19. Skin and Skin Care*
Carolyn Houska Lund and David J. Durand
The skin is a large organ in premature and term infants, making up at least 13% of body weight in contrast to 3% of body weight in adults. 65 Skin functions include thermoregulation, barrier against toxins and infections, water and electrolyte excretion, fat storage and insulation, and tactile sensation.
Like many other organs, the skin of a premature infant is immature. The combination of immaturity with the need for intensive care monitoring and procedures places premature infants at risk for skin trauma and loss of skin integrity. Skin trauma and skin immaturity have serious consequences for infants in the neonatal intensive care unit (NICU), including problems in thermoregulation, fluid and electrolyte balance, diversion of calories for tissue repair, discomfort, potential toxicity from absorbed substances, and increased risk for infection.
This chapter reviews the physiology of term and premature infants’ skin, the differences in structure and function related to skin immaturity, and the prevention and treatment strategies to promote optimal skin integrity for infants in the NICU.
PHYSIOLOGY
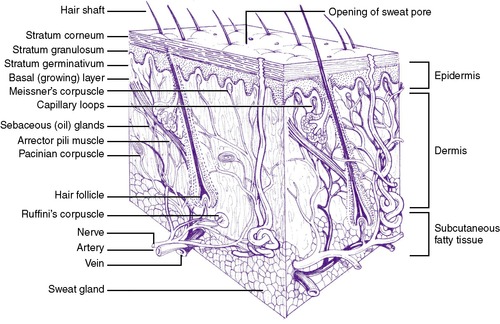
There are three layers to the skin: the epidermis, the dermis, and the subcutaneous layer (Figure 19-1). The epidermis comprises the stratum corneum (a nonliving layer) and the basal layer. The stratum corneum is formed of lipids and protein in “brick and mortar” configuration. The basal layer replaces the stratum corneum with cells called keratinocytes. Approximately every 26 days, keratinocytes migrate from the basal layer to the exfoliated layers of the stratum corneum. In addition to keratinocytes, melanocytes also are found in the basal layer.
 |
| FIGURE 19-1
(From Principles of infant skin care, Skillman, NJ, 1994, Johnson & Johnson.)
|
The dermis, a woven layer of collagen and elastin fibers, is 2 to 4 mm thick at birth. It contains nerves, blood vessels, and hair follicles. Sensations of heat, touch, pressure, and pain originate in the dermal layer. Sebaceous glands and sweat glands are located in the dermis, as well as in the subcutaneous layer of the skin. Sweat glands become mature in term infants during the first week of life, whereas maturation in premature infants occurs between 21 and 33 days and perhaps even longer in extremely premature infants.
The subcutaneous layer is composed of fatty connective tissue, with fat deposition occurring primarily during the last trimester of pregnancy. This layer provides heat insulation and functions as a calorie reservoir.
The skin of a normal term infant is covered with vernix caseosa, a “cheesy” substance composed of water (80%), lipids, and proteins, 113 sebum from sebaceous glands, broken-off lanugo, and desquamated cells from the amnion. Vernix production begins at the end of the second trimester, accumulates on fetal skin in a cephalocaudal manner, 53 and protects the fetus against maceration from the amniotic fluid and chafing caused by crowding in utero. Vernix detaches from fetal skin as the levels of pulmonary surfactant rise, resulting in a progressive increase in the turbidity of the amniotic fluid. 55,91Leaving residual vernix intact may be beneficial after delivery, because the presence of vernix produces earlier acidification of the skin and may act to facilitate colonization by the normal bacterial flora.113,120
The skin of premature infants is thinner than that of term infants and may appear transparent or even gelatinous in extremely immature infants. There is usually a ruddy, red appearance caused by the underdeveloped stratum corneum, making skin color a poor tool for assessing the oxygenation status of very immature infants. There are fewer wrinkles on skin surfaces than in term infants, and the skin is covered by lanugo to varying degrees, depending on maturity; these fine hairs cover the upper back, arms, and forehead. The subcutaneous layer in premature infants is often edematous because of an excess of cutaneous water and sodium (see Chapter 14).
ETIOLOGY
Term Newborn Skin Variations
Although the basic skin structures are the same in all term newborns without dermatologic disease, cutaneous variations may be seen on physical examination. These variations (see the Critical Findings box on p. 484) are not considered pathologic, but it is useful for clinicians to know them, because many parents ask the significance of physical variations as they examine their newborn.
Physiologic and Anatomic Differences in Premature Skin
Developmental differences in skin physiology and anatomy exist between full-term and premature infants when compared with older children and adults. This section discusses these differences and identifies the implications for care.
UNDERDEVELOPMENT OF THE STRATUM CORNEUM
The stratum corneum, the nonliving layer of the epidermis that is responsible for controlling evaporative heat loss and transepidermal water loss (TEWL), contains 10 to 20 layers in adults and term infants. Term infants have been shown to have lower transepidermal water loss than adults, with the lowest levels seen on the first day of life.123Premature infants have fewer layers of stratum corneum, depending on their gestational age. At less than 30 weeks’ gestation, they may have only two or three layers (Figure 19-2); and extremely premature infants of less than 24 weeks’ gestation may have virtually no stratum corneum. 56,92 Another function of the stratum corneum—protection against toxins and infectious agents such as bacteria and viruses—is minimal in premature infants, leaving them vulnerable to transcutaneously transmitted infections and toxicity from topically applied substances.
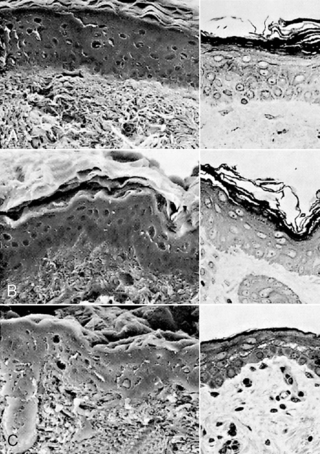 |
| FIGURE 19-2
(From Holbrook KA: A histological comparison of infant and adult skin. In Maibach HI, Boisits EK, editors: Neonatal skin: structure and function, New York, 1982, Marcel Dekker.)
|
Normal Variations of Term Newborn Skin
| Linea nigra | Line of increased pigmentation from umbilicus to genitalia |
| Mongolian spots |
Irregular, blue-gray, bruiselike spots
Usually seen over sacrum and buttocks, may extend over back and shoulders
Caused by pigmented cells in dermis
Most common in infants with darker pigmentation
|
| Lanugo |
Fine, downy hair over back, shoulders, and face
Shed at 32 to 36 weeks’ gestation
|
| Milia |
White, pinhead-size bumps over chin, cheeks, nose, and forehead
Tiny epidermal cysts
If on palate, called Epstein’s pearls
|
| Miliaria |
Caused by retention of sweat from edema in stratum corneum that blocks sweat glands
Most common is rubra (prickly pear), but there are also clear versions
|
| Harlequin sign |
Color of half of body turns deep red while the other half is pale
Caused by immature autoregulation of blood flow
|
| Vernix caseosa |
Gray-white, cheesy substance that protects fetal skin in utero
Gradually diminishes near term
|
| Cutis marmorata | Mottling caused by vasomotor immaturity |
| Erythema toxicum neonatorum |
Small, firm white or yellow pustules with erythematous margin
Most often seen on trunk, arms, and perineal area
Benign condition seen in 30% to 70% of newborns
|
| Acne neonatorum |
Acne-like rash seen in newborns at several weeks of age
Caused by stimulation of sebaceous glands by maternal hormones
More common in males
Instruct caregivers not to use creams, lotion, or ointments because they can worsen the rash
|
| Transient neonatal pustular melanosis |
Resembles miliaria but present at birth
Most frequently found on face, palms of hands, soles of feet
Not infectious or contagious
|
| Café au lait spots |
Irregularly shaped oval lesions
If large size (>4 × >6 cm), or if >6 in number, associated with neurofibromatosis
|
The transition from the aquatic, intrauterine environment to the atmospheric, external environment has been thought to result in accelerated maturation of the stratum corneum and more mature function after the first 10 to 14 days of life. 41,52 However, other authors cite a slower process in premature infants less than 27 weeks’ gestation, with rates of TEWL nearly double adult levels even at 28 days of life.106Premature infants of 23 to 25 weeks’ gestation have losses 10 times higher than term infants initially, and they continue to have elevated heat and water loss resulting from immature barrier function for a longer period.1 The maturation process can take as long as 8 weeks in an infant of 23 weeks’ gestation. 63
DERMAL INSTABILITY
The dermis is made of collagen and elastin fibers in a gel matrix, providing mechanical strength, protection, and elasticity to the skin. The dermis of the term newborn is thinner than the adult dermis and has a higher water content. 56,73 Collagen deposition in the dermis increases with advancing gestational age, preventing fluid from accumulating in this layer. Premature infants have a tendency to become edematous, because they have less collagen and fewer elastin fibers in the dermis.
Both term and premature infants may be prone to necrotic injury from excessive edema because of alteration in blood flow and perfusion to the epidermis. Edematous infants need protection from pressure and ischemic injury, including routine turning and the use of surfaces to minimize pressure points such as water beds and gelled mattresses or pads.
DIMINISHED COHESION BETWEEN EPIDERMIS AND DERMIS
Numerous fibrils connect the epidermis to the dermis at the dermo-epidermal junction. These fibrils are more widely spaced and fewer in number in the premature infant56 (Figure 19-3) but become stronger with advancing gestational and postnatal age. Genetically abnormal fibrils at this junction are found in certain types of the genetic disorder epidermolysis bullosa, a blistering skin condition that occurs with even minimal trauma. Premature infants also are prone to blistering from injury, although this decreases as they mature. This diminished cohesion places premature infants at risk for injury from adhesive removal as well. Particularly if extremely aggressive adhesives are used, there may be a stronger bond of the adhesive to the epidermis than of the epidermis to the dermis, and epidermal stripping may result during adhesive removal.
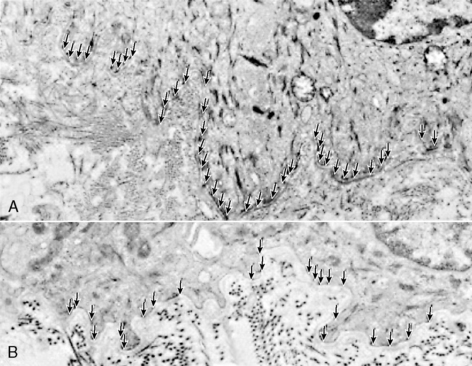 |
| FIGURE 19-3
(From Holbrook KA: A histological comparison of infant and adult skin. In Maibach HI, Boisits EK, editors: Neonatal skin: structure and function, New York, 1982, Marcel Dekker.)
|
SKIN pH
The ability of the skin surface to form and maintain an acid surface is a function of various chemical and biologic processes. Acid skin surfaces with a pH less than 5 have been documented extensively in adults and children. 13 This acid mantle has protective qualities against some pathogens and other microorganisms. Because microbial colonization begins with delivery, the acid skin surface helps keep a state of equilibrium; if the pH shifts from acidic to neutral, there may be an increase in total numbers of bacteria and a shift in species. TEWL also may increase when skin pH rises. 122
Term newborns are born with a relatively alkaline skin surface, measuring a mean pH of 6.34. Within 4 days, the pH declines to a mean of 4.95. 13 Skin pH measurements have been reported in premature infants of varying gestational ages, and the pH was above 6 on the first day, decreasing to 5.5 during the first week, and gradually declining to 5 during the first month. 43Bathing and other skin care practices alter skin pH; it may take an hour or longer to regenerate the acid mantle after bathing with an alkaline soap. Skin that is occluded by wearing diapers has been shown to have a pH of 6, which is known to be a risk factor in the development of diaper dermatitis. 121
NUTRITIONAL DEFICIENCIES
Fat and zinc accumulate in the fetus during the last trimester of pregnancy. Because these nutritional components are necessary for maintaining an intact, healthy skin surface, premature infants born before the last trimester may develop skin problems caused by deficiencies in either of these nutrients. Problems also may be seen in infants who are unable to receive adequate enteral nutrition unless appropriate parenteral supplements are employed.
Essential fatty acid (EFA) deficiency can be seen in premature and postmature infants because of decreased fat stores (see Chapter 17). In this condition, there is a superficial scaling and occasionally desquamation and irritation in the neck, groin, or perianal area. There may be decreased serum levels of EFAs, thrombocytopenia, and impaired platelet aggregation because EFAs are needed to promote platelet function. 44
Providing adequate EFA prevents skin manifestations of EFA deficiency. In infants who are receiving small amounts of enteral nutrients or none at all, administration of intravenous (IV) lipid solutions at a total dose of 0.5 g/kg/day can prevent EFA deficiency (see Chapter 16). Once EFA deficiency occurs, IV lipids can reverse the process in 1 to 2 weeks. Dietary replacement takes longer and is effective only if gastrointestinal function is good. Topical therapy with sunflower seed oil, which is rich in linoleic acid, promotes transdermal absorption of EFA and raises serum levels but is variable in the rate of absorption. In a study of topical application, researchers found that safflower oil failed to yield improvements in patients with EFA deficiency. 57
Zinc, an essential trace mineral, is a cofactor in many areas of metabolism, including lymphocyte transformation and metabolism of protein, nucleic acids, and mucopolysaccharides of skin and subcutaneous tissues and is necessary for normal wound healing. 35 Two thirds of the transfer of zinc from mother to fetus occurs in the last 10 weeks of pregnancy. Zinc deficiency occurs when there are abnormal losses of zinc in stool or urine; when there are low or absent stores, as in premature birth; or during increased demands, such as during rapid growth, stress, or tissue healing. Thus premature infants and infants with pathologic conditions of the intestine (including chronic diarrhea, short bowel syndrome, intestinal diversions such as ileostomy, or intestinal resection) are at increased risk for zinc deficiency. In addition, any infant receiving total parenteral nutrition should receive trace minerals to prevent zinc deficiency (seeChapter 16).
Clinical Features of Zinc Deficiency
• Erythematous, scaly skin
• Excoriations of the groin and perianal areas, neck folds, circumoral area, and at sites of trauma, such as areas of adhesive removal
• Lethargy
• Poor growth
• Alopecia
• Diarrhea
Symptoms of zinc deficiency are listed in the Critical Findings box above. Serum zinc levels of less than 68 mcg/mL accompanied by a low alkaline phosphatase and clinical symptoms are diagnostic of zinc deficiency. Prevention of zinc deficiency for term infants receiving total parenteral nutrition includes zinc supplementation with 100 to 200 mcg/kg/day; premature infants require higher levels of supplementation (400 mcg/kg/day). 127 Premature infants have also been reported to develop zinc deficiency while fed breast milk; they may require an oral zinc sulfate supplement. 126
PREVENTION
During daily skin care practices such as bathing, moisturizing, antimicrobial skin disinfection, and adhesive removal, the skin of newborns is at risk for trauma or disruption of normal barrier function. This is particularly true of newborns in the NICU, who may have been born prematurely or may be critically ill or require surgery.
This section reviews basic skin care practices in terms of impact on skin integrity, preventing potential toxicity, and reducing exposure to potentially sensitizing chemical. Recommendations for preventing trauma, protecting immature barrier function, and promoting skin integrity supported by scientific evidence are presented. These recommendations also are integrated into an evidence-based skin care guideline for health professionals. 7
Bathing
Among the purposes of bathing the newborn are overall hygiene, aesthetics, and protection of health care workers by removing blood and body fluids. Bathing, however, is not an innocuous procedure. During the immediate postbirth period, bathing can result in hypothermia, increased oxygen consumption, and respiratory distress. To prevent hypothermia, increased oxygen consumption, and respiratory distress, the first bath should be delayed until the infant’s temperature has been stabilized in the normal range for 2 to 4 hours95or at 1 hour if radiant heat is provided during the bath.116 With appropriate attention to the environment, there is no difference in heat loss when the bath is performed at the bedside in the mother’s room or in the nursery. 87Bathing also has been shown to destabilize vital signs and temperature in premature infants.96
Bathing with antiseptic soaps and cleansers is still practiced in some nurseries. Studies have shown that although hexachlorophene reduced the number of Staphylococcus aureus strains present on the skin, toxicity was reported, especially in premature infants, associated with absorption through the skin; it should not be used. 3,67,104 Both povidone-iodine and chlorhexidine are sometimes used for the initial bath in newborn nurseries, although the effect on bacterial colonization is transient. 32Chlorhexidine has proved effective in reducing colonization for up to 4 hours32but also can be absorbed.31 Although toxicity from chlorhexidine has not been identified, many nurseries do not use it for routine bathing because of the potential risk. Antimicrobial soap is not recommended by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists4 because of the harshness of the soap and the potentially negative effect it may have on normal skin colonization.
Soaps made with lye and animal fats are alkaline, with a pH above 7.0. Cleansing bars and liquids made with synthetic detergents are formulated to a more neutral pH of 5.5 to 7.0. All soaps and cleansers are at least mildly irritating and drying to skin surfaces114,115and disrupt the skin surface pH.48 In addition, the degree to which the skin is irritated also depends on the length of contact and the frequency of bathing.
The recommendations are (1) to select cleansers that have a neutral pH and minimal dyes and perfumes to reduce risk for potential sensitization to these products, and (2) to bathe the infant no more than every other day.7 The effects of bathing on skin parameters in small premature infants have not been studied to date. To reduce alterations in skin pH, dryness, and irritation in premature infants less than 32 weeks, cleanse with warm-water baths during the first week, using soft cotton cloths, cotton balls, or the caregiver’s hands. It has been shown that skin colonization with bacteria does not increase with bathing as infrequently as every 4 days.98 Less frequent bathing may offer other advantages for premature infants, who have demonstrated physiologic and behavioral disruptions during sponge baths. 96 Immersion bathing, even of stable infants on ventilators or nasal continuous positive airway pressure (NCPAP), may be soothing and less stressful. 2
Immersion bathing places the infant’s entire body, except the head and neck, into warm water (38 ° C [100.4 ° F]), deep enough to cover the shoulders. A recent study of immersion versus sponge bathing in 102 newborns for their first and subsequent baths showed that the immersion-bathed infants had significantly less temperature drop and appeared more content and their mothers reported more pleasure with the bath; there was no difference in cord healing scores with either immersion or sponge bathing. 19 Immersion bathing is also beneficial from a developmental perspective. 2,5 Stable premature infants after umbilical catheters are removed and term infants with umbilical clamps in place can be bathed safely in this way. 7Bathing is an excellent time to educate parents (1) about how to physically care for their baby and (2) about their baby’s neurobehavioral status and social characteristics.64
Emollients
The skin surface of term newborns is drier than that of adults but becomes gradually better hydrated as the eccrine sweat glands mature during the first year of life. 90,103 Maintaining the hydration of the stratum corneum is necessary for an intact skin surface and normal barrier function. Skin that is dry, scaly, or cracking not only is uncomfortable but also can be a portal of entry for microorganisms. Products used to counteract dryness are called moisturizers, emollients, or lubricants. Common emollients include mineral oils, petrolatum, and lanolin and its derivatives. Emollients are sometimes divided into oil-in-water or water-in-oil emulsions.
Emollient use to prevent dermatitis and improve skin integrity has been studied in several randomized, controlled trials in premature infants. In one report, 68 premature infants of 29 to 36 weeks’ gestation were treated with Eucerin cream daily and had less dermatitis as measured by a visual grading scale but no differences in direct measurements of TEWL with an evaporimeter. In a later study, premature infants of both shorter gestation and younger postnatal age were treated with Aquaphor ointment, a water-miscible oil-in-water preparation that contains neither dyes nor perfumes. In this study, there was improvement in both TEWL and visual scale dermatitis. No increases in skin surface temperatures or thermal burns were seen, even when the emollient was applied to infants under radiant heaters or phototherapy lights. In addition, cutaneous cultures revealed no increase in bacterial or fungal colonization on skin treated with emollients. It was noted that fewer treated infants had positive blood or cerebrospinal fluid culture results compared with control subjects, although the study was not large enough to prove this effect. 93
A large, randomized controlled trial of 1191 infants with birth weights of 501 to 1000 g was conducted to determine whether twice-daily application of Aquaphor ointment would reduce combined outcome measures of mortality and sepsis. Although skin integrity appeared improved with routine emollient use, no effect was seen in the outcomes of sepsis plus mortality. Of note, an increase in coagulase-negative Staphylococcus epidermidis bloodstream infections was seen in infants with birth weights below 750 g, although the mechanism and relationship to emollient use are not clearly understood. 40 Although a small case-control study had previously associated petrolatum-based emollients with a higher incidence of fungal infections, 22 this was not seen in the larger trial. The effects of emollients on TEWL or fluid balance were not studied in this trial.
The benefits of emollient use must be carefully weighed against the risk for infection. In general, emollients can be safely used to treat skin with excessive dryness, cracking, or fissures on an “as-needed” basis. They also may be effective in reducing TEWL and evaporative heat loss, although other methods, such as using a high-humidity environment or transparent adhesive dressings, also are available for this purpose. Avoiding products with perfumes or dyes is prudent, because these can be absorbed and are potential contact irritants. 26Small tubes or jars for single-patient use are recommended to prevent contamination with microorganisms.
Skin Disinfectants
Decontamination of skin before invasive procedures such as venipuncture and placement of umbilical catheters and chest tubes is common practice in neonatal intensive care nurseries. However, there are anecdotal reports of skin injury, including blistering, burns, and sloughing, from disinfectants including isopropyl alcohol, povidone-iodine, and alcohol-containing chlorhexidine use in premature infants.51,101,105 There have been case reports of high iodine levels, iodine goiter, and hypothyroidism associated with povidone-iodine use in premature infants. 27,60,97 Several prospective studies of routine povidone-iodine use in intensive care nurseries70,94,107 and one study of presurgical skin preparation of infants younger than 3 months89 found alterations in iodine levels and thyroid effects from povidone-iodine exposure as a result of absorption through the skin. Although one study did not find alterations in thyroid function from iodine absorption in neonates, 49 the study period (10 days) may be too short to see this effect.
Another important aspect of skin disinfection is how effectively disinfectant solutions reduce colonization and infection rates. During skin preparation before blood culture sampling in children and adults, lower rates of microbial colonization were seen with povidone-iodine compared with isopropyl alcohol.30 A larger study of blood culture sampling in adults found fewer contaminated cultures when chlorhexidine had been used compared with cultures from povidone-iodine–cleansed subjects.88
Two studies in premature infants compared skin and peripheral intravenous catheter colonization with bacteria after skin preparation with either chlorhexidine or povidone-iodine. Malathi et al82 found the rate of colonization was no different between disinfectants but the technique of application was important: the authors recommended longer periods of cleansing (>30 seconds) or two consecutive cleansings for maximum reduction of colonization. Garland et al45 reported that chlorhexidine reduced catheter colonization: 4.3% with chlorhexidine compared with 9.3% with povidone-iodine.
A meta-analysis of eight studies involving 4143 central catheters in adult patients found that using chlorhexidine gluconate–containing disinfectants for insertion and routine site care reduced the risk for catheter-related bloodstream infections by 49%28 and has led to recommendations to replace povidone-iodine with chlorhexidine disinfectants. 25 A comparison of isopropyl alcohol, povidone-iodine, and 2% chlorhexidine aqueous solution for disinfection of 668 central venous catheters in adults during insertion and routine dressing changes showed chlorhexidine to be significantly more effective in reducing catheter-related infections. 81 Similar studies have not been conducted in the NICU population. In a sequential study in a single NICU, the rate of positive blood cultures and number of true infections were unchanged when the unit switched from povidone-iodine to chlorhexidine gluconate for skin disinfection.71 Of note, the typical dwell time for central catheters in many of the studies is 7 to 10 days, whereas peripherally inserted central catheters in neonates are often in 3 weeks or longer.
Chlorhexidine gluconate (CHG) is currently available in the United States as a 2% aqueous CHG skin preparation in 4-ounce bottles, as a tincture of 2% CHG in 70% isopropyl alcohol (ChloraPrep) in single-use packaging, and as a wipe containing 0.5% CHG in 70% isopropyl alcohol. The tincture has been approved for infants older than 2 months, although many neonatal units use the tincture “off label” because of the convenience and decreased risk for contamination of bottled products. However, the combination of two disinfectants (CHG and isopropyl alcohol) has a significant potential for skin injury in very-low-birth-weight (VLBW) infants and cannot be recommended for them. All CHG products should not come in contact with the eyes or ears, per manufacturer’s recommendations, because of reports of damage to these structures. However, careful use before scalp intravenous or central line insertion is acceptable if splashing or using excessive amounts of CHG is avoided. CHG is applied in two consecutive wipings or for a 30-second scrubbing period and then is removed with sterile water or saline solution when the procedure is completed.
Many nurseries have chosen to continue the use of povidone-iodine disinfectants because of the lack of single-use CHG products that do not contain isopropyl alcohol. Povidone-iodine is available in a 10% aqueous solution in a variety of single-use applications. It is also applied in two consecutive wipings or for a 30-second scrubbing period and then is allowed to dry for at least 30 seconds before the procedure. Any solution should be completely removed after the procedure, using sterile water or saline solution to prevent any further absorption. Disinfection with isopropyl alcohol is questionable in the NICU, because it is less effective than either povidone-iodine or chlorhexidine and can be irritating and drying to skin surfaces.
The risks and benefits of routine skin antisepsis in infants is a subject that clearly deserves further investigation. Although there are insufficient comparative data on the costs, risks, and benefits of skin antisepsis regimens to mandate standard practice, the use of alcohol pledgets alone provides the least-effective antimicrobial activity. Povidone-iodine and isopropyl alcohol carry significant risks of percutaneous toxicity. The potential for subclinical toxicities must be considered with all products used on small newborns; therefore when several topical therapeutic options are available, the one with the least potential for toxicity should be chosen. In addition, disinfectants should be removed completely from the skin with water or saline to prevent further absorption and contact.
The routine use of antimicrobial sprays, creams, or powders for umbilical cord care has not been shown to be more effective in preventing infection compared with dry cord care.128 The use of antibiotic ointments and antiseptics can prolong the time to cord separation, and it seems to have no beneficial effect on the frequency of infection. 6,62,128 A study of 1811 newborns randomized to receive either routine isopropyl alcohol with each diaper change or natural drying found no umbilical infections in either group, and time to cord separation was reduced from 9.8 days in the alcohol-treated group to 8.16 days in the natural-drying group. 38 Another study randomized 766 newborns to receive either triple dye applied to the umbilical cord immediately after delivery, followed by twice-daily applications of isopropyl alcohol, or “dry care” without any treatment. Infants in the dry-care group were more likely to be colonized with bacteria than those in the treatment group, and one infant in the dry-care group developed omphalitis on the third day of life. The days to cord separation were not reported. 61
Recommendations for umbilical cord care to prevent contamination include the following7:
• Washing hands before handling the cord
• If the cord becomes soiled with urine or stool, cleansing with water and drying with absorbent gauze
• Keeping the diaper folded down and away from the umbilical stump
The development of omphalitis is not necessarily related to cord disinfection, because it occurs also in infants who have received topical disinfectants. However, vigilant attention to the signs and symptoms is necessary by health professionals, and parents need guidance about how to manage the umbilical cord and when to consult their health care provider.37
Adhesive Application and Removal
One of the most common practices in the NICU is the application and removal of adhesives that secure endotracheal tubes, IV devices, and monitoring probes and electrodes. A research utilization project involving 2820 premature and term newborns found that adhesives were the primary cause of skin breakdown among NICU patients.78 Changes in TEWL and skin barrier function are seen in adults after ten consecutive removals of adhesive tape72 and after one removal of adhesive tape in premature infants. 52 Types of damage from adhesive removal include epidermal stripping, tearing, maceration, tension blisters, chemical irritation, sensitization, and folliculitis. 54
Solvents are sometimes used to prevent discomfort and skin disruption from adhesive removal. They contain hydrocarbon derivatives or petroleum distillates that have potential or proven toxicities. Toxicity is a major concern, especially in premature infants with their underdeveloped stratum corneum, increased skin permeability, larger surface-area to body-weight ratio, and immature hepatic and renal function. A case report of toxic epidermal necrosis in a premature infant resulted from the use of a solvent. 59Mineral oil or petrolatum products may be helpful in removing adhesives but cannot be used if the site must be used again for reapplication of adhesives, such as with the retaping of an endotracheal tube. Removing adhesives with water-soaked cotton balls sometimes helps, and gently pulling the adhesive parallel to the skin surface rather than straight up at a 90-degree angle may facilitate removal with less skin trauma. 80
Skin bonding agents promote adherence. Unfortunately, they may create a stronger bond between adhesive and epidermis than the fragile cohesion of the epidermis to the dermis; when the adhesive is removed, epidermal stripping may result. Plastic polymers have been studied and are reported to reduce skin trauma. 42 An alcohol-free skin protectant is available that is less irritating to skin surfaces in adults than are comparable products containing alcohol. 51 This product has been approved for infants older than 30 days to treat mild diaper dermatitis and to prevent skin injury from adhesive removal. 112 A single study from England reports positive effects when using this skin protectant to tape intravenous lines in newborns. 58
Pectin-based skin barriers such as Hollihesive™ and DuoDERM™ are used between skin and adhesive and mold well to curved surfaces while maintaining adherence in moist areas. Studies initially described less visible trauma to skin with pectin barriers. 36,76,86 However, a controlled trial of pectin barrier (Hollihesive), plastic tape (Transpore), and hydrophilic gelled adhesive found that significant skin disruption, as measured by TEWL and visual inspection, occurred after removal of both the pectin barrier and plastic tape. 77 Because the adhesives were left in place 24 hours before removal in this study, a time effect of peak adhesive aggressiveness may have been reached. Significant changes were measured after a single adhesive application and removal in all three weight groups studied (<1000 g; 1001 to 1500 g; and >1500 g), indicating that even larger premature infants are at risk for skin injury from tape removal. Despite this finding, pectin barriers and similar hydrocolloid adhesive products continue to be used in the NICU because they mold well to curved surfaces and adhere even with moisture.
Prevention of skin trauma from adhesive removal includes minimizing tape use when possible by using smaller pieces, backing the adhesive with cotton, and delaying tape removal until adherence is reduced. Pectin barriers and hydrocolloid adhesives may prove helpful, because they mold and adhere well to body contours and often attach better in moist conditions. As with tape, removal of pectin barriers and hydrocolloid should be delayed, if possible, until the adherence lessens. The use of soft gauze wraps to secure probes and hydrogel electrocardiogram electrodes and hydrogel tapes are helpful. Adhesives should be removed slowly and carefully with warm water and cotton balls. Mineral oil or an emollient may facilitate adhesive removal if reapplication of adhesives at the site of removal is not necessary. Silicone-based adhesive products have been shown to improve adherence to wounds and reduce discomfort when removal is necessary39,50 and may prove beneficial if developed for a wider range of adhesive products for neonates.
DATA COLLECTION
History
The gestational age and postnatal age of neonates in the NICU are both important considerations for determining appropriate skin care practices. Premature infants of lower gestational ages have underdeveloped skin layers and function. With advancing postnatal age and maturation, skin integrity and skin barrier function are improved.
Reviewing the maternal history for any dermatologic diseases is also important. Many of the most severe skin diseases, such as forms of congenital ichthyosis or epidermolysis bullosa, are inherited disorders. A positive family history will alert the clinician to the potential for developing these rare disorders.
Signs and Symptoms
A thorough daily examination of all skin surfaces reveals the state of skin integrity for neonates in the NICU. Early signs such as skin abrasions or small excoriations may call for either diagnostic or treatment procedures. A scoring tool, such as the Neonatal Skin Condition Score (NSCS) (see the Critical Findings box below), used in the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN)/National Association of Neonatal Nurses (NANN) research-based practice project, 75,78has been extensively used in both premature and full-term infants, with validity and reliability established.79This scoring system can be integrated into skin care protocols to identify neonates with excessive dryness, erythema, or skin breakdown.7 Risk factors for skin injury in individual patients are listed in the Critical Findings box below. In the first week of life in extremely-low-birth-weight (ELBW) infants (<30 weeks, <1000 g), there may be problems with thermoregulation (see Chapter 6) and dehydration (see Chapter 14) because of the large evaporative heat losses and transepidermal water losses through the immature stratum corneum.
The Neonatal Skin Condition Score
Dryness
1 = Normal, no sign of dry skin
2 = Dry skin, visible scaling
3 = Very dry skin, cracking/fissures
Erythema
1 = No evidence of erythema
2 = Visible erythema <50% body surface
3 = Visible erythema >50% body surface
Breakdown
1 = None evident
2 = Small localized areas
3 = Extensive
note: Perfect score = 3; worst score = 9
Laboratory Data
With the many skin excoriations in both small and large neonates that result from traumatic events such as adhesive removal or pressure necrosis, infection through this portal of entry in the skin is a potential. In VLBW infants, it may be useful to obtain a skin culture, Gram stain, or potassium hydroxide (KOH) preparation11,12 for early detection of microorganisms that can lead to systemic illness in these immunocompromised patients. A skin surface culture is helpful if the skin breakdown cannot be traced to a traumatic injury, because the origin of the breakdown often is linked to infection, especially with fungal infections102 or staphylococcal scalded skin syndrome. A more comprehensive workup for infection may be indicated if there is evidence of clinical deterioration in infants with extensive skin breakdown (see Chapter 22).
TREATMENT
Skin Excoriations
Skin excoriations are cleansed with warmed sterile water or half-normal saline solution; a 20- or 30-mL syringe with a Teflon IV catheter attached can be used to gently débride the excoriation. This technique is effective in flushing out debris and dead tissue from an infected or “dirty” wound, allowing a better surface for healing. Moistening the tissue every 4 to 6 hours aids the healing process, because drying of tissue actually impedes the migration of cells. Once the wound surface is clear, other dressings or ointments can be used.
Risk Factors for Skin Injury
• Gestational age <32 weeks
• Edema
• Use of paralytic agents and vasopressors
• Multiple tubes and lines
• Numerous monitors
• Surgical wounds
• Ostomies
• Technologies that limit movement: high-frequency ventilation; extracorporeal membrane oxygenator
Ointments are sometimes used because of their antibacterial or antifungal properties and also because covering the wound with a semi-occlusive layer promotes healing by facilitating the migration of epithelial cells across the surface. Only if extensive bacterial colonization is suspected, Polysporin, Bacitracin, or Bactroban ointment is used sparingly every 8 to 12 hours. Many dermatologists do not recommend the use of Neosporin because of the potential for developing later sensitization to this ointment, although sensitization to Bacitracin is being reported with increasing frequency. 85 Overuse of antimicrobial ointments can be a problem in promoting more resistant strains of bacteria. If fungal infection is suspected, Nystatin ointment is used, and it can be applied also to surrounding intact skin to prevent extension of the infection. In general, ointments are preferable to creams for this use because of better adherence and healing properties.
Transparent adhesive dressings are made from a polyurethane film backed with adhesive that is impermeable to water and bacteria but allows airflow. A rim of intact skin must be around the wound to attach the dressing. Uses include wound care, dressings for IV devices including central venous lines and percutaneous silicone catheters, and prevention of friction injuries to areas such as the knees or sacrum.
When used for wound care, transparent adhesive dressing promotes “moist healing” that allows the rapid migration of epithelial cells across the site. These dressings should be used only on “clean” (uninfected) wounds because bacteria and fungi can proliferate under the dressing. When the dressing is placed over a clean wound, often a serous or milky exudate composed of leukocytes forms, which actually aids in the prevention of infection. The dressings can be left in place for days at a time or until they become loose. Removing and reattaching the dressings on a daily basis is not recommended because the adhesive can injure the intact skin around the wound and further impede healing.
Another use of transparent adhesive dressings in the NICU is the prevention of excessive TEWL in premature infants.16,21,66,83,117 TEWL, as measured by an evaporimeter, can be reduced by as much as 50% by the creation of this “second skin.” In one study, 83 a nonadherent transparent dressing (not commercially available) was used and the skin under the dressing not only had a lower TEWL while covered but also actually had lower TEWL when removed, suggesting that perhaps a faster maturation of the skin barrier function had occurred. Cultures were obtained both on covered and uncovered skin and showed no increase in either bacterial or fungal colonization under the dressings. Unfortunately, nonadherent dressings are not commercially available and transparent adhesive dressings can cause a significant amount of skin trauma when removed. Alternative ways to reduce TEWL in VLBW infants can be used, including double-walled incubators and heated humidity (see Chapter 6).
Other types of dressings used in wound management include hydrogels (dressings and gel) and hydrocolloid dressings (DuoDERM), both of which promote moist healing.10,109Hydrogel dressings can be used after irrigation of the wound and in conjunction with either antibacterial or antifungal ointment if the wound is infected. These dressings must be changed every 8 to 12 hours, because they can dry out. No adhesive attaches these dressings. It is best to avoid placing hydrogel dressings on intact skin surfaces, because they can macerate the skin and actually reduce barrier function. Hydrocolloid dressings are used over uninfected wounds and can be left in place for 5 to 7 days while healing takes place. Another wound treatment is amorphous hydrogel applied directly onto the wound from a tube. Amorphous hydrogels such as DuoDERM Gel and IntraSITE consist of 80% to 90% water to make it soothing to skin while keeping the wound moist, a cellulose polymer to extract and trap fluid, and propylene glycol to rehydrate tissues. 108.109. and 110.
Surgical wounds that open or dehisce are infrequent but require expert wound management. Nutrition is often a part of the process in getting these wounds to heal, as is the prevention of infection. 46 Often the surgeon or an enterostomal therapist will design the appropriate wound management program for these situations.
Intravenous Extravasations
Prevention of tissue injury from IV extravasations includes taping IV devices with transparent dressings or plastic tape so that the insertion site is clearly visible and observing the site with appropriate documentation every hour. If the IV device is placed in a limb, the tape that secures it to the rigid board should be placed loosely over a bony prominence, such as the elbow or knee, and not on skin in proximity to the insertion site. This allows extravasated fluid and medications to expand over a larger surface and not remain in a small, constricted area, which can result in greater tissue injury. It may be wise to avoid poorly perfused extremities in favor of scalp veins, except the forehead. Using central venous lines such as percutaneous catheters to infuse highly irritating solutions and medications is also recommended. Many nurseries limit the glucose concentrations in peripheral lines to 12.5% and the amino acid concentrations to 2%; calcium and potassium concentrations also are more dilute than those used in central lines.
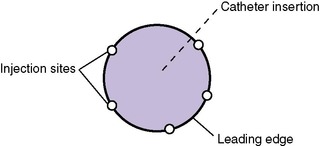
If IV fluid has extravasated into surrounding tissue, the IV device should be removed and the extremity elevated. Use of moisture, heat, or cold is not recommended, because the tissue is vulnerable at this point to further injury.17Hyaluronidase (Amphadase, Vitrase, Hylenex) can be extremely helpful if administered within an hour of extravasation (see Chapter 10). This medication is an enzyme that causes a breakdown of interstitial barrier and allows the diffusion of the extravasated fluid over a larger area to prevent tissue necrosis. 69,100,124The dose of hyaluronidase is 15 to 20 units diluted to 1 mL, although in one study using an animal model, 150 units was used without harmful effects. 69It is administered in five injections, inserted subcutaneously around the periphery of the extravasation site (Figures 19-4and19-5), and ideally is administered within 1 to 2 hours of the extravasation. Extravasations that may benefit from administration of hyaluronidase include any with evidence of blanching, discoloration, or blistering or those involving hypertonic or calcium-containing solutions, even if the site appears relatively undisturbed. Calcium-containing solutions may cause deep tissue injury even when epidermal tissues are not involved. In addition to hyaluronidase administration, creating multiple puncture holes over the area of swelling and gently squeezing or letting the extravasated fluid leak out can facilitate the removal of the infiltrate and prevent skin sloughs. 29Saline washout is another technique described to facilitate the removal of extravasated irritants from tissues surrounding an IV site.24,33,47
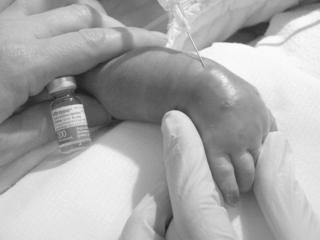 |
| FIGURE 19-5 |
Hyaluronidase is not recommended in the extravasation of vasoconstrictive medications such as dopamine, because the vasoconstriction could extend with its use. Phentolamine (Regitine) is used in this case, because it directly counteracts the action of dopamine. The method of delivery is the same as for hyaluronidase, with the total dose (0.5 mg) diluted to 1 mL, injected in five sites subcutaneously around the periphery of the extravasation.125
When tissue injury occurs after extravasation, treating the wound with techniques using moist healing principles facilitates healing without scarring. There has been success using a generous application of amorphous hydrogel and placing the extremity in a plastic bag, the so-called “bag/boot” method. 111 In most cases, skin grafts can be avoided by the use of appropriate wound healing techniques. In all cases of tissue injury, open wounds should be considered a portal of entry for infection and topical or systemic treatment should be considered.
Diaper Dermatitis
Diaper dermatitis, which has a multitude of causes in infants, affects the perineum, groin, thighs, buttocks, and anal region. The underlying skin condition of the infant contributes to the degree of diaper dermatitis that occurs.
Another factor that influences the development of diaper dermatitis is the degree of wetness of the skin; skin that is moist and macerated becomes more permeable and susceptible to injury.14,15,118 In addition, skin that is moisture-laden becomes more heavily colonized with microorganisms. Skin pH also has an effect; when the skin is exposed to urine, the pH can rise from acid to alkaline ranges and tissues become more vulnerable to injury and penetration by microorganisms.8,118 The alkaline pH also can activate enzymes found in stool, protease, and lipase, which break down protein and fat, the building blocks of the stratum corneum. 20 This is the primary mechanism for direct contact dermatitis from exposure to stool, the most common form of diaper dermatitis.
Strategies for preventing diaper dermatitis include maintaining a skin surface that is dry and has a normal (acidic) skin pH. Frequent diaper changes are recommended, especially in the newborn period.
There is insufficient evidence to support that any specific type of diaper plays a central role in the prevention of diaper dermatitis. 9 However, superabsorbent gelled diapers with breathable covers have been shown to keep skin surfaces dryer by “wicking” the moisture away from the skin and separating urine from feces. 23,34 Use of powders is discouraged because of the risk for inhalation of particles into the respiratory tract. After skin injury from diaper dermatitis has occurred, protecting injured skin to prevent re-injury is the primary goal of treatment. Topical treatment for diaper dermatitis involves ointments and creams containing a variety of ingredients. Most contain zinc oxide or petrolatum and are generally similar in composition. 119Generous application of protective skin barriers that contain zinc oxide can prevent further injury while allowing skin to heal. Once skin excoriations occur, keeping skin open to air may not be effective because the already impaired tissue may be reinjured with fecal contact and dryness is counterproductive to healing. It is not necessary or desirable to completely remove skin barrier products with diaper changes, because this may disrupt healing tissue. Instead, remove as much waste material as possible and reapply the barrier generously to the affected areas with each diaper change. Another class of barrier products is semipermeable barrier film, designed to repel moisture and protect the skin from irritants119; one of these products is approved for use in infants older than 30 days. 112
If Candida albicans is involved in the diaper dermatitis, it is necessary to use an antifungal ointment or cream. Antifungal preparations include Mycostatin, miconazole, clotrimazole, and ketoconazole in ointment or cream forms; ointments are preferable to coat the skin and repel moisture. If the dermatitis is both fungal and a contact irritant dermatitis, it may be necessary to layer the ointment with the antifungal preparation. In this case, Mycostatin powder is used, followed by an application of alcohol-free skin protectant to seal the powder onto the skin surface, followed by a generous application of a skin barrier cream such as zinc oxide or pectin paste.
Occasionally infants may experience extremely severe diaper dermatitis from intestinal malabsorption syndromes or constant dribbling of stool, as in the case of infants who have problems with rectal enervation, such as those with myelomeningocele, bladder exstrophy, or after a “pull-through” procedure for Hirschsprung’s disease. In the case of malabsorption, the stool may have a pH that is higher than normal because of rapid transit through the small intestine and there may be significant amounts of undigested carbohydrates and stool enzymes, as well as increased stool frequency. Severe diaper dermatitis in this case can be a symptom of a more severe nutritional deficiency, or even dehydration, and needs thorough medical evaluation. Stools in these infants should be regularly tested for pH, carbohydrates, and occult blood, and their number and total volume should be measured.
While optimal nutritional therapy is being addressed with special diets or parenteral nutrition, skin protection from injury should be initiated. Products such as pectin paste without alcohol (e.g., Ilex, a non-alcohol pectin paste) may provide a sturdier barrier for these infants than zinc oxide preparations. The skin should be thoroughly cleansed before a very thick application of the pectin paste. Then it is necessary to apply a greasy ointment over this, because the pectin-based paste may adhere to the diaper. When the infant has a stool, it is not necessary to completely remove the barrier paste; the stool can be wiped away as much as possible before reapplying the thick paste barrier. The skin will heal under this protective covering as long as it is protected from re-injury. 74If fungal infection is a component of the dermatitis, antifungal therapy must be instituted in addition to the protective barriers. In this case, Mycostatin powder attached with alcohol-free skin protectant is the first layer; then the barrier cream is applied as described previously.
COMPLICATIONS
Improper handling of newborn skin (and injudicious use of products) can cause damage, prevent healing, and interfere with normal maturation processes. Compromised skin integrity can lead to infection, pain and discomfort, and diversion of calories for tissue repair. Other dangers include toxicity from topically applied substances that are readily absorbed by small infants with a large surface-area to body-weight ratio, as well as immature renal and hepatic function that cannot detoxify chemicals readily.
Injury from infiltrated IV solutions can injure skin and occasionally cause deep tissue necrosis with both muscle and nerve damage. Factors that increase the risk for injury from IV extravasations include length of time between extravasation and treatment; hypertonic solutions, such as those with high calcium, potassium, amino acid, or glucose solutions; medications such as nafcillin that are irritating to veins; and the use of mechanical pumps for infusions. There may be an added risk for injury in patients with poor perfusion to extremities and in limbs that have been secured with restricting adhesives that obstruct venous return.
If the epidermis has been injured, it can easily become a portal of entry for infection. Thus a contact irritant diaper dermatitis can progress to a fungal or staphylococcal infection. Staphylococcus aureus can cause pustule formation at hair follicles and is a rare complication of diaper dermatitis. The mechanism for fungal diaper dermatitis is still debated. Some researchers believe that Candida albicans infection is a secondary invasion to skin that has been previously injured, whereas others see this organism as a primary cause of skin disruption. 99
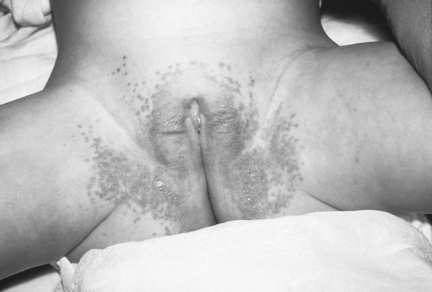
Candida albicans diaper dermatitis causes an intense inflammation that is bright red and sharply demarginated in the inguinal folds, buttocks, thighs, abdomen, and genitalia, often with satellite lesions that extend the rash over the trunk (Figure 19-6). Candida albicans can be harbored in the gastrointestinal tract, necessitating oral therapy if lesions are found in the mouth.
PARENT TEACHING
It is the responsibility of professionals to teach parents informally during caregiving procedures such as bathing, cord care, and diaper changes and to prepare written materials about appropriate skin care practices for their infant after discharge from the NICU (see the Parent Teaching box below). Parents will need education about the normal mechanisms of cord healing, including the range of appearance in umbilical cords, because some cords can appear very moist and soggy. The cord can be cleansed with water if it becomes soiled with urine or stool. 7 Inform parents that minimal use of skin care products is optimal and may reduce the incidence of contact sensitization to chemicals. 18,26,84 It is also extremely useful to educate parents about the mechanisms that are involved in diaper dermatitis so that prevention is stressed and appropriate interventions are selected depending on the underlying cause.
Developmental differences in the anatomy and physiology of neonatal skin affect skin integrity for term and premature infants in the NICU. Prevention is the primary focus of care, and decisions about the best way to provide basic skin care and hygiene based on current research are essential for care providers, both professionals and parents.
Appropriate Skin Care Practices
• Baby needs to be bathed only two or three times per week. Sponge bath with water between tub bathings.
• Use on the skin only products that have as few additives and as little fragrance as possible; minimal skin care products reduce the incidence of contact sensitization of the skin by added chemicals.
• Do not use powder because of inhalation into baby’s lungs.
• Prevent diaper rash by frequently changing wet and soiled diapers, cleansing diaper area, and using diapers that “wick” moisture away from the skin. If baby’s skin becomes red and irritated with one brand of disposable diaper, try another brand.
• Treat diaper rash by using protective skin barriers (e.g., zinc oxide) with each diaper change to prevent further injury and allow skin to heal. Clean waste from skin barrier but do not clean off the skin barrier because this may disrupt skin healing.
• Diaper rash caused by a yeast infection requires antifungal medication.
• Umbilical cord dries and falls off within 7 to 10 days. Turn diaper back away from the cord till it falls off; as the cord separates, a small amount of blood stain may be on the diaper. Keep cord area clean, and rinse with water if it becomes soiled with urine or stool. Call the health care provider immediately if the cord develops an area of red, warm-to-touch skin at the base, a foul odor, or drainage from the base of the cord.
REFERENCES
1. Agren, J.; Sjors, G.; Sedin, G., Transepidermal water loss in infants born at 24 and 25 weeks of gestation, Acta Paediatr 87 (1998) 1185.
2. Als, H.; Lawhon, G.; Brown, E.; et al., Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: neonatal intensive care unit and developmental outcome, Pediatrics 78 (1986) 1123.
3. American Academy of Pediatrics, Red Book: report of the committee on infectious diseases,. ed 27 ( 2006)The Academy, Elk Grove Village, Ill.
4. American Academy of Pediatrics and The American College of Obstetricians and Gynecologists, Guidelines for perinatal care,. ed 6 ( 2007)The Academy, Elk Grove Village, Ill.
5. Anderson, G.M.; Lane, A.; Chang, H., Axillary temperature in transitional newborn infants before and after tub bath, Appl Nurs Res 8 (1995) 123.
6. Arad, I.; Eyal, F.; Fainmesser, P., Umbilical cord care: a study of bacitracin ointment vs. triple dye, Arch Dis Child 56 (1981) 887.
7. Association of Women’s Health, Obstetric and Neonatal Nurses, Evidence-based clinical practice guideline: neonatal skin care. ed 2 ( 2007)The Association, Washington, DC.
8. Atherton, D.J., A review of the pathophysiology, prevention and treatment of irritant diaper dermatitis, Curr Med Res Opin 20 (2004) 645.
9. Baer, E.L.; Davies, M.W.; Easterbrook, K.J., Disposable nappies for preventing napkin dermatitis in infants, Cochrane Database Syst Rev 3 (2006); CD004262.
10. Baharestani, M.M., An overview of neonatal and pediatric wound care knowledge and considerations, Ostomy Wound Manage 53 (2007) 34.
11. Baley, J.; Kliegman, R.M.; Boxerbaum, B.; et al., Fungal colonization in the very low birth weight infant, Pediatrics 78 (1986) 225.
12. Baley, J.; Silverman, R., Systemic candidiasis: cutaneous manifestations in low birth weight infants, Pediatrics 82 (1988) 211.
13. Behrendt, H.; Green, M., Patterns of skin pH from birth through adolescence,. ( 1971)Charles C Thomas, Springfield, Ill.
14. Berg, R., Etiologic factors in diaper dermatitis: a model for development of improved diapers, Pediatrician 14 (1987) 27.
15. Berg, R.; Buckingham, K.; Stewart, R., Etiologic factors in diaper dermatitis: the role of urine, Pediatr Dermatol 3 (1986) 102.
16. Bhandari, V.; Brodsky, N.; Porat, R., Improved outcome of extremely low birth weight infants with Tegaderm application to skin, J Perinatol 25 (2005) 276.
17. Brown, A.; Hoelzer, D.; Piercy, S., Skin necrosis from extravasation of intravenous fluids in children, Plast Reconstr Surg 64 (1979) 145.
18. Bruckner, A.; Weston, W.; Morelli, J., Does sensitization to contact allergens begin in infancy?Pediatrics e3 (2000) 105.
19. Bryanton, J.; Walsh, D.; Barrett, M.; et al., Tub bathing versus traditional sponge bathing for the newborn, J Obstet Gynecol Neonatal Nurs 33 (2004) 704.
20. Buckingham, K.; Berg, R., Etiologic factors in diaper dermatitis: the role of feces, Pediatr Dermatol 3 (1986) 107.
21. Bustamante, S.; Steslow, J., Use of a transparent adhesive dressing in very low birth weight infants, J Perinatol 9 (1989) 165.
22. Campbell, J.; Zaccaria, E.; Baker, C., Systemic candidiasis in extremely low birth weight infants receiving topical petrolatum ointment for care: a case control study, Pediatrics 105 (2000) 1041.
23. Campbell, R.; Seymour, J.L.; Stone, L.C.; et al., Clinical studies with disposable diapers containing absorbent gelling materials: evaluation on infant skin condition, J Am Acad Dermatol 17 (1987) 978.
24. Casanova, D.; Bardot, J.; Magalon, G., Emergency treatment of accidental infusion leakage in the newborn: report of 14 cases, British J Plastic Surgery 54 (2001) 396.
25. Centers for Disease Control and Prevention, Guidelines for the prevention of intravascular catheter related infections, MMWR 51 (2002) 1.
26. Cetta, F.; Lambert, G.; Ros, S., Newborn chemical exposure from over-the-counter skin care products, Clin Pediatr 30 (1991) 286.
27. Chabrolle, J.; Rossier, A., Goiter and hypothyroidism in the newborn after cutaneous absorption of iodine, Arch Dis Child 53 (1978) 495.
28. Chaiyakunapruk, N.; Veenstra, D.L.; Lipsky, B.A.; et al., Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis, Ann Intern Med 136 (2002) 792.
29. Chandavasu, O.; Garrow, E.; Valsa, V.; et al., A new method for the prevention of skin sloughs and necrosis secondary to intravenous infiltration, Am J Perinatol 3 (1986) 4.
30. Choudhuri, J.; McQueen, R.; Inoue, S.; et al., Efficacy of skin sterilization for a venipuncture with the use of commercially available alcohol or iodine pads, Am J Infect Control 18 (1990) 82.
31. Cowen, J.; Ellis, S.; McAinsh, J., Absorption of chlorhexidine from the intact skin of newborn infants, Arch Dis Child 54 (1979) 379.
32. Davies, J.; Babb, J.; Ayliffe, A., The effect on the skin flora of bathing with antiseptic solutions, J Antimicrob Chemother 3 (1977) 473.
33. Davies, J.; Gault, D.; Buchdahl, R., Preventing the scars of neonatal intensive care, Arch Dis Child Fetal Neonatal Ed 70 (1994) F50.
34. Davis, J.; Leyden, J.; Grove, G.; et al., Comparison of disposable diapers with fluff absorbent and fluff plus absorbent polymers: effects on skin hydration, skin pH, and diaper dermatitis, Pediatr Dermatol 6 (1989) 102.
35. Dixon, A., Think zinc, Neonatal Netw 5 (1987) 29.
36. Dollison, E.; Beckstrand, J., Adhesive tape vs. pectin-based barrier use in preterm infants, Neonatal Netw 14 (1995) 35.
37. Donlon, C.R.; Furdon, S.A., Assessment of the umbilical cord outside of the delivery room, Part 2, Adv Neonatal Care 2 (2002) 187.
38. Dore, S.; Buchan, D.; Coulas, S.; et al., Alcohol versus natural drying for newborn cord care, J Obstet Gynecol Neonatal Nurs 27 (1998) 621.
39. Dykes, P.J.; Heggie, R.; Hill, S.A., Effects of adhesive dressings on the stratum corneum of the skin, J Wound Care 10 (2001) 7.
40. Edwards, W.; Conner, J.; Soll, R., The effect of prophylactic ointment therapy on nosocomial sepsis rates and skin integrity in infants of birth weights 501–1000 grams, Pediatrics 113 (2004) 1195.
41. Evans, N.; Rutter, N., Development of the epidermis in the newborn, Biol Neonate 49 (1986) 74.
42. Evans, N.; Rutter, N., Reduction of skin damage from transcutaneous oxygen electrodes using a spray on dressing, Arch Dis Child 61 (1986) 881.
43. Fox, C.; Nelson, D.; Wareham, J., The timing of skin acidification in very low birth weight infants, J Perinatol 18 (1998) 272.
44. Friedman, Z., Essential fatty acids revisited, Am J Dis Child 134 (1980) 397.
45. Garland, J.; Buck, R.; Maloney, P., Comparison of 10% povidone-iodine and 0.5% chlorhexidine gluconate for the prevention of peripheral intravenous catheter colonization in neonates: a prospective trial, Pediatr Infect Dis J 14 (1995) 510.
46. Garvin, G., Wound healing in pediatrics, Nurs Clin North Am 25 (1990) 181.
47. Gault, D., Extravasation injuries, British J Plastic Surgery 46 (1993) 91.
48. Gfatter, R.; Hackl, P.; Braun, F., Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants, Dermatology 195 (1997) 258.
49. Gordon, C.; Rowitch, D.; Mitchell, M.; et al., Topical iodine and neonatal hypothyroidism, Arch Pediatr Adolesc Med 149 (1995) 1336.
50. Gotschall, C.S.; Morrison, M.; Eichelberger, M., Prospective, randomized study of the efficacy of Mepitel on children with partial-thickness scalds, J Burn Care Rehabil 19 (1998) 279.
51. Grove, G.; Leydon, J., Comparison of the skin protectant properties of various film-forming products,. ( 1993)Skin Study Center, KLG, Broomall, Pa.
52. Harpin, V.; Rutter, N., Barrier properties of the newborn infant’s skin, J Pediatr 102 (1983) 419.
53. Haubrich, K., Role of vernix caseosa in the neonate: potential application in the adult population, AACN Clin Issues 14 (2003) 457.
54. Hoath, S.; Narendran, V., Adhesives and emollients in the preterm infant, Semin Neonatol 5 (2000) 112.
55. Hoath, S.; Pickins, W.L., The biology and role of vernix, In: (Editors: Hoath, S.; Maibach, H.) Neonatal skin: structure and function,ed 2 ( 2003)Marcel Dekker, New York.
56. Holbrook, K.A., A histological comparison of infant and adult skin, In: (Editors: Maibach, H.I.; Boisits, E.K.) Neonatal skin: structure and function ( 1982)Marcel Dekker, New York.
57. Hunt, C.; Engel, R.R.; Modler, S.; et al., Essential fatty acid deficiency in neonates: inability to reverse deficiency by topical applications of EFA-rich oil, J Pediatr 92 (1978) 603.
58. Irving, V., Reducing the risk of epidermal stripping in the neonatal population: an evaluation of an alcohol free barrier film, J Neonatal Nurs 7 (2001) 5.
59. Ittman, P.; Bozynski, M.E., Toxic epidermal necrolysis in a newborn infant after exposure to adhesive remover, J Perinatol 13 (1993) 476.
60. Jackson, H.; Sutherland, R., Effect of povidone-iodine on neonatal thyroid function, Lancet 2 (1981) 992.
61. Janssen, P.A.; Selwood, B.L.; Dobson, S.R.; et al., To dye or not to dye: a randomized, clinical trial of a triple dye/alcohol regime versus dry cord care, Pediatrics 111 (2003) 15.
62. Johnson, J.; Malachowshi, N.; Vosti, K.; et al., A sequential study of various modes of skin and umbilical care and the incidence of staphylococcal colonization and infection in the neonate, Pediatrics 58 (1976) 354.
63. Kalia, Y.; Nonato, L.; Lund, C.; et al., Development of the skin barrier function in premature infants, J Invest Dermatol 111 (1998) 320.
64. Karl, D., The interactive newborn bath: using infant behavior to connect parents and newborns, Am J Matern Child Nurs 24 (1999) 280.
65. Klaus, M.H.; Fanaroff, A.A., Yearbook of perinatal/neonatal medicine. ( 1987)Year Book, Chicago.
66. Knauth, A.; Gordin, M.; McNelis, W.; et al., Semipermeable polyurethane membrane as an artificial skin for the premature neonate, Pediatrics 83 (1989) 945.
67. Kopelman, A.E., Cutaneous absorption of hexachlorophene in low-birth-weight infants, J Pediatr 82 (1973) 972.
68. Lane, A.; Drost, S., Effects of repeated application of emollient cream to premature neonates’ skin, Pediatrics 92 (1993) 415.
69. Laurie, S.; Wilson, K.; Kernahan, D.; et al., Intravenous extravasation injuries: the effectiveness of hyaluronidase in their treatment, Ann Plast Surg 13 (1984) 191.
70. Linder, N.; Davidovich, N.; Reichman, B.; et al., Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants, J Pediatr 131 (1997) 434.
71. Linder, N.; Prince, S.; Barzilai, A.; et al., Disinfection with 10% povidone-iodine versus 0.5% chlorhexidine gluconate in 70% isopropanol in the neonatal intensive care unit, Acta Paediatr 93 (2004) 205.
72. Lo, J.; Oriba, H.; Maibach, H.; et al., Transepidermal potassium, ion, and water flux across delipidized and cellophane tape-stripped skin, Dermatologica 180 (1990) 66.
73. Loomis, C.; Koss, T.M.; Chu, D.; et al., Fetal skin development, In: (Editors: Eichenfield, L.; Frieden, I.; Esterly, N.) Neonatal dermatologyed 2 ( 2008)Saunders, Philadelphia.
74. Lund, C., Prevention and management of infant skin breakdown, Nurs Clin North Am 34 (1999) 907.
75. Lund, C.; Kuller, J.; Lane, A.; et al., Neonatal skin care: evaluation of the AWHONN/NANN research-based practice project on knowledge and skin care practices, J Obstet Gynecol Neonatal Nurs 30 (2001) 30.
76. Lund, C.; Kuller, J.M.; Tobin, C.; et al., Evaluation of a pectin-based barrier under tape to protect neonatal skin, J Obstet Gynecol Neonatal Nurs 15 (1986) 39.
77. Lund, C.; Nonato, L.; Kuller, J.; et al., Disruption of barrier function in neonatal skin associated with adhesive removal, J Pediatr 131 (1997) 367.
78. Lund, C.; Osborne, J.; Kuller, J.; et al., Neonatal skin care: clinical outcomes of the AWHONN/NANN evidence-based clinical practice guideline, J Obstet Gynecol Neonatal Nurs 30 (2001) 41.
79. Lund, C.H.; Osborne, J.W., Validity and reliability of the neonatal skin condition score, J Obstet Gynecol Neonatal Nursing 33 (2004) 320.
80. Lund, C.H.; Tucker, J., Skin adhesion, In: (Editors: Hoath, S.; Maibach, H.) Neonatal skin: structure and functioned 2 ( 2003)Marcel Dekker, New York.
81. Maki, D.; Ringer, M.; Alvarado, C., Prospective randomized trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters, Lancet 338 (1991) 339.
82. Malathi, I.; Millar, M.R.; Leeming, J.P.; et al., Skin disinfection in preterm infants, Arch Dis Child 69 (1993) 312.
83. Mancini, A.; Sookdeo-Drost, S.; Madison, K.; et al., Semipermeable dressings improve epidermal barrier function in premature infants, Pediatr Res 36 (1994) 306.
84. Manzini, B.; Ferdani, G.; Simonetti, V.; et al., Contact sensitization in children, Pediatr Dermatol 15 (1998) 12.
85. Marks, J.; Belsito, D.; DeLeo, V.; et al., North American Contact Dermatitis Group: standard tray patch test results, Am J Contact Derm 6 (1995) 160.
86. McLean, S.; Kirchoff, K.T.; Kriynovich, K.; et al., Three methods of securing endotracheal tubes in neonates: a comparison, Neonatal Netw 11 (1992) 17.
87. Medves, J.M.; O’Brien, B., The effect of bather and location of first bath on maintaining thermal stability in newborns, J Obstet Gynecol Neonatal Nurs 33 (2004) 175.
88. Mimoz, O.; Karim, A.; Mercat, A.; et al., Chlorhexidine compared with povidone-iodine as skin preparation before blood culture, Ann Intern Med 131 (1999) 834.
89. Mitchell, I.; Pollock, J.C.; Jamieson, M.P.; et al., Transcutaneous iodine absorption in infants undergoing cardiac operation, Ann Thorac Surg 52 (1991) 1138.
90. Mize, M.; Vila-Coro, A.; Prager, T., The relationship between postnatal skin maturation and electrical skin impedance, Arch Dermatol 125 (1989) 647.
91. Moraille, R.; Pickens, W.; Visscher, M.; et al., A novel role for vernix caseosa as a skin cleanser, Biol Neonate 87 (2005) 8.
92. Nonato, L., Evolution of skin barrier function in neonates. ( 1998)University of California, Berkeley; Unpublished doctoral dissertation, UMI Publication No. AAT9827176.
93. Nopper, A.; Horii, K.; Sookdeo-Drost, S.; et al., Topical ointment therapy benefits premature infants, J Pediatr 128 (1996) 660.
94. Parravicini, E.; Fontana, C.; Paterlini, G.; et al., Iodine, thyroid function, and very low birth weight infants, Pediatrics 98 (1996) 730.
95. Penny-MacGillivray, T., A newborn’s first bath: when?J Obstet Gynecol Neonatal Nurs 25 (1996) 481.
96. Peters, K., Bathing premature infants: physiological and behavioral consequences, Am J Crit Care 7 (1998) 90.
97. Pyati, S.P.; Ramamurthy, R.S.; Krauss, M.T.; et al., Absorption of iodine in the neonate following topical use of povidone-iodine, J Pediatr 91 (1977) 825.
98. Quinn, D.; Newton, N.; Piecuch, R., Effect of less frequent bathing on premature infant skin, J Obstet Gynecol Neonatal Nurs 34 (2005) 741.
99. Rasmussen, J., Classification of diaper dermatitis: an overview, Pediatrician 14 (1987) 6.
100. Raszka, W.; Kueser, T.; Smith, F.; et al., The use of hyaluronidase in the treatment of intravenous extravasation injuries, J Perinatol 10 (1990) 146–149.
101. Reynolds, P.R.; Banerjee, S.; Meek, J.H., Alcohol burns in extremely low birthweight infants: still occurring, Arch Dis Child Fetal Neonatal Ed 90 (2005) F10.
102. Rowen, J.L.; Atkins, J.T.; Levy, M.L.; et al., Invasive fungal dermatitis in the < or = 1000-gram neonate, Pediatrics 95 (1995) 682.
103. Saijo, S.; Tagami, H., Dry skin of newborn infants: functional analysis of the stratum corneum, Pediatr Dermatol 8 (1991) 155.
104. Sarkany, I.; Arnold, L., The effect of single and repeated applications of hexachlorophene on the bacterial flora of the skin of the newborn, Br J Dermatol 82 (1970) 261.
105. Schick, J.B.; Milstein, J.M., Burn hazard of isopropyl alcohol in the neonate, Pediatrics 68 (1981) 587.
106. Sedin, G.; Hammarund, K.; Nilsson, G.E.; et al., Measurements of transepidermal water loss in newborn infants, Clin Perinatol 12 (1985) 79.
107. Smerdely, P.; Lim, A.; Boyages, S.C.; et al., Topical iodine-containing antiseptics and neonatal hypothyroidism in very-low-birth weight infants, Lancet 16 (1989) 661.
108. Sprung, P.; Hou, Z.; Ladin, D., Hydrogels and hydrocolloids: an objective product comparison, Ostomy Wound Manage 44 (1998) 36.
109. Taquino, L., Promoting wound healing in the neonatal setting: process vs. protocol, J Perinat Neonatal Nurs 14 (2000) 104.
110. Thomas, S.; Hay, P., Fluid handling properties of hydrogel dressings, Ostomy Wound Manage 41 (1995) 54.
111. Thomas, S.; Rowe, H.N.; Keats, J.; et al., The management of extravasation injury in neonates, World Wide Wounds, Elect J Wound Heal Manage ( 1997); www.smtl.co.uk; Accessed September 9, 2009.
112. 3M Health Care, 3M Cavilon No Sting Barrier Film (brochure). ( 2001)3M Company, St Paul, Minn.
113. Tollin, M.; Bersson, G.; Kai-Larsen, Y.; et al., Vernix caseosa as a multi-component defense system based on polypeptides, lipids and their interactions, Cell Mol Life Sci 62 (2005) 2390.
114. Tupker, R.A.; Pinnagoda, J.; Coenraads, P.J.; et al., Evaluation of detergent-induced irritant skin reactions by visual scoring and transepidermal water loss measurement, Dermatol Clin 8 (1990) 33.
115. Tupker, R.A.; Pinnagoda, J.; Nater, J.P., The transient and cumulative effect of sodium lauryl sulphate on the epidermal barrier assessed by transepidermal water loss: inter-individual variation, Acta Derm Venereol 70 (1990) 1.
116. Varda, K.; Behnke, R., The effect of timing of initial bath on newborn’s temperature, J Obstet Gynecol Neonatal Nurs 29 (2000) 27.
117. Vernon, H.; Lane, A.T.; Wischerath, L.J.; et al., Semipermeable dressing and transepidermal water loss in premature infants, Pediatrics 86 (1990) 357.
118. Visscher, M.O., Recent advances in diaper dermatitis: etiology and treatment, Pediatric Health 3 (2009) 81.
119. Visscher, M.O., Update on the use of topical agents in neonates, Newborn Infant Nurs Rev 9 (2009) 31.
120. Visscher, M.; Narendran, V.; Pickens, W.; et al., Vernix caseosa in neonatal adaptation, J Perinatol 25 (2005) 440.
121. Visscher, M.O.; Chatterjee, R.; Munson, K.A.; et al., Changes in diapered and nondiapered infant skin over the first month of life, Pediatr Dermatol 17 (2000) 45.
122. Wilhelm, K.; Maibach, H., Factors predisposing to cutaneous irritation, Dermatol Clin 8 (1990) 17.
123. Yosipovitch, G.; Maayan-Metzger, A.; Merlob, P.P.; et al., Skin barrier properties in different body areas in neonates, Pediatrics 106 (2000) 105.
124. Zenk, K., Management of intravenous extravasations, Infusion 5 (1981) 77.
125. Zenk, K.; Sills, J., Management of dopamine-induced perivascular blanching and extravasation in LBW infants, J Perinatol 6 (1986) 82.
126. Zimmerman, A., Acrodermatitis in breast-fed premature infants: evidence for a defect in mammary gland zinc secretion, Pediatrics 69 (1982) 176.
127. Zlotkin, S.; Buchanan, B., Meeting zinc and copper intake requirements in the parenterally fed preterm and full-term infant, J Pediatr 103 (1983) 441.
128. Zupan, J.; Garner, P.; Omari, A.A., Topical umbilical cord care at birth, Cochrane Database Syst Rev 3 (2004); CD001057.