Chapter 8 Sinus Node Dysfunction
General Considerations
Anatomy and Physiology of the Sinus Node
The sinus node is the dominant pacemaker of the heart. Its pacemaker function is determined by its low maximum diastolic membrane potential and steep phase 4 spontaneous depolarization. The molecular mechanisms of pacemaker function of the sinus node are discussed in detail in Chapters 1 and 3.1
The sinus node is a subepicardial specialized muscular structure located laterally within the epicardial groove of the sulcus terminalis of the right atrium (RA) at the junction of the anterior trabeculated appendage with the posterior smooth-walled venous component. The endocardial aspect of the sulcus terminalis is marked by the crista terminalis. Starting epicardially at the junction of the superior vena cava (SVC) and the RA appendage, it courses downward and to the left along the sulcus terminalis, to end subendocardially almost to the inferior vena cava (IVC). The sinus node is a spindle-shaped structure with a central body and tapering ends; the head extends toward the interatrial groove, and the tail extends toward the orifice of IVC. In adults, the sinus node measures 10 to 20 mm long and 2 to 3 mm wide and thick.1–5
The sinus node consists of densely packed specialized myocytes of no definite orientation within a background of extracellular connective tissue matrix.4 The nodal margins can be discrete with fibrous separation from the surrounding atrial myocardium or interdigitate though a transitional zone. Commonly, prongs of nodal (P) cells and transitional (T) cells extend from the nodal body into the atrial myocardium, but actual cell-to-cell interaction is uncertain.6
The pacemaker activity is not confined to a single cell in the sinus node; rather, sinus node cells function as electrically coupled oscillators that discharge synchronously because of mutual entrainment. In fact, it is likely that sinus rhythm results from impulse origin at widely separated sites, with two or three individual wavefronts created that merge to form a single, widely disseminated wavefront. The sinus node is insulated electrically from the surrounding atrial myocytes, except at a limited number of preferential exit sites. Neural and hormonal factors influence both the site of pacemaker activation, likely via shifting points of initial activity, and the point of exit from the sinus node complex. At faster rates, the sinus impulse originates in the superior portion of the sinus node, whereas at slower rates, it arises from a more inferior portion.7 High-density simultaneous endocardial unipolar mapping studies demonstrated the frequent occurrence of spontaneous variations in the P wave and sinus activation sequence in normal individuals. These findings suggested that the sinus node complex in normal hearts displays a dynamic range of activation sites along the posterolateral RA. Furthermore, preferential pathways of conduction were also found to exist between the sinus node and the atrial exit sites, thus potentially contributing to the multicentricity of the sinus node complex.1,5,8
The blood supply to the sinus node region is variable and is therefore vulnerable to damage during operative procedures. The blood supply predominantly comes from a large central artery, the sinus nodal artery, which is a branch of the right coronary artery in 55% to 60% of patients, and from the circumflex artery in 40% to 45%. The sinus nodal artery typically passes centrally through the length of the sinus body, and it is disproportionately large, which is considered physiologically important in that its perfusion pressure can affect the sinus rate. Distention of the artery slows the sinus rate, whereas collapse causes an increase in rate.1,3
The sinus node is densely innervated with postganglionic adrenergic and cholinergic nerve terminals (threefold greater density of beta-adrenergic and muscarinic cholinergic receptors than adjacent atrial tissue), both of which influence the rate of spontaneous depolarization in pacemaker cells and can cause a shift in the principal pacemaker site within the sinus node region, which is often associated with subtle changes in P wave morphology. Enhanced vagal activity can produce sinus bradycardia, sinus arrest, and sinoatrial exit block, whereas increased sympathetic activity can increase the sinus rate and reverse sinus arrest and sinoatrial exit block. Sinus node responses to brief vagal bursts begin after a short latency and dissipate quickly; in contrast, responses to sympathetic stimulation begin and dissipate slowly. The rapid onset and offset of responses to vagal stimulation allow dynamic beat-to-beat vagal modulation of the heart rate, whereas the slow temporal response to sympathetic stimulation precludes any beat-to-beat regulation by sympathetic activity.1
Periodic vagal bursting (as may occur each time a systolic pressure wave arrives at the baroreceptor regions in the aortic and carotid sinuses) induces phasic changes in the sinus cycle length (CL) and can entrain the sinus node to discharge faster or slower at periods identical to those of the vagal burst.3 Because the peak vagal effects on sinus rate and atrioventricular node (AVN) conduction occur at different times in the cardiac cycle, a brief vagal burst can slow the sinus rate without affecting AVN conduction or can prolong AVN conduction time and not slow the sinus rate.1
Pathophysiology of Sinus Node Dysfunction
The cause of sinus node dysfunction (SND) can be classified as intrinsic (secondary to a pathological condition involving the sinus node proper) or extrinsic (caused by depression of sinus node function by external factors such as drugs or autonomic influences).
Intrinsic Sinus Node Dysfunction
Idiopathic degenerative disease is probably the most common cause of intrinsic SND.2 Ischemic heart disease can be responsible for one third of cases of SND. Transient slowing of the sinus rate or sinus arrest can complicate acute myocardial infarction, which is usually seen with acute inferior wall infarction and is caused by autonomic influences. Possible mechanisms for sinus bradycardia after an acute myocardial infarction include neurological reflexes (Bezold-Jarisch reflex), coronary chemoreflexes (vagally mediated), humoral reflexes (enzymes, adenosine, potassium [K+]), oxygen-conserving reflex (“diving” reflex), and infarction or ischemia of the sinus node or the surrounding atrium (e. g., secondary to proximal occlusion of the right or the circumflex coronary artery).
Cardiomyopathy, long-standing hypertension, infiltrative disorders (e.g., amyloidosis and sarcoidosis), collagen vascular diseases, and surgical trauma can also result in SND.9,10 Orthotropic cardiac transplantation with atrial-atrial anastomosis is associated with a high incidence of SND in the donor heart (likely because of sinus nodal artery damage). Musculoskeletal disorders such as myotonic dystrophy or Friedreich ataxia are rare causes of SND. Congenital heart disease, such as sinus venosus and secundum atrial septal defects, can be associated with SND, even though no surgery has been performed.11 Surgical trauma is responsible for most cases of SND in the pediatric population. Most commonly associated with this complication is the Mustard procedure for transposition of the great arteries and repair of atrial septal defects, especially of the sinus venosus type.11
Furthermore, atrial tachyarrhythmias can precipitate SND, likely secondary to remodeling of sinus node function. Although early studies implicated anatomical structural abnormalities in the sinus node, which suggested a fixed SND substrate, more recent evidence implicated a functional, and potentially reversible, component involving remodeling of sinus node ion channel expression and function. This finding was supported clinically by the observation that successful catheter ablation of atrial fibrillation (AF) and atrial flutter can be followed by significant improvements in sinus node function. In particular, downregulation of the funny current (If) and malfunction of the calcium (Ca2+) clock (characterized by reduced sarcoplasmic reticulum Ca2+ release and downregulated ryanodine receptors in the sinus node) seem to account largely for atrial tachycardia–induced remodeling of sinus node. The remodeled atria are associated with more caudal activation of the sinus node complex, slower conduction time along preferential pathways, and only modest shifts within the functional pacemaker complex.5,12,13
On the other hand, SND has been associated with an increased propensity of atrial tachyarrhythmias, AF in particular. The mechanism leading to AF in patients with SND is unlikely to be bradycardia-dependent because AF was found to develop despite pacing in these patients. Importantly, patients with SND appear to have more widespread atrial changes beyond the sinus node, a finding indicating atrial myopathy, as evidenced by increased atrial refractoriness, prolonged P wave duration, delayed conduction, slowing electrogram fractionation, regions of low voltage and scar, and caudal shift of the pacemaker complex with loss of normal multicentric pattern of activation. Furthermore, abnormal atrial electromechanical properties, chronic atrial stretch, and neurohormonal activation are likely contributors to SND and its related atrial myopathy. The diffuse atrial myopathy potentially underlies the increased propensity to AF. The cause of these diffuse atrial abnormalities remains unknown, but there appears to be a relationship between atrial remodeling that predisposes to AF and sinus node remodeling that results in SND.5,14
Genetic defects in ion channels and structural proteins have been shown to contribute to SND, manifesting as sinus bradycardia, sinus arrest, sinoatrial block, or a combination. Mutations in the SCN5A gene (which encodes the alpha subunit of the cardiac sodium [Na+] channel [INa]), the HCN4 gene (which encodes the protein that contributes to formation of If channels), the KCNQ1 gene (which encodes the alpha subunit of the voltage-gated slowly activating delayed rectifier K+ channel responsible for IKs), the GJA5 gene (which encodes for connexin 40, a gap junction protein), the ANK2 gene (which encodes for ankyrin, which links the integral membrane proteins to the underlying cytoskeleton), and the EMD gene (which encodes the nuclear membrane protein emerin) have been associated with familial forms of SND, many of which also exhibit an increased propensity to AF.15–18
Extrinsic Sinus Node Dysfunction
In the absence of structural abnormalities, the predominant causes of SND are drug effects and autonomic influences. Drugs can alter sinus node function by direct pharmacological effects on nodal tissue or indirectly by neurally mediated effects.19 Drugs known to depress sinus node function include beta blockers, calcium channel blockers (verapamil and diltiazem), digoxin, sympatholytic antihypertensive agents (e.g., clonidine), and antiarrhythmic agents (classes IA, IC, and III).
SND can sometimes result from excessive vagal tone in individuals without intrinsic sinus node disease. Hypervagotonia can be seen in hypersensitive carotid sinus syndrome and neurocardiogenic syncope. Well-trained athletes with increased vagal tone occasionally may require some deconditioning to help prevent symptomatic bradyarrhythmias.20 Surges in vagal tone also can occur during Valsalva maneuvers, endotracheal intubation, vomiting, and suctioning. Sinus slowing in this setting is characteristically paroxysmal and may be associated with evidence of AV conduction delay, secondary to effects of the enhanced vagal tone on both the sinus node and AVN. Less common extrinsic causes of SND include electrolyte abnormalities such as hyperkalemia, hypothermia, increased intracranial pressure (the Cushing response), sleep apnea, hypoxia, hypercapnia, hypothyroidism, advanced liver disease, typhoid fever, brucellosis, and sepsis.
Clinical Presentation
More than 50% of the patients with SND are older than 50 years. Patients often are asymptomatic or have symptoms that are mild and nonspecific, and the intermittent nature of these symptoms makes documentation of the associated arrhythmia difficult at times. Symptoms, which may have been present for months or years, include paroxysmal dizziness, presyncope, or syncope, which are predominantly related to prolonged sinus pauses. Episodes of syncope are often unheralded and can manifest in older patients as repeated falls. The highest incidence of syncope associated with SND probably occurs in patients with tachycardia-bradycardia syndrome, in whom syncope typically occurs secondary to a long sinus pause following cessation of the supraventricular tachycardia (usually AF). Occasionally, a stroke can be the first manifestation of SND in patients presenting with paroxysmal AF and thromboembolism.3,19,21
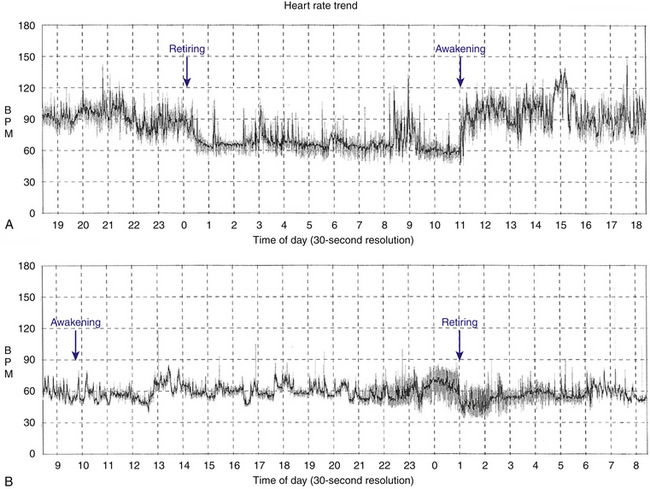
Patients with sinus bradycardia or chronotropic incompetence can present with decreased exercise capacity or fatigue (Fig. 8-1). Chronotropic incompetence is estimated to be present in 20% to 60% of patients with SND.22,23 Other symptoms include irritability, nocturnal wakefulness, memory loss, lightheadedness, and lethargy. More subtle symptoms include mild digestive disturbances, periodic oliguria or edema, and mild intermittent dyspnea. Additionally, symptoms caused by the worsening of conditions such as congestive heart failure and angina pectoris can be precipitated by SND.
Natural History
The natural history of SND can be variable, but slow progression (over 10 to 30 years) is expected. The prognosis largely depends on the type of dysfunction and the presence and severity of the underlying heart disease. The worst prognosis is associated with the tachycardia-bradycardia syndrome (mostly because of the risk for thromboembolic complications), whereas sinus bradycardia is much more benign. The incidence of new-onset AF in patients with SND is about 5.2% per year. New atrial tachyarrhythmias occur with less frequency in patients who are treated with atrial pacing (3.9%) compared with a greatly increased incidence of similar arrhythmias in patients with only ventricular pacing (22.3%).24 Furthermore, thromboembolism occurs in 15.2% among unpaced patients with SND versus 13% among patients treated with only ventricular pacing versus 1.6% among those treated with atrial pacing.19,21,25
The incidence of advanced AV conduction system disease in patients with SND is low (5% to 10%), and, when present, its progression is slow. At the time of diagnosis of SND, approximately 17% of the patients have some degree of AV conduction system disease (PR interval longer than 240 milliseconds, bundle branch block, His bundle–ventricular (HV) interval prolongation, AV Wenckebach rate less than 120 beats/min, or second- or third-degree AV block). New AV conduction abnormalities develop at a rate of approximately 2.7% per year. The incidence of advanced AV block during long-term follow-up is low (approximately 1% per year).21
Diagnostic Evaluation
Generally, the noninvasive methods of ECG monitoring, exercise testing, and autonomic testing are used first. However, if symptoms are infrequent and noninvasive evaluation is unrevealing, invasive electrophysiological (EP) testing may be pursued.26
Electrocardiogram and Ambulatory Monitoring
A 12-lead electrocardiogram (ECG) needs to be obtained in symptomatic patients. However, the diagnosis of SND as the cause of the symptoms is rarely made from the ECG. In patients with frequent symptoms, 24- or 48-hour ambulatory Holter monitoring can be useful. Cardiac event monitoring or implantable loop recorders may be necessary in patients with less frequent symptoms.27 Documentation of symptoms in a diary by the patient while wearing the cardiac monitor is essential for correlation of symptoms with the heart rhythm at the time. In some cases, ambulatory monitoring can exclude SND as the cause of symptoms if normal sinus rhythm (NSR) is documented at the time of symptom occurrence. In contrast, recorded sinus pauses may not be associated with symptoms.
Autonomic Modulation
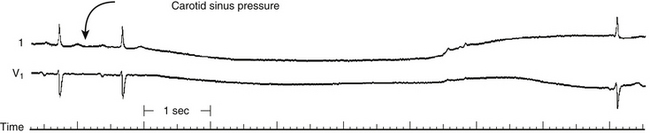
An abnormal response to carotid sinus massage (pause longer than 3 seconds) can indicate SND, but this response can also occur in asymptomatic older individuals. Heart rate response to the Valsalva maneuver (normally decreased) or upright tilt (normally increased) can also be used to verify that the autonomic nervous system itself is intact. Complete pharmacological autonomic blockade is used to determine the intrinsic heart rate (see later).3
Exercise testing
Exercise testing to assess chronotropic incompetence is of value in patients with exertional symptoms (see later).
Electrophysiological Testing
Noninvasive testing is usually adequate in establishing the diagnosis of SND and guiding subsequent therapy. However, invasive EP testing can be of value in symptomatic patients in whom SND is suspected but cannot be documented in association with symptoms. In addition to assessing SND, EP testing can be useful in evaluating other potential causes for symptoms of syncope and palpitations (e.g., AV block, supraventricular tachycardia, and ventricular tachycardia).19,21
Electrocardiographic Features
Sinus Bradycardia
Sinus bradycardia (less than 60 beats/min) is considered abnormal when it is persistent, unexplained, and inappropriate for physiological circumstances. Sinus bradycardia slower than 40 beats/min (not associated with sleep or physical conditioning) is generally considered abnormal.3
Sinus Arrest
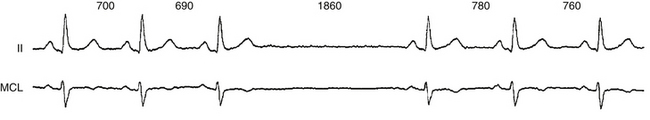
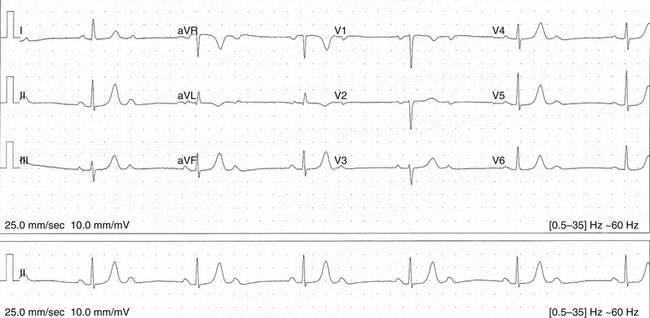
The terms sinus arrest and sinus pause are often used interchangeably; sinus arrest is a result of total cessation of impulse formation within the sinus node. The pause is not an exact multiple of the preceding P-P interval but is random in duration (Fig. 8-2). Although asymptomatic pauses of 2 to 3 seconds can be seen in up to 11% of normal individuals and in one third of trained athletes, pauses longer than 3 seconds are rare in normal individuals and may or may not be associated with symptoms, but they are usually caused by SND.3
Sinoatrial Exit Block
Sinoatrial exit block results when a normally generated sinus impulse fails to conduct to the atria because of delay in conduction or block within the sinus node itself or perinodal tissue. Sinoatrial exit block produces a pause that is eventually terminated by a delayed sinus beat or an atrial or junctional escape beat.28 In theory, sinoatrial exit block can be distinguished from sinus arrest because the exit block pause is an exact multiple of the baseline P-P interval. However, sinus arrhythmia causing normal beat-to-beat variations in the sinus rate often makes the distinction impossible. Furthermore, establishing the diagnosis of sinoatrial exit block versus sinus arrest is often of academic interest only.3
Exit block is classified into three types, analogous to those of AV block: first-degree, second-degree, and third-degree exit block.28 First-degree sinoatrial exit block is caused by abnormal prolongation of the sinoatrial conduction time (SACT). It occurs every time a sinus impulse reaches the atrium, but it is conducted with a delay at a fixed interval. This type of sinoatrial exit block is concealed on the surface ECG and can be diagnosed only by direct sinus node recording or indirect measurement of SACT during an EP study. Second-degree sinoatrial exit block is marked by intermittent failure of the sinus impulse to exit the sinus node. Type I block is viewed as Wenckebach periodicity of the P wave on the surface ECG, and it manifests as progressive delay in conduction of the sinus-generated impulse through the sinus node to the atrium, finally resulting in a nonconducted sinus impulse and absence of a P wave on the surface ECG. Because the sinus discharge is a silent event on the surface ECG, this arrhythmia can be inferred only, because of a missing P wave and the signs of Wenckebach periodicity seen with this type of arrhythmia. The increment in delay in impulse conduction through the sinus node tissue is progressively less; thus, the P-P intervals become progressively shorter until a P wave fails to occur. The pauses associated with this type of sinoatrial exit block are less than twice the shortest sinus cycle. Type II block manifests as an abrupt absence of one or more P waves because of failure of the atrial impulse to exit the sinus node, without previous progressive prolongation of SACT (and without progressive shortening of the P-P intervals). Sometimes, two or more consecutive sinus impulses are blocked within the sinus node, thus creating considerably long pauses. The sinus pause should be an exact multiple of the immediately preceding P-P interval. However, normal variations in the sinus rate caused by sinus arrhythmia can obscure this measurement. Third-degree or complete sinoatrial exit block manifests as absence of P waves, with long pauses resulting in lower pacemaker escape rhythm. This type of block is impossible to distinguish from sinus arrest with certainty without invasive sinus node recordings.21,29,30
Tachycardia-Bradycardia Syndrome
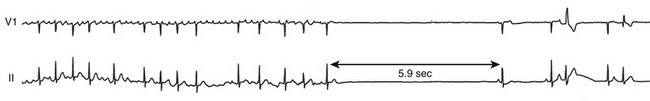
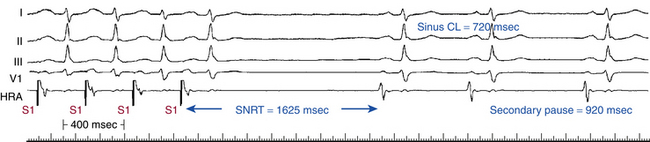
Tachycardia-bradycardia syndrome, frequently referred to as sick sinus syndrome, is a common manifestation of SND, and it refers to the presence of intermittent sinus or junctional bradycardia alternating with atrial tachyarrhythmias (Fig. 8-3). The atrial tachyarrhythmia is most commonly paroxysmal AF, but atrial tachycardia, atrial flutter, and occasionally AVN reentrant tachycardia or AV reentrant tachycardia can also occur.3
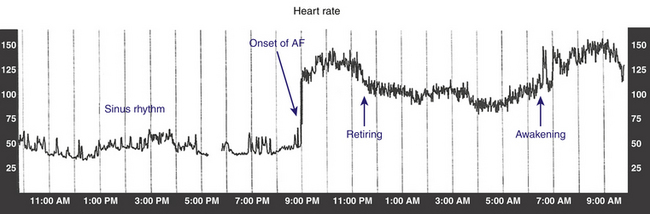
Apart from underlying sinus bradycardia of varying severity, these patients often experience prolonged sinus arrest and asystole on termination of the atrial tachyarrhythmia, resulting from overdrive suppression of the sinus node and secondary pacemakers by the tachycardia. Long sinus pauses that occur following electrical cardioversion of AF constitute another manifestation of SND. Therapeutic strategies to control the tachyarrhythmias often result in the need for pacemaker therapy (Fig. 8-4). On the other hand, atrial tachyarrhythmias can be precipitated by prolonged sinus pauses.2,3,31,32 SND is often caused by functional remodeling from the tachycardia and can be reversible in some patients, thus obviating the need for pacing.
Atrial Fibrillation WITH SLOW VENTRICULAR RESPONSE
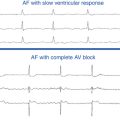
Persistent AF with a slow ventricular response in the absence of AVN blocking drugs is often present in patients with SND. These patients can demonstrate very slow ventricular rates at rest or during sleep and occasionally have long pauses. Occasionally, they can develop complete AV block with a junctional or ventricular escape rhythm. They can also conduct rapidly and develop symptoms caused by tachycardia during exercise. In some cases, cardioversion results in a long sinus pause or junctional escape rhythm before the appearance of sinus rhythm. Although a combination of sinus node and AV conduction disease can be present in many cases, examples of rapid ventricular responses during atrial tachyarrhythmias are frequently found.2
Persistent Atrial Standstill
Atrial standstill is a rare clinical syndrome in which there is no spontaneous atrial activity and the atria cannot be electrically stimulated. The surface ECG usually reveals junctional bradycardia without atrial activity. The atria are generally fibrotic and without any functional myocardium. Lack of mechanical atrial contraction poses a high risk for thromboembolism in these patients.33,34
Chronotropic Incompetence
Treadmill exercise testing can be of substantial value in assessing the chronotropic response (“competence”) to increases in metabolic demands in patients with sinus bradycardia who are suspected of having SND. Although the resting heart rate can be normal, these patients may be unable to increase their heart rate during exercise or may have unpredictable fluctuations in the heart rate during activity. Some patients can initially experience a normal increase in the heart rate with exercise, which then plateaus or decreases inappropriately.22,23,29,30
The definition of chronotropic incompetence is not agreed on, but it is reasonable to designate it as an abnormally slow heart rate response to exercise manifesting as a less than normal increase in the sinus rate at each stage of exercise, with a plateau at less than 70% to 75% of the age-predicted maximum heart rate (220 − age) or an inability to achieve a sinus rate of 100 to 120 beats/min at maximum effort. Irregular (and nonreproducible) increases, and even decreases, in the sinus rate during exercise, can also occur but are rare. Other patients with SND can achieve an appropriate peak heart rate during exercise but may have slow sinus rate acceleration in the initial stage or rapid deceleration of heart rate in the recovery stage.22,23
Carotid Sinus Hypersensitivity
An abnormal response to carotid sinus massage (pause longer than 3 seconds) can indicate SND, but this response may also occur in asymptomatic older individuals (Fig. 8-5).1,28–30
Sinus Arrhythmia
Respiratory sinus arrhythmia, in which the sinus rate increases with inspiration and decreases with expiration, is not an abnormal rhythm and is most commonly seen in young healthy subjects. Sinus arrhythmia is present when the P wave morphology is normal and consistent and the P-P intervals vary by more than 120 milliseconds. Nonrespiratory sinus arrhythmia, in which phasic changes in sinus rate are not related to the respiratory cycle, can be accentuated by the use of vagal agents such as digitalis and morphine; its mechanism is unknown. Patients with nonrespiratory sinus arrhythmia are likely to be older and to have underlying cardiac disease, although the arrhythmia is not a marker for structural heart disease. None of the sinus arrhythmias (respiratory or nonrespiratory) indicate SND. Additionally, respiratory variation in the sinus P wave contour can be seen in the inferior leads and should not be confused with wandering atrial pacemaker, which is unrelated to breathing and therefore is not phasic.29,30
Ventriculophasic sinus arrhythmia is an unusual rhythm that occurs when sinus rhythm and high-grade or complete AV block coexist; it is characterized by shorter P-P intervals when they enclose QRS complexes and longer P-P intervals when no QRS complexes are enclosed (Fig. 8-6). The mechanism is uncertain but may be related to the effects of the mechanical ventricular systole itself: ventricular contraction increases the blood supply to the sinus node, thereby transiently increasing its firing rate. Ventriculophasic sinus arrhythmia is not a pathological arrhythmia and should not be confused with premature atrial complexes or sinoatrial block.
Electrophysiological Testing
Role of Electrophysiological Testing
The diagnosis of SND usually can be made based on clinical and ECG findings, which are typically adequate for deciding subsequent treatment. Once symptoms and SND are correlated with ECG findings, further documentation by invasive studies is not required. Similarly, asymptomatic patients with evidence of SND need not be tested because no therapy is indicated. However, EP testing can be important to assess sinus node function in patients who have had symptoms compatible with SND and in whom no documentation of the arrhythmia responsible for these symptoms has been obtained by prolonged monitoring. In these cases, EP testing can yield information that may be used to guide appropriate therapy. The most useful measures of the overall sinus node function are a combination of the responses to atropine and exercise and the sinus node recovery time (SNRT).28
Sinus Node Recovery Time
The sinus node is the archetype of an automatic focus. Automatic rhythms are characterized by spontaneous depolarization, overdrive suppression, and post-overdrive warm-up or a gradual return to baseline CL. SNRT is the interval between the end of a period of pacing-induced overdrive suppression of sinus node activity and the return of sinus node function, manifest on the surface ECG by a post-pacing sinus P wave. Clinically, SNRT is used to test sinus node automaticity.29,30,35
Technique
Pacing Site
Pacing is performed in the high RA at a site near the sinus node, to decrease the conduction time to and from the sinus node.28
Pacing Cycle Length
SNRT is preferably measured after pacing at multiple CLs. Pacing is started at a CL just shorter than the sinus CL. After a 1-minute rest, pacing is repeated at progressively shorter CLs (with 50- to 100-millisecond decrements) down to a pacing CL of 300 milliseconds.28
Pacing Duration
Pacing is continued for 30 or 60 seconds at a time. Although pacing durations beyond 15 seconds usually have little effect on the SNRT in healthy subjects, patients with SND can have marked suppression after longer pacing durations. It is also preferable to perform pacing at each CL for different durations (30, 60, or 120 seconds), to ensure that sinus entrance block has not obscured the true SNRT.28
Measurements
Several intervals have been used as a measure of SNRT.
Sinus Node Recovery Time
SNRT is the longest pause from the last paced beat to the first sinus return beat at a particular pacing CL. Normally, the SNRT is less than 1500 milliseconds, with a scatter on multiple tests of less than 250 milliseconds (Fig. 8-7). SNRT tends to be shorter with shorter baseline sinus CLs, and therefore various corrections have been introduced.29,30,35
Corrected Sinus Node Recovery Time
Corrected SNRT equals SNRT minus the baseline sinus CL. Normal values of corrected SNRT have been reported from 350 to 550 milliseconds, with 500 milliseconds most commonly used (see Fig. 8-7). However, the use of corrections at slow sinus rates can produce odd results. For example, in a patient with symptomatic bradycardia at a 1500-millisecond CL and an SNRT of 2000 milliseconds, the corrected SNRT is 500 milliseconds. For cases of severe bradycardia, an abnormal uncorrected SNRT of 2000 milliseconds is more accurate; in fact, one does not need SNRT to make the clinical diagnosis.
Ratio of Sinus Node Recovery Time to Sinus Cycle Length
The ratio of [(SNRT/sinus CL) × 100%] is lower than 160% in normal subjects.
Total Recovery Time
On cessation of atrial pacing, the pattern of subsequent beats returning to the basic sinus CL should be analyzed. Various patterns exist.28 Total recovery time equals the time to return to basic sinus CL (normal total recovery time is less than 5 seconds, usually by the fourth to sixth recovery beat).
Secondary Pauses
Normally, following cessation of overdrive pacing, a gradual shortening of the sinus CL is observed until the baseline sinus CL is reached, typically within a few beats. Limited oscillations of recovery CLs before full recovery can be observed, especially at faster pacing rates.28 Secondary pauses are identified when there is an initial shortening of the sinus CL after the SNRT, followed by an unexpected lengthening of the CL (see Fig. 8-7). Sudden and marked secondary pauses occurring during sinus recovery are abnormal. Sinoatrial exit block of variable duration is the primary mechanism of prolonged pauses, with a lesser component of depression of automaticity. Both may, and often do, coexist. However, secondary pauses can be a normal reflex following hypotension induced by pacing at rapid rates or in response to pressure overshoot in the first recovery beat resulting from the prolonged filling time. Because these secondary pauses represent SND and because they occur more frequently following rapid atrial pacing, pacing should be performed at rates up to 200 beats/min.28,29,35,36
Limitations of Sinus Node Recovery Time
Many factors in addition to automaticity are involved in the measurement of SNRT, including proximity of the pacing site to the sinus node and conduction time from the pacing site to the sinus node and vice versa, as well as conduction time in and out of the sinus node. Sinus node entrance block during rapid atrial pacing can lead to a shorter SNRT, whereas sinus node exit block after cessation of pacing can result in marked prolongation of the SNRT.28 Moreover, sometimes SNRT cannot be measured because of atrial ectopic or junctional escape beats that preempt the sinus beat.29,30
Despite these limitations, SNRT is probably the best and most widely used test for sinus node automaticity. Pacing at rates near the baseline sinus CL causes no overdrive suppression, so that the interval between the last paced beat and the next sinus beat is comparable with the baseline CL. If the SNRT after pacing at 500 milliseconds is shorter than after 600 milliseconds, or if there is marked variation (more than 250 milliseconds) in the SNRTs when multiple tests are performed after pacing at 500 milliseconds, this finding can imply that some impulses have not penetrated the sinus node (i.e., some degree of atrial-nodal block exists). At pacing CLs shorter than 500 milliseconds, there is usually little further prolongation of the SNRT; on the contrary, changes in neurohumoral tone may result in shorter SNRTs.29,35
The durations of the maximum SNRT and corrected SNRT are independent of age. Evaluation of the corrected SNRT following pharmacological denervation (see later) can increase the sensitivity of the test.36
Sinus Node Recovery Time in Patients with Sinus Node Dysfunction
The sensitivity of a single SNRT measurement is approximately 35% in patients with SND. This rises to more than 85% when multiple SNRTs at different rates are recorded, along with scatter and total recovery time, with a specificity of more than 90%. A prolonged SNRT or corrected SNRT is found in 35% to 93% of patients suspected of having SND (depending on the population studied). The incidence is lowest in patients with sinus bradycardia. Marked abnormalities in the corrected SNRT usually occur in symptomatic patients with clinical evidence of sinoatrial block or bradycardia-tachycardia syndrome.28,36
The pacing CL at which maximum suppression occurs in patients with SND is unpredictable and, unlike in healthy subjects, tends to be affected by the rate and duration of pacing. However, if sinus entrance block is present, the greatest suppression is likely to occur at relatively long pacing CLs. If the longest SNRT occurs at pacing CLs longer than 600 milliseconds, a normal value can reflect the presence of entrance block. In such cases, a normal SNRT is an unreliable assessment of sinus node automaticity. The fact that the longest SNRT occurs at pacing CLs longer than 600 milliseconds is in itself a marker of SND.28
Marked secondary pauses are another manifestation of SND and can occasionally occur in the absence of prolongation of SNRT, in which case sinoatrial block is the mechanism. Approximately 69% of patients with secondary pauses have clinical evidence of sinoatrial exit block, and 92% of patients with sinoatrial exit block demonstrate marked secondary pauses.28
Sinoatrial Conduction Time
Although the sinus node is the dominant cardiac pacemaker, neither sinus node impulse initiation nor conduction is visible on the surface ECG or on the standard intracardiac recordings because depolarization within the sinus node is of very low amplitude. Sinus node function therefore has usually been assessed indirectly. Normal sinus node function is assumed when the atrial musculature is depolarized at a normal rate and in a normal temporal sequence—so-called normal sinus rhythm. In NSR, the atrial rate is assumed to correspond to the rate of impulse formation within the sinus node; however, the time of impulse conduction from the sinus node to the atrium cannot be ascertained. Several methods have been developed for the assessment of SACT, either indirectly (the Strauss and Narula methods) or by directly recording the sinus node electrogram. Signal-averaging techniques have also been used to measure SACT noninvasively.29,30,36
Direct Recordings
Sinus node depolarization can be recorded directly using high-gain unfiltered electrograms in approximately 50% of patients.28 A catheter with a 0.5- to 1.5-mm interelectrode distance is used. The catheter is placed directly at the SVC-RA junction, or a loop is formed in the RA and the tip of the catheter is then placed at the SVC-RA junction. Optimizing the filter setting can help reduce baseline drift (0.1 to 0.6 Hz to 20 to 50 Hz), with signal gain at 50 to 100 mV/cm.36
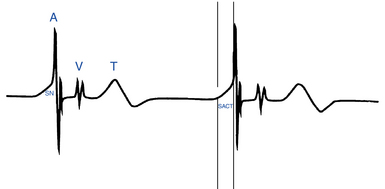
SACT is measured as the interval between the pacemaker prepotential on the local electrogram and the onset of the rapid atrial deflection (Fig. 8-8). When SACT is normal, a smooth upstroke slope merges into the atrial electrogram. When SACT is prolonged, an increasing amount of sinus node potential becomes visible before the rapid atrial deflection. Sinoatrial block is said to occur when the entire sinus node electrogram is seen in the absence of a propagated response to the atrium.28
Strauss Technique
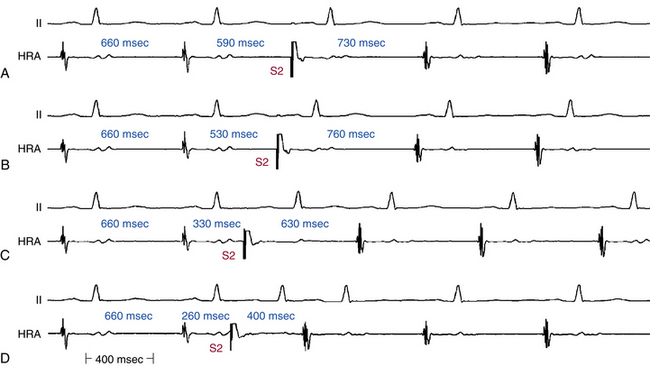
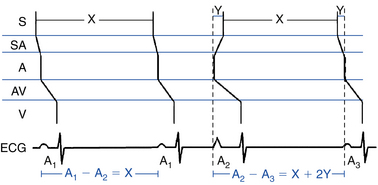
The Strauss technique uses atrial premature stimulation to assess SACT. Baseline sinus beats are designated A1. Progressively premature atrial extrastimuli (AESs; A2) are delivered after every eighth to tenth A1, and the timing of the recovery beat (A3) is measured.28 The Strauss method is useful as part of an overall EP study when information is also sought on conduction system refractoriness or possible dual AVN physiology or bypass tracts during sinus rhythm. Four zones of response of the sinus node to AES have been identified. SACT can be measured only in the zone of reset (Fig. 8-9).29,30,35
1 Zone I: Zone of Collision, Zone of Interference, Nonreset Zone
This zone is defined by the range of A1-A2 intervals at which the A2-A3 interval is fully compensatory (see Fig. 8-9). Very long A1-A2 intervals (with A2 falling in the last 20% to 30% of the sinus CL) generally result in collision of the AES (A2) with the spontaneous sinus impulse (A1). The sinus pacemaker and the timing of the subsequent sinus beat (A3) are therefore unaffected by A2, and a complete compensatory pause occurs—that is, A1-A3 = 2 × (A1-A1).
2 Zone II: Zone of Reset
The range of A1-A2 intervals at which reset of the sinus pacemaker occurs, resulting in a less than compensatory pause, defines the zone of reset (see Fig. 8-9). Shorter A1-A2 intervals result in penetration of the sinus node with resetting so that the resulting pause is less than compensatory—A1-A3 < 2 × (A1-A1)—but without changing sinus node automaticity. This zone typically occupies a long duration (40% to 50% of the sinus CL). In most patients, the A2-A3 interval remains constant throughout zone II, thus producing a plateau in the curve because A2 penetrates and resets the sinus node, but it does so without changing the sinus pacemaker automaticity. Hence, the A2-A3 interval should equal the spontaneous sinus CL (A1-A1) plus the time it takes the AES (A2) to enter and exit the sinus node. The difference between the A2-A3 and A1-A1 intervals therefore has been taken as an estimate of total SACT (Fig. 8-10).36
Conventionally, it is assumed that conduction times into and out of the sinus node are equal (i.e., SACT = [A2-A3 − A1-A1]/2). Data, however, suggest that conduction time into the sinus node is shorter than that out of the sinus node (see Fig. 8-10). The Strauss method for assessment of SACT can be affected by the site of stimulation; the farther the site of stimulation is from the sinus node, the greater the overestimation of SACT will be (because conduction through more intervening atrial and perinodal tissue will be incorporated in the measurement). The value of SACT can also be affected by the prematurity of the AES (A2); the more premature is an A2, the more likely it will be to encroach on perinodal or atrial refractoriness and slow conduction into the sinus node. In addition, an early AES commonly causes pacemaker shift to a peripheral latent pacemaker, which can exit the atrium earlier because of its proximity to the tissue, thus shortening conduction time out of the sinus node.28,29,36
Despite those limitations, for practical purposes, the Strauss method is a reasonable estimate of functional SACT, provided that stimulation is performed as closely as possible to the sinus node and the measurement is taken when a true plateau is present in zone II.28 SACT appears to be independent of the spontaneous sinus CL. However, marked sinus arrhythmia invalidates the calculation of SACT because it is impossible to know whether the return cycle is a result of spontaneous oscillation or is a result of the AES. To eliminate the effects of sinus arrhythmia, multiple tests need to be performed at each coupling interval. Alternatively, atrial pacing drive at a rate just faster than the sinus rate is used instead of delivery of AES during NSR (Narula method; see later). However, this latter method can result in depression of sinus node automaticity, pacemaker shifts, sinus entrance block, sinus acceleration (if the drive pacing CL is within 50 milliseconds of sinus CL), and shortening of sinus action potential and can lead to an earlier onset of phase IV; each of these actions can yield misleading results.35,36
In an occasional patient, in response to progressively premature AESs, the A2-A3 interval prolongs either continuously or after a brief plateau (in both cases, the pause remains less than compensatory). This progressive prolongation of the A2-A3 interval during zone II can be caused by suppression of sinus node automaticity, a shift to a slower latent pacemaker, or an increase in conduction time into the sinus node because of encroachment of A2 on perinodal tissue refractoriness. Thus, it is recommended to use the first third of zone II to measure SACT because it is less likely to introduce such errors.28 Analysis of the A3-A4 interval may provide insights into changes in sinus node automaticity or pacemaker shift. If the A3-A4 interval is longer than the A1-A1 interval, depression of sinus node automaticity is suggested. Therefore, the calculated SACT overestimates the true SACT, and correction of SACT is necessary (in which case the A3-A4 interval is used as the basic sinus CL to which the A2-A3 interval is compared).28
3 Zone III: Zone of Interpolation
This zone is defined as the range of A1-A2 intervals at which the A2-A3 interval is less than the A1-A1 interval and the A1-A3 interval is less than twice the A1-A1 interval (see Fig. 8-9).28 The A1-A2 coupling intervals at which incomplete interpolation is first observed define the relative refractory period of the perinodal tissue. Some investigators refer to this as the sinus node refractory period. In this case, A3 represents delay of A1 exiting the sinus node, which has not been affected.28 The A1-A2 coupling interval at which complete interpolation is observed probably defines the effective refractory period of the most peripheral of the perinodal tissue because the sinus impulse does not encounter refractory tissue on its exit from the sinus node. In this case, (A1-A2) + (A2-A3) = A1-A1, and sinus node entrance block is said to exist.
4 Zone IV: Zone of Reentry
This zone is defined as the range of A1-A2 intervals at which the A2-A3 interval is less than the A1-A1 interval and (A1-A2) + (A2-A3) is less than A1-A1, and the atrial activation sequence and P wave morphology are identical to sinus beats. The incidence of single beats of sinus node reentry is approximately 11% in the normal population.29,30,35
Narula Method
The Narula method for measuring SACT is simpler than the Strauss technique. Instead of atrial premature stimulation, atrial pacing at a rate slightly faster (10 beats/min or more) than the sinus rate is used as A2. It is assumed that such atrial pacing will depolarize the sinus node without significant overdrive suppression. The SACT is then calculated using the same formula as for the Strauss method. The Narula method is the quickest and easiest to perform, but it gives only SACT and does not provide information about the AV conduction system.29,30,35
Sinoatrial Conduction Time in Patients with Sinus Node Dysfunction
The normal SACT is 45 to 125 milliseconds. There is a good correlation between direct and indirect measurements of SACT in a patient with or without SND. However, SACT is an insensitive indicator of SND, especially in patients with isolated sinus bradycardia. SACT is prolonged in only 40% of patients with SND, and more frequently (78%) in patients with sinoatrial exit block or bradycardia-tachycardia syndrome, or both.28 In patients with sinus pauses, corrected SNRT is more commonly abnormal than SACT (80% versus 53%). SACT appears to be directly related to the baseline sinus CL, and the sinus node refractory period is directly related to the drive CL.36
Effects of Drugs
Autonomic Blockade (Intrinsic Heart Rate)
Autonomic blockade is the most commonly used pharmacological intervention for evaluation of SND, and it is used to determine the intrinsic heart rate (i.e., the rate independent of autonomic influences). Autonomic blockade is accomplished by administering atropine, 0.04 mg/kg, and propranolol, 0.2 mg/kg (or atenolol, 0.22 mg/kg). The resulting intrinsic heart rate represents sinus node rate without autonomic influences. The normal intrinsic heart rate is age-dependent and can be calculated using the following equation: intrinsic heart rate (beats/min) = 118.1 − (0.57 × age); normal values are ±14% for age younger than 45 years and ±18% for age older than 45 years. A low intrinsic heart rate is consistent with intrinsic SND. A normal intrinsic heart rate in a patient with known SND suggests extrinsic SND caused by abnormal autonomic regulation. Autonomic blockade with atropine and propranolol also results in shortening of corrected SNRT, as well as sinus CL and SACT.36
Atropine
The normal sinus node responses to atropine are an acceleration of heart rate to more than 90 beats/min and an increase over the baseline rate by 20% to 50%. Atropine-induced sinus rate acceleration is usually blunted in patients with intrinsic SND. Failure to increase the sinus rate to more than the predicted intrinsic heart rate following 0.04 mg/kg of atropine is diagnostic of impaired sinus node automaticity. Atropine (1 to 3 mg) markedly shortens SNRT and, in most cases, corrected SNRT. Atropine also abolishes the marked oscillations frequently observed following cessation of rapid pacing. Atropine occasionally results in the appearance of a junctional escape rhythm on cessation of pacing before sinus escape beats in normal subjects (especially in young men with borderline slow sinus rate) and, more commonly, in patients with SND. When this occurs, the junctional escape rhythm is usually transient (lasting only a few beats). Persistence of junctional rhythm and failure of the sinus rate to increase are indicative of SND.28 Atropine, with or without propranolol, shortens SACT (unrelated to its effects on the sinus rate).35,36
Propranolol
Propranolol (0.1 mg/kg) produces a 12% to 22.5% increase in sinus CL in normal subjects. Patients with SND have a similar chronotropic response to propranolol, a finding suggesting that sympathetic tone or responsiveness, or both, is intact in most patients with SND. Propranolol increases SNRT by 160% in approximately 40% of patients with SND and increases SACT in most patients with SND. The mechanism of these effects is unclear. Effects are minimal in normal subjects.28
Isoproterenol
Isoproterenol (1 to 3 mg/min) produces sinus acceleration of at least 25% in normal subjects. An impaired response to isoproterenol correlates well with a blunted chronotropic response to exercise observed in some patients with SND.28
Digoxin
Digoxin shortens SNRT or corrected SNRT, or both, in some patients with clinical SND, probably because of increased perinodal tissue refractoriness with consequent sinus node entrance block.28
Verapamil and Diltiazem
Verapamil and diltiazem have minimal effects on SNRT and SACT in normal persons. Effects in patients with SND have not been studied, but worsening of the SND is expected.28
Antiarrhythmic Agents
Procainamide, quinidine, mexiletine, dronedarone, and amiodarone can adversely affect sinus node function in patients with SND. Severe sinus bradycardia and sinus pauses are the most common problems encountered. Amiodarone is the worst offender and has even caused severe SND in patients without prior evidence of SND. In general, other drugs have minimal effects on sinus node function in normal persons.29,36
Principles of Management
Once all reversible causes are excluded or treated, correlation of symptoms with ECG evidence of SND is an essential part of the management strategy. Because of the episodic nature of symptomatic arrhythmias, ambulatory monitoring is often required. For the patient with asymptomatic bradycardia or sinus pauses, the long-term prognosis is generally benign, and no treatment is necessary. For symptomatic patients with SND, pacing is the mainstay of treatment (Table 8-1). SND is currently the most common reported diagnosis for pacemaker implantation, and it accounts for 40% to 60% of new pacemaker implants. For symptomatic patients with tachycardia-bradycardia syndrome, pacing may be required to prevent symptomatic bradycardia and allow the use of drug therapy for control of the tachycardia (see Fig. 8-4). These patients are at increased risk for thromboembolism, and the issue of long-term anticoagulation for stroke prevention should be addressed.25,26,29,30,37–39
TABLE 8-1 Guidelines for Permanent Pacing in Sinus Node Dysfunction
| Class I |
• Sinus node dysfunction with heart rate <40 beats/min when a clear association between significant symptoms consistent with bradycardia and the actual presence of bradycardia has not been documented
• Syncope of unexplained origin when clinically significant abnormalities of sinus node function are discovered or provoked in electrophysiological studies
From Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices). Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117:e350-e408, 2008.
Once the decision to pace is made, choosing the optimal pacemaker prescription is essential. For those patients with SND who have normal AV conduction, a single-chamber atrial pacemaker is a reasonable consideration, although in the United States, a dual-chamber pacemaker is usually implanted, largely because of the 1% to 3% annual risk of developing AV block.40 The use of rate-adaptive pacing is important for patients with chronotropic incompetence. For patients with intermittent atrial tachyarrhythmias, atrial pacing has been shown to decrease the incidence of AF and thromboembolism greatly, whereas patients who have only ventricular pacing have not seen a similar benefit.24 Current pacemakers used to treat the tachycardia-bradycardia syndrome follow a special algorithm to switch from a DDD or DDDR mode of operation to a VVI, VVIR, DDI, or DDIR mode on sensing an atrial tachyarrhythmia, and back again to DDD or DDDR mode when a normal atrial rate is sensed. For patients with permanent AF, implantation of a single-chamber ventricular pacemaker is appropriate.3,25,29,30
1. Rubart M., Zipes D.P. Genesis of cardiac arrhythmias: electrophysiological considerations. In: Zipes D.P., Libby P., Bonow R., Braunwald E., editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. ed 7. Philadelphia: Saunders; 2004:653-688.
2. Dobrzynski H., Boyett M.R., Anderson R.H. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921-1932.
3. Line D., Callans D. Sinus rhythm abnormalities. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 4. Philadelphia: Saunders; 2004:479-484.
4. Sanchez-Quintana D., Cabrera J.A., Farre J., et al. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart. 2005;91:189-194.
5. Lau D., Roberts-Thomson K., Sanders P. Sinus node revisited. Curr Opin Cardiol. 2010 November 22. [Epub ahead of print]
6. Basso C., Ho S.Y., Thiene G. Anatomical and histopathological characteristics of the conductive tissues of the heart. In: Gussak I., Antzelevitch C., editors. Electrical diseases of the heart: genetics, mechanisms, treatment, prevention. London: Springer; 2008:37-51.
7. Boullin J., Morgan J.M. The development of cardiac rhythm. Heart. 2005;91:874-875.
8. Stiles M.K., Brooks A.G., Roberts-Thomson K.C., et al. High-density mapping of the sinus node in humans: role of preferential pathways and the effect of remodeling. J Cardiovasc Electrophysiol. 2010;21:532-539.
9. Sanders P., Kistler P.M., Morton J.B., et al. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897-903.
10. Kistler P.M., Sanders P., Fynn S.P., et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109-116.
11. Walsh E.P. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007;115:3224-3234.
12. Yeh Y.H., Burstein B., Qi X.Y., et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576-1585.
13. Joung B., Lin S.F., Chen Z., et al. Mechanisms of sinoatrial node dysfunction in a canine model of pacing-induced atrial fibrillation. Heart Rhythm. 2010;7:88-95.
14. Sweeney M.O., Bank A.J., Nsah E., et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000-1008.
15. Mohler P.J., Anderson M.E. New insights into genetic causes of sinus node disease and atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:516-518.
16. Lei M., Huang C.L., Zhang Y. Genetic Na+ channelopathies and sinus node dysfunction. Prog Biophys Mol Biol. 2008;98:171-178.
17. Lei M., Zhang H., Grace A.A., Huang C.L. SCN5A and sinoatrial node pacemaker function. Cardiovasc Res. 2007;74:356-365.
18. Nof E., Glikson M., Antzelevitch C. Genetics and sinus node dysfunction. J Atr Fibrillation. 2009;1:328-336.
19. Wolbrette D., Naccarelli G. Bradycardias: sinus nodal dysfunction and atrioventricular conduction disturbances. In: Topol E., editor. Textbook of cardiovascular medicine. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2007:1038-1049.
20. Schuchert A., Wagner S.M., Frost G., Meinertz T. Moderate exercise induces different autonomic modulations of sinus and AV node. Pacing Clin Electrophysiol. 2005;28:196-199.
21. Sakai Y., Imai S., Sato Y., et al. Clinical and electrophysiological characteristics of binodal disease. Circ J. 2006;70:1580-1584.
22. Brubaker P.H., Kitzman D.W. Prevalence and management of chronotropic incompetence in heart failure. Curr Cardiol Rep. 2007;9:229-235.
23. Kitzman D.W. Exercise intolerance. Prog Cardiovasc Dis. 2005;47:367-379.
24. Ellenbogen K.A. Pacing therapy for prevention of atrial fibrillation. Heart Rhythm. 2007;4(Suppl):S84-S87.
25. Sweeney M. Sinus node dysfunction. In: Zipes D., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 4. Philadelphia: Saunders; 2004:879-883.
26. Mangrum J.M., DiMarco J.P. The evaluation and management of bradycardia. N Engl J Med. 2000;342:703-709.
27. Maggi R., Menozzi C., Brignole M., et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace. 2007;9:563-567.
28. Josephson M.E. Sinus node function. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:69-92.
29. Miller J.M., Zipes D.P. Diagnosis of cardiac arrhythmias. In: Zipes D., Libby P., Bonow R., Braunwald E., editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. ed 7. Philadelphia: Saunders; 2004:697-712.
30. Benditt D.G., Sakaguchi S., Lurie K., Lu F. Sinus node dysfunction. In: Willerson J., Cohn J., Wellens H.J., Holmes D., editors. Cardiovascular medicine. New York: Springer; 2007:1925-1941.
31. Hocini M., Sanders P., Deisenhofer I., et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003;108:1172-1175.
32. Hadian D., Zipes D.P., Olgin J.E., Miller J.M. Short-term rapid atrial pacing produces electrical remodeling of sinus node function in humans. J Cardiovasc Electrophysiol. 2002;13:584-586.
33. Disertori M., Marini M., Cristoforetti A., et al. Enormous bi-atrial enlargement in a persistent idiopathic atrial standstill. Eur Heart J. 2005;26:2276.
34. Fazelifar A.F., Arya A., Haghjoo M., Sadr-Ameli M.A. Familial atrial standstill in association with dilated cardiomyopathy. Pacing Clin Electrophysiol. 2005;28:1005-1008.
35. Krumerman A., Fisher J.D. Electrophysiological testing. In: Topol E., editor. Textbook of cardiovascular medicine. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2007:1012-1037.
36. Masood A. Techniques of electrophysiological evaluation. In: Fuster V., Alexander R., O’Rourke R., editors. Hurst’s the heart. ed 11. Columbus, Ohio: McGraw-Hill; 2011:935-948.
37. Alboni P., Gianfranchi L., Brignole M. Treatment of persistent sinus bradycardia with intermittent symptoms: are guidelines clear? Europace. 2009;11:562-564.
38. Vardas P.E., Auricchio A., Blanc J.J., et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959-998.
39. Epstein A.E., DiMarco J.P., Ellenbogen K.A., et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices). Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350-e408.
40. Lamas G.A., Lee K.L., Sweeney M.O., et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854-1862.