Chapter 3 Shoulder Ultrasound
![]() Additional videos for this topic are available online at www.expertconsult.com.
Additional videos for this topic are available online at www.expertconsult.com.
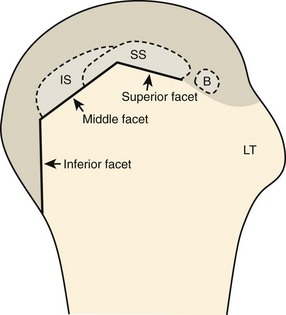
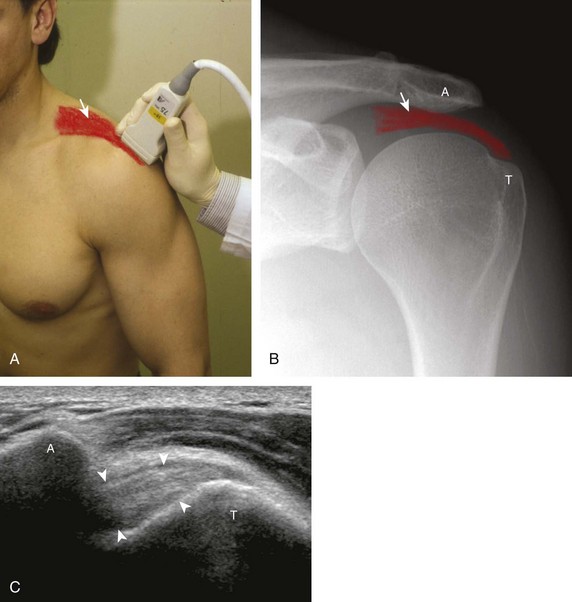
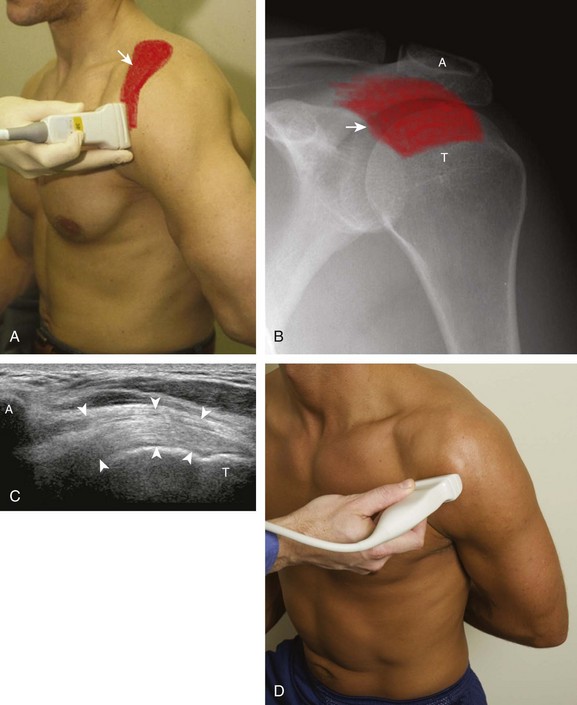
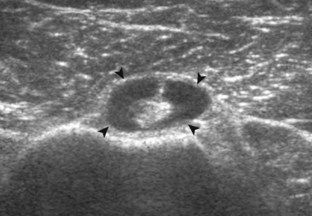
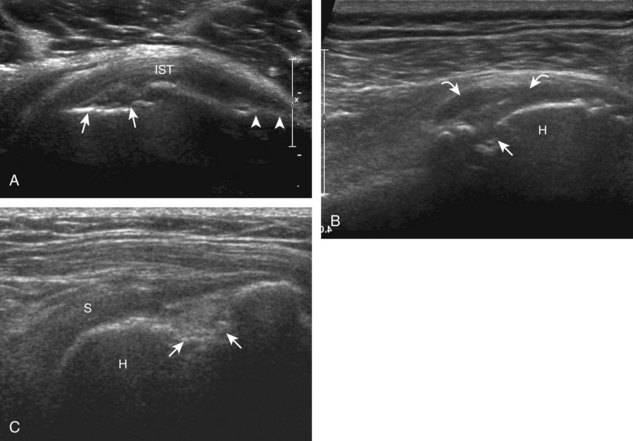
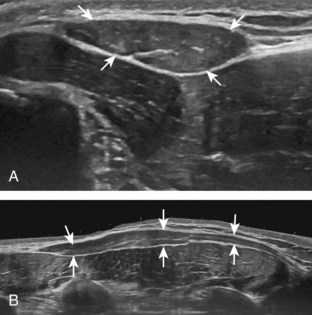
The rotator cuff is composed of four tendons (Fig. 3-1). Anteriorly, the subscapularis with its tendons converges onto the lesser tuberosity. Superiorly, the supraspinatus inserts on the superior aspect of the greater tuberosity; its footprint or attachment averages 2.25 cm anterior to posterior, which covers the superior facet and the anterior portion of the middle facet of the greater tuberosity (Fig. 3-2).1,2 Posterior to the scapula and inferior to the scapular spine, the infraspinatus tendon inserts on the middle facet of the greater tuberosity, and the smaller and more inferior teres minor tendon inserts on the inferior facet of the greater tuberosity. Between the lesser and greater tuberosities anteriorly is the bicipital groove, which contains the long head of the biceps brachii tendon; although not a part of the rotator cuff, its proximal intra-articular portion courses through a space between the supraspinatus and subscapularis tendons, called the rotator interval. At this location, the intra-articular portion of the biceps tendon is stabilized by the biceps reflection pulley made up of the superior glenohumeral ligament and the coracohumeral ligament, which are essentially thickened reflections of the joint capsule. The glenohumeral joint normally communicates with the biceps brachii long head tendon sheath. Several joint recesses also exist and include the axillary recess, which extends inferiorly, and the subscapular recess, which extends medially through the rotator interval to be located inferior to the coracoid process at the superior aspect of the subscapularis tendon in an inverted U shape. The subacromial-subdeltoid bursa is located between the rotator cuff and the overlying deltoid muscle and acromion (see Fig. 3-1). The glenoid labrum represents a rim of fibrocartilage at the periphery of the glenoid.

FIGURE 3-1 Shoulder anatomy.
(A and B, Image courtesy of Carolyn Nowak, Ann Arbor, Michigan. C, From Drake R, Vogl W, Mitchell A: Gray’s anatomy for students, Philadelphia, 2005, Churchill-Livingstone.)
Ultrasound Examination Technique
Table 3-1 is a shoulder ultrasound examination checklist. Examples of diagnostic shoulder ultrasound reports are available online at www.expertconsult.com (see eBox 3-1 and 3-2).
| Step | Structures/Pathologic Features of Interest |
|---|---|
| 1 | Biceps brachii long head |
| 2 | Subscapularis, biceps tendon dislocation |
| 3 | Supraspinatus, infraspinatus |
| 4 | Acromioclavicular joint, subacromial-subdeltoid bursa, dynamic evaluation |
| 5 | Posterior glenohumeral joint, labrum, teres minor, infraspinatus |
eBox 3-1 Sample Diagnostic Shoulder Ultrasound Report
Normal
Examination: Ultrasound of the Shoulder
History: Shoulder pain, evaluate for rotator cuff abnormality
Findings: No evidence of joint effusion. The biceps brachii long head tendon is normal without tendinosis, tear, tenosynovitis, or subluxation/dislocation. The supraspinatus, infraspinatus, subscapularis, and teres minor tendons are also normal. No subacromial-subdeltoid bursal abnormality and no sonographic evidence for subacromial impingement with dynamic maneuvers. The posterior labrum is unremarkable. Additional focused evaluation at site of maximal symptoms was unrevealing.
Impression: Unremarkable ultrasound examination of the shoulder. No rotator cuff abnormality.
eBox 3-2 Sample Diagnostic Shoulder Ultrasound Report
Abnormal
Examination: Ultrasound of the Shoulder
History: Shoulder pain, evaluate for rotator cuff abnormality
Findings: There is a focal anechoic tear of the anterior, distal aspect of the supraspinatus tendon measuring 1 cm short axis by 1.5 cm long axis. The anterior margin of the tear is adjacent to the rotator interval. There is no involvement of the subscapularis, infraspinatus, or rotator interval. A moderate amount of infraspinatus and supraspinatus fatty degeneration is present. There is a small joint effusion distending the biceps brachii tendon sheath and moderate distention of the subacromial-subdeltoid bursa. No biceps brachii long head tendon abnormality and no subluxation/dislocation. Mild osteoarthritis of the acromioclavicular joint. Additional focused evaluation at site of maximal symptoms was unrevealing.
Impression: Focal or incomplete full-thickness tear of the supraspinatus tendon with infraspinatus and supraspinatus muscle atrophy.
General Comments
For ultrasound examination of the shoulder, the patient sits on a stool with low back support but without wheels, and the sonographer sits on a stool with wheels to allow easy maneuvering. For examination of the patient’s left shoulder, the patient faces the ultrasound machine, with the sonographer sitting somewhat between the patient and ultrasound machine if the sonographer is right-handed (Fig. 3-3A, online). For examination of the patient’s right shoulder, the patient turns toward the left and faces the sonographer (see Fig. 3-3B, online). The transducer frequency for the shoulder is generally at least 10 MHz, although one may need to use a lower frequency in evaluation of the deeper structures such as the posterior glenoid labrum or if the patient has a large body habitus. It is important to follow a sequence of steps to ensure a complete and thorough evaluation.3 Although a targeted approach is often used in other peripheral joints, this is not recommended with the shoulder because pain is often diffuse or referred. It is recommended, however, that every sonographic evaluation be followed by targeted evaluation over any area with point tenderness or focal symptoms.
Position No. 1: Long Head of Biceps Brachii Tendon
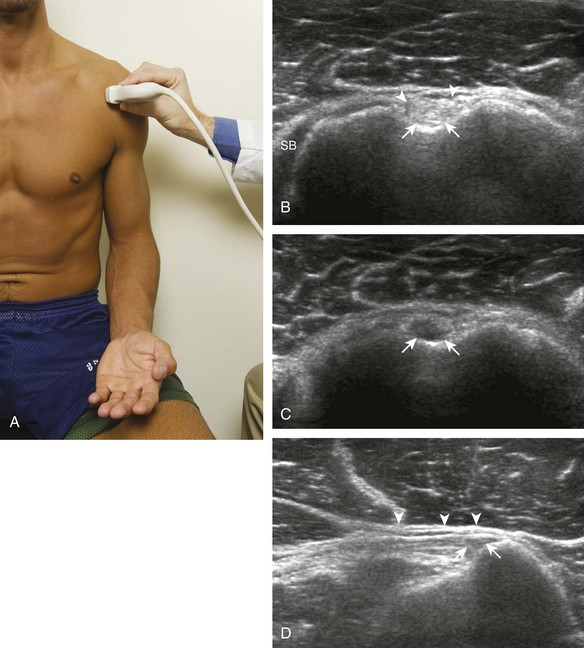
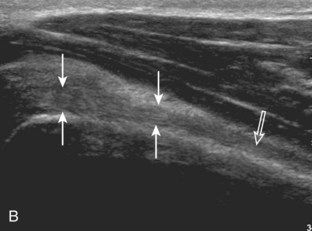
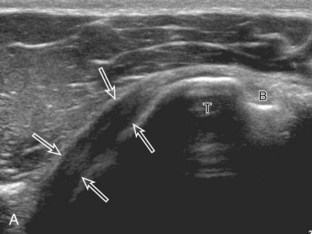
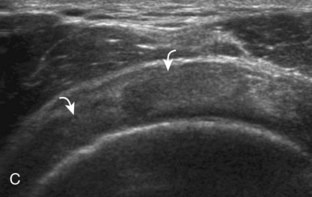
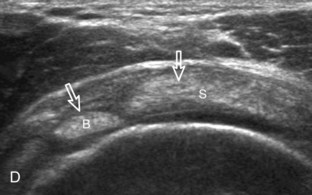
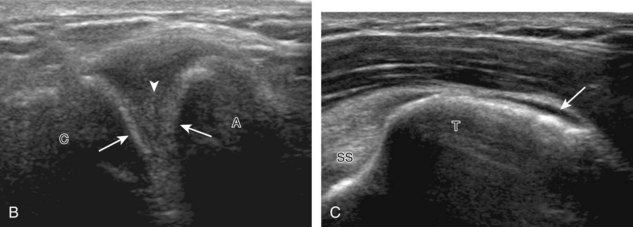
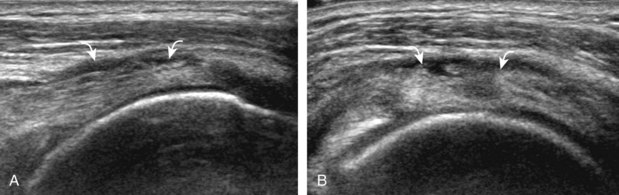
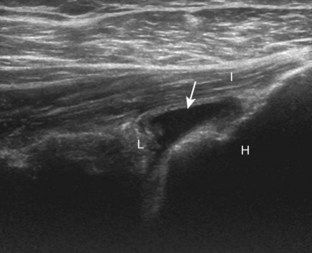
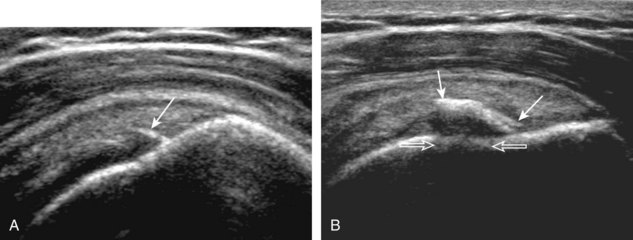
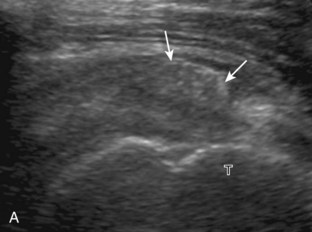
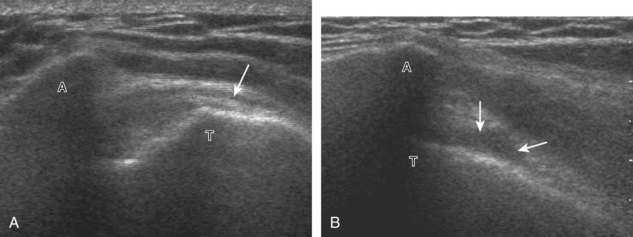
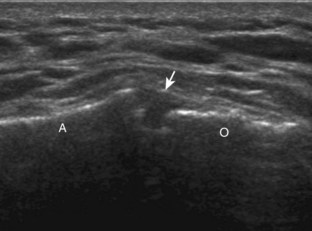
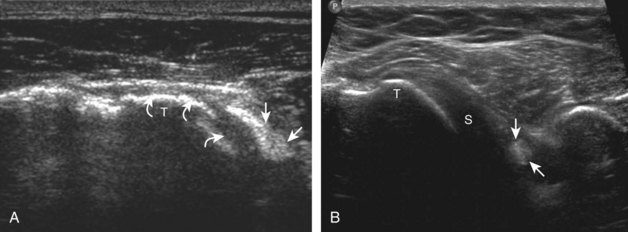
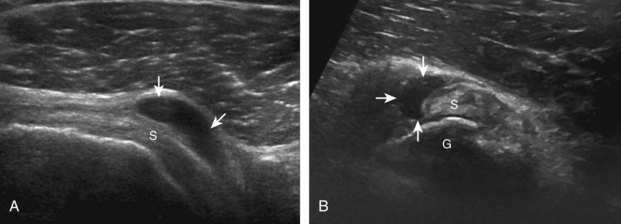
The patient places the hand palm up in supination on his or her leg (Fig. 3-4A). This position rotates the bicipital groove anteriorly, an important bone landmark. The transducer is placed in the transverse plane on the patient, and the long head of the biceps brachii tendon is seen within the bicipital groove in short axis (see Fig. 3-4) (Video 3-1). ![]() Because the distal biceps tendon courses deep, tendon obliquity to the transducer sound beam commonly creates anisotropy and an artifactual hypoechoic appearance of the normal tendon (see Fig. 3-4C). This is corrected by toggling the transducer inferiorly to aim the sound beam superiorly (Video 3-2).
Because the distal biceps tendon courses deep, tendon obliquity to the transducer sound beam commonly creates anisotropy and an artifactual hypoechoic appearance of the normal tendon (see Fig. 3-4C). This is corrected by toggling the transducer inferiorly to aim the sound beam superiorly (Video 3-2).![]() A hyperechoic and well-defined humeral cortex in the floor of the bicipital groove indicates that the sound beam is perpendicular to the overlying biceps tendon. The biceps brachii tendon is evaluated in short axis from proximal to distal. It is important to evaluate the most proximal aspect where the biceps tendon courses over the humeral head because this is a common site for tendon pathology.4 Evaluation is also continued inferiorly to the level of the pectoralis tendon (see Fig. 3-4D) to assess the pectoralis and biceps because complete biceps brachii long head tendon tears may retract to this level. The transducer is then turned 90 degrees to visualize the tendon in long axis from the humeral head to the pectoralis tendon (Fig. 3-5A) (Video 3-3).
A hyperechoic and well-defined humeral cortex in the floor of the bicipital groove indicates that the sound beam is perpendicular to the overlying biceps tendon. The biceps brachii tendon is evaluated in short axis from proximal to distal. It is important to evaluate the most proximal aspect where the biceps tendon courses over the humeral head because this is a common site for tendon pathology.4 Evaluation is also continued inferiorly to the level of the pectoralis tendon (see Fig. 3-4D) to assess the pectoralis and biceps because complete biceps brachii long head tendon tears may retract to this level. The transducer is then turned 90 degrees to visualize the tendon in long axis from the humeral head to the pectoralis tendon (Fig. 3-5A) (Video 3-3).![]() Asymmetrical pressure on the distal aspect of the transducer (or heel-toe maneuver) is typically needed to bring the biceps tendon fibers perpendicular to the transducer sound beam to eliminate anisotropy (see Fig. 3-5B and C) (Video 3-4).
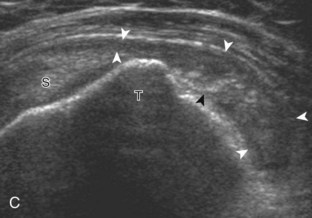
Asymmetrical pressure on the distal aspect of the transducer (or heel-toe maneuver) is typically needed to bring the biceps tendon fibers perpendicular to the transducer sound beam to eliminate anisotropy (see Fig. 3-5B and C) (Video 3-4).![]() An additional method to visualize the biceps tendon in long axis is to identify the characteristic pyramid shape of the lesser tuberosity (see Fig. 3-5D); movement of the transducer laterally from this point will visualize the bicipital groove and biceps long head tendon (Video 3-5).
An additional method to visualize the biceps tendon in long axis is to identify the characteristic pyramid shape of the lesser tuberosity (see Fig. 3-5D); movement of the transducer laterally from this point will visualize the bicipital groove and biceps long head tendon (Video 3-5).![]()
Position No. 2: Subscapularis and Biceps Tendon Dislocation
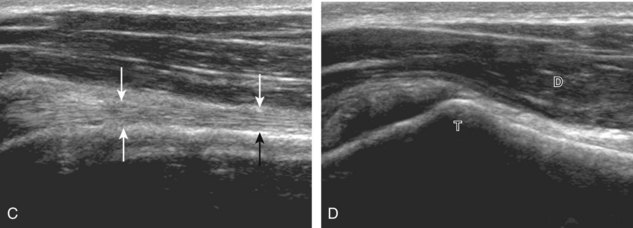
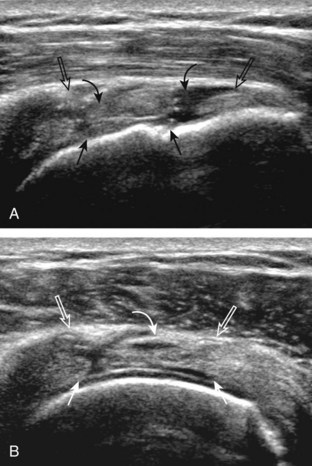
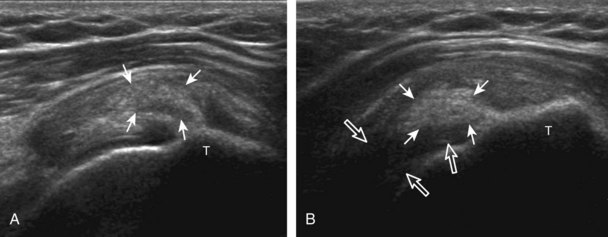
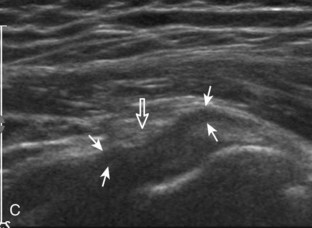
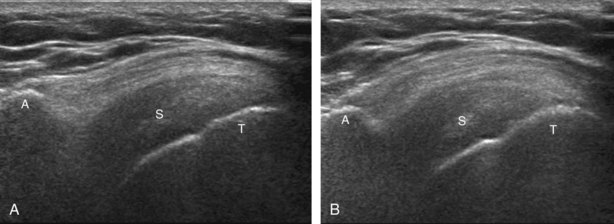
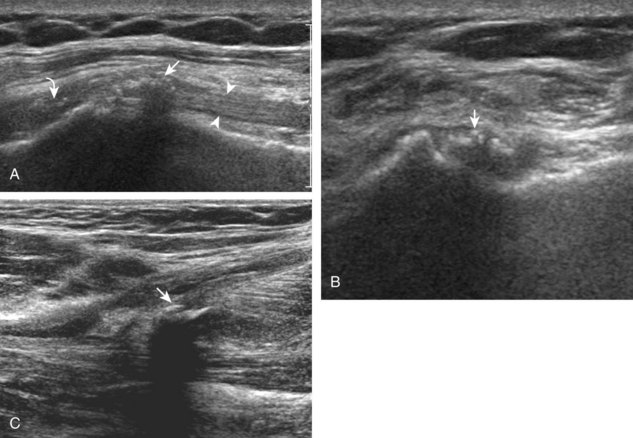
The transducer is placed in the transverse plane, as before, to visualize the bicipital groove and to center the field of view over the lesser tuberosity (see Fig. 3-4A). In this neutral position, although the subscapularis tendon can be seen in long axis, there is significant anisotropy (Fig. 3-6A). Ask the patient to rotate the shoulder externally (see Fig. 3-6B), and this will bring the subscapularis tendon fibers into view perpendicular to the transducer sound beam and will eliminate anisotropy (see Fig. 3-6C) (Video 3-6).![]() It is important to move the transducer superiorly and inferiorly over the lesser tuberosity to ensure complete evaluation of the subscapularis tendon. The transducer should also be moved laterally over the bicipital groove to evaluate for potential biceps brachii tendon subluxation or dislocation, which may be present only in external rotation (see Biceps Tendon, Subluxation and Dislocation).5 Center the transducer over the distal subscapularis tendon again, rotate the transducer 90 degrees, and assess the subscapularis tendon in short axis (Fig. 3-7A and B). In this view, it is common to see vertical hypoechoic striations of muscle or interfaces between the several tendon bundles, especially when spatial compound sonography is not used (see Fig. 3-7C) (Video 3-7).
It is important to move the transducer superiorly and inferiorly over the lesser tuberosity to ensure complete evaluation of the subscapularis tendon. The transducer should also be moved laterally over the bicipital groove to evaluate for potential biceps brachii tendon subluxation or dislocation, which may be present only in external rotation (see Biceps Tendon, Subluxation and Dislocation).5 Center the transducer over the distal subscapularis tendon again, rotate the transducer 90 degrees, and assess the subscapularis tendon in short axis (Fig. 3-7A and B). In this view, it is common to see vertical hypoechoic striations of muscle or interfaces between the several tendon bundles, especially when spatial compound sonography is not used (see Fig. 3-7C) (Video 3-7).![]()
Position No. 3: Supraspinatus and Infraspinatus
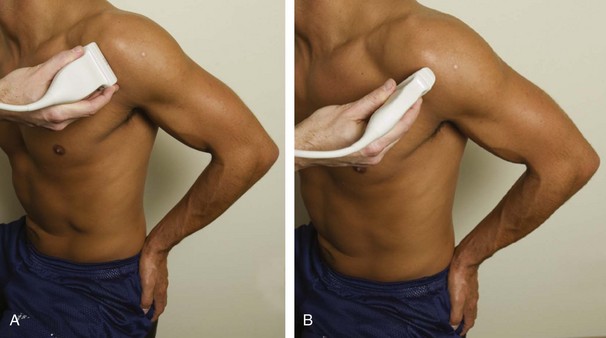
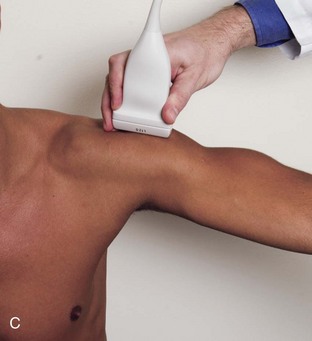
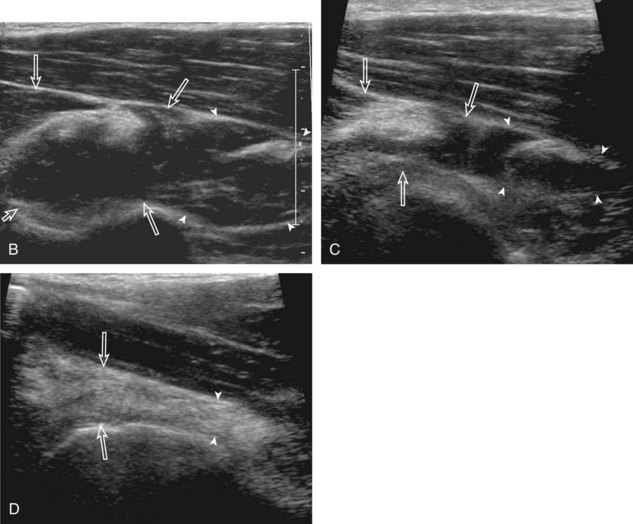
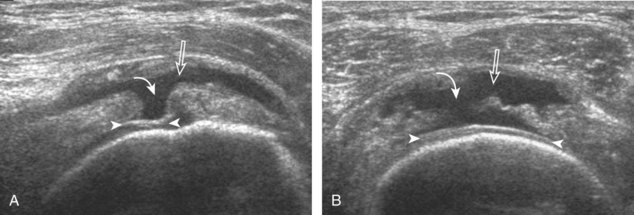
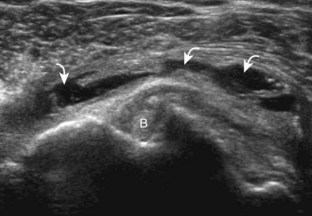
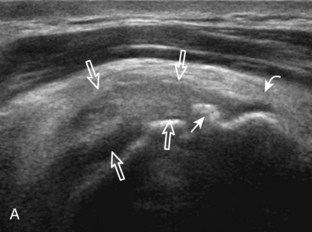
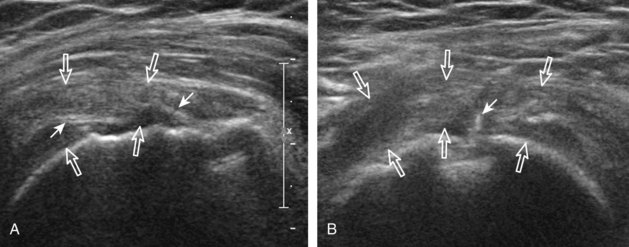
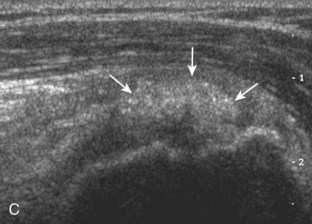
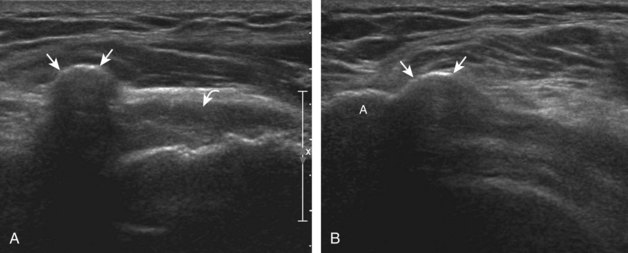
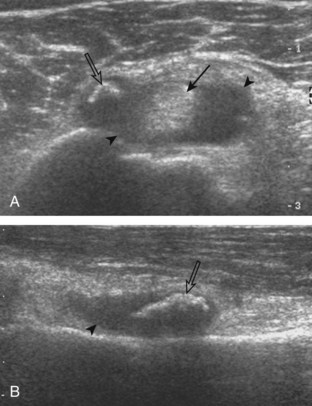
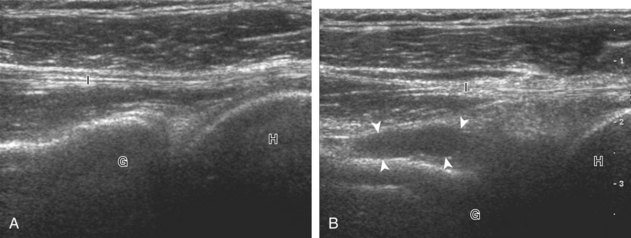
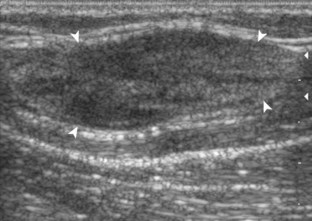
The goal when imaging the supraspinatus is to evaluate the tendon in long and short axis. This will avoid numerous diagnostic pitfalls and is an indicator that the operator has a thorough understanding of anatomy and shoulder ultrasound technique. The key to obtaining such images is to understand the anatomy of the greater tuberosity and the effects of various shoulder positioning. If one wanted to assess the supraspinatus tendon in long axis with the shoulder in neutral position, the transducer would be placed in the coronal place over the greater tuberosity; however, in this position, much of the tendon is hidden beneath the acromion, which could hide more proximal cuff tears (Fig. 3-8). One way to correct this is to ask the patient to place the back of his or her ipsilateral hand in the lower lumbar region and to keep the elbow close to the body (called the Crass position) (Fig. 3-9).6 In this position, the humerus is rotated internally such that the greater tuberosity is located anteriorly on the patient. By placing the transducer in the sagittal plane on the patient over the greater tuberosity, a long axis view of the supraspinatus tendon is demonstrated. Rotating the transducer 90 degrees (or transverse on the patient) will produce a short axis image of the supraspinatus. The Crass position is helpful when one is first learning shoulder ultrasound technique in that a long and short axis views are easily obtained; however, the significant disadvantages of this position include limited view of the rotator interval (see later) and, often, significant patient discomfort. Because of this, the modified Crass position is used (I primarily use the modified Crass position and uncommonly the Crass position for problem solving) (Fig. 3-10).7 To obtain the modified Crass position, the patient is asked to place his or her hand on the ipsilateral hip area. The elbow should be pointed posteriorly to ensure some degree of shoulder external rotation compared with the Crass position; otherwise, the rotator interval may not be visible. The greater tuberosity is now located between the locations in the neutral and Crass positions; therefore, to obtain a long axis view of the supraspinatus tendon, the transducer is placed over the greater tuberosity and pointed superior and oblique toward the patient’s ear. Usually, the axis of the transducer is parallel to the proximal biceps tendon and humeral shaft regardless of each position when imaging the supraspinatus in long axis. The transducer is then turned 90 degrees to evaluate the supraspinatus tendon in short axis.
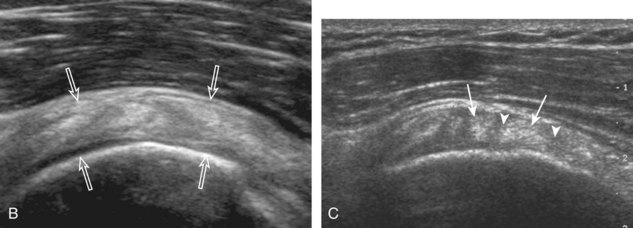
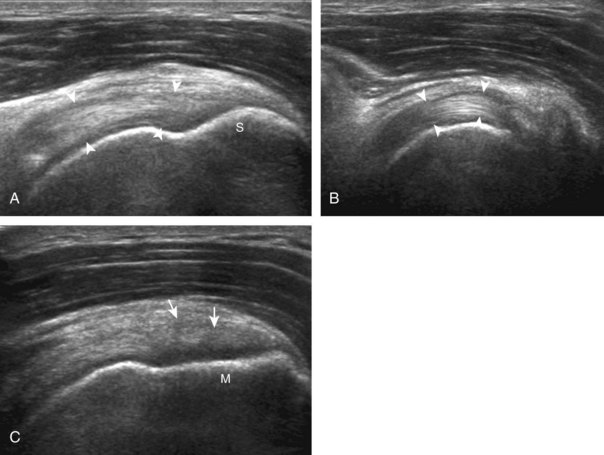
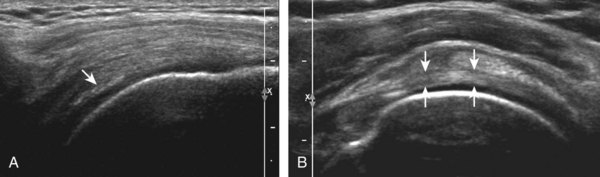
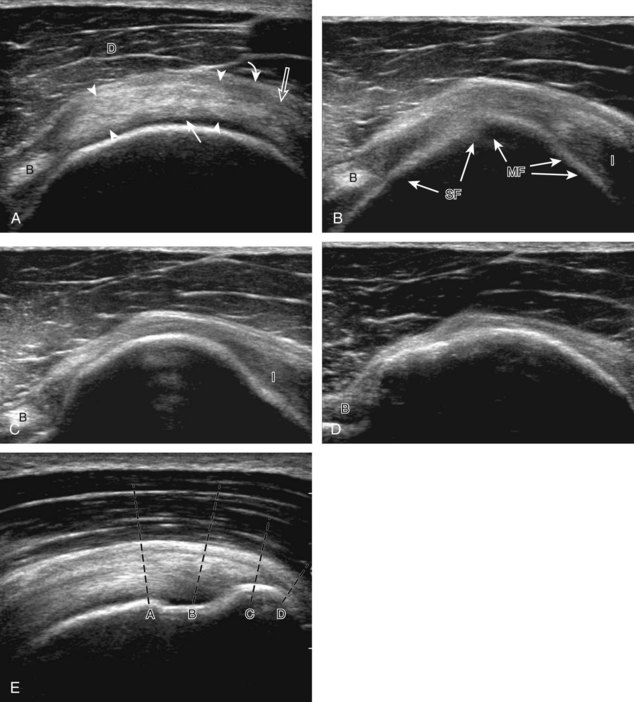
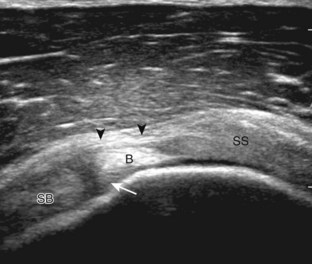
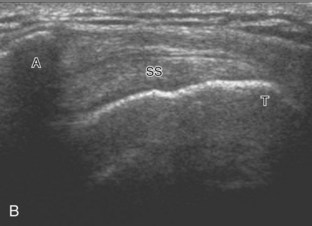
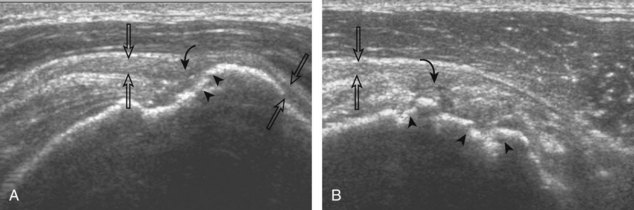
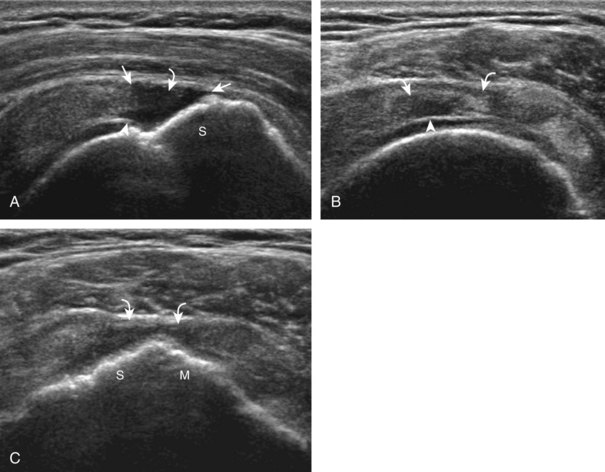
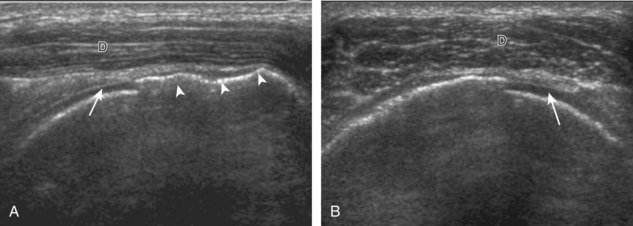
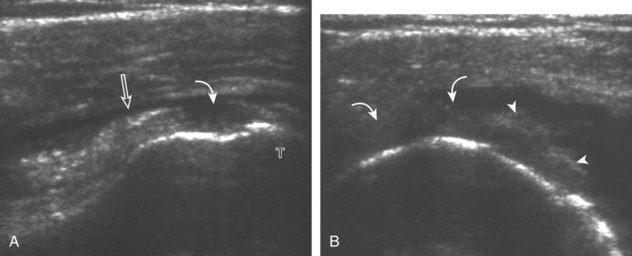
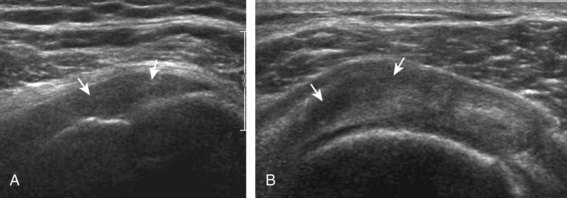
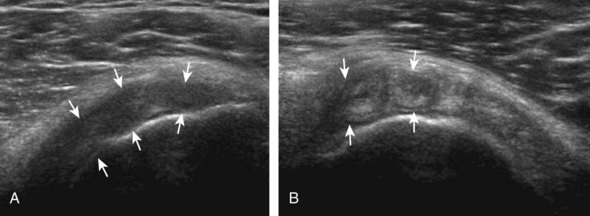
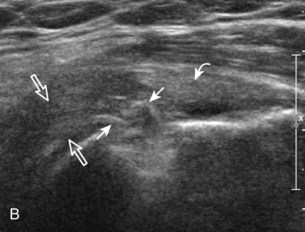
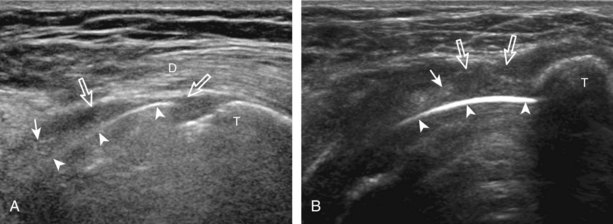
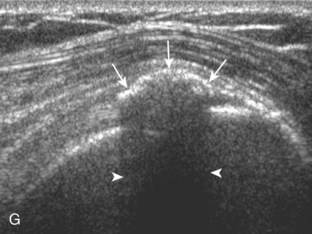
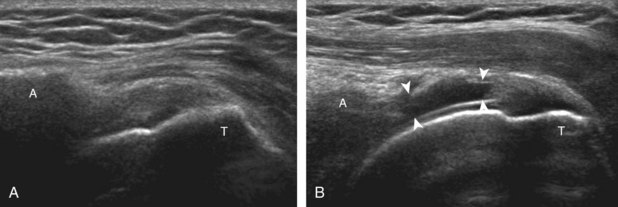
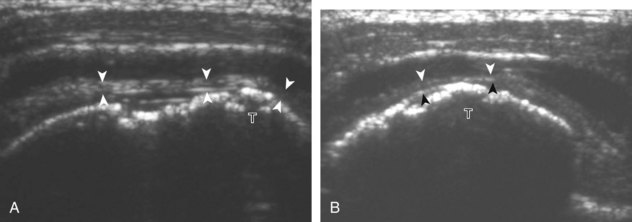
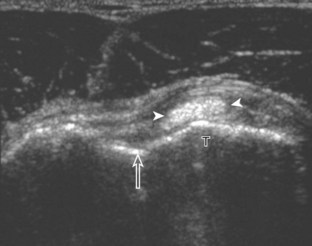
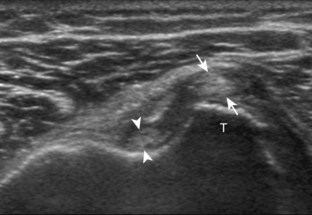
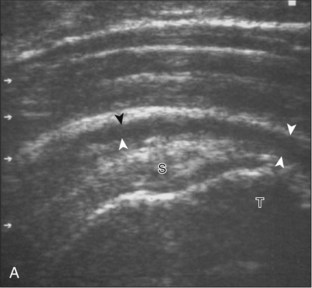
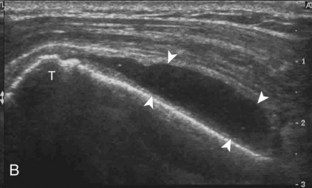
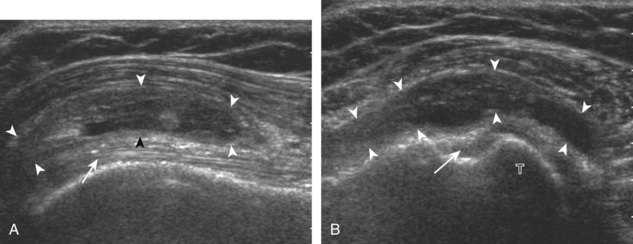
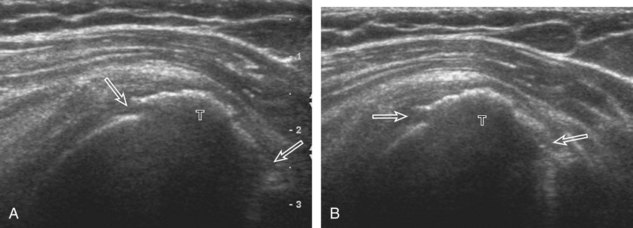
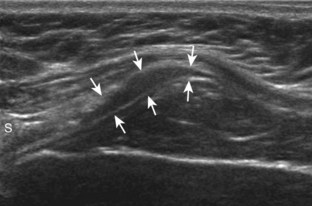
Regardless of patient positioning in the Crass or modified Crass position, supraspinatus evaluation begins with evaluation in long axis because this important view allows visualization of the three surfaces of the supraspinatus tendon. In long axis, the normal supraspinatus will appear hyperechoic and fibrillar, with a convex superior margin (Fig. 3-11) (Video 3-8).![]() The thin hypoechoic layer over the curved humeral head represents the hyaline articular cartilage. At times, a thin hypoechoic layer over the greater tuberosity, which represents the fibrocartilage transition zone between the tendon and bone at the enthesis, may be seen and should not be confused with hyaline articular cartilage over the rounded humeral head. One must be aware that the distal fibers of the tendon curve downward at the greater tuberosity near the articular surface, and the transducer orientation should be adjusted using the heel-toe maneuver to eliminate anisotropy (Fig. 3-12A and B) (Video 3-9).
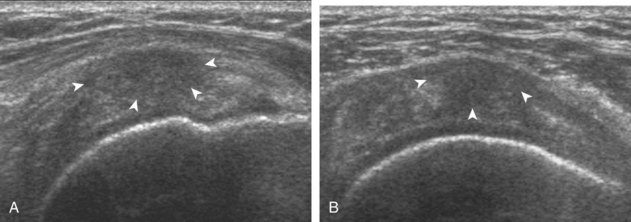
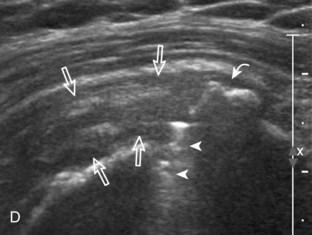
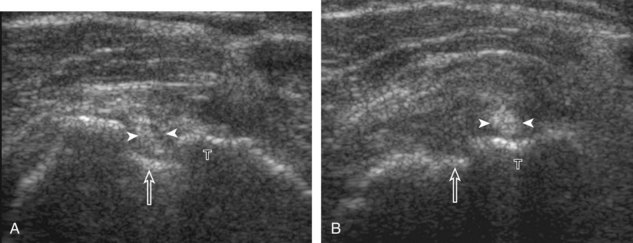
The thin hypoechoic layer over the curved humeral head represents the hyaline articular cartilage. At times, a thin hypoechoic layer over the greater tuberosity, which represents the fibrocartilage transition zone between the tendon and bone at the enthesis, may be seen and should not be confused with hyaline articular cartilage over the rounded humeral head. One must be aware that the distal fibers of the tendon curve downward at the greater tuberosity near the articular surface, and the transducer orientation should be adjusted using the heel-toe maneuver to eliminate anisotropy (Fig. 3-12A and B) (Video 3-9).![]() A hyperechoic and well-defined humeral head cortex indicates that the sound beam is perpendicular to the bone and overlying tendon. The footprint of the supraspinatus tendon inserts over approximately 2.25 cm of the greater tuberosity shelf, so the transducer should be moved anterior and posterior over the greater tuberosity (or medial and lateral on the patient in the modified Crass position), to ensure complete evaluation.2 It is important to continue scanning anteriorly along the greater tuberosity until the intra-articular portion of the biceps tendon is seen because this would indicate that the full anterior extent of the supraspinatus was evaluated, a location where supraspinatus tendon tears commonly occur.8,9 Including a long axis image of the intra-articular portion of the long head biceps brachii tendon will document that the most anterior aspect of the supraspinatus was evaluated (see Fig. 3-11B). As the transducer is moved posteriorly over the middle facet of the greater tuberosity, the infraspinatus is also evaluated (see Fig. 3-11C). At the middle facet, the angle between the greater tuberosity and the articular surface of the humeral head flattens, and alternating hypoechoic linear areas representing anisotropy of the infraspinatus tendon fibers can be seen over the supraspinatus tendon (Video 3-10).
A hyperechoic and well-defined humeral head cortex indicates that the sound beam is perpendicular to the bone and overlying tendon. The footprint of the supraspinatus tendon inserts over approximately 2.25 cm of the greater tuberosity shelf, so the transducer should be moved anterior and posterior over the greater tuberosity (or medial and lateral on the patient in the modified Crass position), to ensure complete evaluation.2 It is important to continue scanning anteriorly along the greater tuberosity until the intra-articular portion of the biceps tendon is seen because this would indicate that the full anterior extent of the supraspinatus was evaluated, a location where supraspinatus tendon tears commonly occur.8,9 Including a long axis image of the intra-articular portion of the long head biceps brachii tendon will document that the most anterior aspect of the supraspinatus was evaluated (see Fig. 3-11B). As the transducer is moved posteriorly over the middle facet of the greater tuberosity, the infraspinatus is also evaluated (see Fig. 3-11C). At the middle facet, the angle between the greater tuberosity and the articular surface of the humeral head flattens, and alternating hypoechoic linear areas representing anisotropy of the infraspinatus tendon fibers can be seen over the supraspinatus tendon (Video 3-10).![]() Minimal thinning of the cuff over this region may be seen.10 In addition, the rotator cable may be seen as a distinct hyperechoic structure (see Fig. 3-15).
Minimal thinning of the cuff over this region may be seen.10 In addition, the rotator cable may be seen as a distinct hyperechoic structure (see Fig. 3-15).
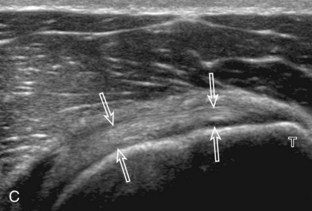
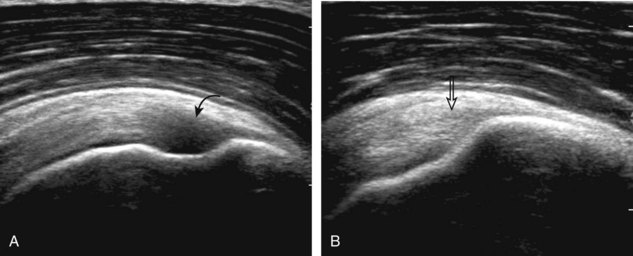
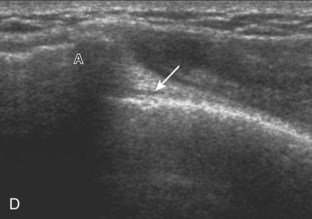
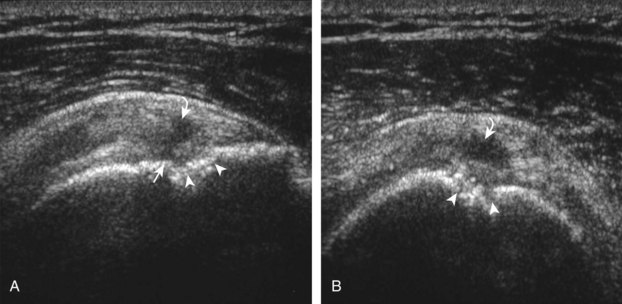
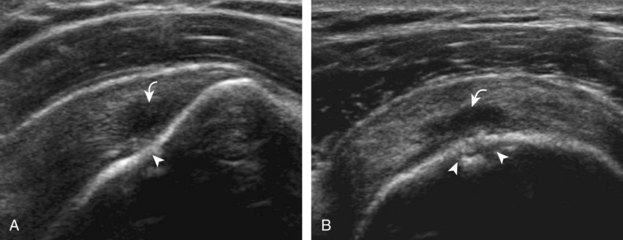
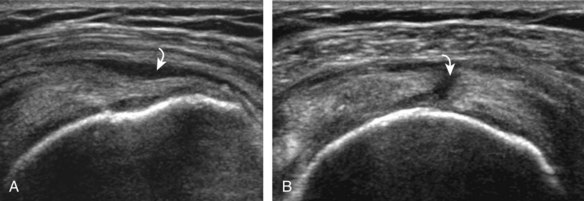
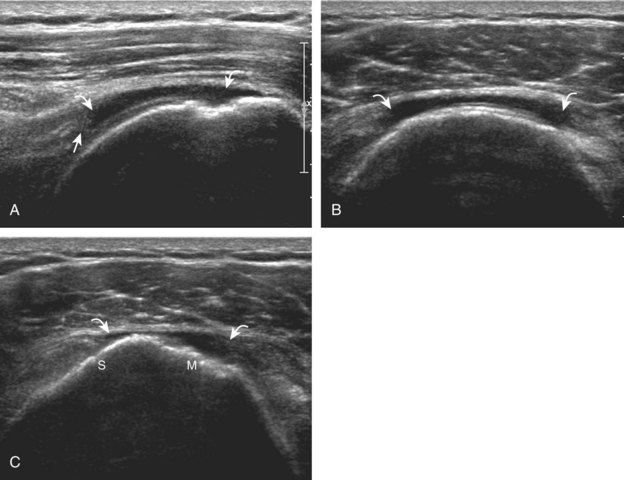
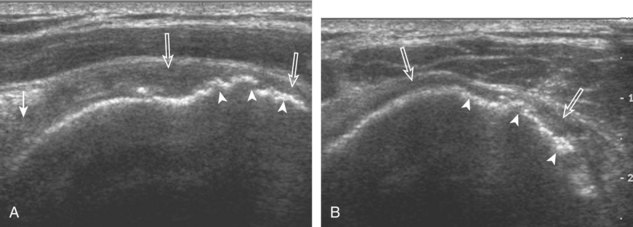
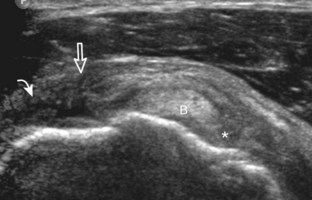
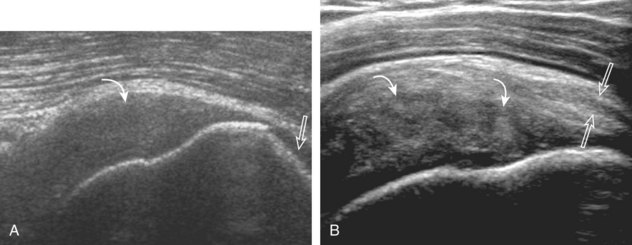
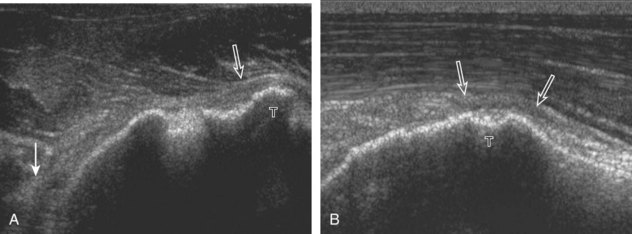
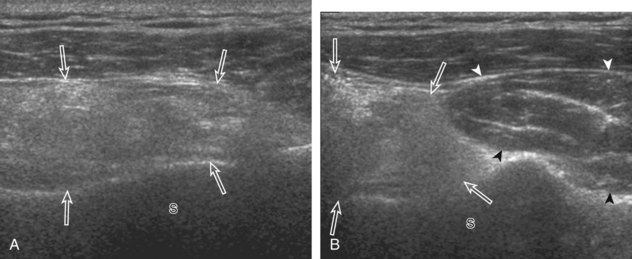
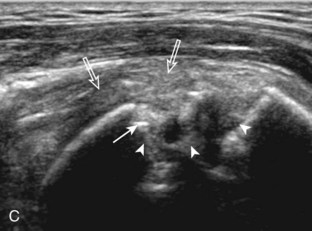
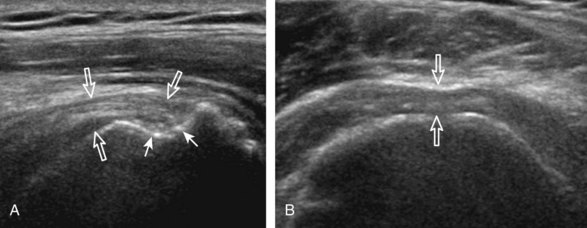
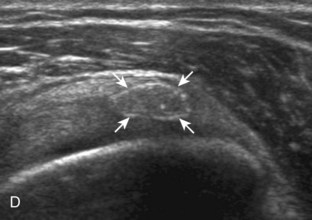
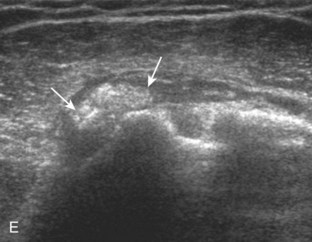
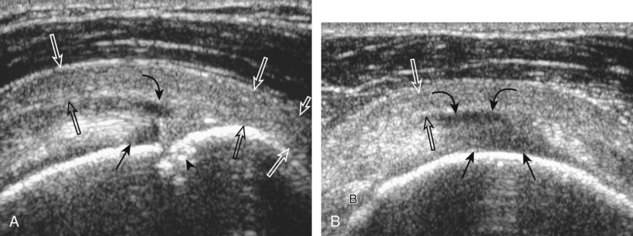
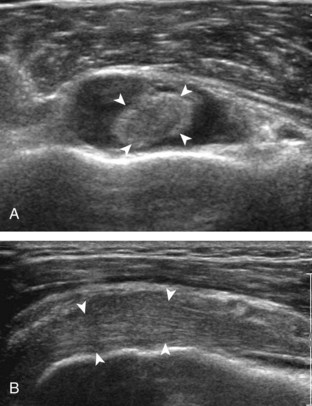
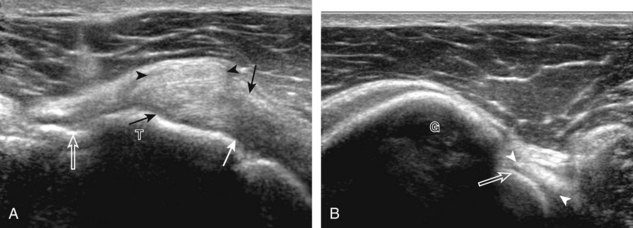
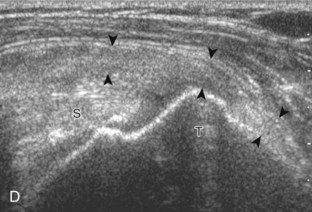
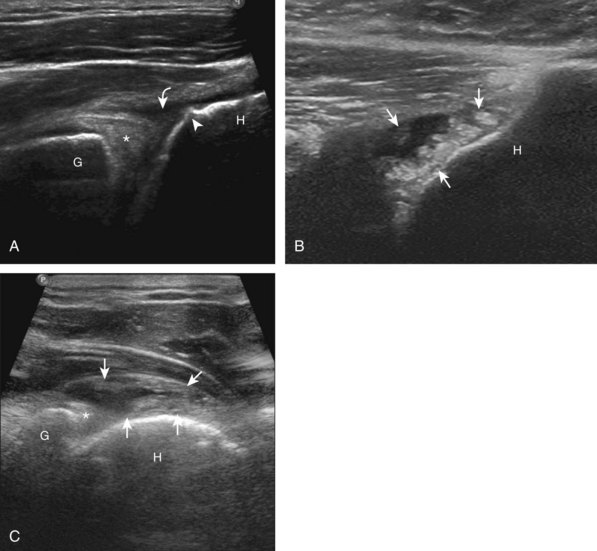
After assessment of the supraspinatus in long axis, the transducer is turned 90 degrees to evaluate the tendon in short axis (Fig. 3-13) (Video 3-11).![]() First, beginning over the proximal aspect of the supraspinatus, the humeral head should be seen as a round echogenic line with overlying hypoechoic hyaline cartilage (see Fig. 3-13A). The transducer should be toggled until the bone cortex and overlying tendon are hyperechoic and well defined to eliminate anisotropy (see Fig. 3-12C and D). At this level, the rotator cuff should be of fairly uniform thickness, similar to a tire on a wheel, measuring on average 6 ± 1.1 mm.11 This appearance indicates that the transducer is in the true short axis plane relative to the supraspinatus tendon and not in an oblique plane. The transducer is then moved distally relative to the supraspinatus tendon. As the hyaline cartilage disappears from view, the round humeral head surface will be replaced with the angulated surface of the greater tuberosity facets. At this point, the tendon uniformly becomes thinner, an indication that the transducer position is now beyond the articular surface. The facets of the greater tuberosity from anterior to posterior appear as three flat surfaces: the superior, middle, and inferior facets.1 The supraspinatus tendon inserts on the superior facet and the superior half of the middle facet, the infraspinatus inserts on the middle facet (overlapping the supraspinatus tendon superficially), and the teres minor inserts on the inferior facet.1 At this point, both the distal supraspinatus and infraspinatus are assessed. Similar to long axis imaging, alternating hypoechoic lines are seen over the middle facet, which represent anisotropy of the infraspinatus tendon fibers over the supraspinatus (Video 3-12).
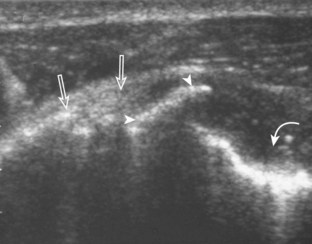
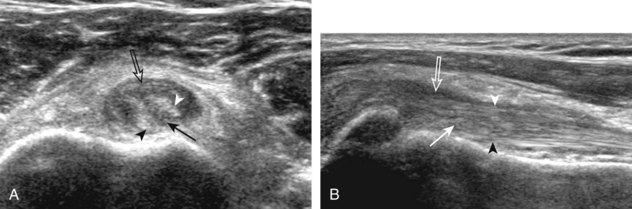
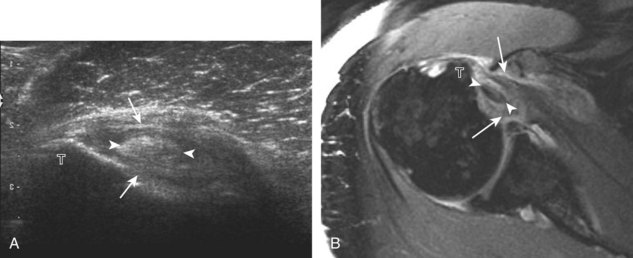
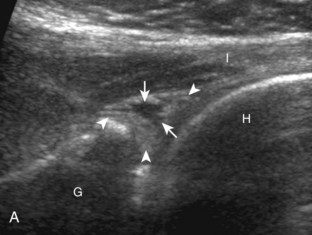
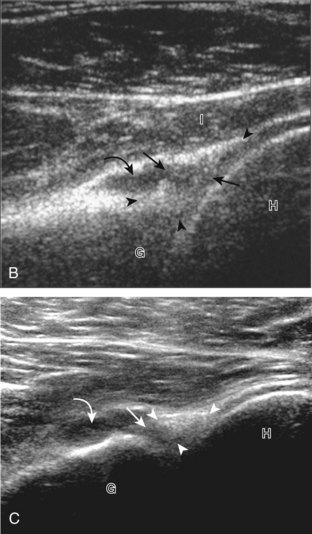
First, beginning over the proximal aspect of the supraspinatus, the humeral head should be seen as a round echogenic line with overlying hypoechoic hyaline cartilage (see Fig. 3-13A). The transducer should be toggled until the bone cortex and overlying tendon are hyperechoic and well defined to eliminate anisotropy (see Fig. 3-12C and D). At this level, the rotator cuff should be of fairly uniform thickness, similar to a tire on a wheel, measuring on average 6 ± 1.1 mm.11 This appearance indicates that the transducer is in the true short axis plane relative to the supraspinatus tendon and not in an oblique plane. The transducer is then moved distally relative to the supraspinatus tendon. As the hyaline cartilage disappears from view, the round humeral head surface will be replaced with the angulated surface of the greater tuberosity facets. At this point, the tendon uniformly becomes thinner, an indication that the transducer position is now beyond the articular surface. The facets of the greater tuberosity from anterior to posterior appear as three flat surfaces: the superior, middle, and inferior facets.1 The supraspinatus tendon inserts on the superior facet and the superior half of the middle facet, the infraspinatus inserts on the middle facet (overlapping the supraspinatus tendon superficially), and the teres minor inserts on the inferior facet.1 At this point, both the distal supraspinatus and infraspinatus are assessed. Similar to long axis imaging, alternating hypoechoic lines are seen over the middle facet, which represent anisotropy of the infraspinatus tendon fibers over the supraspinatus (Video 3-12).![]() As the transducer is moved more distally, the greater tuberosity becomes somewhat square, and the rotator cuff thins even more and eventually disappears as the transducer moves beyond the greater tuberosity and beyond the rotator cuff (see Fig. 3-13C and D). Similar to evaluation of the supraspinatus tendon in long axis, it is critical that the intra-articular portion of the biceps tendon (the rotator interval) is identified to indicate that the most anterior aspect of the supraspinatus tendon is evaluated. This is one of the advantages of the modified Crass position because this important landmark is well visualized. In addition, the biceps reflection pulley is identified with the superior glenohumeral ligament seen at the subscapularis aspect of the biceps tendon adjacent to the humerus, and the coracohumeral ligament is identified over the biceps tendon as it courses lateral to merge with the supraspinatus tendon (Fig. 3-14).12
As the transducer is moved more distally, the greater tuberosity becomes somewhat square, and the rotator cuff thins even more and eventually disappears as the transducer moves beyond the greater tuberosity and beyond the rotator cuff (see Fig. 3-13C and D). Similar to evaluation of the supraspinatus tendon in long axis, it is critical that the intra-articular portion of the biceps tendon (the rotator interval) is identified to indicate that the most anterior aspect of the supraspinatus tendon is evaluated. This is one of the advantages of the modified Crass position because this important landmark is well visualized. In addition, the biceps reflection pulley is identified with the superior glenohumeral ligament seen at the subscapularis aspect of the biceps tendon adjacent to the humerus, and the coracohumeral ligament is identified over the biceps tendon as it courses lateral to merge with the supraspinatus tendon (Fig. 3-14).12
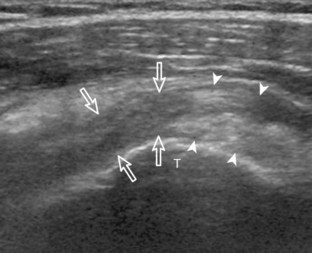
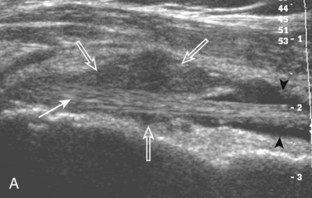
Another structure of the rotator cuff is the rotator cable, which may be identified by its characteristic shape and position (Fig. 3-15).13 The rotator cable has a U shape, with each limb attaching to the greater tuberosity. The curved aspect of the U is visualized with its fibers perpendicular to the supraspinatus at the articular surface. The rotator cable is more prominent in some individuals (termed cable dominant) and outlines an area of the rotator cuff within the U, termed the rotator crescent.
Position No. 4: Acromioclavicular Joint, Subacromial-Subdeltoid Bursa, and Dynamic Evaluation
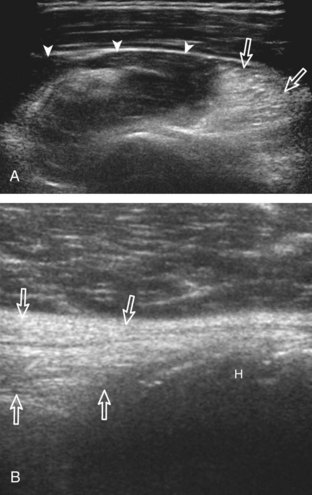
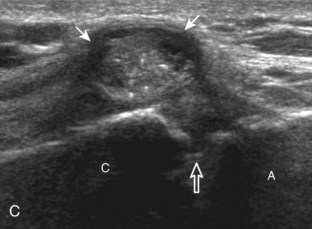
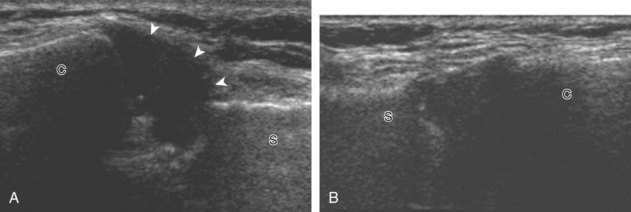
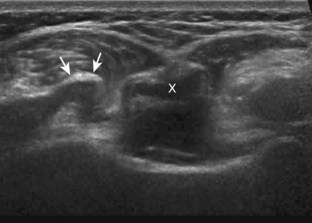
The acromioclavicular joint can be located with palpation of the clavicle and placement of the transducer in the coronal-oblique plane over the distal clavicle (Fig. 3-16) or by moving the transducer superiorly in the transverse plane from the bicipital groove region. The acromioclavicular joint is identified by the bone landmarks and hypoechoic joint space, although a hyperechoic fibrocartilage disk may be seen.14 If the acromioclavicular joint is widened, the patient can place his or her hand on the opposite shoulder to assess for acromioclavicular joint widening or, conversely, narrowing, which may be associated with pain.15 The transducer is then moved laterally in the coronal place over the proximal humerus beyond the greater tuberosity to assess for fluid within the dependent portion of the subacromial-subdeltoid bursa (see Fig. 3-16C).
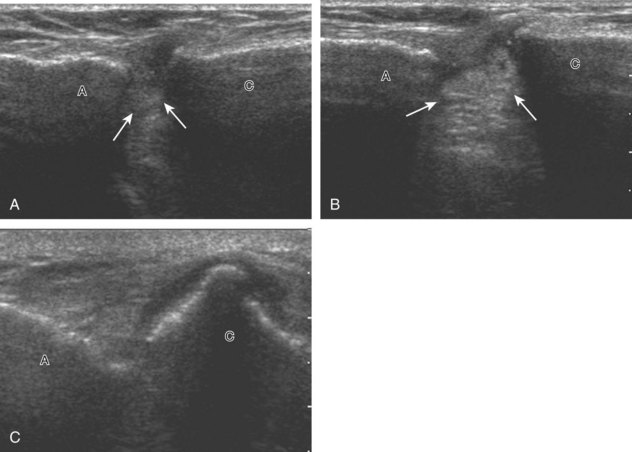
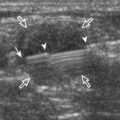
To dynamically assess for subacromial impingement, the transducer is positioned in the coronal or coronal-oblique plane to visualize the lateral border of the acromion and the adjacent greater tuberosity (Fig. 3-17). The examiner assesses the supraspinatus tendon and subacromial-subdeltoid bursa dynamically first by passively abducting the arm (with or without elbow flexion). This allows the examiner to slow or stop the patient’s movement if the bone landmarks are not visualized to allow repositioning of the transducer and also trains the patient to abduct the arm at a particular speed. The movement is then repeated actively (see Fig. 3-17C and D). Subsequent pooling of fluid in the subacromial-subdeltoid bursa indicates subacromial impingement, although more advanced cases can show additional upward movement of the humeral head.16,17 The finding of incomplete sliding of the supraspinatus beneath the acromion during this dynamic maneuver indicates adhesive capsulitis.18 When assessing for subacromial impingement, the transducer should also be moved anterior to the acromion to assess the region of the coracoacromial ligament for abnormal distention of the subacromial-subdeltoid bursa as well.
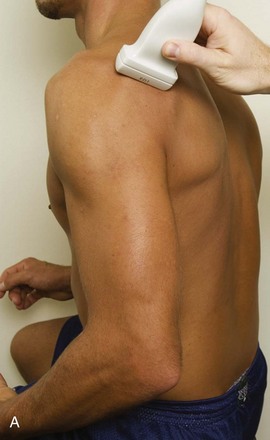
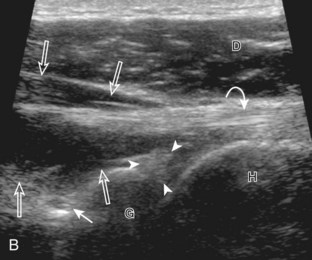
Position No. 5: Infraspinatus, Teres Minor, and Posterior Glenoid Labrum
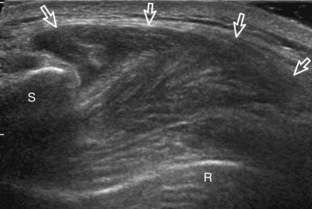
The patient rotates on the stool to permit visualization of the posterior structures of the shoulder; initially, the patient keeps his or her hand palm up on the thigh. Place the transducer in the oblique axial plane angled superiorly toward the humeral head parallel and just inferior to the scapular spine (Fig. 3-18). Position the transducer to visualize the well-defined central tendon of the infraspinatus tendon within the infraspinatus muscle at the musculotendinous junction posterior to the glenoid to ensure an imaging plane that is long axis to the infraspinatus (see Fig. 3-18B). The infraspinatus tendon can then be followed distally to its insertion on the middle facet at the posterior aspect of the greater tuberosity. Evaluation of the distal infraspinatus tendon supplements earlier evaluation from the modified Crass position (see Figs. 3-11 and 3-13). If the infraspinatus tendon is not visible because of shadowing beneath the acromion (which is not common), then the patient can place the hand on the opposite shoulder to improve visualization; this maneuver is less ideal because the infraspinatus tendon, seen linear and perpendicular to the sound beam in neutral shoulder position, becomes curved, introducing anisotropy. The transducer can then be moved inferiorly to visualize the smaller teres minor, with its tendon more superficial over the muscle compared with the infraspinatus tendon (see Fig. 3-18C). The transducer is then turned 90 degrees to evaluate the infraspinatus and teres minor in short axis (Fig. 3-19). An alternate approach in identification of the infraspinatus and teres minor is to palpate the scapular spine, place the transducer sagittal on the patient over the scapular spine, and then move the transducer inferiorly. The first structure identified inferior to the scapular spine is the infraspinatus. Once the infraspinatus and teres minor are identified, the transducer is turned long axis to the infraspinatus tendon to evaluate the hyperechoic triangle-shaped posterior glenoid labrum (see Fig. 3-18B). It is important to slide the transducer medially from the glenohumeral joint to assess the spinoglenoid notch, a site where paralabral cysts may be found. The patient can actively internally and externally rotate the shoulder to assess the infraspinatus tendon and posterior glenoid labrum dynamically (Video 3-13).![]() This maneuver is also important in the evaluation for posterior glenohumeral joint recess fluid, which also facilitates evaluation of potential paralabral tears (see Glenoid Labrum and Paralabral Cyst). In shoulder external rotation, the suprascapular vein may dilate, and this can simulate a paralabral cyst (see Glenoid Labrum and Paralabral Cyst) (Video 3-14).
This maneuver is also important in the evaluation for posterior glenohumeral joint recess fluid, which also facilitates evaluation of potential paralabral tears (see Glenoid Labrum and Paralabral Cyst). In shoulder external rotation, the suprascapular vein may dilate, and this can simulate a paralabral cyst (see Glenoid Labrum and Paralabral Cyst) (Video 3-14).![]()
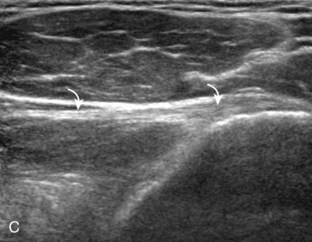
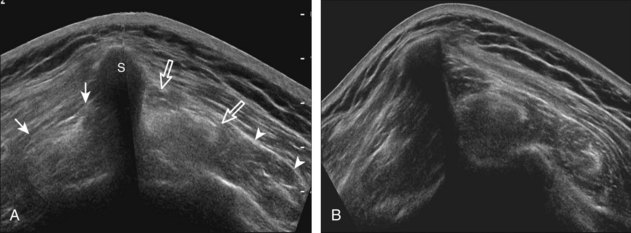
To complete the posterior shoulder examination, the transducer is turned 90 degrees and moved medial to assess the infraspinatus and teres minor in short axis globally at the musculotendinous junctions for atrophy or fatty degeneration; the infraspinatus muscle should be nearly twice the size of the teres minor over the scapular body, with normal muscle appearing relatively hypoechoic compared with hyperechoic tendon (see Fig. 3-19B). At this site, a ridge is often seen in the scapula, which forms a concave surface beneath each muscle and aids in their identification. The transducer can be moved superiorly to similarly assess for atrophy of the supraspinatus muscle. An extended field of view image may be considered (if available on the ultrasound machine) (see Fig. 3-19E).19
Rotator Cuff Abnormalities
Supraspinatus Tears and Tendinosis
General Comments
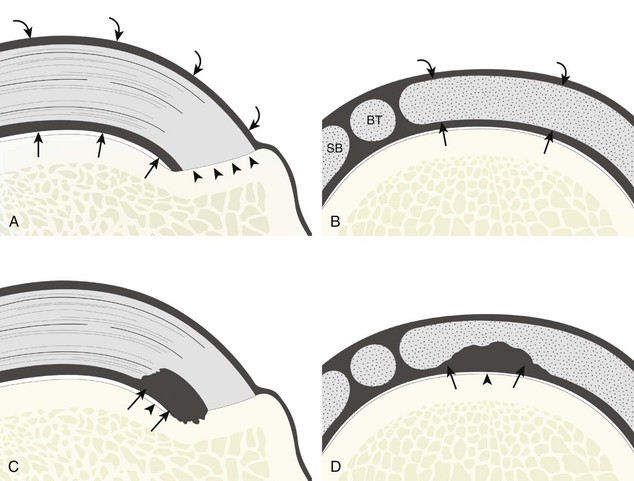
Most rotator cuff tears involve the supraspinatus tendon, although they may extend posterior to involve the infraspinatus and anterior to involve the biceps reflection pulley and subscapularis tendons.8 The anterior aspect of the distal supraspinatus is a common site of tear, often near the rotator interval, although a more posterior location near the supraspinatus-infraspinatus junction has been described with degenerative cuff tears.20 Most tendon tears are the result of chronic attrition and possible superimposed injury, and they typically occur after the age of 40 years. Such chronic supraspinatus tears occur distally and are associated with cortical irregularity of the greater tuberosity, an important indirect sign of supraspinatus tendon tear.21,22 Acute tears may occur more proximally and may or may not have associated cortical irregularity, depending on the age of the patient and the state of the underlying rotator cuff. Accurate localization of a tendon tear is essential to classify the tear properly (Fig. 3-20). For example, partial-thickness tears could involve either the articular or bursal surface of the tendon. A tear that is localized within the tendon or that extends only to the greater tuberosity surface (or footprint) of the supraspinatus attachment is called an interstitial or intrasubstance tear because it would not be visible at arthroscopy or bursoscopy.2,8 A tear that extends from articular to bursal surfaces is a full-thickness tear. Correct description and nomenclature are also essential. A full-thickness tear may be focal or incomplete, whereas a full-thickness tear that involves the entire width of a tendon can be termed a complete or full-width full-thickness tear. Initial evaluation in long axis of the supraspinatus is ideal in that the three surfaces of the rotator cuff (articular, bursal, greater tuberosity) are visible and easily identified.23 Most tears are anechoic or hypoechoic. As a supraspinatus tendon tear becomes larger, tendon retraction and volume loss of the tendon occur, with loss of the normal superior convex shape. Ultrasound and magnetic resonance imaging (MRI) have comparable accuracies in detection and measurement of rotator cuff tears.24 A meta-analysis of 65 articles has also shown that ultrasound and MRI are comparable in sensitivity and specificity for the diagnosis of rotator cuff tear.25
Partial-Thickness Tear
Partial-thickness supraspinatus tendon tears are characterized by a well-defined hypoechoic or anechoic abnormality that disrupts the tendon fibers.21,26 Such tears may be articular-side or bursal-side partial-thickness tears determined by which surface of the tendon is involved. An intrasubstance or interstitial tear may also be considered a form of partial-thickness tear, but one that does not extend to the articular or bursal surface.
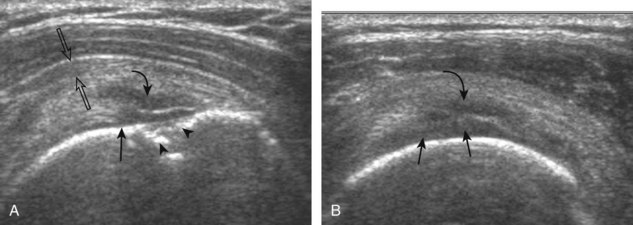
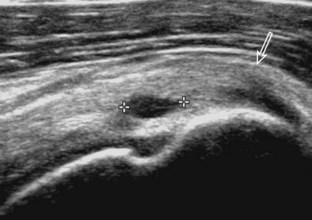
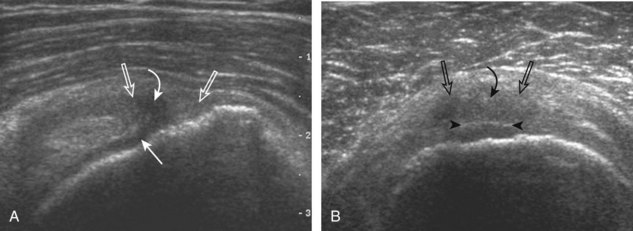
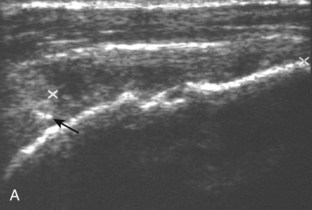
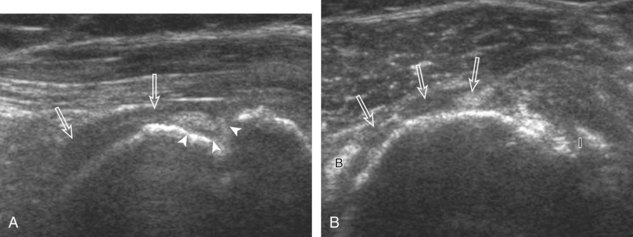
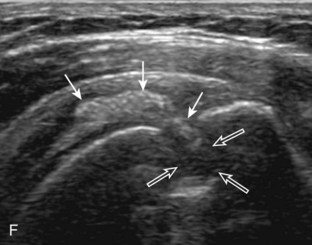
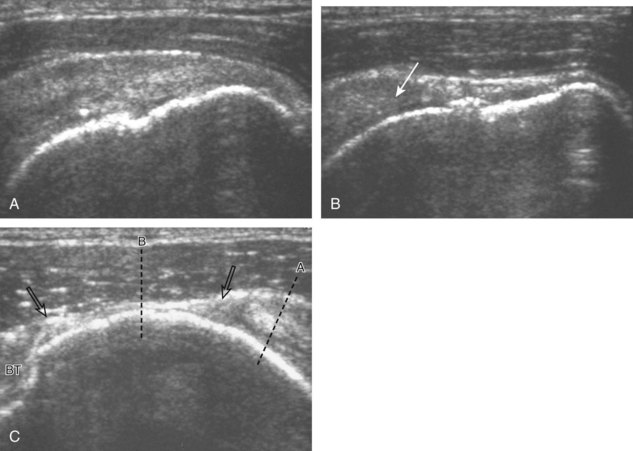
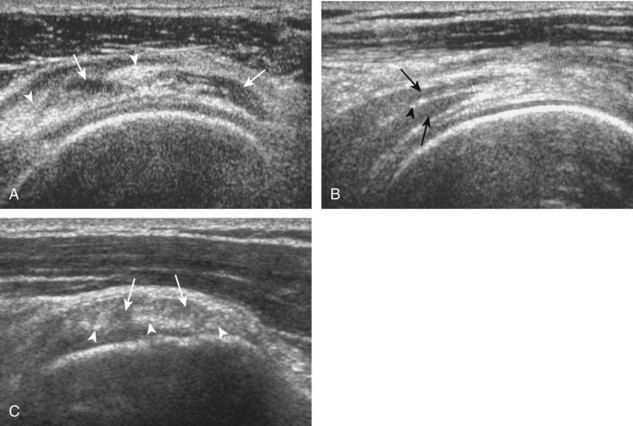
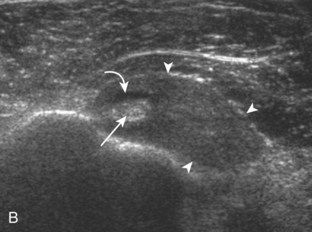
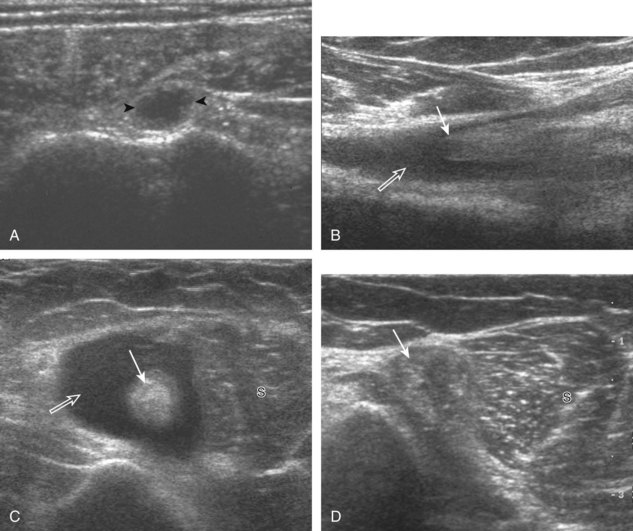
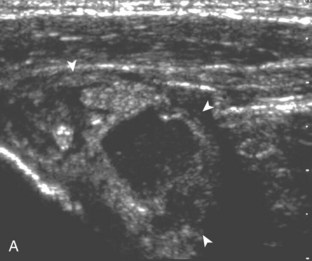
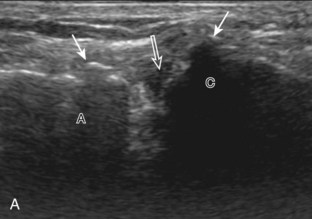
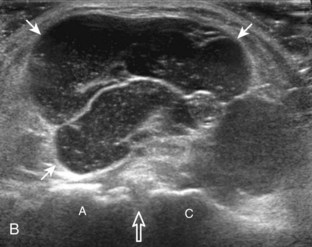
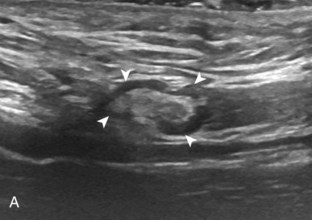
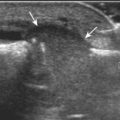
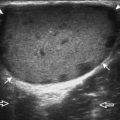
Articular-side partial-thickness tears most commonly involve the supraspinatus anteriorly and distally at the greater tuberosity and are seen with increased frequency in patients younger than 40 years.8,9 A mixed hyperechoic-hypoechoic appearance may be present, which represents hypoechoic fluid that surrounds the hyperechoic torn tendon stump (Figs. 3-21, 3-22, and 3-23).26 Cortical irregularity of the greater tuberosity immediately adjacent to the tendon tear is common, related to the chronic cuff attrition at the site of the tear.21,22 An acute tear of a previously normal cuff or a proximal tear (see Fig. 3-23) does not demonstrate cortical irregularity and more likely appears anechoic from fluid, although these types of tears are less common. With an articular-side partial-thickness tear, the superior surface of the tendon remains convex because global tendon volume loss is usually absent. Articular surface extension of a tear is suggested when the tear is in direct contact with the hypoechoic hyaline cartilage. The hyperechoic interface between the tendon tear and the hyaline cartilage may be accentuated in this situation (called the cartilage interface sign).27 The terms rim-rent tear and PASTA (partial articular-sided supraspinatus tendon avulsion) lesion are used specifically to describe a far-distal articular-side partial-thickness tear, immediately adjacent to the greater tuberosity surface (Video 3-15).8,9 ![]()
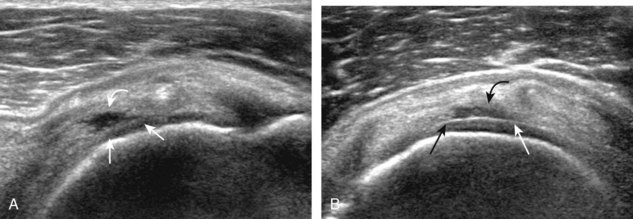
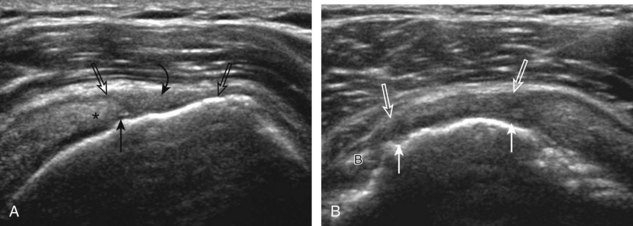
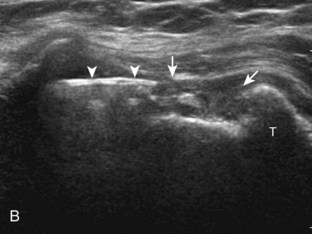
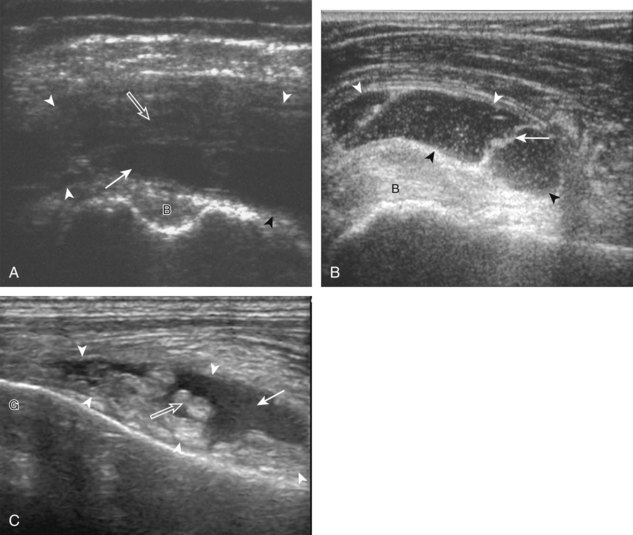
A bursal-side partial-thickness supraspinatus tendon tear is also hypoechoic or anechoic, but it is localized to the bursal surface (Figs. 3-24, 3-25, 3-26, and 3-27).21 Tear extension from the bursal surface to the greater tuberosity surface (or tendon footprint), without extension to the articular surface, is still considered a bursal-side partial-thickness tear. Because of the superficial location of the tear, tendon thinning and volume loss of the cuff are usually present. This situation results in loss of the normal superior convexity of the supraspinatus tendon surface, with dipping of the deltoid muscle and subacromial-subdeltoid bursa into the torn tendon gap. Similar to other supraspinatus tendon tears, greater tuberosity cortical irregularity is typically present because the tear extends from the bursal surface to the greater tuberosity surface. If adjacent subacromial-subdeltoid bursal synovial hypertrophy is present, hypoechoic or isoechoic synovial tissue may fill the torn tendon gap, making the tear and tendon thinning less conspicuous (see Fig. 3-27) (Video 3-16).![]()
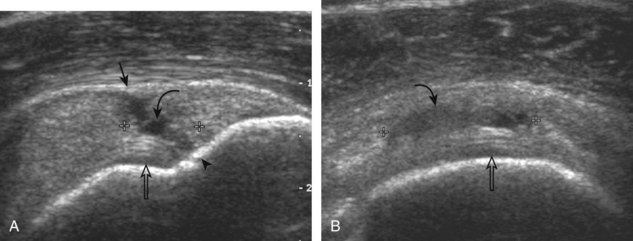
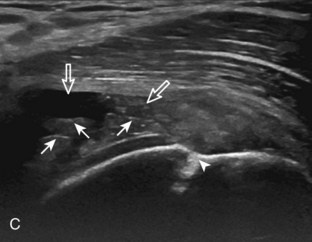
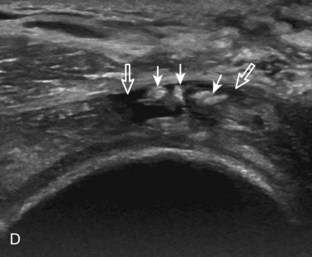
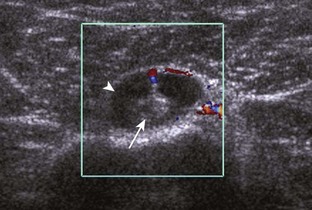
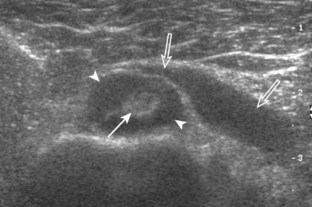
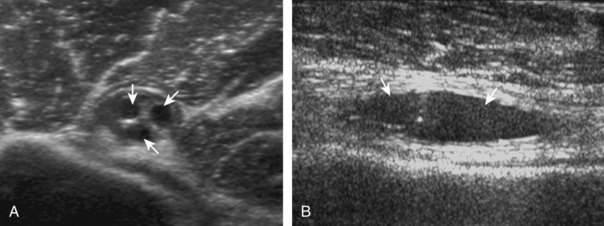
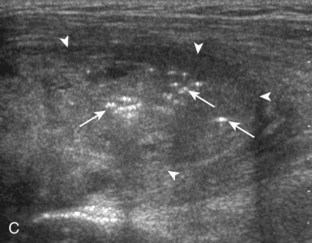
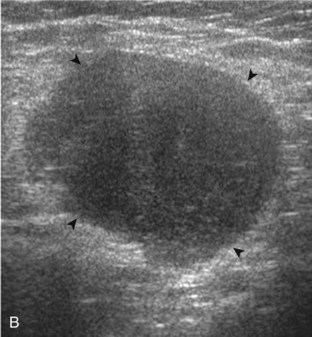
A tendon tear that does not contact the articular or bursal side of the supraspinatus is termed an intrasubstance or interstitial tear.8,9 Such tears may be anechoic or hypoechoic, located within the tendon substance or in contact with the greater tuberosity surface (Fig. 3-28). Cortical irregularity is often seen in the latter situation. Volume loss of the tendon is absent. Extensive intrasubstance tears may either represent or be precursors of a more extensive delamination tear. The presence of a well-defined anechoic cyst within the rotator cuff is usually associated with a supraspinatus articular-side tear (Fig. 3-29).28
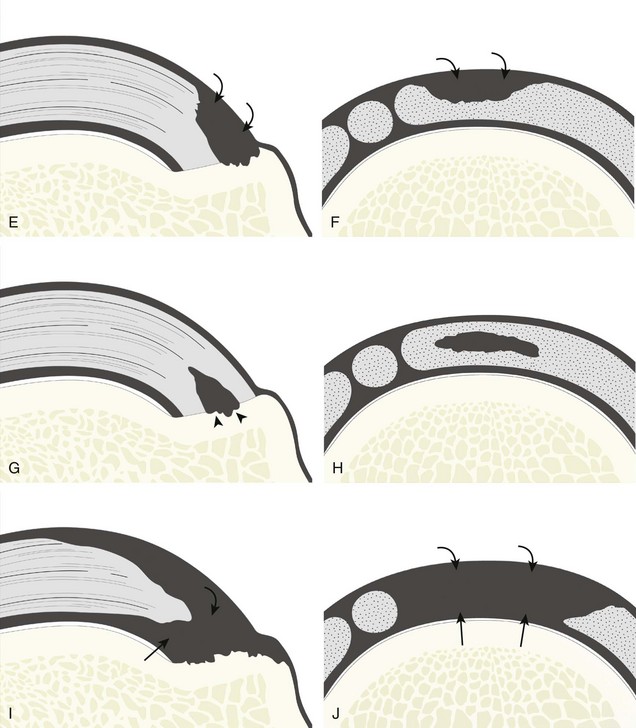
Full-Thickness Tear
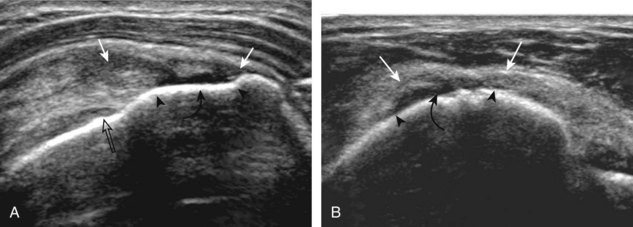
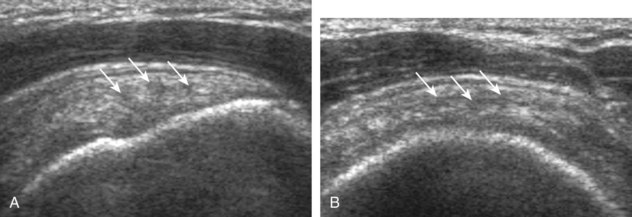
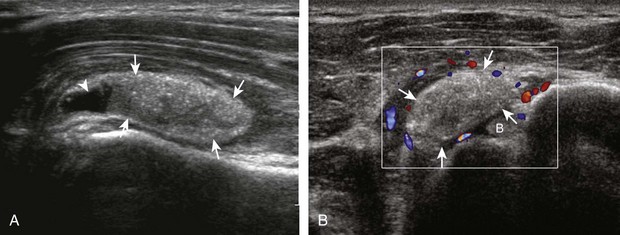
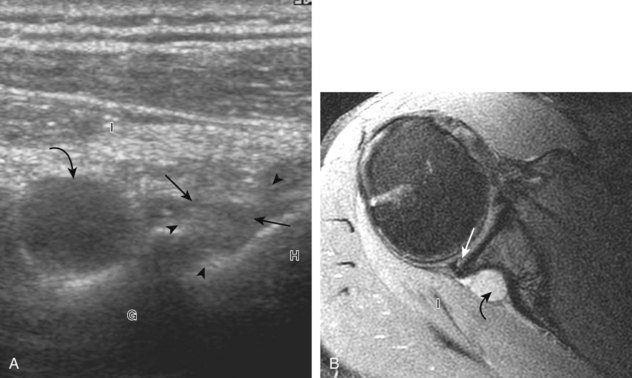
A full-thickness supraspinatus tendon tear is characterized by a well-defined hypoechoic or anechoic abnormality that disrupts the hyperechoic tendon fibers and extends from the articular to bursal surfaces of the tendon (Figs. 3-30 through 3-38).21,24 Anterior and distal location is common, although degenerative supraspinatus tears may be isolated more posterior at the supraspinatus-infraspinatus junction.8,20 Associated cortical irregularity of the adjacent greater tuberosity surface is usually present at a supraspinatus tear in patients older than 40 years. Identification of a hyperechoic interface between the hypoechoic hyaline cartilage and anechoic or hypoechoic tendon tear (cartilage interface sign) assists in the identification of the articular extent.27 This finding is only seen when the sound beam is perpendicular to the hyaline cartilage; the heel-toe maneuver when imaging the tendon in long axis and toggling the transducer while in short axis is helpful (see Chapter 1). Small full-thickness tears may not be associated with volume loss of the tendon, especially if filled with fluid. Narrow longitudinal tears are best visualized with the tendon in short axis (see Fig. 3-31). As a tear becomes larger, flattening or concavity of the superior supraspinatus tendon surface with volume loss is typical (Video 3-17). ![]() Acute tears may occur more proximally, and more commonly they are anechoic and are filled with fluid (see Fig. 3-34).29 An acute tear in a patient younger than 40 years or a proximal tear does not demonstrate cortical irregularity, although these types of tears are less common. It is important to describe the location of the tendon tear, the dimensions of the tear in long axis and short axis, and extension to other adjacent tendons. A full-thickness tear that is focal may be termed an incomplete full-thickness tear, whereas a tear that involves the entire width of a tendon may be termed a complete or full-width full-thickness tear. Chronic tears may be associated with extensive remodeling of the greater tuberosity, and the distal torn tendon may be tapered without adjacent fluid but possibly with isoechoic or hyperechoic synovial hypertrophy (see Fig. 3-38). With regard to tear extension to other tendons, a supraspinatus tear that extends posterior to the rotator interval beyond 2.5 cm involves the infraspinatus tendon.2 In addition, imaging the cuff in short axis over the greater tuberosity facets assists in this determination because a tear that extends over the posterior aspect of the middle facet indicates infraspinatus involvement as well. A supraspinatus tendon tear may also extend anteriorly through the rotator interval to involve the cephalad fibers of the subscapularis tendon (see Subscapularis Tears and Tendinosis). A bicep reflection pulley tear and long head of biceps brachii tendon subluxation or dislocation may also occur in this situation (Fig. 3-39) (Video 3-18).12
Acute tears may occur more proximally, and more commonly they are anechoic and are filled with fluid (see Fig. 3-34).29 An acute tear in a patient younger than 40 years or a proximal tear does not demonstrate cortical irregularity, although these types of tears are less common. It is important to describe the location of the tendon tear, the dimensions of the tear in long axis and short axis, and extension to other adjacent tendons. A full-thickness tear that is focal may be termed an incomplete full-thickness tear, whereas a tear that involves the entire width of a tendon may be termed a complete or full-width full-thickness tear. Chronic tears may be associated with extensive remodeling of the greater tuberosity, and the distal torn tendon may be tapered without adjacent fluid but possibly with isoechoic or hyperechoic synovial hypertrophy (see Fig. 3-38). With regard to tear extension to other tendons, a supraspinatus tear that extends posterior to the rotator interval beyond 2.5 cm involves the infraspinatus tendon.2 In addition, imaging the cuff in short axis over the greater tuberosity facets assists in this determination because a tear that extends over the posterior aspect of the middle facet indicates infraspinatus involvement as well. A supraspinatus tendon tear may also extend anteriorly through the rotator interval to involve the cephalad fibers of the subscapularis tendon (see Subscapularis Tears and Tendinosis). A bicep reflection pulley tear and long head of biceps brachii tendon subluxation or dislocation may also occur in this situation (Fig. 3-39) (Video 3-18).12![]() It is also important to assess for supraspinatus and infraspinatus atrophy in the setting of a rotator cuff tear because this finding is associated with poor surgical outcome after repair (see Rotator Cuff Atrophy).30
It is also important to assess for supraspinatus and infraspinatus atrophy in the setting of a rotator cuff tear because this finding is associated with poor surgical outcome after repair (see Rotator Cuff Atrophy).30
Tendinosis
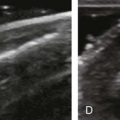
The term tendinosis (or tendinopathy) is used rather than tendinitis because there are no active inflammatory cells in this condition. This represents a degenerative process with eosinophilic, fibrillar, and mucoid degeneration and possible chondroid metaplasia.31,32 At ultrasound, focal tendinosis is characterized by a heterogeneous, somewhat ill-defined, hypoechoic area in the tendon without a tendon defect (Fig. 3-40).21,33 It is important to distinguish this abnormality from anisotropy because both may appear hypoechoic (see Fig. 3-12). Unlike tendon tear, tendinosis is usually less defined, it may be associated with tendon swelling, and it is usually not associated with adjacent cortical irregularity of the greater tuberosity. Diffuse tendinosis may cause the entire tendon to appear hypoechoic, equal in echogenicity to adjacent muscle (Fig. 3-41). Unlike a massive tendon tear, a normal convex superior surface of the supraspinatus is seen.
Indirect Signs of Supraspinatus Tendon Tear
Tendon Thinning.
Thinning or volume loss of the supraspinatus tendon and flattening or superior concavity of the superior supraspinatus tendon surface typically indicate tendon fiber loss. This condition can be seen with full-thickness tendon tears, especially moderate size or larger (see Figs. 3-33 and 3-38), and bursal-sided partial-thickness supraspinatus tendon tears (see Figs. 3-26 and 3-27). The presence of tendon thinning helps to exclude tendinosis because this latter condition, in contrast, shows normal tendon thickness or swelling (see Figs. 3-40 and 3-41) (Video 3-19).![]()
Cortical Irregularity.
When there is cortical irregularity of the greater tuberosity immediately adjacent to a defined hypoechoic or anechoic tendon abnormality of the supraspinatus, this increases the likelihood that the tendon abnormality represents a tear (see Figs. 3-21 and 3-22).21,22 This finding is most helpful in the differentiation between tendon tear and tendinosis because both may appear as a hypoechoic tendon abnormality. With tendinosis, the hyperechoic greater tuberosity surface is typically smooth, as in the normal state (see Fig. 3-41). Cortical irregularity is common with chronic attrition tears of the supraspinatus, and it may be absent with an acute tendon tear in a younger individual or with proximal tears (see Fig. 3-34). The significance of greater tuberosity cortical irregularity is specific to the attachment of the supraspinatus tendon. Cortical irregularity of the posterior aspect of the greater tuberosity involving the bare area (an area of intra-articular cortex without hyaline cartilage) beneath the infraspinatus tendon is a common finding, possibly a normal variant, and is usually without significance.34 However, if cortical irregularity at this site is extensive, this too can be associated with articular surface partial-thickness infraspinatus tendon tear and posterior labral tear in the setting of posterosuperior impingement syndrome.35 Finally, cortical irregularity of the lesser tuberosity of the subscapularis tendon insertion is also a common finding and is of little clinical significance in the absence of an adjacent tendon abnormality.
Joint Effusion and Bursal Fluid.
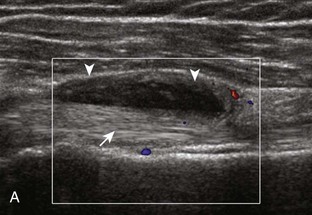
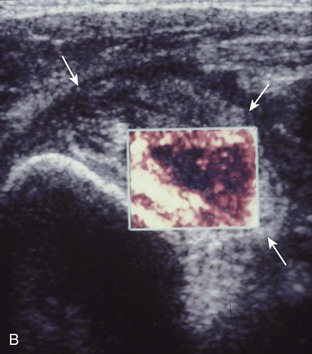
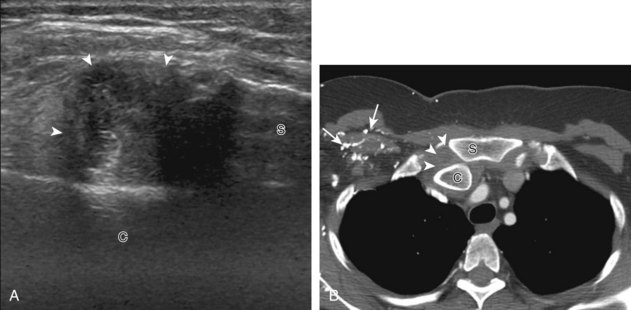
Investigators have shown that the findings of both glenohumeral joint effusion and subacromial-subdeltoid fluid suggest rotator cuff tear with a positive predictive value of 95%.36 To diagnose joint effusion, the long head of the biceps brachii tendon is evaluated in the bicipital groove for anechoic fluid (Fig. 3-42). The long head of the biceps brachii tendon sheath normally communicates with the glenohumeral joint, so increased joint fluid will collect in this dependent extension of the joint. A tiny sliver of fluid at one side of the biceps tendon is often seen normally, but fluid greater than this is considered abnormal, especially if it is seen circumferential to the biceps tendon. With regard to the posterior glenohumeral joint recess, small effusions may only be visible with the shoulder in external rotation (Video 3-20).![]() With larger joint effusions, fluid in the posterior shoulder joint recess can be seen even in neutral position deep to the infraspinatus tendon (Fig. 3-43). Subacromial-subdeltoid bursal fluid is diagnosed when the hyperechoic walls of the bursa are separated by more than 1 or 2 mm of anechoic fluid.37 Both joint fluid surrounding the biceps brachii long head tendon and distention of the subacromial-subdeltoid bursa may be seen over the anterior shoulder (Fig. 3-44) (Video 3-21).
With larger joint effusions, fluid in the posterior shoulder joint recess can be seen even in neutral position deep to the infraspinatus tendon (Fig. 3-43). Subacromial-subdeltoid bursal fluid is diagnosed when the hyperechoic walls of the bursa are separated by more than 1 or 2 mm of anechoic fluid.37 Both joint fluid surrounding the biceps brachii long head tendon and distention of the subacromial-subdeltoid bursa may be seen over the anterior shoulder (Fig. 3-44) (Video 3-21). ![]() Bursal fluid may also collect dependently, so it is important to evaluate the most inferior aspect of the bursa to visualize small quantities of fluid (see Fig. 3-16C) (Video 3-22).
Bursal fluid may also collect dependently, so it is important to evaluate the most inferior aspect of the bursa to visualize small quantities of fluid (see Fig. 3-16C) (Video 3-22).![]() Although simple joint and bursal fluid is commonly anechoic, complex fluid may appear hypoechoic or even isoechoic to adjacent muscle tissue.
Although simple joint and bursal fluid is commonly anechoic, complex fluid may appear hypoechoic or even isoechoic to adjacent muscle tissue.
Cartilage Interface Sign.
Normally, the surface of the hypoechoic hyaline cartilage that covers the humeral head is hyperechoic when the sound beam is perpendicular to the cartilage and underlying bone cortex. When an adjacent tendon is abnormally hypoechoic, or especially if there is adjacent anechoic fluid, this hyperechoic interface becomes more pronounced and is termed the cartilage interface sign.27 The presence of this sign is helpful in that it indicates a tendon abnormality does extend to the articular surface (see Fig. 3-23) (Video 3-23).![]() This sign may be seen with a hypoechoic tendon abnormality, but it is most striking in the presence of an anechoic fluid-filled tendon tear (see Fig. 3-30).
This sign may be seen with a hypoechoic tendon abnormality, but it is most striking in the presence of an anechoic fluid-filled tendon tear (see Fig. 3-30).
Infraspinatus Tears and Tendinosis
Similar to the supraspinatus tendon, tears of the infraspinatus tendon may be partial-thickness tears (extending only to the articular or bursal surface, or intrasubstance and not in contact with either the articular or bursal surface) or full-thickness tears (that extend from the bursal to the articular surface) (Fig. 3-45). Tendon tears can appear hypoechoic or anechoic with possible tendon thinning, whereas tendinosis is typically hypoechoic with tendon swelling (Fig. 3-46). Partial-thickness articular-side tears of the infraspinatus have been described in the setting of internal or posterosuperior impingement syndrome.35 This syndrome relates to impingement between the posterior aspect of the humerus and glenoid when the shoulder is externally rotated and abducted to 90 degrees, thus causing posterosuperior labral tear, marked cortical irregularity of the posterior aspect of the greater tuberosity, and partial-thickness articular infraspinatus tendon tear. Unlike the cortical irregularity of the superior aspect of the greater tuberosity associated with supraspinatus tendon tears, some degree of cortical irregularity of the posterior aspect of the greater tuberosity in the bare area devoid of cartilage is considered a variation of normal.34 When this irregularity is marked and associated with infraspinatus and adjacent labral disorders, posterosuperior impingement syndrome should be considered. Full-thickness tears of the infraspinatus tendon usually represent posterior extension of a supraspinatus tendon tear and are uncommonly isolated. An infraspinatus tear is present when a supraspinatus tear extends greater than 2.5 cm posterior to the intra-articular portion of the long head of the biceps brachii tendon or if the tear is visible over the posterior aspect of the greater tuberosity middle facet (see Fig. 3-37B). When the entire width of the infraspinatus tendon is torn and retracted, this represents a complete full-thickness tear.
Subscapularis Tears and Tendinosis
Similar to infraspinatus tendon tears, isolated tears of the subscapularis tendon are uncommon.38,39 Isolated full-thickness, complete, or full-width tears appear as complete tendon discontinuity, usually at the lesser tuberosity attachment (Fig. 3-47). Significant tendon retraction is common, and this becomes more obvious with the shoulder positioned in external rotation (Video 3-24).![]() If a fragment of bone is avulsed, this will appear as hyperechoic and shadowing, attached to the subscapularis tendon (Fig. 3-48). The biceps brachii long head tendon may be dislocated into the glenohumeral joint with full-thickness subscapularis tendon tear. More commonly, subscapularis tears are isolated to the cephalad aspect in association with an anterior supraspinatus tendon (Fig. 3-49).39 A subscapularis tear that extends from the bursal to articular surface that is isolated to the superior aspect would still be described as a focal or incomplete full-thickness tear. In this setting, the long head of the biceps brachii tendon may be dislocated over the lesser tuberosity or into the substance of the subscapularis tendon at the site of the tear (see Biceps Tendon, Subluxation and Dislocation). Tendinosis may also involve the subscapularis, which appears as heterogeneous abnormal hypoechogenicity and possible tendon swelling (Fig. 3-50).
If a fragment of bone is avulsed, this will appear as hyperechoic and shadowing, attached to the subscapularis tendon (Fig. 3-48). The biceps brachii long head tendon may be dislocated into the glenohumeral joint with full-thickness subscapularis tendon tear. More commonly, subscapularis tears are isolated to the cephalad aspect in association with an anterior supraspinatus tendon (Fig. 3-49).39 A subscapularis tear that extends from the bursal to articular surface that is isolated to the superior aspect would still be described as a focal or incomplete full-thickness tear. In this setting, the long head of the biceps brachii tendon may be dislocated over the lesser tuberosity or into the substance of the subscapularis tendon at the site of the tear (see Biceps Tendon, Subluxation and Dislocation). Tendinosis may also involve the subscapularis, which appears as heterogeneous abnormal hypoechogenicity and possible tendon swelling (Fig. 3-50).
Rotator Cuff Atrophy
In the setting of a rotator cuff tear, the supraspinatus and infraspinatus may undergo fatty degeneration or infiltration and possible atrophy.40 This is important information because the presence of both fatty infiltration and muscle atrophy is a negative prognostic factor when considering rotator cuff repair.41 The degree of rotator cuff atrophy relates to size (and therefore retraction and likely chronicity) and location (most are anterior) of the rotator cuff tear.20,41 Isolated or more pronounced atrophy of the infraspinatus muscle is also possible, even if the large or chronic rotator cuff tear is limited to the supraspinatus, possibly because of compromised suprascapular nerve from altered biomechanics.30 The subscapularis and teres minor are usually unaffected. Paralabral cyst formation from a labral tear is another potential cause of both supraspinatus and infraspinatus muscle denervation when located in the suprascapular notch, or isolated to the infraspinatus when located in the spinoglenoid notch.
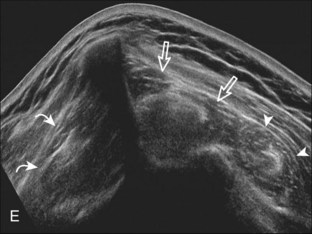
At ultrasound, fatty degeneration or infiltration and muscle atrophy will appear as increased echogenicity of the muscle and resultant poor differentiation between the tendon and muscle.30 The hyperechoic tendon may appear relatively enlarged with ill-defined borders in the setting of fatty infiltration, best appreciated at the musculotendinous junction in short axis. Fatty atrophy will also result in decreased muscle bulk.40 Imaging of the involved muscle in short axis is especially helpful in identifying decreased muscle size. One landmark when imaging the infraspinatus for atrophy is the posterior scapular cortex at the level of the musculotendinous junction, where a ridge is typically seen separating the minimal concavities of the scapula, which help to define the infraspinatus and adjacent teres minor muscles. This site is useful in that the infraspinatus muscle is usually about twice the area compared with the adjacent teres minor. In addition, the echogenicity of the infraspinatus muscle can be compared with the teres minor, which is routinely normal even in the setting of a rotator cuff tear. Infraspinatus atrophy is diagnosed when the muscle echogenicity is greater than the teres minor and the size is less than twice the area (Fig. 3-51). The supraspinatus should also be assessed for atrophy by moving the transducer superiorly from the infraspinatus between the clavicle and scapular spine.40 Extended field of view imaging may be helpful to demonstrate both supraspinatus and infraspinatus muscle atrophy compared with the teres minor on one image (Fig. 3-52).19 Muscle echogenicity should not be compared with the overlying deltoid because this muscle is commonly echogenic in older individuals.
Teres minor atrophy may be seen in up to 3% of shoulders, which will appear as increased echogenicity and possible decreased muscle size compared with the infraspinatus.42 This finding is often asymptomatic and may be due to the presence of a fibrous band or variation in teres minor innervation that predisposes to nerve compression.42–44 Uncommonly, teres minor atrophy may relate to quadrilateral space syndrome. The quadrilateral space is defined by the borders of the humerus, the long head of the triceps muscle, and the teres minor and teres major tendons. The axillary nerve and posterior circumflex humeral artery and veins traverse this space, and compression of these structures by fibrous bands, adjacent paralabral cyst, or mass may result in quadrilateral space syndrome.45 At sonography, the teres minor or deltoid muscle, or both, may appear hyperechoic and small as a result of atrophy (Fig. 3-53).46 To diagnose subtle abnormalities in size and echogenicity, it is helpful to make comparisons with the contralateral side or to compare the teres minor with the adjacent infraspinatus at the level of the muscle belly because normally the infraspinatus is about twice the size of the teres minor at this level.
Postoperative Shoulder
Evaluation of the rotator cuff after surgery can be challenging; however, ultrasound has been shown to be effective in the evaluation of the postoperative cuff with 89% accuracy.47 One must be familiar with the types of rotator cuff repairs and aware of the appearances of the repaired and intact rotator cuff. For repair of a rotator cuff tear, a low-grade partial-thickness tear is commonly débrided, whereas a high-grade partial-thickness tear is converted to a full-thickness tear and repaired. Transosseous suture or suture anchors may be used, with the latter being single, multiple, single row, or double row.48 After rotator cuff repair, the tendon may appear thin and heterogeneous, whereas at other times, the tendon may be thickened and heterogeneously hypoechoic (Figs. 3-54, 3-55, and 3-56).49–51 The general trend is for the repaired cuff to become more homogenous and hyperechoic over time, with a normal-appearing cuff present by 9 to 12 months. Hyperechoic suture material and suture anchors may be seen, as may the implantation trough, which appears as an angulated contour defect at the greater tuberosity (see Fig. 3-56A). Because suture may cause shadowing of the underlying tendon that may simulate a tendon defect, imaging the cuff in short axis is helpful to show that the anechoic area is narrow and corresponds to the area directly under a suture, which is unlike a rotator cuff defect. If the repaired tendon is attached by suture passing through drill holes in the greater tuberosity, then suture may be seen at the lateral aspect of the greater tuberosity as well. A hyperechoic focus attached to bone with reverberation can be seen with a metallic suture anchor. The area of the subacromial-subdeltoid bursa is commonly hypoechoic and thickened, and many times this bursa has been débrided or resected. Bone irregularity of the acromion could indicate changes related to acromioplasty.
To diagnose recurrent rotator cuff tear after repair, the most important finding is visualization of a tendon defect (Figs. 3-57 and 3-58).49–51 Compressibility of the anechoic or hypoechoic tendon abnormality helps to differentiate tear from postoperative changes. Most recurrent rotator cuff tears are large or become large, with at least moderate retraction. Identification of retracted suture that is not continuous with the implantation site is additional evidence for a recurrent tear. Another feature of a recurrent cuff is visible suture without surrounding rotator cuff tendon (see Fig. 3-58C and D). Be aware that the many tendon defects are asymptomatic, although larger defects tend to have symptoms. An equivocal ultrasound finding, especially within the first 6 to 9 months, should be reimaged after several weeks or months because such findings may improve over time, whereas a true tear often enlarges.
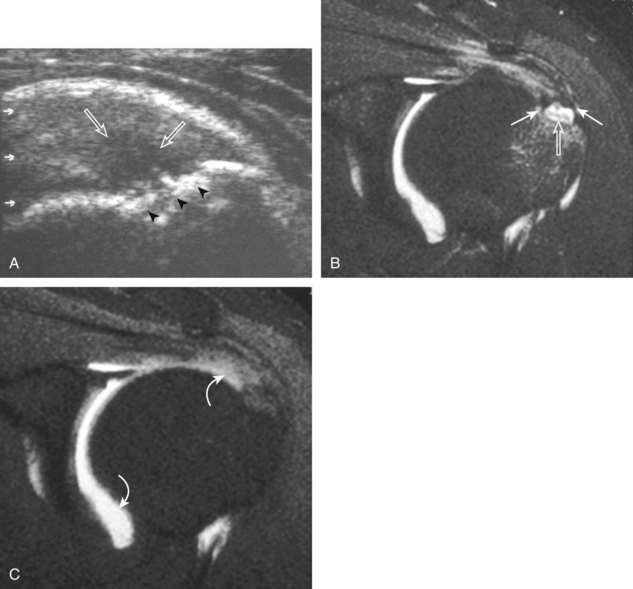
Shoulder arthroplasty or joint replacement involves resection of the humeral head, placement of a metal component, and possibly resection and replacement of the glenoid surface.48 With a conventional shoulder arthroplasty, the greater tuberosity is not resected, so the rotator cuff can be seen with ultrasound attaching normally to the humerus using routine bone landmarks. This is unlike a reverse total shoulder arthroplasty, used when there is an underlying rotator cuff tear before surgery and the tuberosities are resected. At ultrasound, the metal humeral component appears hyperechoic and smooth, with reverberation artifact, in the expected location of the humeral head (Fig. 3-59).52,53The normal rotator cuff should be identified over the humeral surface attaching to the tuberosities, and therefore tendon discontinuity or nonvisualization similar to the native shoulder is consistent with rotator cuff tear (Fig. 3-60). Ultrasound is ideal in evaluation of the rotator cuff after shoulder arthroplasty because artifact from the joint replacement occurs deep to the components, and the overlying rotator cuff region is easily visualized.
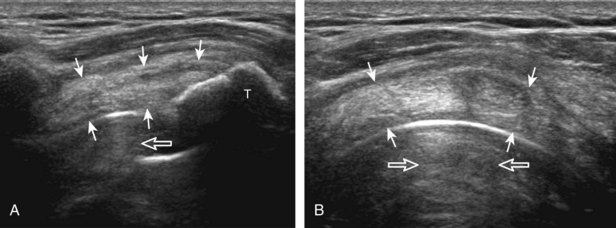
FIGURE 3-59 Rotator cuff after shoulder arthroplasty.
(From Jacobson JA, Miller B, Bedi A, Morag Y: Imaging of the postoperative shoulder. Semin Musculoskelet Radiol 15:320-339, 2011.)
Calcific Tendinosis
Calcific deposits occur in the rotator cuff primarily as calcium hydroxyapatite deposition, possibly from decreased oxygen tension and fibrocartilaginous metaplasia.54 The underlying rotator cuff is typically intact. While the supraspinatus is most commonly involved, other rotator cuff tendon involvement is not unusual. The calcific deposits most commonly are hyperechoic with posterior acoustic shadowing (Video 3-25),![]() although appearances may vary (Fig. 3-61). Small calcifications may be linear along the axis of the tendon fibers (see Fig. 3-61A and B) (Video 3-26),
although appearances may vary (Fig. 3-61). Small calcifications may be linear along the axis of the tendon fibers (see Fig. 3-61A and B) (Video 3-26),![]() whereas others have an amorphous appearance or are globular with minimal or no shadowing (see Fig. 3-61C, D, and E). Cortical erosions and osseous involvement may also be present (see Fig. 3-61F).55 In approximately 7% of cases, tendon calcification shadowing is absent, and radiographs may be normal if calcifications are in the form of a thick fluid or slurry.56 When calcific deposit echogenicity is isoechoic to tendon without shadowing, the amorphous echotexture during real-time scanning can be identified, and this replaces the normal fibrillar tendon appearance (Video 3-27).
whereas others have an amorphous appearance or are globular with minimal or no shadowing (see Fig. 3-61C, D, and E). Cortical erosions and osseous involvement may also be present (see Fig. 3-61F).55 In approximately 7% of cases, tendon calcification shadowing is absent, and radiographs may be normal if calcifications are in the form of a thick fluid or slurry.56 When calcific deposit echogenicity is isoechoic to tendon without shadowing, the amorphous echotexture during real-time scanning can be identified, and this replaces the normal fibrillar tendon appearance (Video 3-27). ![]() An additional method to help in this distinction is the use of anisotropy. With angulation of the transducer so that the sound beam is not perpendicular to the tendon fibers, the adjacent tendon will appear artifactually hypoechoic (Fig. 3-62) (see Video 3-27).
An additional method to help in this distinction is the use of anisotropy. With angulation of the transducer so that the sound beam is not perpendicular to the tendon fibers, the adjacent tendon will appear artifactually hypoechoic (Fig. 3-62) (see Video 3-27).![]() This does not significantly change the appearance of the calcifications; therefore, the hyperechoic calcific deposits become more conspicuous when they are surrounded by the hypoechoic tendon. Calcifications may be multiple in one or many tendons. In addition, large calcifications may contact the acromion during arm elevation as a form of impingement (Video 3-28).
This does not significantly change the appearance of the calcifications; therefore, the hyperechoic calcific deposits become more conspicuous when they are surrounded by the hypoechoic tendon. Calcifications may be multiple in one or many tendons. In addition, large calcifications may contact the acromion during arm elevation as a form of impingement (Video 3-28).![]()
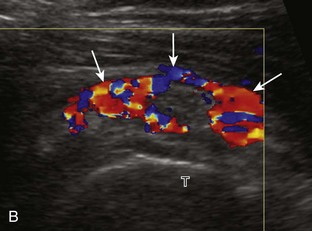
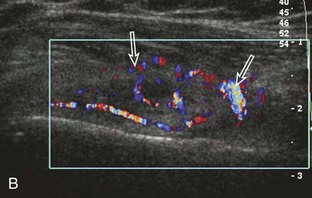
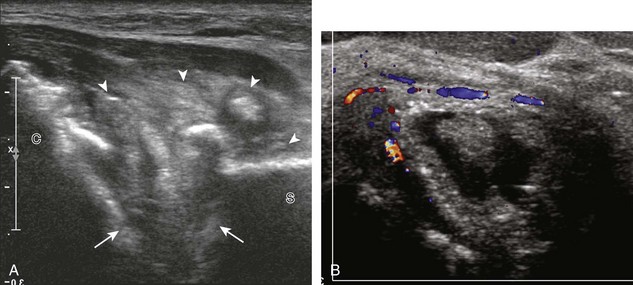
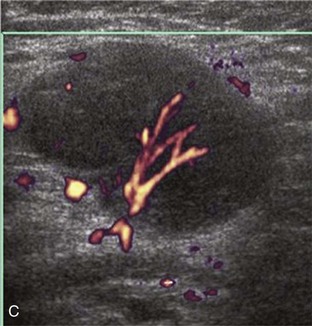
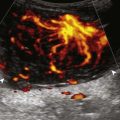
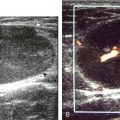
Calcifications that are amorphous without shadowing are associated with acute symptoms, whereas well-defined calcifications with shadowing are associated with subacute or chronic symptoms.57 Larger calcifications associated with an abnormal subacromial-subdeltoid bursa are also more likely symptomatic.58 Increased flow on color and power Doppler imaging around the calcifications may be evident, a finding that correlates with patients’ symptoms and a higher likelihood of the resorptive phase (Fig. 3-63).57 Calcifications may be identified at the bursal edge of the involved tendon with possible extension into the subacromial-subdeltoid bursa (see Subacromial-Subdeltoid Bursa). Ultrasound-guided percutaneous lavage and aspiration of the calcifications have been shown to be effective and improve symptoms, although clinical outcome at 5 and 10 years may be similar regardless of treatment (see Chapter 9).54
Impingement Syndrome
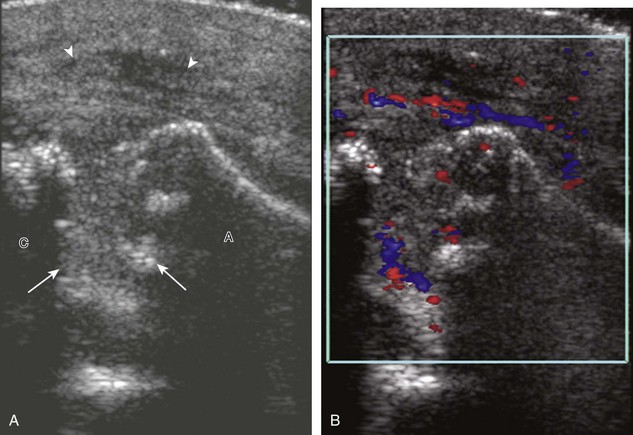
Of the rotator cuff tendons, the supraspinatus is prone to impingement. This is because the supraspinatus passes through a confined space between the scapula and the coracoacromial arch, which consists of the acromion, the distal clavicle, the acromioclavicular joint, the coracoid process, and the coracoacromial ligament. The subacromial-subdeltoid bursa also traverses this space over the supraspinatus tendon. Any abnormality that decreases the size of this space, such as an inferior acromioclavicular osteophyte or subacromial enthesophyte, can predispose to tendon impingement. The effect on the supraspinatus tendon is that of tendinosis and possible tear, whereas the overlying subacromial-subdeltoid bursa may be thickened with fluid or synovial hypertrophy. Sonography can suggest the diagnosis of early subacromial impingement when the gradual pooling of subacromial-subdeltoid bursal fluid at the acromion tip during active arm elevation is present (Fig. 3-64) (Video 3-29).17 ![]() This sign is most important when other causes of bursal fluid are excluded, such as primary inflammatory bursitis. For diagnosis of subacromial impingement, the shoulder is dynamically assessed with the transducer in the coronal-oblique plane showing the bone landmarks of the acromion and the greater tuberosity (see Fig. 3-17). Sliding the transducer anterior to the acromion may show bursal thickening beneath or adjacent to the coracoacromial ligament not visible at the level of the acromion because of shadowing (see Fig. 3-64C) (Video 3-30).
This sign is most important when other causes of bursal fluid are excluded, such as primary inflammatory bursitis. For diagnosis of subacromial impingement, the shoulder is dynamically assessed with the transducer in the coronal-oblique plane showing the bone landmarks of the acromion and the greater tuberosity (see Fig. 3-17). Sliding the transducer anterior to the acromion may show bursal thickening beneath or adjacent to the coracoacromial ligament not visible at the level of the acromion because of shadowing (see Fig. 3-64C) (Video 3-30).![]() A subacromial enthesophyte spur may also be seen at the acromion attachment of the coracoacromial ligament, which points anterior and medial from the acromion toward the coracoid process (Fig. 3-65). In addition to pooling of fluid, other findings of subacromial impingement include gradual distention of the subacromial-subdeltoid bursa with synovial tissue or snapping of a thickened bursa (Video 3-31).
A subacromial enthesophyte spur may also be seen at the acromion attachment of the coracoacromial ligament, which points anterior and medial from the acromion toward the coracoid process (Fig. 3-65). In addition to pooling of fluid, other findings of subacromial impingement include gradual distention of the subacromial-subdeltoid bursa with synovial tissue or snapping of a thickened bursa (Video 3-31).![]() Another finding described with subacromial impingement is superior bulging of the coracoacromial ligament when imaging the supraspinatus in short axis.59 Later stages of subacromial impingement include abnormal upward migration of the humeral head.16 Bone impingement may occur between the acromion and the greater tuberosity, usually in the setting of a rotator cuff tear (Video 3-32).
Another finding described with subacromial impingement is superior bulging of the coracoacromial ligament when imaging the supraspinatus in short axis.59 Later stages of subacromial impingement include abnormal upward migration of the humeral head.16 Bone impingement may occur between the acromion and the greater tuberosity, usually in the setting of a rotator cuff tear (Video 3-32).![]() The presence of an os acromiale has also been associated with symptoms of cuff impingement (Fig. 3-66).
The presence of an os acromiale has also been associated with symptoms of cuff impingement (Fig. 3-66).
Another form of rotator cuff impingement involves the subscapularis tendon and overlying subacromial-subdeltoid bursa between the coracoid process and lesser tuberosity of the proximal humerus. Ultrasound findings in this condition include decreased distance between the coracoid process and the lesser tuberosity (5.9 to 9.6 mm) compared with the asymptomatic side (7.8 to 17.5 mm) with the ipsilateral hand placed on the opposite shoulder.60 An additional finding is abnormal distention of the anterior aspect of the subacromial-subdeltoid bursa in the region of the subscapularis tendon and coracoid, which further distends with extension and internal rotation, correlating with anteromedial pain.61
Adhesive Capsulitis
Adhesive capsulitis or frozen shoulder is characterized by shoulder pain and limitation of motion.62 Although often of unclear etiology, this condition is associated with diabetes mellitus, trauma, and immobilization.62 At sonography, adhesive capsulitis can be initially suggested when the patient has limited external shoulder rotation while evaluating the subscapularis. Adhesive capsulitis is also suggested when there is continuous limitation of the sliding movement of the supraspinatus tendon beneath the acromion with active arm elevation (Fig. 3-67) (Video 3-33).18![]() Abnormal hypoechogenicity and hyperemia in the rotator interval, as well as thickening of the coracohumeral ligament, are other described findings with adhesive capsulitis.63,64
Abnormal hypoechogenicity and hyperemia in the rotator interval, as well as thickening of the coracohumeral ligament, are other described findings with adhesive capsulitis.63,64
Pitfalls in Rotator Cuff Ultrasound
Errors in Scanning Technique
Improper Positioning of the Shoulder
With the shoulder in neutral position, much of the supraspinatus is hidden beneath the acromion. Although the distal 1 to 2 cm of the tendon may be seen at its footprint, hyperextension of the shoulder (e.g., the Crass or modified Crass position) is needed to expose the supraspinatus tendon for evaluation (Fig. 3-68). In a supine patient, the supraspinatus may also be visualized by having the patient hyperextend the arm posterior to the shoulder.65
Incomplete Evaluation of the Supraspinatus Tendon
Many supraspinatus tendon tears occur anteriorly, often near the rotator interval.8,9 It is important that the entire extent of the supraspinatus tendon, especially the anterior portion, be completely evaluated. This can be ensured with visualization of the intra-articular portion of the biceps brachii tendon in the rotator interval (Fig. 3-69). When imaging the supraspinatus tendon in long axis, the transducer should be moved anteriorly on the greater tuberosity until the biceps tendon is seen. When imaging the supraspinatus tendon in short axis, the biceps tendon should again be visualized to ensure that the anterior aspect of the supraspinatus tendon is evaluated.
Imaging of the Rotator Cuff Too Distally
This can occur when imaging the supraspinatus tendon in short axis. If the transducer is located too distally over the greater tuberosity beyond the rotator cuff attachment, the image of deltoid muscle lying over the proximal humerus may simulate a massive rotator cuff tear because no cuff is visible (see Fig. 3-13D). This diagnosis is easily excluded by turning the transducer 90 degrees, long axis to the supraspinatus tendon, to indicate the improper transducer location. This is another reason that evaluation of the supraspinatus begins in its long axis.
Misinterpretation of Normal Structures
Misinterpretation of the Rotator Interval
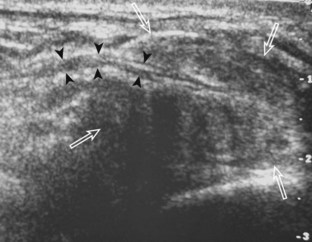
The rotator interval is a space between the superior margin of the subscapularis tendon and the anterior margin of the supraspinatus tendon. Within this interval, the intra-articular portion of the long head of the biceps brachii tendon is located, along with the biceps pulley system of capsular thickening and reflections and the superior glenohumeral and coracohumeral ligaments (see Fig. 3-14).12 The superior glenohumeral ligament is located at the subscapularis side of the biceps brachii tendon with fibers merging with the coracohumeral ligament, which is located superficial to the biceps tendon. The rotator interval also is the site where the glenohumeral joint communicates with the more medial subscapularis recess. The rotator interval is easily seen when the transducer is transverse to the supraspinatus tendon, although the modified Crass position may be needed to optimize visualization. The biceps tendon appears hyperechoic and is separated from the adjacent supraspinatus by a thin hypoechoic interface, which should not be misinterpreted for tendon tear.10
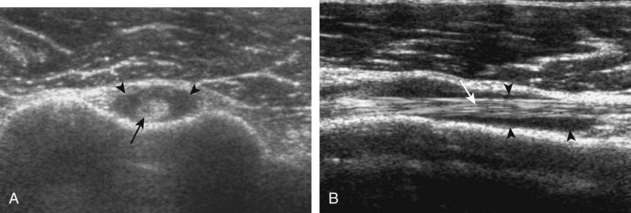
Misinterpretation of the Musculotendinous Junction
The musculotendinous junction represents a transition from hypoechoic muscle to hyerechoic tendon. Because this transition is not uniform, a mixed hyperechoic-hypoechoic appearance may be seen. One example is the proximal aspect of the supraspinatus tendon when one visualizes the oval anterior or central tendon of the supraspinatus and the small and flatter posterior tendon.66 When imaged in short axis, the intervening hypoechoic areas representing muscle tissue should not be misinterpreted as tendon disease, such as tendinosis (Fig. 3-70A and B). This pitfall is easily avoided by imaging the tendon in long axis, where the tapering appearance of the hypoechoic muscle tissue can be appreciated. A similar effect also involves the subscapularis, where both hypoechoic muscle and hyperechoic tendon are seen (see Fig. 3-70C). This appearance is most obvious in short axis, where several hyperechoic tendon bundles can be seen within hypoechoic muscle. With use of transducers with spatial compound imaging, this normal heterogeneous appearance becomes less conspicuous (see Fig. 3-7B).
Misinterpretation of the Supraspinatus-Infraspinatus Junction
Distally, the fibers of the supraspinatus and infraspinatus converge to form a common cuff of tendon. Over the anterior portion of the middle greater tuberosity facet, infraspinatus tendon fibers overlap supraspinatus tendon fibers.1 At ultrasound, this produces a series of uniform linear hypoechoic bands superficial to the posterior aspect of the supraspinatus. These bands appear hypoechoic as a result of infraspinatus anisotropy when imaging the supraspinatus in long or short axis (Fig. 3-71). This striped appearance is more conspicuous during real-time scanning because the fairly equally spaced hypoechoic oblique lines uniformly move through the tendon (see Video 3-8). These hypoechoic areas should not be misinterpreted for tendon pathology. This characteristic appearance can be used to locate the supraspinatus-infraspinatus junction, which is important in the localization of a rotator cuff tear and determination of tear extent. Another finding to assist in identification of the supraspinatus-infraspinatus tendon junction is visualization of slight cuff thinning on transverse imaging at this site. Finally, one can measure 2.5 cm posterior from the biceps brachii tendon at transverse imaging of the supraspinatus because this point indicates the posterior margin of the supraspinatus tendon.2 The bulk of the infraspinatus tendon is located posterior to this point, although there is overlap of the infraspinatus tendon superficial to the supraspinatus tendon over the middle greater tuberosity facet (see Fig. 3-2).1
Misinterpretation of Pathology
Subacromial-Subdeltoid Bursa Simulating Tendon
Although the abnormal subacromial-subdeltoid bursa is often distended with anechoic or hypoechoic fluid, the bursa may contain complex fluid or synovial hypertrophy. In these latter conditions, the echogenicity within the bursa may be isoechoic or even hyperechoic to adjacent muscle, and it may be nearly equal in echogenicity to tendon (Fig. 3-72). In the presence of a bursal-side partial-thickness tear (see Fig. 3-27) or a full-thickness tendon tear (see Fig. 3-37), the subacromial-subdeltoid bursa may lie within the torn tendon gap. When the bursa is hyperechoic and similar in echogenicity to tendon, it is important not to mistake the bursal contents for intact tendon fibers. The thickened bursa can be differentiated from tendon by the lack of fibrillar architecture and identification of the bursal tissue that extends beyond the greater tuberosity, unlike the rotator cuff. A similar situation may occur in the setting of a massive cuff tear, in which the thin hyperechoic wall of the subacromial-subdeltoid bursa may simulate intact fibers (Fig. 3-73). Because the bursal wall extends beyond the greater tuberosity distal to the rotator cuff tendon attachment to bone, this too excludes tendon fibers as the cause.
Rim-Rent Tear Versus Intrasubstance Tear
A rim-rent tear is an articular-side partial-thickness supraspinatus tendon tear that involves the most distal aspect of the tendon at the greater tuberosity insertion (see Fig. 3-21).8,9 When a well-defined hypoechoic or anechoic tendon abnormality is at this location, it is important to determine whether the abnormal echogenicity is in contact with the articular surface (representing a rim-rent tear) or is only the greater tuberosity surface within the supraspinatus tendon (an intrasubstance tear) (see Fig. 3-28). In the latter situation, an intrasubstance tear is not seen at arthroscopy or bursoscopy. Therefore, it is critical to determine whether intra-articular extension is seen, which appears as contact between the tendon tear and the hypoechoic hyaline cartilage with a possible cartilage interface sign (Fig. 3-74).
Tendinosis Versus Tendon Tear
Because tendinosis and tendon tear may both appear hypoechoic, one must rely on other sonographic findings to help with this distinction (Table 3-2).21 If a tendon abnormality is hypoechoic, ill defined, and heterogeneous, then tendinosis is suggested (see Fig. 3-40). In contrast, if a tendon abnormality is more anechoic and well defined, then tendon tear is suggested (see Fig. 3-30). In addition, tendon swelling suggests tendinosis, whereas tendon thinning suggests either bursal-side partial-thickness tear or full-thickness tear. The most important sign that assists in the differentiation between supraspinatus tear and tendinosis in patients older than 40 years is cortical irregularity of the greater tuberosity. A supraspinatus tendon abnormality immediately adjacent to cortical irregularity of the greater tuberosity likely represents a tear. This association is not necessarily found with other aspects of the rotator cuff. For example, cortical irregularity often is seen in the lesser tuberosity without adjacent subscapularis tendon abnormality. In addition, some degree of cortical irregularity of the posterior humerus beneath the infraspinatus (termed the bare area because it is devoid of cartilage) is considered a variation of normal.34 However, extensive irregularity in this region, coexisting with posterior labrum tear and partial-thickness infraspinatus tendon tear, indicates posterosuperior impingement syndrome.67
| Tear | Tendinosis |
|---|---|
| Anechoic | Hypoechoic |
| Well defined | Ill defined |
| Homogeneous | Heterogeneous |
| Thin | Swollen |
| Bone irregularity* | Smooth cortex* |
* Specific to greater tuberosity attachment of supraspinatus in patients older than 40 years.
Biceps Tendon
Joint Effusion and Tenosynovitis
Because the tendon sheath of the long head of the biceps brachii tendon normally communicates with the glenohumeral joint, increased joint fluid can be found in this tendon sheath adjacent to the long head of the biceps brachii tendon at the level of the bicipital groove (Fig. 3-75).68 More than a small sliver of fluid on one side of the biceps tendon is considered abnormal. Joint effusion, if simple, can be anechoic, whereas complex fluid may be hypoechoic, isoechoic, or hyperechoic relative to muscle, and it may resemble synovial hypertrophy (Fig. 3-76). The findings of flow on color or power Doppler imaging and the lack of internal movement with transducer pressure suggest synovial hypertrophy rather than complex fluid.69 It is also important to differentiate communicating joint effusion from tenosynovitis. If tendon sheath distention is focal, symptomatic with transducer pressure, with hyperemia, this suggests tenosynovitis (Fig. 3-77). Fluid that remains focal or loculated at the level of the bicipital groove with palpation under ultrasound visualization also suggests tenosynovitis (Video 3-34).![]() In contrast, a long segment of asymptomatic tendon sheath distention associated with distention of another shoulder joint recess, such as the posterior glenohumeral joint or subscapular recesses, suggests joint effusion. Normal flow seen in a branch of the anterior circumflex humeral artery should not be misinterpreted as abnormal vascularity (Fig. 3-78).69 It is also important not to mistake distention of the subacromial-subdeltoid bursa for biceps sheath abnormalities because the bursal distention is often seen superficial to the biceps tendon anteriorly (Fig. 3-79; see Fig. 3-44). Intra-articular bodies from the glenohumeral joint are commonly seen within the biceps brachii long head tendon sheath (Fig. 3-80). The finding of joint effusion alone within the long head of the biceps brachii tendon sheath has a positive predictive value of 60% for diagnosis of rotator cuff tear; the additional finding of subacromial-subdeltoid bursal fluid increases the positive predictive value to 95%.36
In contrast, a long segment of asymptomatic tendon sheath distention associated with distention of another shoulder joint recess, such as the posterior glenohumeral joint or subscapular recesses, suggests joint effusion. Normal flow seen in a branch of the anterior circumflex humeral artery should not be misinterpreted as abnormal vascularity (Fig. 3-78).69 It is also important not to mistake distention of the subacromial-subdeltoid bursa for biceps sheath abnormalities because the bursal distention is often seen superficial to the biceps tendon anteriorly (Fig. 3-79; see Fig. 3-44). Intra-articular bodies from the glenohumeral joint are commonly seen within the biceps brachii long head tendon sheath (Fig. 3-80). The finding of joint effusion alone within the long head of the biceps brachii tendon sheath has a positive predictive value of 60% for diagnosis of rotator cuff tear; the additional finding of subacromial-subdeltoid bursal fluid increases the positive predictive value to 95%.36
Tendon Tear and Tendinosis
Tendinosis of the biceps brachii long head tendon appears as hypoechoic enlargement of the tendon, but without tendon fiber disruption (Fig. 3-81). It is important not to misinterpret refraction shadowing from prominent deltoid fascia as abnormality of the biceps brachii tendon (Video 3-35).![]() Pathology of the biceps tendon most commonly occurs within 3.5 cm of the tendon origin, which may be seen proximal to and at the bicipital groove.4 Assessment of the most proximal biceps is completed with the shoulder in the modified Crass position when the anterior aspect of the supraspinatus tendon and the rotator interval are assessed. When the involved tendon shows additional anechoic clefts or an irregular superficial surface, then superimposed partial-thickness tear is present, especially when the bicipital groove is irregular from osseous spurs (Fig. 3-82).70 Rarely, an intratendinous ganglion cyst may be seen within the biceps tendon (Fig. 3-83).71 A full-thickness tear appears as complete fiber disruption and usually results in retraction at the torn tendon stump; therefore, the primary finding is lack of visualization of the long head of biceps tendon or an empty bicipital groove (Fig. 3-84A).70 There is often isoechoic or hyperechoic synovial hypertrophy or collapsed tendon sheath in the bicipital groove, which should not be misinterpreted as tendon fibers; imaging distally at the pectoralis tendon and proximally in the rotator interval for biceps tendon may be helpful. When there is full-thickness biceps brachii tendon tear, imaging more distally in short axis demonstrates the thickened and retracted distal stump, usually at the pectoralis tendon when chronic, which may or may not be surrounded by hypoechoic or anechoic fluid (see Fig. 3-84B to D). The normal muscle of the short head of the biceps brachii is seen just medially. Refraction shadowing deep to the torn and retracted tendon stump is another indirect sign of full-thickness tear. Often, the proximal aspect of the biceps tear is not seen in the bicipital groove or in the rotator interval because the tear has occurred proximally at the biceps anchor at the glenoid labrum. When the biceps brachii long head tendon is not seen in the bicipital groove, in addition to the possibility of a full-thickness tear, one must also consider the diagnosis of biceps tendon dislocation (see next paragraph). It is also important to inquire about prior surgery because the intra-articular portion of the biceps brachii long head tendon may be surgically transected (termed tenotomy) or transected and attached to the humerus (termed tenodesis) at the level of the bicipital groove (Fig. 3-85).
Pathology of the biceps tendon most commonly occurs within 3.5 cm of the tendon origin, which may be seen proximal to and at the bicipital groove.4 Assessment of the most proximal biceps is completed with the shoulder in the modified Crass position when the anterior aspect of the supraspinatus tendon and the rotator interval are assessed. When the involved tendon shows additional anechoic clefts or an irregular superficial surface, then superimposed partial-thickness tear is present, especially when the bicipital groove is irregular from osseous spurs (Fig. 3-82).70 Rarely, an intratendinous ganglion cyst may be seen within the biceps tendon (Fig. 3-83).71 A full-thickness tear appears as complete fiber disruption and usually results in retraction at the torn tendon stump; therefore, the primary finding is lack of visualization of the long head of biceps tendon or an empty bicipital groove (Fig. 3-84A).70 There is often isoechoic or hyperechoic synovial hypertrophy or collapsed tendon sheath in the bicipital groove, which should not be misinterpreted as tendon fibers; imaging distally at the pectoralis tendon and proximally in the rotator interval for biceps tendon may be helpful. When there is full-thickness biceps brachii tendon tear, imaging more distally in short axis demonstrates the thickened and retracted distal stump, usually at the pectoralis tendon when chronic, which may or may not be surrounded by hypoechoic or anechoic fluid (see Fig. 3-84B to D). The normal muscle of the short head of the biceps brachii is seen just medially. Refraction shadowing deep to the torn and retracted tendon stump is another indirect sign of full-thickness tear. Often, the proximal aspect of the biceps tear is not seen in the bicipital groove or in the rotator interval because the tear has occurred proximally at the biceps anchor at the glenoid labrum. When the biceps brachii long head tendon is not seen in the bicipital groove, in addition to the possibility of a full-thickness tear, one must also consider the diagnosis of biceps tendon dislocation (see next paragraph). It is also important to inquire about prior surgery because the intra-articular portion of the biceps brachii long head tendon may be surgically transected (termed tenotomy) or transected and attached to the humerus (termed tenodesis) at the level of the bicipital groove (Fig. 3-85).
Subluxation and Dislocation
When the biceps brachii long head tendon is not normally identified in the bicipital groove, one must consider medial subluxation or dislocation.5 With subluxation of the long head of the biceps brachii tendon, the tendon is partially out of the bicipital groove and medially displaced so that the medial aspect of the tendon is superficial to the lesser tuberosity (Fig. 3-86). The biceps may also dislocate medially and superficially over the lesser tuberosity and subscapularis (Fig. 3-87) (Video 3-36),![]() superficial and medial to the subscapularis (Fig. 3-88), into a subscapularis tendon tear (Fig. 3-89), or through a subscapularis tendon tear into the glenohumeral joint.12 In these situations, the supporting structures of the biceps tendon at the rotator interval are usually abnormal (see Fig. 3-39) (see Video 3-18). With medial biceps tendon dislocation, the intra-articular location of the biceps brachii long head tendon may be difficult to identify because it may simulate the glenoid labrum or other intra-articular structure; distal imaging in short axis shows the dislocated biceps tendon coursing out of the joint and returning to a normal position lateral to the biceps brachii short head muscle. It is also important to evaluate for abnormal tendon displacement dynamically because abnormal tendon position may only occur with the shoulder in external rotation (Fig. 3-90) (see Video 3-36).5
superficial and medial to the subscapularis (Fig. 3-88), into a subscapularis tendon tear (Fig. 3-89), or through a subscapularis tendon tear into the glenohumeral joint.12 In these situations, the supporting structures of the biceps tendon at the rotator interval are usually abnormal (see Fig. 3-39) (see Video 3-18). With medial biceps tendon dislocation, the intra-articular location of the biceps brachii long head tendon may be difficult to identify because it may simulate the glenoid labrum or other intra-articular structure; distal imaging in short axis shows the dislocated biceps tendon coursing out of the joint and returning to a normal position lateral to the biceps brachii short head muscle. It is also important to evaluate for abnormal tendon displacement dynamically because abnormal tendon position may only occur with the shoulder in external rotation (Fig. 3-90) (see Video 3-36).5 ![]() The contrary is also true, whereby medial dislocation of the biceps brachii long head tendon over the lesser tuberosity in neutral shoulder position may relocate into the bicipital groove, with shoulder internal rotation associated with a painful snap (see Fig. 3-87) (Video 3-37).
The contrary is also true, whereby medial dislocation of the biceps brachii long head tendon over the lesser tuberosity in neutral shoulder position may relocate into the bicipital groove, with shoulder internal rotation associated with a painful snap (see Fig. 3-87) (Video 3-37).![]() Subluxation or dislocation of the biceps brachii tendon can be associated with biceps tendon tear (Fig. 3-91).
Subluxation or dislocation of the biceps brachii tendon can be associated with biceps tendon tear (Fig. 3-91).
Subacromial-Subdeltoid Bursa
The normal subacromial-subdeltoid bursa is a synovial space, separate from the glenohumeral joint, located between the rotator cuff and the overlying acromion and deltoid muscle (see Fig. 3-1). At ultrasound it appears as a thin, uniform, 1- to 2-mm hypoechoic layer of synovial fluid surrounded by hyperechoic bursal wall and peribursal fat layers.11,37 Abnormal distention of the subacromial-subdeltoid bursa may appear anechoic or hypoechoic from simple fluid, or it may range from hypoechoic to hyperechoic as a result of complex fluid or synovial hypertrophy (Fig. 3-92). Color or power Doppler imaging may differentiate complex fluid from synovitis because blood flow suggests synovial hypertrophy (Fig. 3-93). The term bursitis is often reserved for cases in which inflammation is truly present. Causes of subacromial-subdeltoid bursal distention include impingement, rotator cuff tear, hemorrhage (Fig. 3-94A), and amyloidosis. Inflammatory conditions should also be considered, such as gout (see Fig. 3-94B), rheumatoid arthritis (see Fig. 3-94C), and infection.37 Identification of hyperechoic foci with ring-down artifact within a complex bursal fluid collection raises concern for gas-producing infection (see Fig. 3-93C). Calcium hydroxyapatite deposition may be located in the bursa (Fig. 3-95) (Video 3-38), ![]() or it may extend from the adjacent rotator cuff (see Fig. 3-63). Focal thickening of the subacromial-subdeltoid bursa can indicate chronic impingement; dynamic imaging during active elevation of the arm should be completed to evaluate for impingement of the thickened bursa beneath the acromion, possibly associated with a snapping sensation (see Videos 3-30 and 31). With regard to rotator cuff tears, it has been shown that the presence of subacromial-subdeltoid bursal fluid has a 70% positive predictive value for rotator cuff tear; a combination of joint fluid distention of the long head of the biceps brachii tendon sheath and subacromial-subdeltoid fluid increases the positive predictive value to 95%.36 Other causes of subacromial-subdeltoid bursal distention include synovial proliferative disorders, such as pigmented villonodular synovitis and synovial osteochondromatosis.
or it may extend from the adjacent rotator cuff (see Fig. 3-63). Focal thickening of the subacromial-subdeltoid bursa can indicate chronic impingement; dynamic imaging during active elevation of the arm should be completed to evaluate for impingement of the thickened bursa beneath the acromion, possibly associated with a snapping sensation (see Videos 3-30 and 31). With regard to rotator cuff tears, it has been shown that the presence of subacromial-subdeltoid bursal fluid has a 70% positive predictive value for rotator cuff tear; a combination of joint fluid distention of the long head of the biceps brachii tendon sheath and subacromial-subdeltoid fluid increases the positive predictive value to 95%.36 Other causes of subacromial-subdeltoid bursal distention include synovial proliferative disorders, such as pigmented villonodular synovitis and synovial osteochondromatosis.
When the subacromial-subdeltoid bursa is distended, fluid often collects dependently, such as over and beyond the greater tuberosity (see Fig. 3-92B) (see Video 3-22).37 In this situation, it is important to evaluate this dependent portion of the subacromial-subdeltoid bursa because it may not be readily visualized when evaluation is focused over the rotator cuff at or proximal to the greater tuberosity. Another dependent area of the subacromial-subdeltoid bursa that may become distended is anteriorly over the bicipital groove, often seen in evaluation of the biceps brachii long head tendon (Fig. 3-96). It is important not to mistake this bursal distention for a biceps tendon sheath abnormality (see Video 3-21). More proximal evaluation in the sagittal plane assists in this distinction because bursal disease extends proximally over the rotator cuff, whereas biceps tendon sheath disease either terminates proximally or extends into the joint with the biceps long head tendon. Focal anterior distention of the subacromial-subdeltoid bursa with extension and internal rotation of the shoulder can be associated with symptoms related to coracoid impingement.61
Glenohumeral Joint and Recesses
The glenohumeral joint has several recesses that preferentially distend with joint fluid or other joint processes, which include the biceps brachii long head tendon sheath, the posterior glenohumeral joint recess, the subscapularis recess, and the axillary recess. These recesses should be assessed for pathology and also serve as potential sites for joint aspiration or injection. The joint recess where even small amounts of joint fluid can be seen is the biceps brachii long head tendon sheath (see Fig. 3-75).68 As discussed previously, the differential diagnosis for abnormality surrounding the biceps tendon at the bicipital groove includes both localized biceps tenosynovitis (see Fig. 3-77) and a more diffuse joint process related to the glenohumeral joint. Assessing the other joint recess assists in this differential. A pathologic process surrounding the biceps tendon that is out of proportion to findings in other glenohumeral joint recesses suggests a localized process. Another glenohumeral joint recess includes the posterior recess, which is assessed with transducer placement over the infraspinatus tendon, where joint fluid, synovial hypertrophy and intra-articular bodies may be identified (Fig. 3-97) (Videos 3-39 and 3-40).![]() Small amounts of joint fluid may only become visible at this site with the shoulder in external rotation (see Video 3-20).68 The subscapularis recess also is commonly distended and has a characteristic shape of an inverted “U” over the top of the subscapularis tendon near the coracoid process (Fig. 3-98). This shape distinguishes the subscapularis recess from the uncommon subcoracoid bursa, which is located anterior the subscapularis tendon directly inferior to the coracoid but is not located over the superior edge of the subscapularis tendon and does not communicate with the glenohumeral joint.72 Another feature of the subscapularis bursa is its change in shape and degree of distention with shoulder movement, increasing with internal rotation and decreasing in external rotation (Video 3-41)
Small amounts of joint fluid may only become visible at this site with the shoulder in external rotation (see Video 3-20).68 The subscapularis recess also is commonly distended and has a characteristic shape of an inverted “U” over the top of the subscapularis tendon near the coracoid process (Fig. 3-98). This shape distinguishes the subscapularis recess from the uncommon subcoracoid bursa, which is located anterior the subscapularis tendon directly inferior to the coracoid but is not located over the superior edge of the subscapularis tendon and does not communicate with the glenohumeral joint.72 Another feature of the subscapularis bursa is its change in shape and degree of distention with shoulder movement, increasing with internal rotation and decreasing in external rotation (Video 3-41)![]() . The axillary recess is located inferior to the glenohumeral joint imaged from the axilla. In addition to assessing for joint fluid, other joint processes such as intra-articular bodies and synovial hypertrophy can be visible in any of the above described joint recesses. Cortical irregularity involving the articular surface of the humerus could represent osteophytes (see Fig. 3-97A), an osteochondral abnormality, subchondral fracture or collapse from osteonecrosis, a Hill Sachs impaction fracture (Fig. 3-99A), or possibly a true erosion if adjacent synovial hypertrophy (Fig. 3-99B and C). The latter should be differentiated anatomically from cortical irregularity of the greater tuberosity, which is not an inflammatory erosion but rather related to rotator cuff tear (see Figs. 3-21 and 3-22). The adjacent hyaline cartilage and humeral head can also be assessed for layering of monosodium urate crystals (called the double contour sign) in gout.73
. The axillary recess is located inferior to the glenohumeral joint imaged from the axilla. In addition to assessing for joint fluid, other joint processes such as intra-articular bodies and synovial hypertrophy can be visible in any of the above described joint recesses. Cortical irregularity involving the articular surface of the humerus could represent osteophytes (see Fig. 3-97A), an osteochondral abnormality, subchondral fracture or collapse from osteonecrosis, a Hill Sachs impaction fracture (Fig. 3-99A), or possibly a true erosion if adjacent synovial hypertrophy (Fig. 3-99B and C). The latter should be differentiated anatomically from cortical irregularity of the greater tuberosity, which is not an inflammatory erosion but rather related to rotator cuff tear (see Figs. 3-21 and 3-22). The adjacent hyaline cartilage and humeral head can also be assessed for layering of monosodium urate crystals (called the double contour sign) in gout.73
Glenoid Labrum and Paralabral Cyst
The glenoid labrum is a fibrocartilaginous structure located at the rim of the glenoid, which serves to help stabilize the glenohumeral joint. The normal labrum appears as a hyperechoic, triangular structure attached to the bony glenoid (see Fig. 3-18B).74 Heterogeneous hypoechogenicity of the labrum indicates degeneration, whereas a well-defined hypoechoic or anechoic cleft indicates labral tear (Fig. 3-100) (Video 3-42).67![]() Ultrasound is most helpful when the labrum appears normal; an abnormal-appearing labrum at ultrasound should be followed with MRI or preferably magnetic resonance arthrography to define the labral abnormality. One major limitation of ultrasound in evaluation of the labrum is difficultly in visualizing the entire extent of the labrum. The anterior labrum is more difficult to demonstrate compared with the posterior labrum because of the thickness of the overlying soft tissues; evaluation with dynamic imaging may be helpful.75 Similarly, it is extremely difficult to visualize the superior labrum because of the overlying osseous structures. Nonetheless, routine evaluation of the posterior labrum is easily accomplished during evaluation of the infraspinatus tendon, an area where paralabral cyst formation may occur. It is helpful to evaluate the posterior labrum with the shoulder in external rotation because this position causes joint fluid to locate around the labrum in the posterior glenohumeral joint recess, thereby making a labral tear more conspicuous. The transducer can also be placed over the supraspinatus muscle between the clavicle and scapular spine long axis to the supraspinatus to evaluate for suprascapular notch paralabral cyst.
Ultrasound is most helpful when the labrum appears normal; an abnormal-appearing labrum at ultrasound should be followed with MRI or preferably magnetic resonance arthrography to define the labral abnormality. One major limitation of ultrasound in evaluation of the labrum is difficultly in visualizing the entire extent of the labrum. The anterior labrum is more difficult to demonstrate compared with the posterior labrum because of the thickness of the overlying soft tissues; evaluation with dynamic imaging may be helpful.75 Similarly, it is extremely difficult to visualize the superior labrum because of the overlying osseous structures. Nonetheless, routine evaluation of the posterior labrum is easily accomplished during evaluation of the infraspinatus tendon, an area where paralabral cyst formation may occur. It is helpful to evaluate the posterior labrum with the shoulder in external rotation because this position causes joint fluid to locate around the labrum in the posterior glenohumeral joint recess, thereby making a labral tear more conspicuous. The transducer can also be placed over the supraspinatus muscle between the clavicle and scapular spine long axis to the supraspinatus to evaluate for suprascapular notch paralabral cyst.
When a cystic abnormality is seen adjacent to the glenoid labrum, the diagnosis of paralabral cyst should be considered (Fig. 3-101) (Video 3-43).76![]() In this setting, an underlying labral tear is usually present. Ultrasound may show the joint fluid extension through the labral tear, which communicates with the paralabral cyst analogous to a parameniscal cyst in the knee. One must be aware that paralabral cysts may extend away from the labrum. This may occur at the suprascapular notch (at the superior margin of the scapula) and at the spinoglenoid notch (between the scapula and base of the scapular spine). It is important to scan medial to the glenoid routinely when imaging the infraspinatus tendon in long axis to evaluate for spinoglenoid notch paralabral cyst. One must not mistake a dilated suprascapular vein transiently present in shoulderexternal rotation for a paralabral cyst in the spinoglenoid notch (Fig. 3-102) (see Video 3-14); however, fixed dilation or venous varix may cause suprascapular nerve compression.77 One potential complication of a posterior paralabral cyst is suprascapular nerve entrapment, which may cause denervation and atrophy of the infraspinatus muscle (when spinoglenoid) or of both supraspinatus and infraspinatus muscles (when suprascapular in location), associated with shoulder pain and weakness (see Fig. 3-52). Although visualization of the normal suprascapular nerve may be difficult, identification of the adjacent suprascapular artery with color or power Doppler imaging assists in its localization. The suprascapular nerve and artery extend inferiorly along the posterior margin of the scapular through the spinoglenoid notch. Ultrasound-guided percutaneous aspiration of a paralabral cyst can be performed (see Chapter 9), although the cyst may recur unless the underlying labral tear is repaired or treated.
In this setting, an underlying labral tear is usually present. Ultrasound may show the joint fluid extension through the labral tear, which communicates with the paralabral cyst analogous to a parameniscal cyst in the knee. One must be aware that paralabral cysts may extend away from the labrum. This may occur at the suprascapular notch (at the superior margin of the scapula) and at the spinoglenoid notch (between the scapula and base of the scapular spine). It is important to scan medial to the glenoid routinely when imaging the infraspinatus tendon in long axis to evaluate for spinoglenoid notch paralabral cyst. One must not mistake a dilated suprascapular vein transiently present in shoulderexternal rotation for a paralabral cyst in the spinoglenoid notch (Fig. 3-102) (see Video 3-14); however, fixed dilation or venous varix may cause suprascapular nerve compression.77 One potential complication of a posterior paralabral cyst is suprascapular nerve entrapment, which may cause denervation and atrophy of the infraspinatus muscle (when spinoglenoid) or of both supraspinatus and infraspinatus muscles (when suprascapular in location), associated with shoulder pain and weakness (see Fig. 3-52). Although visualization of the normal suprascapular nerve may be difficult, identification of the adjacent suprascapular artery with color or power Doppler imaging assists in its localization. The suprascapular nerve and artery extend inferiorly along the posterior margin of the scapular through the spinoglenoid notch. Ultrasound-guided percutaneous aspiration of a paralabral cyst can be performed (see Chapter 9), although the cyst may recur unless the underlying labral tear is repaired or treated.
Greater Tuberosity
It is not uncommon to identify a greater tuberosity fracture during routine evaluation of the rotator cuff after trauma. This is because a greater tuberosity fracture may be overlooked at radiography, and the patient may then present for ultrasound to evaluate for rotator cuff tear. At sonography, a greater tuberosity fracture appears as a cortical step-off and discontinuity at the junction of the humeral articular surface and greater tuberosity (Fig. 3-103) (Video 3-44).78 ![]() The distal aspect of the fracture is often seen over the lateral aspect of the greater tuberosity or near the humeral metaphysis. It is important not to mistake the step-off deformity of fracture for cortical irregularity of the greater tuberosity related to rotator cuff tear. In the latter situation, focal pitting and cortical irregularity are present at the supraspinatus footprint, whereas fracture is characterized by a long segment cortical step-off and discontinuity at the margins of the greater tuberosity. Often, there is point tenderness directly over the fracture.
The distal aspect of the fracture is often seen over the lateral aspect of the greater tuberosity or near the humeral metaphysis. It is important not to mistake the step-off deformity of fracture for cortical irregularity of the greater tuberosity related to rotator cuff tear. In the latter situation, focal pitting and cortical irregularity are present at the supraspinatus footprint, whereas fracture is characterized by a long segment cortical step-off and discontinuity at the margins of the greater tuberosity. Often, there is point tenderness directly over the fracture.
Pectoralis Major
Evaluation of the pectoralis major muscle tendon may not be a part of the routine shoulder examination but rather a focused examination directed by a patient’s history or symptoms. The pectoralis muscle consists of two heads, a clavicular head that originates from the medial two thirds of the clavicle, and a sternal head that consists of manubrial and abdominal laminae that originate from the sternum and a portion of the costal cartilage, ribs, and abdominal fascia. As the tendons of the two pectoralis muscular heads extend toward the humerus, they twist 180 degrees so that the clavicular head moves anterior and inferior to the sternal head, which results in the sternal head being superior to the clavicular head at the humerus. The tendon’s attachment is 4 to 6 cm in sagittal length just lateral to the bicipital groove of the humeral diaphysis.79,80
Evaluation of the pectoralis begins with the biceps brachii long head tendon in short axis over the bicipital groove. The transducer is then moved inferior to the subscapularis tendon attachment, where the pectoralis tendon is identified as it extends over the biceps tendon to attach on the humerus (see Fig. 3-4D). It is important to scan superiorly and inferiorly through the entire 4- to 6-cm tendon attachment to ensure complete evaluation, keeping in mind that the sternal head is superior to the clavicular head. Once the pectoralis major tendon is identified, the transducer can be moved medially to visualize the musculotendinous junction and muscle belly.
Tendon tears appear as hypoechoic or anechoic tendon disruption (Fig. 3-104). Tendon retraction indicates full-thickness tear of at least one of the pectoralis heads. The distinction between musculotendinous junction tear and a more distal tendon tear or bone avulsion is important because the latter requires surgery. Hypoechoic edema with a hyperechoic fracture fragment at the humerus indicates avulsion, whereas nonvisualization of the tendon over the biceps brachii long head tendon indicates a distal tendon tear. A musculotendinous junction abnormality with visible distal tendon is characteristic of a musculotendinous tear. Most pectoralis major tears are partial-thickness tears (one head is torn while the other is intact) located at the musculotendinous junction, most commonly of the sternal head. In this situation, the full-thickness tear of the sternal head may only minimally retract as the clavicular head remains intact. Most full-thickness tears usually involve the distal tendon or the attachment of tendon to the humerus.
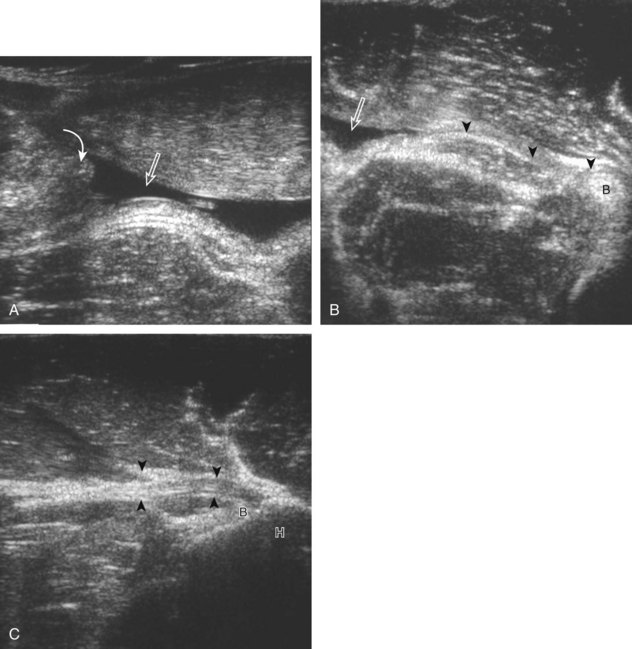
FIGURE 3-104 Pectoralis major tear.
(B and C, From Weaver JS, Jacobson JA, Jamadar DA, et al: Sonographic findings of pectoralis major tears with surgical, clinical, and magnetic resonance imaging correlation in 6 patients. J Ultrasound Med 24:28, 2005. Reproduced with permission from the American Institute of Ultrasound in Medicine.)
Acromioclavicular Joint
The acromioclavicular joint is routinely evaluated in shoulder sonography. If there is difficulty finding this structure, it can be palpated directly or found by following the clavicle laterally. The acromioclavicular joint can also be found by scanning superiorly from the bicipital groove region in the transverse plane. The normal acromioclavicular joint has smooth cortical margins with less than 3 mm of hypoechoic joint capsule distention.81 The intra-articular fibrocartilage disk appears hyperechoic but may be difficult to identify. Several pathologic processes involve the acromioclavicular joint.14 The most common pathologic condition is degenerative osteoarthritis (Fig. 3-105A), seen frequently after the age of 40 years. In this situation, the capsule may be distended, and later there will be bone irregularity, osteophyte formation, and joint space narrowing (often seen with dynamic imaging). The intra-articular fibrocartilage disk is disintegrated in most individuals after the age of 40 years from routine overuse and degenerative change.82 Cysts may be associated with the acromioclavicular joint, which can produce a mass on physical examination (see Fig. 3-105B). This may be the result of glenohumeral joint fluid that tracks into the acromioclavicular joint in the setting of a chronic and massive supraspinatus tendon tear, called the geyser sign (see Fig. 3-105C). If there is widening of the acromioclavicular joint, considerations include trauma and inflammation. In the setting of the acute trauma, one may see widening of the joint, possible elevation of the clavicle, and possible hyperechoic step-off fracture. If symptomatic, it is important to evaluate the acromioclavicular joint dynamically for ligament abnormality (Video 3-45),![]() as described earlier in the section on ultrasound examination techniques (Fig. 3-106).14,15 With more chronic injury, the acromioclavicular joint may be widened as a result of distal clavicular resorption, termed post-traumatic osteolysis, often seen in weight lifters. When widening and cortical irregularity of the distal clavicle and adjacent acromion are associated with fluid or synovial distention of the joint capsule and hyperemia, inflammatory conditions, such as rheumatoid arthritis and infection, should be considered. Infection is more common in intravenous drug abusers and those with septicemia, and ultrasound-guided joint aspiration should be completed to exclude the diagnosis (see Chapter 9) (Fig. 3-107).
as described earlier in the section on ultrasound examination techniques (Fig. 3-106).14,15 With more chronic injury, the acromioclavicular joint may be widened as a result of distal clavicular resorption, termed post-traumatic osteolysis, often seen in weight lifters. When widening and cortical irregularity of the distal clavicle and adjacent acromion are associated with fluid or synovial distention of the joint capsule and hyperemia, inflammatory conditions, such as rheumatoid arthritis and infection, should be considered. Infection is more common in intravenous drug abusers and those with septicemia, and ultrasound-guided joint aspiration should be completed to exclude the diagnosis (see Chapter 9) (Fig. 3-107).
Sternoclavicular Joint
Ultrasound may be used to evaluate the sternoclavicular joint.14 A septic joint appears as distention of the joint capsule, with variable-echogenicity fluid and synovitis (Fig. 3-108). Bone erosions, as well as joint space widening or subluxation, may occur. The findings of joint distention greater than 10 mm that extends over both the clavicle and sternum, elevated Westergren red blood cell sedimentation rate, and fever are strong indicators of infection.83 Ultrasound-guided percutaneous aspiration is recommended if there is any concern for infection. In older women, it is not uncommon for degenerative changes of a sternoclavicular joint with capsular thickening to present as a soft tissue mass. Ultrasound can also diagnose subluxation or dislocation of the sternoclavicular joint (Fig. 3-109). It is important to include dynamic evaluation because subluxation may be dependent on patient arm positioning. Comparison with the contralateral side is important in diagnosing subtle subluxation. Any suspected posterior subluxation or dislocation should be evaluated with computed tomography to assess for adjacent vascular abnormalities (Fig. 3-110).
Miscellaneous Disorders
Lymph node enlargement and other masses can be seen during ultrasound evaluation of the shoulder. A normal axillary lymph node appears oval, with a hypoechoic cortical rim (which decreases in thickness in older age), hyperechoic hilus, and a hilar pattern of vascularity if any. The central hyperechoic area is the result not of fat but rather of the reflective interfaces between the sinusoids and fat (Fig. 3-111A). An axillary lymph node that demonstrates eccentric thickening of the cortex, diffuse thickening of the cortex with hilar thinning, absence of the hilum, roundness, or a peripheral vascular pattern on color or power Doppler imaging suggests malignancy.84 A strict size criterion is not effective because even lymph nodes smaller than 5 mm (largest diameter) have almost 10% risk for malignancy in the setting of breast cancer.85 Often, ultrasound-guided percutaneous or excisional biopsy is required (see Fig. 3-111B and C; Fig. 3-112). Other soft tissue masses, if not originating from a synovial joint, are often not specific for one diagnosis, and ultrasound-guided percutaneous biopsy is usually required.
The identification of a soft tissue mass at ultrasound is often not specific for one diagnosis, although it limits the differential diagnosis by determining whether a mass is cystic or solid. A heterogeneous fluid collection not representing a bursal fluid collection could represent hematoma or abscess (see Chapter 2). One relatively common mass includes a soft tissue lipoma (see Chapter 2). In the setting of limb amputation, a solid soft tissue mass could represent a neuroma. These masses are often hypoechoic but heterogeneous, and continuity between the mass and a peripheral nerve is essential to the diagnosis (Fig. 3-113). Because neuromas normally develop at a transected peripheral nerve, transducer palpation is important to determine which neuroma is symptomatic, to guide appropriate treatment.
There is one tumor-like abnormality that is specific to the shoulder, which is the elastofibroma.86 This is not a true tumor, but rather a pseudotumor of fibroelastic tissue, possibly resulting from mechanical friction between the chest wall and the scapula. At ultrasound, elastofibroma appears heterogeneous and hyperechoic, with interspersed curvilinear hypoechoic strands at ultrasound (Fig. 3-114) (Video 3-46).![]() The key to the diagnosis is the location because 99% of these lesions occur at the scapular tip, deep to the serratus anterior and latissimus dorsi muscles. This pseudotumor is most common in older women and may be bilateral in up to 66%.
The key to the diagnosis is the location because 99% of these lesions occur at the scapular tip, deep to the serratus anterior and latissimus dorsi muscles. This pseudotumor is most common in older women and may be bilateral in up to 66%.
Patients may also present with a palpable abnormality of the chest wall. One such etiology is a normal variant called the sternalis muscle. This variant occurs in 2% to 11% of the population and is located over the most medial aspect of the pectoralis at the sternum, just lateral to midline and elongated parallel to the rectus abdominis.87 At ultrasound, the expected location of a sternalis muscle and the sonographic characteristics of normal muscle tissue allow a correct diagnosis (Fig. 3-115, online). A more common cause of a palpable nodule just below the sternum is the xiphoid process. The variable size, ossification, and shape of the xiphoid process can create a palpable mass. At ultrasound, the location, shape, and ultrasound appearance of either bone or cartilage is characteristic of a xiphoid process (Fig. 3-116). Some individuals present a painful snap associated with the chest wall during activities. The dynamic capabilities of ultrasound are useful to diagnose slipping rib syndrome, in which the abnormal mobility of a lower anterior rib end can cause snapping when it abruptly slips over an adjacent rib or xiphoid process (Fig. 3-117) (Video 3-47).88 ![]()
1 Minagawa H, Itoi E, Konno N, et al. Humeral attachment of the supraspinatus and infraspinatus tendons: an anatomic study. Arthroscopy. 1998;14:302–306.
2 Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20:246–249.
3 Jacobson JA. Shoulder US: anatomy, technique, and scanning pitfalls. Radiology. 2011;260:6–16.
4 Buck FM, Grehn H, Hilbe M, et al. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193:1367–1375.
5 Farin PU, Jaroma H, Harju A, et al. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology. 1995;195:845–848.
6 Crass JR, Craig EV, Feinberg SB. The hyperextended internal rotation view in rotator cuff ultrasonography. J Clin Ultrasound. 1987;15:416–420.
7 Ferri M, Finlay K, Popowich T, et al. Sonography of full-thickness supraspinatus tears: comparison of patient positioning technique with surgical correlation. AJR Am J Roentgenol. 2005;184:180–184.
8 Schaeffeler C, Mueller D, Kirchhoff C, et al. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477–1484.
9 Tuite MJ, Turnbull JR, Orwin JF. Anterior versus posterior, and rim-rent rotator cuff tears: prevalence and MR sensitivity. Skeletal Radiol. 1998;27:237–243.
10 Middleton WD, Reinus WR, Melson GL, et al. Pitfalls of rotator cuff sonography. AJR Am J Roentgenol. 1986;146:555–560.
11 Bretzke CA, Crass JR, Craig EV, et al. Ultrasonography of the rotator cuff: normal and pathologic anatomy. Invest Radiol. 1985;20:311–315.
12 Petchprapa CN, Beltran LS, Jazrawi LM, et al. The rotator interval: a review of anatomy, function, and normal and abnormal MRI appearance. AJR Am J Roentgenol. 2010;195:567–576.
13 Morag Y, Jacobson JA, Lucas D, et al. US appearance of the rotator cable with histologic correlation: preliminary results. Radiology. 2006;241:485–491.
14 Ferri M, Finlay K, Popowich T, et al. Sonographic examination of the acromioclavicular and sternoclavicular joints. J Clin Ultrasound. 2005;33:345–355.
15 Peetrons P, Bedard JP. Acromioclavicular joint injury: enhanced technique of examination with dynamic maneuver. J Clin Ultrasound. 2007;35:262–267.
16 Bureau NJ, Beauchamp M, Cardinal E, et al. Dynamic sonography evaluation of shoulder impingement syndrome. AJR Am J Roentgenol. 2006;187:216–220.
17 Farin PU, Jaroma H, Harju A, et al. Shoulder impingement syndrome: sonographic evaluation. Radiology. 1990;176:845–849.
18 Ryu KN, Lee SW, Rhee YG, et al. Adhesive capsulitis of the shoulder joint: usefulness of dynamic sonography. J Ultrasound Med. 1993;12:445–449.
19 Kavanagh EC, Koulouris G, Parker L, et al. Does extended-field-of-view sonography improve interrater reliability for the detection of rotator cuff muscle atrophy? AJR Am J Roentgenol. 2008;190:27–31.
20 Kim HM, Dahiya N, Teefey SA, et al. Location and initiation of degenerative rotator cuff tears: an analysis of three hundred and sixty shoulders. J Bone Joint Surg Am. 2010;92:1088–1096.
21 Jacobson JA, Lancaster S, Prasad A, et al. Full-thickness and partial-thickness supraspinatus tendon tears: value of US signs in diagnosis. Radiology. 2004;230:234–242.
22 Wohlwend JR, van Holsbeeck M, Craig J, et al. The association between irregular greater tuberosities and rotator cuff tears: a sonographic study. AJR Am J Roentgenol. 1998;171:229–233.
23 Arend CF, da Silva TR. Comparison between exclusively long-axis and multiple-axis sonographic protocols for screening of rotator cuff lesions in symptomatic shoulders. J Ultrasound Med. 2010;29:1725–1732.
24 Teefey SA, Rubin DA, Middleton WD, et al. Detection and quantification of rotator cuff tears: comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86:708–716.
25 de Jesus JO, Parker L, Frangos AJ, et al. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR Am J Roentgenol. 2009;192:1701–1707.
26 van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology. 1995;197:443–446.
27 van Holsbeeck M, Introcaso JH, Kolowich PA. Sonography of tendons: patterns of disease. Instr Course Lect. 1994;43:475–481.
28 Kassarjian A, Torriani M, Ouellette H, et al. Intramuscular rotator cuff cysts: association with tendon tears on MRI and arthroscopy. AJR Am J Roentgenol. 2005;185:160–165.
29 Teefey SA, Middleton WD, Bauer GS, et al. Sonographic differences in the appearance of acute and chronic full-thickness rotator cuff tears. J Ultrasound Med. 2000;19:377–378. quiz 83
30 Strobel K, Hodler J, Meyer DC, et al. Fatty atrophy of supraspinatus and infraspinatus muscles: accuracy of US. Radiology. 2005;237:584–589.
31 Buck FM, Grehn H, Hilbe M, et al. Magnetic resonance histologic correlation in rotator cuff tendons. J Magn Reson Imaging. 2010;32:165–172.
32 Kjellin I, Ho CP, Cervilla V, et al. Alterations in the supraspinatus tendon at MR imaging: correlation with histopathologic findings in cadavers. Radiology. 1991;181:837–841.
33 Bachmann GF, Melzer C, Heinrichs CM, et al. Diagnosis of rotator cuff lesions: comparison of US and MRI on 38 joint specimens. Eur Radiol. 1997;7:192–197.
34 Jin W, Ryu KN, Park YK, et al. Cystic lesions in the posterosuperior portion of the humeral head on MR arthrography: correlations with gross and histologic findings in cadavers. AJR Am J Roentgenol. 2005;184:1211–1215.
35 Tirman PF, Bost FW, Garvin GJ, et al. Posterosuperior glenoid impingement of the shoulder: findings at MR imaging and MR arthrography with arthroscopic correlation. Radiology. 1994;193:431–436.
36 Hollister MS, Mack LA, Patten RM, et al. Association of sonographically detected subacromial/subdeltoid bursal effusion and intraarticular fluid with rotator cuff tear. AJR Am J Roentgenol. 1995;165:605–608.
37 van Holsbeeck M, Strouse PJ. Sonography of the shoulder: evaluation of the subacromial-subdeltoid bursa. AJR Am J Roentgenol. 1993;160:561–564.
38 Farin P, Jaroma H. Sonographic detection of tears of the anterior portion of the rotator cuff (subscapularis tendon tears). J Ultrasound Med. 1996;15:221–225.
39 Morag Y, Jamadar DA, Miller B, et al. The subscapularis: anatomy, injury, and imaging. Skeletal Radiol. 2011;40:255–269.
40 Khoury V, Cardinal E, Brassard P. Atrophy and fatty infiltration of the supraspinatus muscle: sonography versus MRI. AJR Am J Roentgenol. 2008;190:1105–1111.
41 Melis B, DeFranco MJ, Chuinard C, et al. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res. 2010;468:1498–1505.
42 Sofka CM, Lin J, Feinberg J, et al. Teres minor denervation on routine magnetic resonance imaging of the shoulder. Skeletal Radiol. 2004;33:514–518.
43 Friend J, Francis S, McCulloch J, et al. Teres minor innervation in the context of isolated muscle atrophy. Surg Radiol Anat. 2010;32:243–249.
44 McClelland D, Paxinos A. The anatomy of the quadrilateral space with reference to quadrilateral space syndrome. J Shoulder Elbow Surg. 2008;17:162–164.
45 Cothran RL, Jr., Helms C. Quadrilateral space syndrome: incidence of imaging findings in a population referred for MRI of the shoulder. AJR Am J Roentgenol. 2005;184:989–992.
46 Brestas PS, Tsouroulas M, Nikolakopoulou Z, et al. Ultrasound findings of teres minor denervation in suspected quadrilateral space syndrome. J Clin Ultrasound. 2006;34:343–347.
47 Prickett WD, Teefey SA, Galatz LM, et al. Accuracy of ultrasound imaging of the rotator cuff in shoulders that are painful postoperatively. J Bone Joint Surg Am. 2003;85:1084–1089.
48 Jacobson JA, Miller B, Bedi A, et al. Imaging of the postoperative shoulder. Semin Musculoskelet Radiol. 2011;15:320–339.
49 Fealy S, Adler RS, Drakos MC, et al. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34:120–127.
50 Miller BS, Downie BK, Kohen RB, et al. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39:2064–2070.
51 Nho SJ, Adler RS, Tomlinson DP, et al. Arthroscopic rotator cuff repair: prospective evaluation with sequential ultrasonography. Am J Sports Med. 2009;37:1938–1945.
52 Miller TT. Sonography of joint replacements. Semin Musculoskelet Radiol. 2006;10:79–85.
53 Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol. 2003;180:1117–1120.
54 Serafini G, Sconfienza LM, Lacelli F, et al. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle US guided percutaneous treatment—nonrandomized controlled trial. Radiology. 2009;252:157–164.
55 Flemming DJ, Murphey MD, Shekitka KM, et al. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol. 2003;181:965–972.
56 Farin PU. Consistency of rotator-cuff calcifications: observations on plain radiography, sonography, computed tomography, and at needle treatment. Invest Radiol. 1996;31:300–304.
57 Chiou HJ, Chou YH, Wu JJ, et al. Evaluation of calcific tendonitis of the rotator cuff: role of color Doppler ultrasonography. J Ultrasound Med. 2002;21:289–295. quiz 96–97
58 Le Goff B, Berthelot JM, Guillot P, et al. Assessment of calcific tendonitis of rotator cuff by ultrasonography: comparison between symptomatic and asymptomatic shoulders. Joint Bone Spine. 2010;77:258–263.
59 Wang YC, Wang HK, Chen WS, et al. Dynamic visualization of the coracoacromial ligament by ultrasound. Ultrasound Med Biol. 2009;35:1242–1248.
60 Tracy MR, Trella TA, Nazarian LN, et al. Sonography of the coracohumeral interval: a potential technique for diagnosing coracoid impingement. J Ultrasound Med. 2010;29:337–341.
61 Stallenberg B, Destate N, Feipel V, et al. Involvement of the anterior portion of the subacromial-subdeltoid bursa in the painful shoulder. AJR Am J Roentgenol. 2006;187:894–900.
62 Griesser MJ, Harris JD, Campbell JE, et al. Adhesive capsulitis of the shoulder: a systematic review of the effectiveness of intra-articular corticosteroid injections. J Bone Joint Surg Am. 2011;93:1727–1733.
63 Homsi C, Bordalo-Rodrigues M, da Silva JJ, et al. Ultrasound in adhesive capsulitis of the shoulder: is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006;35:673–678.
64 Lee JC, Sykes C, Saifuddin A, et al. Adhesive capsulitis: sonographic changes in the rotator cuff interval with arthroscopic correlation. Skeletal Radiol. 2005;34:522–527.
65 Turrin A, Cappello A. Sonographic anatomy of the supraspinatus tendon and adjacent structures. Skeletal Radiol. 1997;26:89–93.
66 Seibold CJ, Mallisee TA, Erickson SJ, et al. Rotator cuff: evaluation with US and MR imaging. Radiographics. 1999;19:685–705.
67 Tirman PF, Feller JF, Janzen DL, et al. Association of glenoid labral cysts with labral tears and glenohumeral instability: radiologic findings and clinical significance. Radiology. 1994;190:653–658.
68 Zubler V, Mamisch-Saupe N, Pfirrmann CW, et al. Detection and quantification of glenohumeral joint effusion: reliability of ultrasound. Eur Radiol. 2011;21:1858–1864.
69 Breidahl WH, Stafford Johnson DB, Newman JS, et al. Power Doppler sonography in tenosynovitis: significance of the peritendinous hypoechoic rim. J Ultrasound Med. 1998;17:103–107.
70 Skendzel JG, Jacobson JA, Carpenter JE, et al. Long head of biceps brachii tendon evaluation: accuracy of preoperative ultrasound. AJR Am J Roentgenol. 2011;197:942–948.
71 Rutten MJ, de Jong MD, van Loon T, et al. Intratendinous ganglion of the long head of the biceps tendon: US and MRI features (2010: 9b). Intratendinous ganglion. Eur Radiol. 2010;20:2997–3001.
72 Grainger AJ, Tirman PF, Elliott JM, et al. MR anatomy of the subcoracoid bursa and the association of subcoracoid effusion with tears of the anterior rotator cuff and the rotator interval. AJR Am J Roentgenol. 2000;174:1377–1380.
73 Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30:495–503.
74 Taljanovic MS, Carlson KL, Kuhn JE, et al. Sonography of the glenoid labrum: a cadaveric study with arthroscopic correlation. AJR Am J Roentgenol. 2000;174:1717–1722.
75 Rasmussen OS. Anterior shoulder instability: sonographic evaluation. J Clin Ultrasound. 2004;32:430–437.
76 Hashimoto BE, Hayes AS, Ager JD. Sonographic diagnosis and treatment of ganglion cysts causing suprascapular nerve entrapment. J Ultrasound Med. 1994;13:671–674.
77 Carroll KW, Helms CA, Otte MT, et al. Enlarged spinoglenoid notch veins causing suprascapular nerve compression. Skeletal Radiol. 2003;32:72–77.
78 Patten RM, Mack LA, Wang KY, et al. Nondisplaced fractures of the greater tuberosity of the humerus: sonographic detection. Radiology. 1992;182:201–204.
79 Rehman A, Robinson P. Sonographic evaluation of injuries to the pectoralis muscles. AJR Am J Roentgenol. 2005;184:1205–1211.
80 Weaver JS, Jacobson JA, Jamadar DA, et al. Sonographic findings of pectoralis major tears with surgical, clinical, and magnetic resonance imaging correlation in 6 patients. J Ultrasound Med. 2005;24:25–31.
81 Alasaarela E, Tervonen O, Takalo R, et al. Ultrasound evaluation of the acromioclavicular joint. J Rheumatol. 1997;24:1959–1963.
82 Simovitch R, Sanders B, Ozbaydar M, et al. Acromioclavicular joint injuries: diagnosis and management. J Am Acad Orthop Surg. 2009;17:207–219.
83 Johnson MC, Jacobson JA, Fessell DP, et al. The sternoclavicular joint: can imaging differentiate infection from degenerative change? Skeletal Radiol. 2010;39:551–558.
84 Esen G, Gurses B, Yilmaz MH, et al. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur Radiol. 2005;15:1215–1223.
85 Obwegeser R, Lorenz K, Hohlagschwandtner M, et al. Axillary lymph nodes in breast cancer: is size related to metastatic involvement? World J Surg. 2000;24:546–550.
86 Bianchi S, Martinoli C, Abdelwahab IF, et al. Elastofibroma dorsi: sonographic findings. AJR Am J Roentgenol. 1997;169:1113–1115.
87 Nuthakki S, Gross M, Fessell D. Sonography and helical computed tomography of the sternalis muscle. J Ultrasound Med. 2007;26:247–250.
88 Meuwly JY, Wicky S, Schnyder P, et al. Slipping rib syndrome: a place for sonography in the diagnosis of a frequently overlooked cause of abdominal or low thoracic pain. J Ultrasound Med. 2002;21:339–343.