Skull and brain
12.1
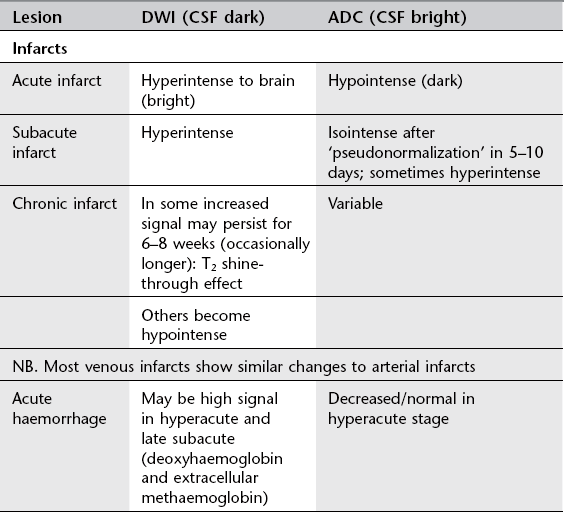
Acute arterial infarct: CT
1. Initial appearances often normal in first few hours. Larger infarcts more conspicuous.
(a) Low density with reduced grey/white differentiation (cell and tissue swelling): wedge shaped, involving grey and white matter; ‘insular ribbon sign’ (loss of grey/white differentiation in perisylvian region; loss of normal anatomical differentiation of basal ganglia).
(b) Mass effect – local (sulcal effacement).
(c) Hyperdense vessels due to thrombus (luminal/mural); usually middle cerebral or basilar arteries (mural calcification may mimic hyperdense artery [string] sign).
(a) More extensive area of low attenuation or progressive decreased attenuation.
(b) Generalized mass effect (ventricular or basal cistern effacement ± midline shift [subfalcine herniation] or other herniation syndromes: uncal, transtentorial).
(c) Secondary haemorrhagic transformation (usually occurs after a few days unless anticoagulated; reperfusion injury) if haemorrhage from outset consider embolus or venous stroke.
4. Contrast enhancement usually unhelpful and often confusing; simply reflects breakdown of blood–brain barrier. Any enhancement pattern possible.
5. CT perfusion study may demonstrate decreased perfusion in wider area of brain (ischaemic penumbra) suggesting further tissue at risk.
6. CT angiography can demonstrate dissection/stenosis/occlusion (embolic or thrombotic).
12.2
Acute arterial infarct: MRI
1. More sensitive than CT but signs similar (reduced attenuation on CT = increased T2 signal on MRI). Wedge shaped; grey and white matter involvement; often initially cortical change). Signal change usually (but not always) evident within 3–6 hours (within minutes on DWI). Increased T2 signal due to cytotoxic oedema (cell swelling; restricted diffusion).
2. Acute infarcts show increased DWI signal and low ADC. Low ADC signal ‘pseudonormalizes’, i.e. becomes bright from around 5–10 days postinfarct.
3. Mass effect – local and/or general as on CT.
4. Absent flow voids in affected major vessels; increased signal on T2 and FLAIR; most often seen in MCA (check carotid canal at skull base) or basilar.
5. Contrast enhancement may be confusing as on CT; prolonged transit time of contrast through distal or collateral vessels may be seen on postgadolinium T1 sequences.
6. Perfusion-weighted imaging may show poor perfusion in wider territory than changes on standard sequences: diffusion/perfusion mismatch may demonstrate ischaemic penumbra representing potentially salvageable brain tissue.
7. MR angiography may show vessel stenosis/occlusion in extracranial or intracranial vessels.
12.3
Venous infarcts
1. Usually secondary to venous sinus thrombosis:
(a) CT – hyperdense and expanded sinus precontrast; peripheral enhancement around luminal thrombus postcontrast (delta sign).
(b) MRI – absence of normal flow void: acute thrombus high signal on T1 (methaemoglobin); variable signal on T2 (beware normal variation in venous sinus anatomy with areas of hypoplasia).
(c) Check for evidence of underlying cause on scans (mastoid infection, paranasal sinus infection, etc.).
2. If an area of infarction is seen which is not in arterial distribution, consider sinus thrombosis.
3. Venous infarction often haemorrhagic (but this is not a contraindication to anticoagulation; aim of anticoagulation is to stop propagation of thrombus).
4. Beware symmetrical low attenuation in deep grey matter structures (especially thalami); suggests involvement of deep cerebral veins which may not otherwise be seen on scans.
12.4
Indications for CT and MRI in acute stroke
Indications for MRI in suspected stroke
1. If CT normal but clinical suspicion high, MRI more sensitive for early detection.
2. Assessment of diffusion/perfusion mismatch and suitability for thrombolysis.
3. Detection of stroke in distribution not well seen on CT (vertebrobasilar circulation in posterior fossa especially).
4. Detection of underlying cause such as arterial dissection or thrombosis (absent flow voids on standard sequences; narrowing/occlusion on MR angiography).
5. Assessment of intracranial and extracranial vessels by MR angiography.
12.5
Differentiation between infarct and tumour
1. Clinical history – abrupt vs gradual onset and development of symptoms.
2. Distribution – tumours not confined to vascular territory.
3. Shape – infarcts usually wedge shaped with base at periphery; tumours tend to be spherical/ovoid.
4. Tissue involvement – infarcts involve grey and white matter; most metastases or higher-grade gliomas involve white matter primarily; lower-grade primary tumours may involve grey matter.
5. Advanced imaging techniques such as DWI or MR spectroscopy may be useful in cases that remain unclear on standard sequences.
12.6
Appearances of blood on scans
CT
1. Acute = higher attenuation than underlying brain (soon after episode of bleeding for up to 7–10 days).
2. Subacute = similar attenuation to underlying brain (transition usually occurs between 1 and 2 weeks).
3. Chronic = lower attenuation than underlying brain (suggests at least 2–3 weeks old but could be older).
MRI
1. Oxyhaemoglobin – iso/hypointense T1; hyperintense T2 (less than 24 hours approx.).
2. Deoxyhaemoglobin – iso/hypointense T1; hypointense T2 (1–3 days approx.).
3. Intracellular methaemoglobin – hyperintense T1; hypointense T2 (3–7 days approx.).
4. Extracellular methaemoglobin – hyperintense T1; hyperintense T2 (1–2+ weeks approx.).
5. Haemosiderin – isointense/hypointense T1; hypointense T2.
12.7
Subarachnoid haemorrhage
Causes
1. Ruptured intracranial aneurysm (75%): potentially devastating condition with 50% immediate mortality and high long-term morbidity.
2. Bleeding from vascular malformation (cerebral or spinal) (5%).
3. Trauma – tends to have peripheral distribution in sulci rather than concentrated in basal cisterns.
4. Extension from parenchymal haematoma (often hypertensive bleed) (5%).
5. Perimesencephalic haemorrhage – low pressure probable venous haemorrhage; few symptoms and signs and good prognosis.
Diagnosis and investigation
1. CT – most sensitive in first few days (98% on day 1, only 50% positive by 7 days). Check review areas on scans: anterior interhemispheric fissure, sylvian fissure, posterior horns of lateral ventricles, third ventricle, basal cisterns, foramen magnum. CT angiography may be performed.
2. Lumbar puncture – negative CT scan does not exclude SAH, especially if scan performed days after ictus; therefore lumbar puncture mandatory if CT negative.
3. Late MRI more sensitive than CT – proton density, gradient echo T2 and FLAIR sequences most sensitive.
4. Catheter angiography now used less often in initial work-up as CT angiography often used at time of diagnosis of aneurysmal SAH and for planning therapy (neurointerventional vs surgical).
12.8
Intracranial aneurysms
Presentation
1. Sudden onset of severe headache ± neurological signs ± reduced level of consciousness with scan findings of:
(b) Parenchymal haemorrhage (usually with associated SAH).
(c) Intraventricular haemorrhage (most often secondary to bleeding in the general subarachnoid space, occasionally primary).
Incidence in general population ~2–3%. Overall risk of rupture = 0.5–1.5% per annum: variable according to size and position of aneurysm, sex, smoking, etc.
Diagnosis
1. CT and CT angiography – extra-axial mass in subarachnoid space; enhances if patent; may be thrombosed and/or have calcification (especially giant aneurysms). CT angiography demonstrates site and morphology of aneurysm and may allow planning of treatment (neurointervention vs surgery) without need for catheter angiography.
2. MR and MR angiography – patent aneurysm will show flow void; giant or partially thrombosed aneurysms can show complex flow patterns with heterogeneous signals on standard sequences. Not reliable for treatment planning.
3. Catheter angiography – invasive with 0.1–0.5% inherent stroke risk. Still considered gold standard but may soon be superseded by CT angiography.
12.9
Vascular malformations
Two main types: those with arteriovenous shunts and those without shunts.
Malformations with AV shunts
1. Arteriovenous malformations – present in young to middle-aged adults with one or combination of haemorrhage (40%), seizures (30%), neurological deficit or headache (20%). Annual cumulative rupture risk ~3% per year. Consist of one or more arterial pedicles draining directly to enlarged draining veins through nidus. Multiple lesions in various syndromes: e.g. Osler–Weber–Rendu and Wyburn–Mason.
(a) CT – hyperdense enlarged serpiginous vessels; often speckled calcification; enhance strongly.
(b) MRI – serpiginous flow voids; may be evidence of local atrophy and gliosis or previous haemorrhage.
(c) Catheter angiography – gold standard for assessment of morphology and nidal architecture including presence of associated arterial or venous aneurysms, varices and stenoses.
2. Dural arteriovenous fistulae – acquired lesions presenting in older population (50–70 years) compared to AVMs (20–40 years). Occur following damage to venous structures (post-thrombosis, surgery, trauma). Symptoms and signs secondary to arterialization of venous system: bruit, venous hypertension, pulsatile tinnitus (if primary involvement is sinuses); haemorrhage, focal neurology, seizures (if primary or major secondary involvement of cortical veins). Caroticocavernous fistula may give rise to proptosis and chemosis.
(a) CT – often normal unless complications, e.g. haemorrhage; enlargement of cavernous sinus and superior ophthalmic veins if caroticocavernous fistula.
(b) MRI – standard sequences often normal unless complication; dynamic subtraction angiography demonstrates lesions well.
(c) Catheter angiography – still gold standard for diagnosis and demonstration of morphology on which classification and treatment planning based.
Malformations without AV shunts
1. Cavernous angioma (cavernoma) – sinusoidal spaces lined with endothelium; occur anywhere in CNS, commonest pons. Present with small haemorrhages (usually not associated with large haemorrhages) or seizures; often incidental findings. Multiple cavernomas may be familial.
(a) CT – iso/hyperdense lesion, often calcification.
(b) MRI – characteristic appearance is heterogeneous signal centre with low T2 signal (haemosiderin) rim. Gradient echo most sensitive sequence for detection.
2. Developmental venous anomaly (venous angioma) – probably due to persistent embryonic veins which drain normal brain. Not thought to have increased haemorrhage risk.
12.10
CNS infection
Meningitis
1. Bacterial meningitis – CT usually normal. May see generalized brain swelling and/or focal or generalized ischaemia. Scanning only postcontrast may mask pathology. If contrast to be used, precontrast and postcontrast scans should be obtained. Lack of enhancement does not exclude meningitis. Hydrocephalus may be communicating and/or obstructive.
2. Subdural effusion or empyema – low attenuation extra-axial collection ± rim enhancement.
3. Cerebritis – diffuse area of parenchymal low attenuation which may develop into abscess.
4. Abscess – thin-walled rim-enhancing lesion; may be very little systemic disturbance.
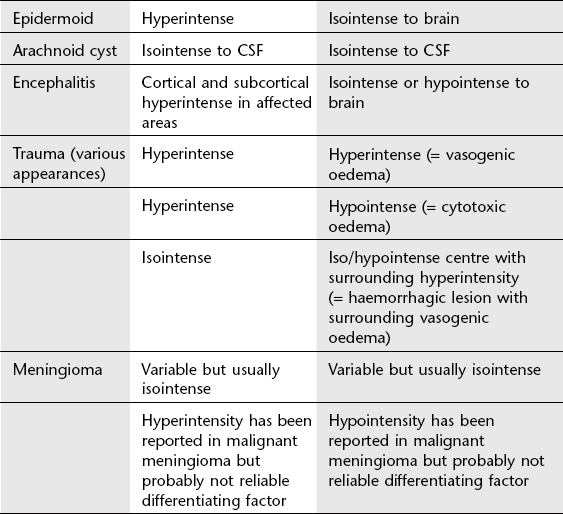
Encephalitis and encephalitis-like disorders
1. CT – less sensitive than MRI, often normal in early HSV encephalitis; low attenuation in medial temporal lobes, later usually unilateral; may be haemorrhagic.
2. MRI – increased T2 signal in medial temporal lobes, insula, cingulate gyrus; usually bilateral but asymmetrical. Atrophy, gliosis and encephalomalacia long term.
Slow viruses
1. Subacute sclerosing panencephalitis (SSPE) – progressive increased T2 signal and atrophy several years after primary measles infection.
2. Rasmussen’s encephalitis – progressive neurological deficits and intractable seizures in children; increased T2 signal and atrophy in one cerebral hemisphere.
Prion diseases
1. Sporadic CJD – rapidly progressive dementing illness associated with myoclonus, ataxia, pyramidal and extrapyramidal signs, cortical blindness.
MRI – increased T2 and FLAIR signal in caudate and putamen (corpus striatum); less often signal change in thalamus, globus pallidus and periaqueductal grey.
2. Variant CJD – sensory disturbances, depression, abnormal eye movements and involuntary movements.
Encephalitis and encephalitis-like disorders
Aiken, A. H. Central nervous system infection. Neuroimaging Clin N Am. 2010; 20(4):557–580.
Foerster, B. R., Thurnher, M. M., Malani, P. N., et al. Intracranial infections: clinical and imaging characteristics. Acta Radiol. 2007; 48(8):875–893.
Hughes, D. C., Raghavan, A., Mordekar, S. R., et al. Role of imaging in the diagnosis of acute bacterial meningitis and its complications. Postgrad Med J. 2010; 86(1018):478–485.
12.11
HIV and the brain
Infections
Viral
1. HIV – progressive dementia and atrophy due to subacute encephalitis.
(a) CT – diffuse white matter low attenuation.
(b) MRI – diffuse/patchy white matter high T2 signal, may involve basal ganglia. Mass effect and contrast enhancement usually absent.
2. Cytomegalovirus – typically signal abnormalities seen in periventricular distribution.
3. Progressive multifocal leukencephalopathy; papovavirus infection.
12.12
Congenital CNS infections
1. CMV – damages germinal matrix causing periventricular calcification. Earlier infection leads to more extensive damage.
2. Toxoplasmosis – focal areas of calcification more widespread than with CMV and involve basal ganglia, periventricular regions and cortex.
3. Rubella – microcephaly, parenchymal calcification and atrophy.
4. HIV – diffuse cerebral atrophy; may cause calcification of basal ganglia after first year.
12.13
Head injury
Primary effects
1. Fracture – impact head injury; commonest = linear; complex fractures (diastatic, stellate, depressed) tend to occur with mechanisms involving greater degrees of force; skull base fractures may be occult (look for secondary clues such as fluid in sphenoid sinus or mastoid air cells; pneumocephalus); facial nerve palsy or ossicular disruption in temporal fractures.
2. Extradural haemorrhage – usually arterial bleed (middle meningeal); biconvex lentiform haematoma (limited by coronal and lambdoid sutures as inner layer of dura bound to sutures but may cross sagittal suture); mixed attenuation haematoma may mean ongoing bleeding.
3. Subdural haemorrhage – usually venous low-pressure bleed; crescentic biconcave collection over cerebral hemisphere (can cross coronal and lambdoid but not usually sagittal sutures).
4. Subarachnoid haemorrhage – post-traumatic SAH usually low-volume scattered bleeds; peripheral distribution.
5. Contusions – larger mixed attenuation (CT) or signal (MRI) intra-axial lesions; tend to occur in inferior frontal and anterior temporal lobes (impact against bony anterior walls of anterior and middle cranial fossae); usually surrounding oedema adding to mass effect.
6. Diffuse axonal injury – smaller lesions than contusions; shearing injury secondary to rotational or acceleration/deceleration forces; tend to be widespread occurring at grey–white junction, corpus callosum, internal capsule and brainstem.
Secondary effects
1. Cerebral oedema and ischaemia – may lead to cerebral herniation syndromes.
2. Subfalcine – midline shift which can give rise to ischaemia in distribution of anterior cerebral artery; contralateral hydrocephalus with obstruction at foramen of Monro.
3. Uncal – medial aspect of medial temporal lobe presses on third nerve (fixed dilated pupil).
4. Transtentorial – descending transtentorial herniation causes brainstem distortion and ischaemia in distribution of posterior cerebral artery.
Delayed effects
1. Atrophy – focal (following contusion, etc.); generalized (following DAI or large extra-axial haematomas which required surgical evacuation).
2. CSF leak – secondary to fractures of frontal sinus, anterior cranial fossa, sphenoid sinus, temporal bone (secondary meningitis).
3. Arteriovenous fistula – direct caroticocavernous fistula.
4. Pseudoaneurysm – following arterial wall tear.
5. Leptomeningeal cyst – dura trapped within fracture line, CSF pulsation prevents fracture healing, leading to ‘growing fracture’.
Kemp, A. M., Jaspan, T., Griffiths, J., et al. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 2011; 96(12):1103–1112.
Kubal, W. S. Updated imaging of traumatic brain injury. Radiol Clin North Am. 2012; 50(1):15–41.
12.14
Hydrocephalus
1. Commensurate enlargement of temporal horns.
2. Ventricles disproportionately enlarged compared to sulci.
12.15
Pneumocephalus
1. Trauma – compound fractures of vault, fractures involving paranasal sinuses or mastoid air cells.
3. Osteoma of paranasal sinus (especially ethmoid) may erode through sinus wall.
4. Other sinus or skull base erosive tumours.
5. Empty sella – rare complication = development of communication between sella and sphenoid sinus.
12.16
CT attenuation of cerebral masses
Relative to normal brain (masses with variable appearances not included).
Hyperdense
12.17
Differential diagnosis of a solitary intracerebral mass
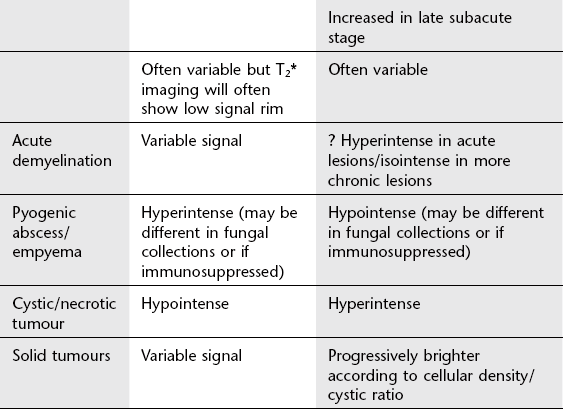
1. Primary brain tumour – high-grade tumours tend to have most mass effect (tumour and surrounding oedema), heterogeneous with areas of necrosis (glioblastoma); may infiltrate and involve/cross corpus callosum; variable enhancement but tends to increase with increased grade.
2. Metastasis – appearance variable on scans depending on primary; often considerable associated oedema (vasogenic, white matter), multiple/solitary, often located grey–white junction.
3. Arterial infarct – developing low attenuation (CT), high T2 signal (MRI) wedge-shaped lesion with variable mass effect; various enhancement patterns if contrast given.
4. Venous infarct – area of low attenuation (CT), high signal (MRI) not in arterial distribution, often associated mass, often haemorrhagic.
5. Abscess – homogeneous, thin enhancing rim, usually considerable vasogenic oedema.
6. Acute demyelinating plaque – may be very large with minimal clinical signs; low attenuation (CT) and high T2 signal (MRI); variable enhancement.
7. Haematoma – subacute to chronic.
8. Cerebritis/encephalitis – poorly defined area of low attenuation (CT); HSV predilection for limbic system; variable enhancement.
9. Aneurysm – may give rise to mass effect by itself but also often associated oedema in surrounding brain; appearance varies according to whether patent or associated intramural thrombus ± calcification.
Essig, M., Anzalone, N., Combs, S. E., et al. MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice. AJNR Am J Neuroradiol. 2012; 33:803–817.
Omuro, A. M., Leite, C. C., Mokhtari, K., et al. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006; 5(11):937–948.
Tang, Y. Z., Booth, T. C., Bhogal, P., et al. Imaging of primary central nervous system lymphoma. Clin Radiol. 2011; 66:768–777.
12.18
Intracranial calcification
Normal variant
1. Pineal – after 10 years of age on SXR, earlier on CT.
2. Choroid plexus – trigones of lateral ventricles; unusual in third and fourth ventricles.
4. Basal ganglia – globus pallidus; usually bilateral.
5. Habenular commissure – C-shaped calcification in tela choroidea of third ventricle.
Tumours
2. Oligodendroglioma (50% calcify).
3. Astrocytoma (lower incidence of calcification than oligodendroglioma but higher incidence than oligodendroglioma).
6. Metastases – adenocarcinoma (gastrointestinal and breast, especially after therapy in breast).
7. Pineal region tumours – teratoma and germinoma.
8. Chordoma and chondrosarcoma.
9. Fatty midline tumours – dermoid and lipoma of corpus callosum.
12.19
Basal ganglia calcification
2. Endocrine – hypoparathyroidism, pseudohypoparathyroidism, hypothyroidism.
3. Metabolic – mitochondrial disorders (Leigh’s disease), Fahr’s disease, Cockayne’s syndrome.
4. Toxins – carbon monoxide, lead, posthypoxic.
5. Post-therapeutic – mineralizing angiopathy following chemotherapy or radiation in basal ganglia, dentate nuclei of cerebellum and corticomedullary junction.
12.20
Meningeal enhancement on CT and MRI
Dural
1. Infection – skull base osteomyelitis, paranasal sinuses.
2. Tumour – meningioma (dural tail); metastasis (especially breast); lymphoma.
5. Intracranial hypotension – also often subdural effusions, dural sinus engorgement and brainstem descent.
7. Idiopathic pachymeningitis.
12.21
Enhancement of ependymal and subarachnoid space on CT and MRI
Ependymal
12.22
Multiple ring-enhancing lesions
1. Metastases – solid/ring-enhancing (usually thicker irregular wall than abscess); grey–white junction; commonest primary tumours = lung, breast, kidney, colon, melanoma; multiple in 80%; commonest infratentorial mass in adults.
2. Abscess – usually thin, uniform wall; homogeneous centre; high signal on DWI and low signal ADC (cystic tumours in absence of haemorrhage usually low signal DWI and high signal ADC).
3. Demyelination – acute demyelinating plaques may enhance (breakdown of blood–brain barrier).
5. Lymphoma – solid tumour (pretreatment) in immunocompetent patients; may be ring enhancing in immunocompromised.
6. Infarcts – multiple suggest emboli.
7. Contusion/haematoma – breakdown of blood–brain barrier can lead to peripheral enhancement.
12.23
Basal ganglia: bilateral abnormalities
Caudate nucleus; putamen; globus pallidus; subthalamic nucleus, substantia nigra, ventral tegmentum.
Vascular
1. Lacunar (small, deep) infarcts – well-defined low-attenuation lesions (CT), high T2 signal (MRI).
2. Acute near total hypoxic insults (especially in perinatal period); high T2 signal in posterior putamina, ventrolateral thalami, perirolandic white matter and cortex and hippocampal formations.
3. Cardiac arrest, near-miss drowning and opiate overdose can cause increased T2 signal in globus pallidus and putamen.
4. Deep cerebral vein thrombosis (although thalami usually affected first/as well).
Toxins
1. Kernicterus – increased signal in globus pallidus on T1 and T2 (MRI).
2. Hepatocellular degeneration – high T1 signal.
3. Prolonged total parenteral nutrition can lead to excess manganese deposition in basal ganglia: increased signal on T1.
4. Exogenous toxins – carbon monoxide, methanol, cyanide, hydrogen sulphide.
Inherited metabolic disease
1. Wilson’s disease (striatum).
2. Kearns–Sayre disease (globus pallidus).
3. Mitochondrial cytopathies – Leigh’s disease (striatum and globus pallidus primarily).
4. Leukodystrophies – Krabbe’s (thalami and periventricular white matter).
5. Amino acid disorders – methylmalonic aciduria (globus pallidus).
6. Lipidoses – Tay–Sachs disease (striatum, thalami).
7. Neurodegeneration with brain iron accumulation (NBIA, formerly called Hallervorden–Spatz syndrome); low signal in central globus pallidus on T2 (MRI) with surrounding high signal: ‘eye of the tiger’.
12.25
Thalamus: bilateral abnormalities
Vascular
1. Lacunar (small, deep) infarcts.
2. Arterial infarct – perforating arteries from tip of basilar artery may supply both thalami.
3. Venous ischaemic and infarction – thrombosis of straight sinus/vein of Galen/deep cerebral veins: bilateral symmetrical low attenuation (CT) or high T2 signal (MRI) ± haemorrhagic transformation.
Metabolic
1. Carbon monoxide poisoning – basal ganglia, especially globus pallidus, also involved.
2. Wernicke’s encephalopathy – thiamine deficiency in chronic alcoholism: involvement of mesial thalamic nuclei, mamillary bodies, midbrain and floor of third ventricle with increased T2 signal.
3. Inherited metabolic conditions – mitochondrial cytopathies (Leigh’s disease); certain leukodystrophies (Krabbe’s disease).
12.26
Inherited metabolic white matter disease
Dysmyelination
Lysosomal disorders
1. Metachromatic leukodystrophy – arylsulphatase A deficiency; AR; presentation aged 2–3 years (usually), can be later; MRI: diffuse symmetrical increased white matter signal sparing subcortical U fibres; may involve cerebellum; cerebral atrophy later.
2. Krabbe’s (globoid) leukodystrophy – galactosylceramide β-galactosidase deficiency; AR; early presentation (within 6 months) usually; MRI: symmetrical increased T2 signal posteriorly with involvement of thalami and caudate; severe atrophy late.
Peroxisomal disorders
1. Adrenoleukodystrophy – different phenotypes
(a) Cerebral adrenoleukodystrophy (40%).
(b) Adrenomyeloneuropathy (46%) – progressive spastic diplegia in adults.
(c) Primary adrenocortical deficiency without CNS involvement.
2. Zellweger’s (cerebrohepatorenal) syndrome – presents in neonatal period with extensive white matter changes and cortical dysplasia (polymicrogyria type).
Mitochondrial disorders
Respiratory chain enzyme disorders causing myopathies or multisystem disorders with encephalopathy.
1. Leigh’s disease – usually increased T2 signal in central grey matter (mainly corpus striatum) but white matter can be involved and any pattern of abnormality can be seen.
2. MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes): focal cortical and brainstem white matter changes with basal ganglia calcification; atrophy later.
3. MERRF (myoclonic epilepsy with ragged red fibres).
4. Kearns–Sayre syndrome – progressive external ophthalmoplegia and pigmentary retinal degeneration ± heart block, elevated CSF protein and cerebellar dysfunction. White matter abnormalities seen in association with cerebral and/or cerebellar atrophy and calcification in deep grey and deep white matter.
Amino organic acid abnormalities
12.27
Multiple sclerosis and its differential diagnosis
Vascular
1. Small vessel ischaemia – usually deep and subcortical white matter; discrete or confluent; commoner if hypertension and/or diabetes.
2. Infarct – solitary abnormality with little mass effect involving white matter and adjacent cortex; may be difficult to distinguish from solitary plaque MS; acute infarct will have high signal on DWI and low signal ADC.
Other demyelinating conditions
1. Acute disseminated encephalomyelitis (ADEM): monophasic autoimmune disorder, usually follows viral infection or immunization; ADEM usually fewer, larger lesions than MS and more often also affects grey matter; mass effect unusual.
2. Central pontine (osmotic) myelinolysis – MRI low T1, high T2 signal in central pons with sparing of periphery, pons swollen; clinically most usually follows intravenous fluid correction of chronic hyponatraemia.
12.28
Cerebral atrophy
Focal
1. Postischaemia/postinfarction.
2. Post-trauma – contusion, haematoma.
3. Postinfective – encephalitis and meningitis.
4. Alzheimer’s disease – commonest dementia; hippocampal atrophy usually most severe but may be generalized.
6. Parkinson’s disease – generalized atrophy and atrophy of substantia nigra; increased putaminal iron (reduced T2 signal).
7. Progressive supranuclear palsy – atrophy of tectum, globus pallidus and frontal lobes.
8. Pick’s disease – severe frontal and anterior temporal atrophy.
9. Huntington’s disease – atrophy of caudate heads.
10. Corticobasal degeneration – atrophy of posterior parietal ± frontal lobes.
12.29
Diffuse cerebellar atrophy
12.30
Intracranial manifestations of well-known neurocutaneous disorders
Neurofibromatosis type 1
12.35
Intrasellar mass
Neoplastic
1. Pituitary microadenoma – < 10 mm; enhance more slowly than normal pituitary, therefore low signal on enhanced studies (will show enhancement if imaged late); often different signal to normal gland on unenhanced scans; distort outline of gland.
2. Pituitary macroadenoma – > 10 mm; solid/cystic enhancing mass; MRI most sensitive for diagnosis and demonstration of tumour spread: suprasellar (chiasm), parasellar (cavernous sinus; significant if > 50% of carotid encased), retroclival.
3. Meningioma – usually extend into sella; origin in sella very rare.
Non-neoplastic
1. Pituitary (pars intermedia) cyst – similar to microadenoma but may be lower T1; higher T2 signal.
2. Pituitary hyperplasia – peripubertal, pregnancy.
3. Internal carotid aneurysm – medially placed flow void.
5. Rathke’s cleft cyst – may be intrasellar, suprasellar or involve both compartments.
6. Lymphocytic hypophysitis – lymphocytic infiltration of anterior pituitary, infundibulum and floor of hypothalamus; typically during pregnancy and peripartum period but also occurs in males; enhances postcontrast with hypothalamic tail.
7. Langerhans’ cell histiocytosis – typically enlarged enhancing infundibulum.
12.37
Suprasellar mass
Neoplastic
1. Extension of pituitary macroadenoma.
2. Meningioma – arising in and extending from anterior cranial fossa, sphenoid wing or diaphragma sella: homogeneous mass with uniform enhancement (unless cystic); pituitary should be visible as separate structure.
3. Craniopharyngioma – sellar/suprasellar/both; solid/cystic.
6. Hypothalamic hamartoma – uniform mass in patients with precocious puberty or gelastic seizures.
Non-neoplastic
1. Ectatic or aneurysmal carotid artery.
3. Epidermoid cyst – non-enhancing lobulated mass; signal usually higher than that of CSF on T1 FLAIR and DWI.
4. Dermoid – midline mass with fat and calcification; rupture gives rise to disseminated small areas of high T1 signal fat in subarachnoid space.
12.38
Cavernous sinus/parasellar mass
Neoplastic
Non-neoplastic
1. Ectatic or aneurysmal carotid artery.
2. Cavernous sinus thrombosis – sinus expanded, abnormal signal; usually secondary to perifacial/orbital sepsis.
3. Caroticocavernous fistula – direct (via internal carotid artery) or indirect (via dura) causes enlargement of sinus and draining veins (especially superior ophthalmic vein) leading to ophthalmoplegia, proptosis and chemosis. Drainage routes other than orbit may predominate (therefore normal orbit does not exclude caroticocavernous fistula).
4. Invasive sinusitis – Aspergillus in immunocompromised patients.
8. Tolosa–Hunt syndrome – painful ophthalmoplegia caused by non-specific granulomatous infiltration of cavernous sinus and superior orbital fissure; usually steroid responsive.
12.39
Pineal region mass
Pineal gland
1. Simple pineal cyst – < 1 cm, often slightly higher signal than CSF; no enhancement; common incidental finding of no significance if no mass effect or symptoms.
2. Germinoma – commonest pineal germ cell tumour; M : F = 10 : 1; check infundibulum/suprasellar region for synchronous tumour (10%); CT: hyperdense, calcification; MRI: image whole neuraxis for spread; serum markers often positive (α-fetoprotein); tend to occur in first two decades.
3. Teratoma – second commonest pineal germ cell tumour: heterogeneous mass (fat, calcification, cystic change); spectrum of malignant potential.
12.40
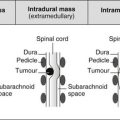
Intraventricular mass in adults
Lateral ventricles
12.41
Cerebellopontine angle mass
1. Vestibular schwannoma (acoustic neuroma) – commonest (90%); intracanalicular component often expands porus acousticus of internal auditory meatus but may be all extracanalicular; may cause distortion of brainstem (middle cerebellar peduncle) and obstructive hydrocephalus (but hydrocephalus may be present in absence of obstruction).
2. Meningioma (9%) – broad base against petrous bone, may extend into but usually do not expand porus.
3. Epidermoid (1%) – low attenuation (CT); lobulated mass (MRI) of similar signal to CSF on most sequences but increased signal on FLAIR and DWI. Growing epidermoids insinuate themselves around surrounding vessels and nerves.
12.43
Middle/external ear mass
Inflammatory
1. Acquired cholesteatoma – expanding mass of epithelial debris in epitympanum of middle ear cavity; erodes and invades surrounding bone leading to:
(a) Cerebral abscess/meningitis by erosion of tegmen.
(b) Conductive deafness by erosion of ossicles.
(d) Vertigo and deafness by labyrinthine erosion and endolymph leak.
2. Acute otitis media – may result in mastoiditis.
3. Malignant otitis externa – acute osteomyelitis of temporal bone in elderly, diabetics, immunocompromised: local bone erosion and extensive soft-tissue swelling.
4. Cholesterol granuloma – non-specific chronic inflammation of middle ear and mastoid; high signal T1 and T2.
5. Serous otitis media – sterile fluid in middle ear cavity.
6. Middle ear effusion – secondary to blockage of Eustachian tube, e.g. nasopharyngeal carcinoma.
7. Tympanosclerosis – deposits of fibrotic/calcified tissue in middle ear, epitympanum, tympanic membrane; areas of high density on high-resolution CT.
12.44
Temporal bone mass
Neoplastic
1. Glomus tumour – jugulare and tympanicum.
3. Metastasis – breast, lung, renal, prostate.
5. Lymphoma – but more commonly involves orbits or paranasal sinuses.
6. Nasopharyngeal carcinoma – direct extension.
7. Rhabdomyosarcoma – commonest soft-tissue sarcoma in children; usually involves orbit, paranasal sinuses and pharynx.
8. Carcinoma of the parotid – direct extension into floor of external auditory meatus/mastoid; infiltration along facial nerve.
12.45
Temporal bone sclerosis
1. Otosclerosis – condition characterized by periods of demineralization followed by sclerotic repair; ill-defined bone resorption around oval window or cochlea (lucent halo around cochlea) followed by irregular bone deposition; both processes may be simultaneous rather than sequential.
2. Paget’s disease – initial changes at petrous apex with demineralization and irregular bone deposition leading to hypertrophied, irregularly mineralized bone; can involve otic capsule, labyrinth and internal auditory canal.
3. Fibrous dysplasia – thickening of outer table of squamous temporal bone with obliteration of mastoid air cells.
4. Osteopetrosis – homogeneous sclerotic temporal bone with obliteration of air cells; progressive narrowing of internal auditory meatus can cause facial palsy.
12.46
Pulsatile tinnitus
Anatomical
1. Large/dehiscent jugular bulb – normal flow may be perceived especially if thin plate of bone normally between wall of jugular vein and middle ear absent; may also give rise to protrusion of jugular bulb into middle ear (not to be mistaken for glomus).
2. Aberrant carotid artery – inferior compartment of middle ear filled by carotid running posterior and lateral to its normal course.
3. Persistent stapedial artery – failure of regression of embryonic stapedial artery runs through lumen of stapes.
12.47
Jugular foramen mass
Neoplastic
1. Glomus jugulare – erosion/lysis and expansion of jugular foramen and surrounding structures; intratumoral vessels seen as flow voids on T2 MRI (pepper-pot appearance); arterial blush on angiography with AV shunting.
2. Schwannoma – foramen enlarged but no erosion or lysis; well-defined, lobulated tumour which enhances postcontrast.
3. Direct invasion from local tumour.
12.49
Diffuse skull base abnormality
Neoplastic
1. Metastases – commonest = breast, bronchus, prostate; four common clinical syndromes:
12.50
Skull vault lucency without sclerotic edge
12.52
Generalized increase in density of skull vault
1. Paget’s disease – multiple islands of dense bone, loss of differentiation of inner and outer tables, thickening of skull vault; basilar invagination can occur.
2. Sclerotic metastases – breast (post-treatment), prostate.
3. Fibrous dysplasia – younger age group than Paget’s.
5. Renal osteodystrophy – osteosclerosis in 25%; looks similar to Paget’s.
6. Acromegaly – enlarged frontal sinuses, prognathism, enlarged sella, thickened skull vault.
7. Chronic haemolytic anaemias.
12.55
Thin skull
Generalized
5. Chronically raised intracranial pressure.
6. Lacunar skull – bone dysplasia of membranous skull; indentations or pits in frontal and parietal regions that may be full thickness; defects separated by thin rims of bone; usually disappear by 6 months; not associated with raised intracranial pressure.
12.58
‘Hair-on-end’ skull vault
Haemolytic anaemias
1. Sickle-cell anaemia – initially in frontal region but can involve whole skull where diploic space present (marrow cavity), i.e. above level of internal occipital protuberance.
2. Thalassaemia – marrow hyperplasia more marked in this than other anaemias.
3. Hereditary spherocytosis and elliptocytosis.