Chapter 130 Smoking and the Spine
Tobacco is responsible for one in five deaths, and tobacco use is the single largest preventable cause of death and disability in the United States.1 Smoking has been linked to numerous health conditions, including cancer, respiratory disease, cardiovascular disease, and peripheral vascular disease1; however, the delayed onset of these diseases makes it difficult for users to realize the potential consequences of tobacco use until significant damage has been done. The addictive properties of tobacco use, both chemical and psychological, make cessation difficult for even the most determined quitter. Despite numerous public health efforts, tobacco use continues to have significant adverse effects on the health of people everywhere.2
Tobacco use affects the entire body and has been identified as a risk factor for six of the eight leading causes of death in the world: malignancies, heart disease, cerebrovascular disease, lower respiratory infections, chronic obstructive pulmonary disease, and tuberculosis2–5 (Table 130-1). Associated with increased osteoclast and decreased osteoblast activity, smoking has been implicated as a risk factor for osteoporosis, delayed fracture healing, and fusion pseudarthrosis. In general, multiple studies have indicated that spine-related disorders are more common in smokers, and spine surgeries are up to four times more frequent in cigarette smokers.6 Symptomatic fusion pseudarthrosis is believed to be three to five times more common in smokers than nonsmokers.6
TABLE 130-1 Common Adverse Health Effects due to Tobacco Use
| Adverse Effect | Signs |
|---|---|
| Cancer | Lung, trachea, bronchus, oral, esophageal, stomach, liver, pancreatic, bladder, kidney, and cervical cancers; leukemia |
| Cardiovascular | Atherosclerosis, cerebrovascular disease, coronary artery disease, abdominal aortic aneurysm |
| Dental | Implant complications and failure |
| Dermatologic | Advanced skin aging, wrinkles |
| Gastrointestinal | Peptic ulcer disease |
| Immunologic | Infectious disease susceptibility, tuberculosis |
| Ophthalmologic | Cataracts |
| Orthopaedic | Decreased bone mineral density, delayed fracture healing, increased fracture risk |
| Respiratory | Chronic obstructive pulmonary disease, pneumonia, impaired lung development |
| Reproductive | Maternal infertility, miscarriage, low birth weight, still birth, sudden infant death syndrome |
| Spinal | Back and neck pain, prolonged disability, poor surgical outcomes |
| Surgical | Surgical site infections, delayed healing |
Data from Brunnhuber K, Cummings KM, Feit S, et al: Putting evidence into practice: smoking cessation, 2007. www.clinicalevidence.bmj.com/downloads/smoking-cessation.pdf; and Wipfli H, Samet JM: Global economic and health benefits of tobacco control: part 1. Clin Pharmacol Ther 86:263–271, 2009.
Adverse Health Effects of Tobacco Use
Initially linked to lip, mouth, and throat cancers in the 18th and 19th centuries, tobacco use has been shown to result in significant damage throughout the body.7 Typically associated with lung cancer, smoking actually results in more deaths from cardiovascular and respiratory diseases.8 Lung diseases linked to smoking include cancer, bronchitis, emphysema, and a predisposition for pneumonia.1 Injuries to the cardiovascular system resulting from smoking include coronary artery disease, myocardial infarction, cerebral vascular attack, aneurysm, and peripheral vascular disease.1 Coronary artery disease is two to four times more common in smokers than nonsmokers.1 In nonsmokers, exposure to secondhand smoke has been associated with a 25% to 30% increased risk for the development of coronary artery disease.9 Smoking has also been associated with the development of insulin resistance, cataracts, and bacterial infections.1,10,11 Smokeless tobacco has been associated with increased risk of oral cancers, gingival recession, and oral leukoplakia.5
Spine-Specific Consequences of Tobacco Use
Although smoking has been the form of tobacco use most often studied, any type of tobacco use has been shown to affect the spine adversely in multiple ways (Table 130-2). Not only are tobacco users more likely to have back and neck pain or injuries, they are less likely to have significant improvement following treatment.
Back Pain
An estimated 15% to 20% of adults have at least one episode of low back pain every year, and 50% to 80% experience low back pain at least once in a lifetime.12 Similarly, neck pain is experienced by approximately 20% of the adult population in one year and by 66% of the population in a lifetime.12 A majority of evidence on the epidemiology of neck and back pain indicates that cigarette use is associated with higher rates of pain.13–18 Although authorities have suggested the existence of a causal relationship between smoking and neck and back pain, a relative lack of prospective studies makes it difficult to confirm this theory.
Compared with nonsmokers, smokers who have back pain are often younger, report more severe symptoms, and indicate that the symptoms were present for a larger portion of the day.16 Smokers are also more often extremely dissatisfied by their current health, more likely to report depression, more pessimistic regarding the resolution of their pain, and less likely to show a trend toward improvement following surgery.16 Adolescent smokers are significantly more likely to develop back pain than nonsmokers, with evidence supporting a dose-response relationship.17,18
Disc Degeneration
Degeneration of the intervertebral disc is believed to be pivotal in the development of lower back pain. In addition to the effects of aging, variable dynamic and biologic stresses contribute to disc degeneration.19 Following exposure to nicotine, catecholamine-mediated vasoconstriction results in decreased blood flow to the intervertebral discs.19 Elevated carboxyhemoglobin levels in smokers and the expected presence of free radicals and toxic substances could further contribute to inadequate nutrition of intervertebral disc tissues. Alterations in cell morphology, increased levels of proinflammatory cytokines, decreased glycosaminoglycan levels, and decreased production of collagen with a shift toward production of type I collagen have been demonstrated following exposure of intervertebral disc cells to nicotine.19,20
Chronic cough has been linked to the development of back pain in the general population, and cigarette smokers are three times more likely to have a chronic cough than people who have never smoked or previous smokers.21 Vertebral body or end-plate fractures resulting from smoking-induced decreased bone mineral density are also likely to lead to back pain.22
Fusion
Smoking has been associated with decreased fracture healing rates and increased incidence of nonunion; thus, it is not surprising that smokers also tend to have similar difficulties following arthrodesis procedures.23–30 A rabbit model of intertransverse process fusion found reduced fusion rates in those receiving nicotine up to the day of surgery and a 0% fusion rate in those continuing to receive nicotine postoperatively.31 Nicotine exposure resulted in decreased expression of genes for vascular endothelial growth factor, basic fibroblast growth factor, types I and II collagen, and bone morphogenetic protein (BMP)-2, BMP-4, and BMP-6.32
Following dorsal lumbar fusion procedures, smokers have been found to have pseudarthrosis rates increased up to 40% higher than nonsmokers.26–29 Smokers able to stop prior to surgery and remain abstinent in the postoperative period have improved rates of fusion compared with people who continue to smoke.27 The use of rhBMP-2 has been shown to significantly increase fusion rates in smokers.28 Following surgery, smokers were less likely to return to full-time work, more likely to be disabled, and less satisfied with the outcome.27
Analysis of results from ventral cervical arthrodesis procedures indicate that there is an increased pseudarthrosis rate following multilevel discectomy and interbody grafting using autogenous grafts in smokers compared with nonsmokers.30 The clinical outcome is significantly better for nonsmokers than smokers.30
Surgical Complications
Surgical site infections have been reported to be more common in smokers following spine surgery,33 and chronic hypoxia decreases collagen synthesis and deposition in healing wounds.34,35 Published results are mixed, but smoking has also been suggested as a risk factor for the development of deep venous thromboses following surgical procedures.36
Tissue oxygenation is compromised in smokers due to multiple factors, including increased levels of carboxyhemoglobin, atherosclerosis, and vasoconstriction. Subcutaneous tissue oxygen tension begins to decrease 10 minutes after initiation of cigarette smoking, reaches a low of 22% to 48% below baseline after approximately 30 minutes, and remains below normal for up to one hour.35 The combination of nicotine-mediated vasoconstriction and increased levels of carbon monoxide in smokers results in an estimated 70% decrease in subcutaneous tissue oxygenation in regular smokers.35
The presence of oxygen is crucial for effective prevention of infection,26,34 and smokers have demonstrated increased susceptibility to a number of bacterial infections, including meningitis, periodontitis, otitis media, and infection of the respiratory and urinary tracts.11 Reactive oxygen species produced by neutrophils and monocytes are necessary for effective functioning of the immune system, and levels are decreased in smokers.34 Increased subcutaneous oxygen levels postoperatively result in decreased surgical site infection rates, and smokers quitting smoking 4 to 8 weeks prior to surgery have shown a decreased incidence of surgical site infection.34
Following fusion with segmental fixation for adult spinal deformity, smoking was found to be a risk factor for adjacent-segment problems; the overall relative risk of reoperation for any reason was 2.59 in smokers, with 25.8% requiring another procedure.37 Prior to elective general surgery or orthopaedic procedures, smokers randomized to receive smoking cessation counseling and offered nicotine replacement therapy had cessation rates significantly higher than the control group, up to 58%, and significantly lower postoperative complication rates.38,39 Cessation had the largest effect on reducing surgical site complications.39 The number needed to treat to prevent one complication has ranged from three to five patients.38,39
Overall Outcomes
After surgical procedures for isthmic lumbosacral spondylolisthesis or chronic low back pain, smokers were more likely to be dissatisfied and have worse results.40–42 Patients actively serving in the military were significantly less likely to return to full-time work following a lumbar microdiscectomy if they were smokers.43
Musculoskeletal Consequences of Tobacco Use
General Adverse Effects
The remainder of the musculoskeletal system suffers similar consequences due to tobacco use. Smokers experience decreased rates of healing of fractures, fusions, and tissues, which results in poorer outcomes overall and an increased incidence of posttraumatic and postoperative infections. Postoperative smoking predicts failure of fingertip replantations44 and reduced success of anterior cruciate ligament reconstruction.45 Following tibial and femoral shaft fractures, smokers are more likely to experience delayed union or nonunion than nonsmokers.46,47 Smokers are also at increased risk for rotator cuff tears48 and are likely to have delayed tendon to bone healing following rotator cuff repair based on findings in a rat model.49
Osteoporosis
Smoking has long been associated with the development of osteoporosis and increased risk of fractures. Evidence indicates that smoking is an independent, dose-dependent risk factor for decreased bone density.50,51 This finding is not limited to older patients. A study of young male military recruits found smoking to be a risk factor for decreased spine and hip bone mineral density.52 Research has also shown that smokers have reduced bone size and amounts of cancellous and cortical bone. Estradiol activity is routinely decreased in smokers due to multiple mechanisms. This results in decreased intestinal absorption of calcium and increased risk of osteoporosis.53,54 Smoking-related alterations in body mass and composition, estrogen metabolism, calcium absorption, and osteocyte function are thought to have important effects on bone health.53,54
Smoking is believed to increase fracture risk through multiple mechanisms; low bone mineral density cannot account for the entire increase in risk.55 Effects are seen in both premenopausal and postmenopausal women, although effects are often more significant following menopause and in women with decreased body mass.56,57 Men who smoke are also affected and have been found to have a 4% to 15.3% lower bone mineral density than men who had never smoked.58 Twin studies of same-sex pairs discordant for smoking indicate a possible dose-dependent relationship.56,59
Smokers can have significantly reduced bone mineral density at the lumbar spine, forearm, and calcaneus, with more substantial deficits noted in the hip.50 Smoking has been shown to increase the lifetime risk of vertebral and hip fractures in both men and women who smoke.50,55,57,60–62 Current smoking has also been implicated as a risk factor for proximal humerus and distal forearm fractures.63 Smokers have been shown to be at risk for sustaining fractures up to 5.2 years earlier than nonsmokers, and fracture risk increased as tobacco consumption increased.63 Smoking cessation results in a rapid drop in fracture risk during the first 10 years, but the risk of fracture remains elevated for more than 30 years.63
There is also evidence that smokers may be more likely to fall than nonsmokers due to relative weakness, poorer balance, and impaired neuromuscular performance.54 It has been estimated that smokers have the physical and neuromuscular function of someone 5 years older.54
Epidemiology of Tobacco Use
Worldwide
Tobacco use, which is linked to 100 million deaths in the 20th century and currently accounts for 5.4 million deaths a year, has been classified as an epidemic by the World Health Organization.2 At current rates, tobacco use will result in over 8 million deaths a year by the year 2030 and an estimated 1 billion deaths in the 21st century.64 Tobacco kills one third to one half of all people who use it—one person every 6 seconds—resulting in death approximately 8 to 16 years prematurely65 More than 40% of men and approximately 12% of women smoke tobacco worldwide.4
In the United States
In the United States, cigarette smoking kills an estimated 440,000 people each year. This is more than the number of people killed as a result of car accidents, alcohol abuse, illegal drug use, homicide, and AIDS combined.1 In the 40 years between 1964 and 2004, more than 12 million Americans died prematurely due to smoking-related illnesses. At the current rate, 25 million smokers alive today will die due to the adverse effects of smoking.1 Cigarette-related residential fires result in more than 1000 deaths each year, making cigarettes the number one cause of residential fire fatalities.1
The 2007 National Survey on Drug Use and Health found that 70.9 million Americans 12 years of age and older were current tobacco users.66 As shown in Table 130-3, a majority of these, 60.1 million, were current cigarette smokers.66 The rate of tobacco use varies greatly between age groups, with people 18 to 25 years of age having the highest rate of tobacco use in the previous 30 days, 41.8%.66 Of youths 12 to 17 years of age, 12.4% (3.1 million) had used a tobacco product in the past 30 days.66
TABLE 130-3 Current Tobacco Use among Americans 12 Years of Age and Older in 2007
| Number of People (in millions) | Percent of U.S. Population | |
|---|---|---|
| Cigarette smokers | 60.1 | 24.2 |
| Cigar smokers | 13.3 | 5.4 |
| Smokeless tobacco users | 8.1 | 3.2 |
| Pipe smokers | 2 | 0.8 |
| Any tobacco use | 70.9 | 28.6 |
Data from Substance Abuse and Mental Health Services Administration: Results from the 2007 national survey on drug use and health: national findings, Rockville, MD, 2008, Office of Applied Studies.
Financial Costs
The financial burden of tobacco use on society is overwhelming. It is estimated that tobacco use is directly responsible for more than 96 billion dollars of the U.S. health care budget each year.1 An additional burden of 97 billion dollars annually can be attributed to lost productivity due to tobacco use.1 By not accounting for other costs associated with tobacco use, such as diseases due to secondhand smoke and burns related to tobacco use, the estimated annual cost associated with tobacco use of 193 billion dollars is likely a significant underestimate.1
Secondhand Smoke
Passive, or secondhand, smoke has also been shown to increase the risk of smoking-related illness. It is estimated that approximately 3000 lung cancer deaths and 46,000 deaths from coronary artery disease are due to passive smoke exposure each year.67 Children exposed to tobacco smoke are at increased risk for sudden infant death syndrome and respiratory and ear infections. In addition, they are more likely to develop asthma and to have more severe symptoms.1,9
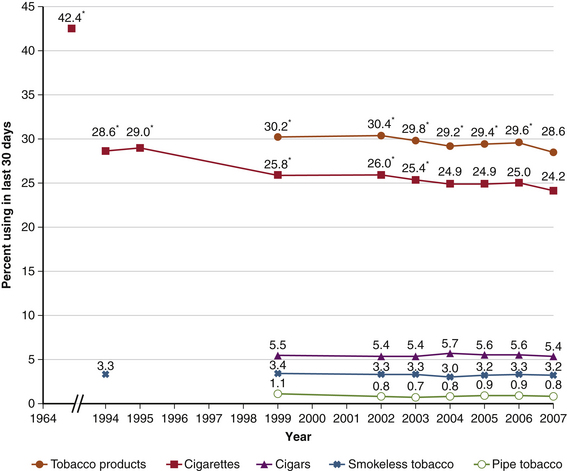
Recent Trends of Tobacco Use
As of 1960, approximately 50% of adult men in the United States were cigarette smokers.4 Since the 1946 release of the Surgeon General’s report on smoking and health, increased awareness of the harms of tobacco use have led to an almost 50% decrease in the number of Americans using tobacco1,66 (Fig. 130-1). Between 2000 and 2007, there was an 18% decline in cigarette sales in the United States. The average price of a pack of cigarettes increased from $2.93 to $3.93 during the same period.68 Although the rates of cigarette use and overall tobacco use by 18- to 25-year-olds decreased from 2002 to 2007, the rates of cigar and smokeless tobacco use slightly increased.66
In 2007, there were approximately 6100 new cigarette smokers 12 years of age and older every day, for a total of 2.2 million new smokers.66 Of these, 59.7% were younger than 18 years of age.66 In that same year, a higher percentage of males than females (53.2% vs. 22.4%) reported tobacco use during the previous 30 days.66
There is significant variability in the rate of tobacco use between the different ethnic groups in the United States. In 2007, the prevalence of past month tobacco use in those 12 years of age and older was 15.4% for Asians, 22.7% for Hispanics, 26.8% for blacks, 30.7% for whites, 35.2% for groups reporting two or more races, and 41.8% for American Indians or Alaska Natives.66 Educational level inversely influences cigarette use, with use decreasing as education increases.66
As of 2006, Utah had the lowest smoking rates, with 10.4% of men and 9.3% of women 18 years of age or older reporting current cigarette use.8 Kentucky had the highest smoking rates, with 29.1% of men and 28.0% of women, for the same period.8
People with mental illness compose one group that has not seen an appreciable decrease in smoking rates. Although smoking rates have been reported near 90% in people with schizophrenia, smoking rates are two to four times greater in people with bipolar disorder, major depression, posttraumatic stress disorder, and other mental illnesses than in the general population.69 People with psychiatric disorders are believed to purchase more than 40% of cigarettes sold in the United States.1
Also associated with increased cigarette use, current illicit drug use was reported in 20.1% of smokers compared with 4.1% of those not currently using cigarettes.66 Similarly, alcohol use was reported by 66.9% of cigarette smokers compared with 46.1% of nonsmokers.66 Cigarette users were also more likely to be heavy drinkers and participate in binge drinking than those not currently using cigarettes.66
A corresponding increase in sales of other tobacco products negated approximately 30% of the decline in cigarette sales. For example, cigar sales increased by 37% from 2000 to 2007,68 and use of noncigarette smoked tobacco products, including bidis, kreteks, and shisha, has also increased.2
Tobacco and Tobacco Smoke
The composition and behavior of tobacco smoke is a complex subject that is not fully understood. The identities, behaviors, and health consequences of many of the components of tobacco smoke have yet to be well defined. Tobacco and tobacco smoke contain approximately 6700 compounds, about 4000 of which have been identified. At least 63 of these compounds are known to be carcinogenic in mammals, and 11 are known to be human carcinogens.70 An average commercially produced cigarette in the United States is filled with 700 mg of tobacco, which contains 10 to 14 mg of nicotine.71,72 Nicotine (C10H14N2, 1-methyl-2-(3-pyridyl)-pyrrolidine) is a naturally occurring plant alkaloid and is responsible for a majority of the addictive properties of tobacco.
Some compounds in tobacco smoke that are considered hazardous include formaldehyde, acetaldehyde, benzene, carbon monoxide, arsenic, isoprene, toluene, acetone, styrene, ammonia, hydrogen cyanide, and vinyl chloride.73 Almost all of the common elements have been identified in tobacco, including a number of radioactive isotopes and free radicals.73 Many, including cadmium, mercury, titanium, and lead, are also found in tobacco smoke. When inhaled, tobacco smoke is an aerosol with a gas phase containing approximately 8% particulate material on a weight basis.74,75 There are approximately 109 to 1010 particles/cm3 in tobacco smoke, with 50% to 90% being retained in the lungs after expiration.73
Although cigarettes are a roll of tobacco wrapped in a substance not containing tobacco, cigars consist of tobacco wrapped in leaf tobacco or in any substance containing tobacco.70 Cigar tobacco is high in nitrate, and the fermenting process unique to cigars converts the nitrate to very high levels of carcinogenic nitrogen-containing compounds.70 Cigar smoke also contains elevated levels of tar, ammonia, nitrosamines, and nitrogen oxides.70 Having a higher pH than cigarette smoke, cigar smoke contains higher concentrations of free nicotine in the particulate and vapor phases.70 This is absorbed through the oral mucosa and does not require the cigar to be lit. Some cigars contain as much tar and nicotine as a pack of cigarettes.
Nicotine
Cigarettes are very efficient means of drug delivery. With typical use, 10 puffs over a 5-minute period, an average smoker receives 1 to 2 mg of nicotine per cigarette.1,76 When inhaled, nicotine is quickly absorbed into the venous circulation, avoiding first-pass metabolism and reaching peak concentrations within 10 seconds.1,71,77 With smokeless tobacco and most cigar and pipe use, nicotine levels peak more slowly as the nicotine is absorbed through the mucosal membranes. To facilitate absorption of nicotine, a weak base, through the mucous membranes, chewing tobacco and snuff are buffered to an alkaline pH, near 7 or 8.77,78 Peak nicotine concentrations following the use of chewing tobacco are reached in approximately 30 minutes and are maintained for about 2 hours.71 Air-cured tobaccos, commonly used in pipes and cigars, produce smoke with a relatively alkaline pH, facilitating mucosal absorption of nicotine.71
Nicotine distributes throughout the body; plasma levels of nicotine in regular smokers range from 50 nmol/L in light smokers to 300 nmol/L in heavy smokers.20,71 Nicotine concentrations in breast milk are approximately three times greater than maternal serum concentrations, and nicotine accumulates in amniotic fluid and fetal tissues.71
Specific Adverse Health Effects of Tobacco Use
Cancer
Approximately one third of all cancer deaths can be attributed to smoking, and rates of death from cancer are twice as high in smokers and four times as high in heavy smokers than in nonsmokers.1,8 Smoking is linked to 90% of all lung cancer cases, and lung cancer accounts for 80% of cancer deaths attributed to smoking.1,8 Other cancers associated with smoking include oropharyngeal, laryngeal, esophageal, stomach, pancreatic, cervical, renal, bladder, and acute myelogenous leukemia.1,8 Of all possible carcinogens present in tobacco, the seven tobacco-specific nitrosamines have emerged as some of the most active based on rodent and preliminary human studies.79 Nonsmoking adults living with a smoker have been found to have a 20% to 30% increased risk of lung cancer compared with the general population.9
Numerous rodent studies have confirmed the ability of oral snuff to cause oral cancers.78 Many suspected carcinogens, including the tobacco-specific N-nitrosamines, are present in oral tobacco.78 Snuff also contains the radioactive isotope polonium-210, formaldehyde, and acetaldehyde.78
Although nicotine investigators have not shown that smoking is a direct carcinogen, animal studies suggest that it acts as a tumor promoter by inhibiting apoptosis and preventing the killing of malignant cells.77 Increased angiogenesis seen in animals receiving nicotine may contribute to tumor growth and eventual metastasis.77
Cardiovascular Effects
Cigarette smoking increases the risk of coronary artery disease by 80%. It also increases the incidence of stroke, angina, myocardial infarction, and fatal coronary artery disease.10 Both primary and secondhand tobacco smoke are associated with the development of atherosclerosis. Passive smoking increases the risk of coronary artery disease by 30%10
Proposed mechanisms leading to the increased incidence of acute myocardial infarction seen in smokers include increased coronary artery vascular resistance, stimulation of coronary vasospasm, increased risk of plaque rupture or erosion, and induction of thrombosis.10 Cigarette smoking is associated with decreased availability of nitric oxide, a free radical responsible for initiating vasodilation, especially in the coronary arteries and microvascular beds.10
Cigarette smoke has been associated with an increased peripheral blood leukocyte count and increased markers of inflammation, including C-reactive protein, interleukin-6, and tumor necrosis factor alpha.10 This results in increased leukocyte recruitment, adherence to the endovascular endothelium, and transendothelial migration of monocytes.10 The lipid profiles of smokers traditionally show decreased levels of high-density lipoprotein with increased levels of low-density lipoprotein, serum cholesterol, and triglycerides.10 Platelets from smokers have an increased propensity to aggregate, both spontaneously and following stimulation.10
Pulmonary Effects
Cigarette smoke leads to pulmonary emphysema through the initiation of inflammatory responses in lung tissues and impairment of repair processes.80 Following exposure to cigarette smoke, appropriate recruitment and proliferation of lung fibroblasts and epithelial cells is inhibited.80 Similarly, cigarette smoke inhibits lysyl oxidase, the enzyme responsible for cross-linking and polymerizing elastin.80
Pregnancy
Nicotine easily crosses the placenta and can reach fetal concentrations that are 15% higher than maternal levels.1 Additionally, elevated levels of maternal and fetal carbon monoxide can decrease fetal oxygen delivery.1 Adverse outcomes associated with smoking during pregnancy include fetal growth retardation, decreased birth weight, spontaneous abortion, and sudden infant death.81 In addition to an estimated 910 infant deaths annually due to smoking,1 costs due to smoking-related neonatal care are believed to be more than 350 million dollars a year.82 As a later consequence, children of mothers who smoked more than one pack a day during pregnancy have almost twice the risk of developing nicotine dependence.83
Addiction and Dependence
The Diagnostic and Statistic Manual of Mental Disorders-IV (DSM-IV)84 defines substance dependence as a maladaptive pattern of substance use leading to clinically significant impairment or distress (Box 130-1). Dependence on nicotine is more prevalent than on any other substance of abuse.85 The most basic explanation of nicotine’s addictive properties, like those of other drugs of abuse, is that it activates reward pathways1 (Table 130-4).
BOX 130-1 Definition of Substance Dependence
• Substance is taken in larger amounts or over a longer period than was intended
• Persistent desire or unsuccessful efforts to decrease substance use
• Great deal of time spent in activities necessary to obtain the substance, use it, or recover from its effects
• Important social, occupational, or recreational activities that are given up or reduced because of substance use
From American Psychiatric Association Task Force on DSM-IV: Diagnostic and statistical manual of mental disorders: DSM-IV-TR, ed 4, Washington DC, 2000, American Psychiatric Association.
TABLE 130-4 Neurotransmitter Levels Increased through Activation of Nicotinic Receptors and Resulting Effects
| Neurotransmitter | Effect(s) |
|---|---|
| Acetylcholine | Arousal, cognitive improvement |
| Beta-endorphin | Decreased anxiety, relaxation |
| Dopamine | Appetite suppression, enjoyment |
| Gamma-aminobutyric acid | Decreased anxiety, relaxation |
| Glutamate | Memory improvement |
| Norepinephrine | Appetite suppression, arousal |
| Serotonin | Appetite suppression, mood regulation |
Data from Benowitz NL: Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med 121:S3–S10, 2008.
Rodent studies indicate that acetaldehyde, present in tobacco smoke and a metabolite of ethanol, increases the reinforcing properties of nicotine and has a more significant effect on adolescents than adults.86 These findings may indicate why adolescents are more likely to become addicted to tobacco. Twin studies have found that up to 40% to 70% of the risk of nicotine addiction may be due to a genetic predisposition.1 A number of genes, a majority located on chromosome 15, that may place people at increased risk for nicotine addiction have been identified.87–90
Tobacco users also commonly acquire a psychological addiction to tobacco use. Smokers frequently develop compulsive behavior, including the need to handle, light, feel, or smell cigarettes, and they associate certain behaviors, people, and situations with the pleasurable effects of tobacco use.77 These conditioned cues are major factors preventing people from halting smoking and often lead to relapse.77
Certain populations appear to be more susceptible to tobacco addiction, including alcoholics and people with psychiatric disorders. Studies have shown that nicotinic acetylcholine receptors (nAChRs) play a role in regulating the effects of alcohol consumption, and 80% of alcoholics smoke regularly.1,69 In tobacco users with psychiatric disorders, it is difficult to ascertain whether increased smoking rates in these people are due to self-medication with nicotine, an intrinsic predisposition to smoking, or, most likely, a combination of the two.
It is important to note that nicotine is more addictive than alcohol, heroine, and cocaine.
Withdrawal
Box 130-2 lists diagnostic criteria for nicotine withdrawal.84 Often beginning within a few hours of the last nicotine use, common nicotine withdrawal symptoms include nicotine craving, anxiety, depression, cognitive and attention deficits, sleep disturbances, irritability, insomnia, and increased appetite.1,77 Withdrawal symptoms are typically more significant in people who smoke cigarettes than in people using other forms of nicotine.84 Peaking within a few days, withdrawal symptoms often decrease after a few weeks but may continue for months.1
BOX 130-2 Diagnostic Criteria for Nicotine Withdrawal
Daily use of nicotine for at least several weeks
Abrupt cessation of nicotine use, or reduction in the amount of nicotine used, followed within 24 hours by four (or more) of the following signs:
Symptoms that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. (The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder.)
From American Psychiatric Association Task Force on DSM-IV: Diagnostic and statistical manual of mental disorders: DSM-IV-TR, ed 4, Washington DC, 2000, American Psychiatric Association.
Cessation
Rates
A majority of tobacco users, approximately 35 million people in the United States, desire to quit each year.1,91 Of those people who try to quit on their own, more than 85% relapse—most within 1 week—and only 3% are abstinent at 6 months.1,92 Cessation studies have found that women are less likely to start the quitting process and, if they are able to quit, they are more likely to relapse than men.1
Benefits
A 35-year-old man who quits smoking increases his life expectancy by 5 years on average.1 Surgeons have been found to significantly underestimate the potential benefits of preoperative smoking cessation, reported to be up to a 65% reduction in complications.93 Blood pressure and myocardial infarction risk begin to decrease within 24 hours of smoking cessation, and the resting heart rate decreases by 5 to 12 beats per minute within the first few days.1,84 Coughing and shortness of breath typically improve within the first 1 to 9 months. The excess risk for the development of cardiovascular disease drops by 50% within the first year of cessation and drops to approximately the same level as in a person who has never smoked after 5 years.4 The risk of smoking-related cancers declines after cessation but never reaches that of people who have never smoked.4 Weight increases 2 to 3 kg on average within the first year of cessation.84
Counseling
The American Cancer Society has identified four steps involved in smoking cessation: making the decision to quit, setting a quit date and choosing a quit plan, dealing with withdrawal, and continuing not to smoke.1 Predictors of successful smoking cessation have been suggested to help providers effectively target counseling efforts94 (Box 130-3).
BOX 130-3 Suggested Predictors of Successful Attempts at Smoking Cessation
Absence of excessive alcohol use
Absence of other smokers in the home or workplace
From Caponnetto P, Polosa R: Common predictors of smoking cessation in clinical practice. Respir Med 102:1182–1192, 2008.
New patients presenting to a group of spine practitioners who were identified as smokers received either usual care, consisting of a suggestion that they should quit and a handout on the effects of smoking on spinal disorders, or aggressive treatment including the handout, education on the relationship between smoking and the patient’s condition, and instruction that they must quit smoking.95 This message was restated at every visit for members of the aggressive treatment group, and elective surgery was not completed if the patient was still smoking.95 The effect of the practitioner’s intervention was highly significant, with 35.6% of patients in the aggressive treatment group quitting smoking compared with only 19.5% in the usual care group.95 Younger patients, patients with greater daily cigarette consumption, and patients smoking for a longer period of time were less likely to stop smoking.95 There was not any difference in patients returning for follow-up care between the two groups, indicating that patients receiving aggressive treatment were not more likely to seek care elsewhere.95 In addition to decreasing preoperative smoking rates, cessation counseling can have long-term effects on tobacco use.29,96
Approaches
The tobacco use status of each patient should be assessed at every opportunity. Once tobacco uses have been identified, they should be advised to quit, and their willingness to quit should be assessed. The “Five As” model guides clinicians in treating addiction and has been recommended as a component of smoking cessation counseling97 (Box 130-4). The patient’s willingness to quit using tobacco can then be categorized using the “stages of change” to follow the patient’s progress and identify how efforts should be focused to best encourage cessation98 (Box 130-5).
BOX 130-4 “Five As” Model for Treating Addiction
Ask about tobacco use at every visit
Assess the patient’s willingness to quit
From Tobacco Use and Dependence Guideline Panel: Treating tobacco use and dependence: 2008 update, Rockville, MD, 2008, U.S. Dept. of Health and Human Services, Public Health Service.
BOX 130-5 Stages of Change for Smoking Cessation Counseling
Precontemplation: not planning to quit within the next 6 months
Contemplation: considering quitting within the next 6 months
Preparation: planning on quitting within the next 30 days
From Prochaska JO, DiClemente CC: Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 51:390–395, 1983.
Behavioral Therapy
For many tobacco users, the most difficult things to overcome are the behavioral and social aspects associated with tobacco use. Triggers such as being around certain people, performing particular tasks, or consuming specific beverages or food items can often make cravings worse and reinforce any withdrawal symptoms. Behavioral therapies should be customized to each person to help recognize high-risk situations, improve problem-solving capabilities, identify social supports, and develop coping strategies.1 Women having weight concerns addressed through cognitive-behavioral therapy have been found to be more successful at quitting than those not receiving therapy.1
Behavioral assistance is now available to most people over the telephone and Internet. Although typical intervention programs run from 1 to 3 months, 75% to 80% of smokers who quit relapse within 6 months.1 Studies have found that extending the length of the smoking cessation program can result in cessation rates as high as 50% at 1 year.1
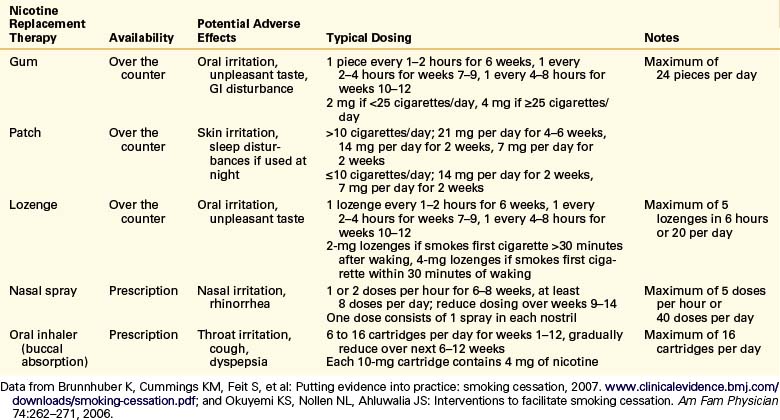
Pharmacologic Therapy
Pharmacologic agents currently used for tobacco use cessation include nicotine replacement therapies (NRTs) and medications with various mechanisms of action (Tables 130-5 and 130-6). NRTs are used to ease withdrawal symptoms and typically produce lower nicotine levels that do not result in the same pleasurable effects as tobacco products.1 Although NRTs still contain nicotine, they do not contain the many other chemicals, including carcinogens, found in tobacco products.1 The full nicotine content of NRT products is usually higher than that actually absorbed.71 Transdermal nicotine patches provide sustained levels of nicotine throughout the day, and nicotine gums, nasal sprays, and inhalers provide more rapid increases in blood levels to help alleviate acute cravings.77
NRT is contraindicated for use by people concurrently using tobacco products due to concerns related to increased blood levels of nicotine. However, studies evaluating NRT use by people continuing to use tobacco products did not identify an increased risk of adverse cardiovascular events.77,99,100 NRT also does not appear to increase the risk of adverse cardiovascular events in patients with a history of cardiovascular disease.101
All forms of NRT are effective and increase the long-term cessation rate by 50% to 70% when used by smokers motivated to quit who used more than 10 to 15 cigarettes a day.101,102 There is not sufficient evidence to recommend one form of NRT over another, but therapy recommendations should be customized to meet each patient’s needs.101 Evidence does suggest that combining a nicotine patch with a more rapid-acting NRT may be more effective than either alone.101
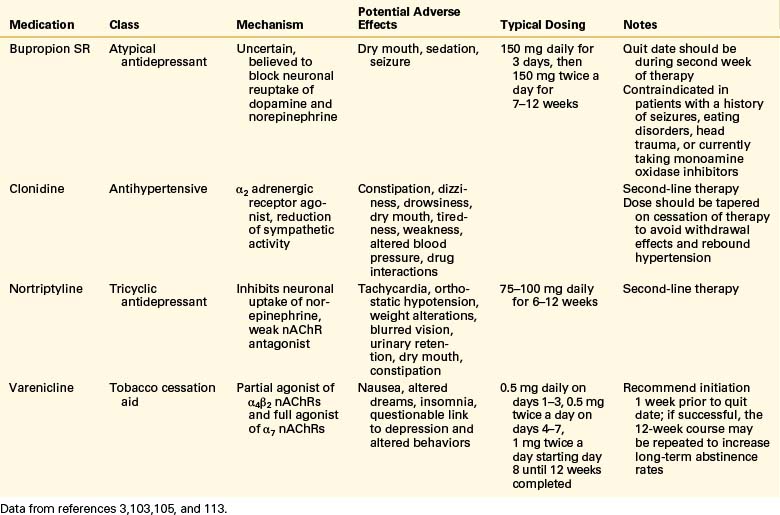
The antidepressant bupropion (Wellbutrin) was approved in 1997 as a smoking cessation aid and is marketed for this indication under the trade name Zyban. Bupropion is believed to facilitate smoking cessation through two mechanisms. First, it may block some of the nAChRs and decrease the positive reinforcement received from tobacco use.77 Second, it acts similarly to nicotine by increasing brain levels of norepinephrine and dopamine.77,103
More recently, varenicline tartrate (Chantix) has been approved for use in smoking cessation. An analogue of cytosine, varenicline is a selective nicotine receptor partial agonist targeted to the α4β2 nAChR.77 As a partial agonist, varenicline leads to less dopamine release than nicotine and is able to block the effects of additional nicotine consumption.77 This action helps decrease withdrawal symptoms and prevent positive reinforcement associated with continued tobacco use.77 Varenicline can reduce relapse rates in smokers who have been able to quit and was shown to have better cessation rates compared with placebo and bupropion.77,102,104
Nortriptyline is a tricyclic antidepressant that inhibits neuronal uptake of norepinephrine and acts as a weak nAChR antagonist.102 Clinical trials indicate that when used with NRT, nortriptyline increases smoking cessation rates compared with NRT alone.102 The α2-adrenergic receptor agonist clonidine decreases sympathetic outflow from the brain and may work to decrease anxiety commonly experienced during cessation.92
Self-reported smoking status has been found to be fairly accurate with the overall sensitivity and specificity for self-report near 90%.105 To assess compliance with tobacco use cessation, levels of cotinine, the primary metabolite of nicotine, can be measured. Levels greater than 3.08 ng/mL are believed to indicate current tobacco use in adults living in the United States.71 However, plasma levels of cotinine can be increased by exposure to secondhand smoke.
Resources
The availability of resources for tobacco use cessation varies from community to community. Numerous health organizations and government agencies provide a wealth of information on tobacco use and cessation appropriate for both health care providers and tobacco users (Table 130-7). Many have also developed cessation guidelines to help guide clinical interventions and provide support to those contemplating or attempting to halt tobacco use.
TABLE 130-7 Selected Organizations That Provide Tobacco-Related Resources
| Organization | Web Address(es) |
|---|---|
| American Cancer Society | www.cancer.org |
| American Lung Association | www.lungusa.org |
| Centers for Disease Control and Prevention | www.cdc.gov/tobacco |
| National Cancer Institute | www.nci.nih.gov www.smokefree.gov |
| National Institute on Drug Abuse | www.nida.nih.gov |
| Office of the Surgeon General | www.surgeongeneral.gov |
| World Health Organization | www.who.int |
Telephone-based quitlines providing telephone-based cessation counseling are available to residents of all 50 states, the District of Columbia, U.S. territories, and all 10 Canadian provinces. Quitlines are operated by various vendors under contract from government organizations and are available to anyone with access to a telephone. In the United States, a quitline database is organized by the North American Quitline Consortium (www.naquitline.org). They also operate 1-800-QUIT-NOW, a toll-free number connecting callers in the United States to the appropriate local quitline. The database contains extensive information on each quitline and can help providers identify available resources. The National Cancer Institute operates another national quitline at 1-877-44U-QUIT.
Current Research
Nicotine vaccines have been developed to stimulate production of nicotine antibodies that bind to nicotine and delay or prevent its passage through the blood-brain barrier, decreasing nicotine’s reinforcing effects.1,77 Currently in clinical trials, early results are encouraging.102 Early clinical trials of a cannabinoid receptor antagonist, Rimonabant (Acomplia), resulted in decreased smoking rates, but the trials were recently halted.77 Oral nicotine formulations, including drops and ingestible beads, are currently undergoing testing. A nicotine-delivering pulmonary inhaler has yet to be developed.102
Taking advantage of already identified pathways, new selective nAChR agonists and antagonists are being investigated. Building on the finding that slow metabolizers of nicotine smoke less and are more likely to quit, inhibitors of cytochrome P450 (CYP) enzyme CYP2A6 have been developed. The CYP2A6 inhibitors developed thus far induce significant toxicity and have not been shown safe for human use.77 Evidence supporting a genetic component to nicotine addiction has fueled research efforts to identify the specific genes involved and accurately predict a person’s response to specific treatment plans.1
Public Policy
Increasing the price of tobacco through higher taxes is one of the most effective ways to decrease consumption and encourage cessation.106 In addition to discouraging tobacco use, tobacco taxes also raise revenue that can be used for tobacco control and cessation programs.2 Increasing tobacco taxes by 10% has been found to decrease tobacco use by approximately 4% in high-income countries and 8% in low- and middle-income countries. This scenario still results in a 7% increase in tobacco tax revenue.2 Increases in tobacco costs are especially effective in preventing and decreasing tobacco use by younger people.107
The largest federal cigarette excise tax increase on record in the United States went into effect on April 1, 2009, resulting in a combined federal and average state cigarette excise tax of $2.21 per pack.108 This completed a 321% increase in the federal excise tax from $0.39 per pack in 1995 to $1.01 per pack in 2009.108 Similarly, the average state excise tax on April 1, 2009, was $1.20 per pack, a 267% increase since 1995.108 The state excise taxes in 2009 ranged from $0.07 per pack in South Carolina to $2.75 in New York.108 Additional taxes applied by some local governments can significantly increase the cost of cigarettes, such as the additional $2.68 tax per pack of cigarettes assessed by Chicago-Cook County.108
As of July 2009, 17 states, plus Puerto Rico and the District of Columbia, had laws in effect that required workplaces, restaurants, and bars to be 100% smoke free.109 An additional 14 states had laws in place that required workplaces, restaurants, and/or bars to be smoke free.109 Eliminating smoking in the workplace has been associated with a 3.8% decrease in the number of smokers and a 29% decrease in cigarette consumption by people in the workplace.110
Implemented in 1989, California was the first state to have a comprehensive statewide tobacco control program consisting of antitobacco advertising, tobacco tax increases, and smoking cessation assistance.111 This program rapidly expanded to include a smokers’ help line and the first statewide workplace smoking ban.111 As a result, California has made significant progress in reducing smoking rates and experienced the largest decrease in lung cancer death rates in the United States between 1975 and 2005.8
American Cancer Society. Guide to quitting smoking. 2009. www.cancer.org/docroot/PED/content/PED_10_13X_Guide_for_Quitting_Smoking. Accessed October 17, 2009
Hilibrand A.S., Fye M.A., Emery S.E., et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg [Am]. 2001;83:668-673.
Moller A.M., Villebro N., Pedersen T., et al. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114-117.
Rechtine G.R.II, Frawley W., Castellvi A., et al. Effect of the spine practitioner on patient smoking status. Spine (Phila Pa 1976). 2000;25:2229-2233.
Ward K.D., Klesges R.C. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259-270.
1. American Cancer Society. Guide to quitting smoking. 2009. www.cancer.org/docroot/PED/content/PED_10_13X_Guide_for_Quitting_Smoking. Accessed October 17, 2009
2. World Health Organization. Research for International Tobacco Control: WHO report on the global tobacco epidemic, 2008: the MPOWER package. Geneva: World Health Organization; 2008.
3. Brunnhuber K., Cummings K.M., Feit S., et al. Putting evidence into practice: smoking cessation. 2007. www.clinicalevidence.bmj.com/downloads/smoking-cessation.pdf.
4. Wipfli H., Samet J.M. Global economic and health benefits of tobacco control: part 1. Clin Pharmacol Ther. 2009;86:263-271.
5. Giovino G.A. The tobacco epidemic in the United States. Am J Prev Med. 2007;33:S318-S326.
6. Hadley M.N., Reddy S.V. Smoking and the human vertebral column: a review of the impact of cigarette use on vertebral bone metabolism and spinal fusion. Neurosurgery. 1997;41:116-124.
7. Proctor R.N. The global smoking epidemic: a history and status report. Clin Lung Cancer. 2004;5:371-376.
8. Jemal A., Thun M.J., Ries L.A., et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672-1694.
9. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, Coordinating Center for Health Promotion: The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon general—executive summary. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006.
10. Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731-1737.
11. Bagaitkar J., Demuth D.R., Scott D.A. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12.
12. Rubin D.I. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353-371.
13. Hogg-Johnson S., van der Velde G., Carroll L.J., et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33:S39-S51.
14. Goldberg M.S., Scott S.C., Mayo N.E. A review of the association between cigarette smoking and the development of nonspecific back pain and related outcomes. Spine (Phila Pa 1976). 2000;25:995-1014.
15. Leboeuf-Yde C. Smoking and low back pain. A systematic literature review of 41 journal articles reporting 47 epidemiologic studies. Spine (Phila Pa 1976). 1999;24:1463-1470.
16. Vogt M.T., Hanscom B., Lauerman W.C., Kang J.D. Influence of smoking on the health status of spinal patients: the National Spine Network database. Spine (Phila Pa 1976). 2002;27:313-319.
17. Feldman D.E., Rossignol M., Shrier I., et al. Smoking. A risk factor for development of low back pain in adolescents. Spine (Phila Pa 1976). 1999;24:2492-2496.
18. Mikkonen P., Leino-Arjas P., Remes J., et al. Is smoking a risk factor for low back pain in adolescents? A prospective cohort study. Spine (Phila Pa 1976). 2008;33:527-532.
19. Oda H., Matsuzaki H., Tokuhashi Y., et al. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. J Orthop Sci. 2004;9:135-141.
20. Akmal M., Kesani A., Anand B., et al. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine (Phila Pa 1976). 2004;29:568-575.
21. Pavord I.D., Chung K.F. Management of chronic cough. Lancet. 2008;371:1375-1384.
22. Wai E.K., Rodriguez S., Dagenais S., Hall H. Evidence-informed management of chronic low back pain with physical activity, smoking cessation, and weight loss. Spine J. 2008;8:195-202.
23. Hamilton G.A., Mullins S., Schuberth J.M., et al. Revision lapidus arthrodesis: rate of union in 17 cases. J Foot Ankle Surg. 2007;46:447-450.
24. Krannitz K.W., Fong H.W., Fallat L.M., Kish J. The effect of cigarette smoking on radiographic bone healing after elective foot surgery. J Foot Ankle Surg. 2009;48:525-527.
25. Haverstock B.D., Mandracchia V.J. Cigarette smoking and bone healing: implications in foot and ankle surgery. J Foot Ankle Surg. 1998;37:69-74. discussion 8
26. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976). 1986;11:942-943.
27. Glassman S.D., Anagnost S.C., Parker A., et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976). 2000;25:2608-2615.
28. Glassman S.D., Dimar J.R.III, Burkus K., et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine (Phila Pa 1976). 2007;32:1693-1698.
29. Andersen T., Christensen F.B., Laursen M., et al. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine (Phila Pa 1976). 2001;26:2623-2628.
30. Hilibrand A.S., Fye M.A., Emery S.E., et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg [Am]. 2001;83:668-673.
31. Wing K.J., Fisher C.G., O’Connell J.X., Wing P.C. Stopping nicotine exposure before surgery. The effect on spinal fusion in a rabbit model. Spine (Phila Pa 1976). 2000;25:30-34.
32. Theiss S.M., Boden S.D., Hair G., et al. The effect of nicotine on gene expression during spine fusion. Spine (Phila Pa 1976). 2000;25:2588-2594.
33. Veeravagu A., Patil C.G., Lad S.P., Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009;34:1869-1872.
34. Rodriguez P.G., Felix F.N., Woodley D.T., Shim E.K. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34:1159-1169.
35. Jensen J.A., Goodson W.H., Hopf H.W., Hunt T.K. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126:1131-1134.
36. Platzer P., Thalhammer G., Jaindl M., et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77:755-760.
37. Mok J.M., Cloyd J.M., Bradford D.S., et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine (Phila Pa 1976). 2009;34:832-839.
38. Lindstrom D., Sadr Azodi O., Wladis A., et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg. 2008;248:739-745.
39. Moller A.M., Villebro N., Pedersen T., Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114-117.
40. Hanley E.N.Jr., Levy J.A. Surgical treatment of isthmic lumbosacral spondylolisthesis. Analysis of variables influencing results. Spine (Phila Pa 1976). 1989;14:48-50.
41. Wilson-MacDonald J., Fairbank J., Frost H., et al. The MRC spine stabilization trial: surgical methods, outcomes, costs, and complications of surgical stabilization. Spine (Phila Pa 1976). 2008;33:2334-2340.
42. LaCaille R.A., DeBerard M.S., Masters K.S., et al. Presurgical biopsychosocial factors predict multidimensional patient: outcomes of interbody cage lumbar fusion. Spine J. 2005;5:71-78.
43. Dewing C.B., Provencher M.T., Riffenburgh R.H., Kerr S., Manos R.E. The outcomes of lumbar microdiscectomy in a young, active population: correlation by herniation type and level. Spine (Phila Pa 1976). 2008;33:33-38.
44. Li J., Guo Z., Zhu Q., et al. Fingertip replantation: determinants of survival. Plast Reconstr Surg. 2008;122:833-839.
45. Kowalchuk D.A., Harner C.D., Fu F.H., Irrgang J.J. Prediction of patient-reported outcome after single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25:457-463.
46. Gaston M.S., Simpson A.H. Inhibition of fracture healing. J Bone Joint Surg [Br]. 2007;89:1553-1560.
47. Taitsman L.A., Lynch J.R., Agel J., et al. Risk factors for femoral nonunion after femoral shaft fracture. J Trauma. 2009;67:1389-1392.
48. Baumgarten K.M., Gerlach D., Galatz L.M., et al. Cigarette smoking increases the risk for rotator cuff tears. Clin Orthop Relat Res. 2010;468:1534-1541.
49. Galatz L.M., Silva M.J., Rothermich S.Y., et al. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg [Am]. 2006;88:2027-2034.
50. Ward K.D., Klesges R.C. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259-270.
51. Gerdhem P., Obrant K.J. Effects of cigarette-smoking on bone mass as assessed by dual-energy x-ray absorptiometry and ultrasound. Osteoporos Int. 2002;13:932-936.
52. Ruffing J.A., Cosman F., Zion M., et al. Determinants of bone mass and bone size in a large cohort of physically active young adult men. Nutr Metab (Lond). 2006;3:14.
53. Demirbag D., Ozdemir F., Ture M. Effects of coffee consumption and smoking habit on bone mineral density. Rheumatol Int. 2006;26:530-535.
54. Wong P.K., Christie J.J., Wark J.D. The effects of smoking on bone health. Clin Sci (Lond). 2007;113:233-241.
55. Kanis J.A., Johnell O., Oden A., et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155-162.
56. MacInnis R.J., Cassar C., Nowson C.A., et al. Determinants of bone density in 30- to 65-year-old women: a co-twin study. J Bone Miner Res. 2003;18:1650-1666.
57. Law M.R., Hackshaw A.K. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841-846.
58. Kiel D.P., Zhang Y., Hannan M.T., et al. The effect of smoking at different life stages on bone mineral density in elderly men and women. Osteoporos Int. 1996;6:240-248.
59. Hopper J.L., Seeman E. The bone density of female twins discordant for tobacco use. N Engl J Med. 1994;330:387-392.
60. van der Klift M., de Laet C.E., McCloskey E.V., et al. Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res. 2004;19:1172-1180.
61. Paganini-Hill A., Chao A., Ross R.K., Henderson B.E. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology. 1991;2:16-25.
62. Melhus H., Michaelsson K., Holmberg L., et al. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14:129-135.
63. Olofsson H., Byberg L., Mohsen R., et al. Smoking and the risk of fracture in older men. J Bone Miner Res. 2005;20:1208-1215.
64. Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442.
65. Peto R., Lopez A.D., Boreham J., et al. Mortality from smoking worldwide. Br Med Bull. 1996;52:12-21.
66. Substance Abuse and Mental Health Services Administration: Results from the 2007 national survey on drug use and health: national findings. Rockville, MD: Office of Applied Studies; 2008.
67. National Institute on Drug Abuse. National Insitutes of Health: Topics in brief—tobacco addiction. November, 2007. Bethesda, MD
68. Connolly G.N., Alpert H.R. Trends in the use of cigarettes and other tobacco products, 2000–2007. JAMA. 2008;299:2629-2630.
69. Kalman D., Morissette S.B., George T.P. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106-123.
70. Baker F., Ainsworth S.R., Dye J.T., et al. Health risks associated with cigar smoking. JAMA. 2000;284:735-740.
71. Benowitz N.L., Hukkanen J., Jacob P.III. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29-60.
72. Stanfill S.B., Calafat A.M., Brown C.R., et al. Concentrations of nine alkenylbenzenes, coumarin, piperonal and pulegone in Indian bidi cigarette tobacco. Food Chem Toxicol. 2003;41:303-317.
73. Rodgman A., Perfetti T.A. The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press; 2009.
74. Adam T., Mitschke S., Streibel T., et al. Quantitative puff-by-puff-resolved characterization of selected toxic compounds in cigarette mainstream smoke. Chem Res Toxicol. 2006;19:511-520.
75. Borgerding M., Klus H. Analysis of complex mixtures—cigarette smoke. Exp Toxicol Pathol. 2005;57(Suppl 1):43-73.
76. Pankow J.F. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14:1465-1481.
77. Benowitz N.L. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57-71.
78. Hoffmann D., Djordjevic M.V. Chemical composition and carcinogenicity of smokeless tobacco. Adv Dent Res. 1997;11:322-329.
79. Hecht S.S. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160-171.
80. Liu X.D., Zhu Y.K., Umino T., et al. Cigarette smoke inhibits osteogenic differentiation and proliferation of human osteoprogenitor cells in monolayer and three-dimensional collagen gel culture. J Lab Clin Med. 2001;137:208-219.
81. Ayadi M.F., Adams E.K., Melvin C.L., et al. Costs of a smoking cessation counseling intervention for pregnant women: comparison of three settings. Public Health Rep. 2006;121:120-126.
82. Adams E.K., Miller V.P., Ernst C., et al. Neonatal health care costs related to smoking during pregnancy. Health Econ. 2002;11:193-206.
83. Buka S.L., Shenassa E.D., Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160:1978-1984.
84. American Psychiatric Association Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR, ed 4. Washington DC: American Psychiatric Association; 2000.
85. Markou A: Review. neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159-3168.
86. Cao J., Belluzzi J.D., Loughlin S.E., et al. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025-2035.
87. Thorgeirsson T.E., Stefansson K. Genetics of smoking behavior and its consequences: the role of nicotinic acetylcholine receptors. Biol Psychiatry. 2008;64:919-921.
88. Saccone S.F., Hinrichs A.L., Saccone N.L., et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36-49.
89. Bergen A.W., Conti D.V., Van Den Berg D., et al. Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology. 2009;34:2252-2264.
90. Perkins K.A., Lerman C., Coddington S., et al. Gene and gene by sex associations with initial sensitivity to nicotine in nonsmokers. Behav Pharmacol. 2008;19:630-640.
91. Wipfli H., Samet J.M. Global economic and health benefits of tobacco control: part 2. Clin Pharmacol Ther. 2009;86:272-280.
92. Benowitz N.L. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:S3-S10.
93. Owen D., Bicknell C., Hilton C., et al. Preoperative smoking cessation: a questionnaire study. Int J Clin Pract. 2007;61:2002-2004.
94. Caponnetto P., Polosa R. Common predictors of smoking cessation in clinical practice. Respir Med. 2008;102:1182-1192.
95. Rechtine G.R.II, Frawley W., Castellvi A., et al. Effect of the spine practitioner on patient smoking status. Spine (Phila Pa 1976). 2000;25:2229-2233.
96. Walker N.M., Morris S.A., Cannon L.B. The effect of pre-operative counselling on smoking patterns in patients undergoing forefoot surgery. Foot Ankle Surg. 2009;15:86-89.
97. Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service; 2008.
98. Prochaska J.O., DiClemente C.C. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
99. Joseph A.M., Norman S.M., Ferry L.H., et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792-1798.
100. Wennberg P., Eliasson M., Hallmans G., et al. The risk of myocardial infarction and sudden cardiac death amongst snuff users with or without a previous history of smoking. J Intern Med. 2007;262:360-367.
101. Stead L.F., Perera R., Bullen C., et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;3:CD000146.
102. Buchhalter A.R., Fant R.V., Henningfield J.E. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067-1088.
103. Fowler J.S., Logan J., Wang G.J., Volkow N.D. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75-82.
104. Garrison G.D., Dugan S.E. Varenicline: a first-line treatment option for smoking cessation. Clin Ther. 2009;31:463-491.
105. Patrick D.L., Cheadle A., Thompson D.C., et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84:1086-1093.
106. Meier K.J., Licari M.J. The effect of cigarette taxes on cigarette consumption, 1955 through 1994. Am J Public Health. 1997;87:1126-1130.
107. Carpenter C., Cook P.J. Cigarette taxes and youth smoking: new evidence from national, state, and local Youth Risk Behavior Surveys. J Health Econ. 2008;27:287-299.
108. Centers for Disease Control and Prevention. Federal and state cigarette excise taxes—United States, 1995–2009. MMWR. 2009;58:524-527.
109. American Nonsmokers’ Rights Foundation: Overview list—how many smokefree laws? www.no-smoke.org/pdf/mediaordlist.pdf. Accessed September 25, 2009
110. Fichtenberg C.M., Glantz S.A. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ. 2002;325:188.
111. Messner K., Pierce J.P., Zhu S.H., et al. The California Tobacco Control Program’s effect on adult smokers: (1) smoking cessation. Tob Control. 2007;16:85-90.
112. Okuyemi K.S., Nollen N.L., Ahluwalia J.S. Interventions to facilitate smoking cessation. Am Fam Physician. 2006;74:262-271.