery to the lungs (Chapter 22). The most efficient of these mechanisms combines CO2 with H2O to form carbonic acid, which in turn dissociates into hydrogen ions and bicarbonate ions.
The reactions are reversible and subject to the law of mass action:
< ?xml:namespace prefix = "mml" />
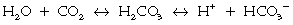
Metabolic acidosis
Several causes of metabolic acidosis (Box 95-1) may occur simultaneously in patients with critical illness. Acidemia may impair cardiac contractile function, constrict pulmonary arteries, and reduce adrenergic receptor responsiveness to catecholamines; therefore, many clinicians often restore the HCO3−/PCO2 ratio to 20 by administering bicarbonate. Treatment of the underlying problem is then undertaken to ultimately restore and maintain a pH of 7.4.
Therapeutic uses of sodium bicarbonate
Lactate acidosis
Lactate acidosis caused by anaerobic glycolysis due to inadequate O2 delivery (see Box 95-1) is probably the most common cause of metabolic acidosis faced by the anesthesia provider. If the pH is below 7.2 to 7.25 or is decreasing to those levels despite all measures to restore O2 delivery (e.g., normalization of blood volume and composition, restoration of adequate ventilation, or pharmacologic or mechanical support of cardiac function), many clinicians will administer NaHCO3.