Shock, Sepsis, and Multiple Organ Dysfunction Syndrome
Beverly Carlson, Lorraine Fitzsimmons and Christopher Walker
Objectives
• Describe the generalized shock response and systemic inflammatory response.
• Explain the pathophysiology of the five forms of shock and MODS.
• Identify the clinical manifestations of the five forms of shock and MODS.
• Summarize the nursing priorities for managing a patient with each type of shock or MODS.
![]()
Be sure to check out the bonus material, including free self-assessment exercises, on the Evolve web site at
http://evolve.elsevier.com/Urden/priorities/.
Shock is an acute, widespread process of impaired tissue perfusion that results in cellular, metabolic, and hemodynamic alterations. Ineffective tissue perfusion occurs when an imbalance develops between cellular oxygen supply and cellular oxygen demand. This imbalance can occur for a variety of reasons and eventually results in cellular dysfunction and death. This chapter presents an overview of the general shock response, or shock syndrome, followed by a discussion of the various shock states.
Shock Syndrome
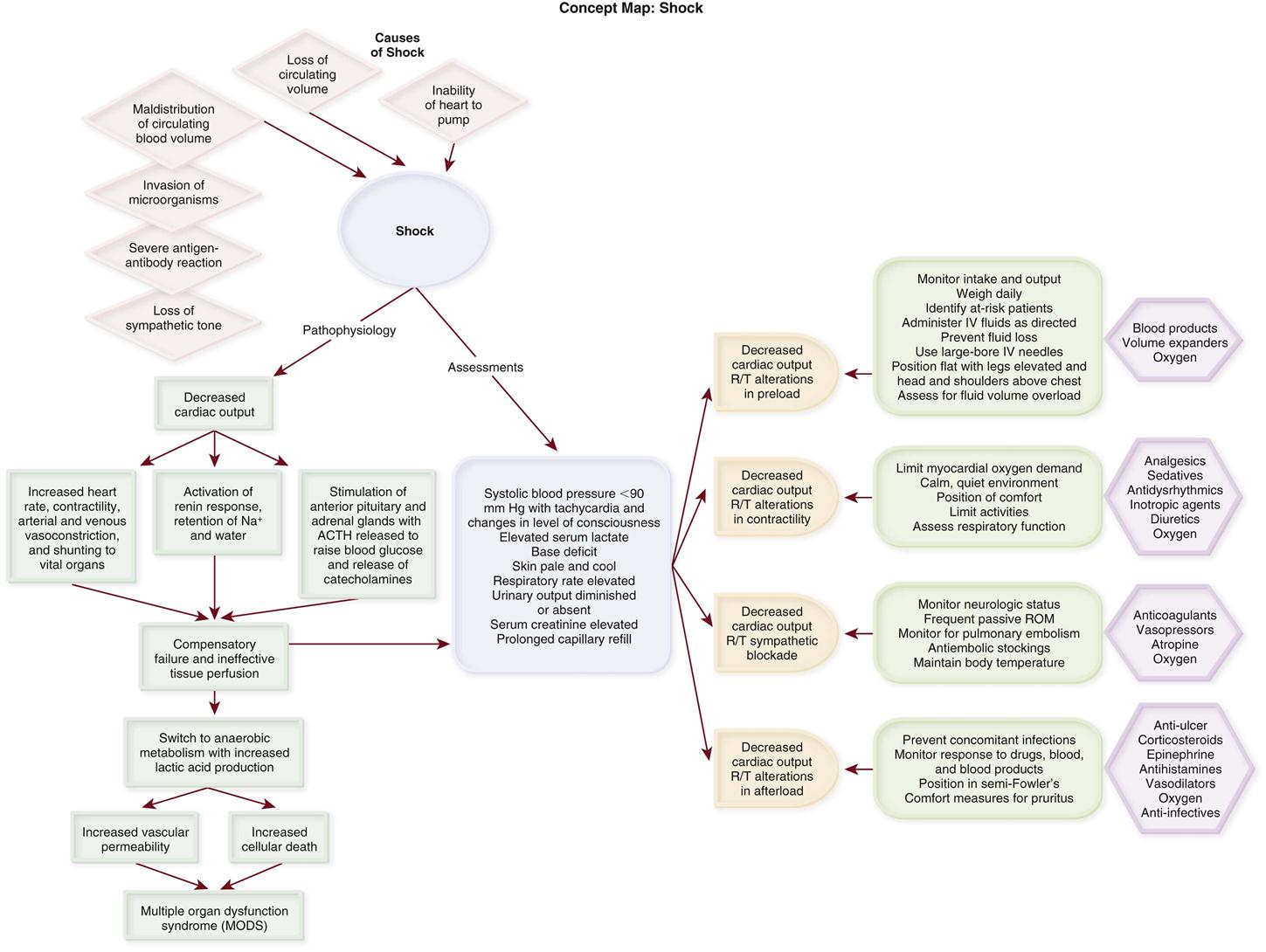
Shock is a complex pathophysiological process that often results in multiple organ dysfunction syndrome (MODS) and death. All types of shock eventually result in ineffective tissue perfusion and acute circulatory failure. The shock syndrome is a pathway involving a variety of pathological processes that may be categorized as four stages: initial, compensatory, progressive, and refractory. Progression through each stage varies with the patient’s prior condition, duration of initiating event, response to therapy, and correction of underlying cause.
Etiology
Shock can be classified as hypovolemic, cardiogenic, or distributive, depending on the pathophysiological cause and hemodynamic profile. Hypovolemic shock results from a loss of circulating or intravascular volume. Cardiogenic shock results from the impaired ability of the heart to pump. Distributive shock results from maldistribution of circulating blood volume and can be further classified as septic, anaphylactic, or neurogenic. Septic shock is the result of microorganisms entering the body. Anaphylactic shock is the result of a severe antibody-antigen reaction. Neurogenic shock is the result of the loss of sympathetic tone.
Pathophysiology
During the initial stage, cardiac output (CO) is decreased, and tissue perfusion is threatened. Almost immediately, the compensatory stage begins as the body’s homeostatic mechanisms attempt to maintain CO, blood pressure, and tissue perfusion. The compensatory mechanisms are mediated by the sympathetic nervous system (SNS) and consist of neural, hormonal, and chemical responses. The neural response includes an increase in heart rate and contractility, arterial and venous vasoconstriction, and shunting of blood to the vital organs. Hormonal compensation includes activation of the renin response and stimulation of the anterior pituitary and adrenal medulla. Activation of the renin response results in the production of angiotensin II, which causes vasoconstriction and the release of aldosterone and antidiuretic hormone (ADH), leading to sodium and water retention. Stimulation of the anterior pituitary results in the secretion of adrenocorticotropic hormone (ACTH), which stimulates the adrenal cortex to produce glucocorticoids, causing a rise in blood glucose levels. Stimulation of the adrenal medulla causes the release of epinephrine and norepinephrine, which further enhance the compensatory mechanisms.
During the progressive stage, the compensatory mechanisms begin failing to meet tissue metabolic needs, and the shock cycle is perpetuated (Concept Map: Shock). As tissue perfusion becomes ineffective, the cells switch from aerobic to anaerobic metabolism to produce energy. Anaerobic metabolism produces small amounts of energy but large amounts of lactic acid, producing lactic acidemia. Increased vascular permeability from endothelial and epithelial hypoxia and inflammatory mediators results in intravascular hypovolemia, tissue edema, and further decline in tissue perfusion.1,2 A systemic release of inflammatory mediators in response to tissue hypoxia, especially in gut tissue, produces microcirculatory impairment and derangement of cellular metabolism, facilitating progression of the shock cycle.1–3 The patient is experiencing the systemic inflammatory response syndrome (SIRS), and irreversible damage begins to occur. Some cells die as a result of apoptosis, an injury-activated, preprogrammed cellular suicide. Others die as the sodium-potassium pump in the cell membrane fails, causing the cell and its organelles to swell. Cellular energy production comes to a complete halt as the mitochondria swell and rupture. At this point, the problem becomes one of oxygen use instead of oxygen delivery. Even if the cell were to receive more oxygen, it would be unable to use it because of damage to the mitochondria. The cell’s digestive organelles swell and leak destructive enzymes into the cell, accelerating cell death.3
Every system in the body is affected by this process (Box 26-1). Cardiac dysfunction develops as a result of the release of myocardial depressant cytokines.1,2 Ventricular failure eventually occurs, further perpetuating the entire process. Central nervous system (CNS) dysfunction develops as a result of cerebral hypoperfusion, leading to failure of the SNS, cardiac and respiratory depression, and thermoregulatory failure. Endothelial injury from hypoxia and inflammatory cytokines and impaired blood flow result in microvascular thrombosis. Hematological dysfunction occurs as a result of consumption of clotting factors, release of inflammatory cytokines, and dilutional thrombocytopenia. Disseminated intravascular coagulation (DIC) eventually may develop. Pulmonary dysfunction occurs as a result of increased pulmonary capillary membrane permeability, pulmonary microemboli, and pulmonary vasoconstriction. Ventilatory failure and acute lung injury (ALI) develop. Renal dysfunction develops as a result of renal vasoconstriction and renal hypoperfusion, leading to acute tubular necrosis (ATN). Gastrointestinal dysfunction occurs as a result of splanchnic vasoconstriction and hypoperfusion and leads to failure of the gut organs. Disruption of the intestinal epithelium releases gram-negative bacteria into the system, which further perpetuates the entire shock syndrome.4
During the refractory stage, shock becomes unresponsive to therapy and is considered irreversible. As the individual organ systems die, MODS—defined as failure of two or more body systems—occurs. Death is the final outcome. Regardless of the etiological factors, death occurs from ineffective tissue perfusion because of the failure of the circulation to meet the oxygen needs of the cell.3
Assessment and Diagnosis
The patient with a mean arterial blood pressure (MAP) less than 60 mm Hg or with evidence of multisystem organ hypoperfusion is considered to be in a shock state.1,5 Because shock is a dynamic physiological phenomenon, hypotension may occur late in the process.6 Clinical manifestations vary according to the underlying cause of shock, the stage of the shock, and the patient’s response to shock.
Compensatory mechanisms may produce normal hemodynamic values even when tissue perfusion is compromised.4–7 Global indicators of systemic perfusion and oxygenation include serum lactate, arterial base deficit, serum bicarbonate, and central or mixed venous oxygen saturation levels. Inadequate cellular oxygenation with anaerobic metabolism and increased metabolic lactate production increase the serum lactate level.8 The level and duration of this hyperlactatemia are predictive of morbidity and mortality.7–11 The base deficit derived from arterial blood gas (ABG) values also reflects global tissue acidosis and is frequently used to assess the severity of shock.6,7,9 Studies have demonstrated serum bicarbonate to be an equivalent alternative to arterial base deficit in predicting mortality in surgical and trauma patients.12,13 The use of mixed venous oxygen saturation (SvO2) measured by means of a pulmonary artery catheter or central venous oxygen saturation (SCvO2) measured with a central venous catheter allows assessment of the balance of oxygen delivery and oxygen consumption and the ratio of oxygen extraction.14–16 After years of recommended use to guide the care of patients with severe sepsis, this measure of global oxygen balance is being evaluated for use in other critically ill populations.15–21 The sections on different types of shock discuss clinical assessment and diagnosis of the patient in shock.
Medical Management
The major focus of the treatment of shock is the improvement and preservation of tissue perfusion. Adequate tissue perfusion depends on an adequate supply of oxygen being transported to the tissues and the cell’s ability to use it. Oxygen transport is influenced by pulmonary gas exchange, CO, and hemoglobin level. Oxygen use is influenced by the internal metabolic environment. Management of the patient in shock focuses on supporting oxygen delivery.1,22
Adequate pulmonary gas exchange is critical to oxygen transport. Establishing and maintaining an adequate airway are the first steps in ensuring adequate oxygenation. After the airway is patent, emphasis is placed on improving ventilation and oxygenation. Therapies include administration of supplemental oxygen and mechanical ventilatory support.
An adequate CO and hemoglobin level are crucial to oxygen transport. CO depends on heart rate, preload, afterload, and contractility. A variety of fluids and drugs are used to manipulate these parameters. The types of fluids used include crystalloids and colloids. The categories of drugs used include vasoconstrictors, vasodilators, positive inotropes, and antidysrhythmics.
Fluid administration is indicated for decreased preload related to intravascular volume depletion, and it can be accomplished by use of a crystalloid or colloid solution, or both. Crystalloids are balanced electrolyte solutions that may be hypotonic, isotonic, or hypertonic. Examples of crystalloid solutions used in shock situations are normal saline and lactated Ringer’s solution. Colloids are protein- or starch-containing solutions. Examples of colloid solutions are blood and blood components, such as albumin, and pharmaceutical plasma expanders, such as hetastarch, dextran, and mannitol.
The choice of fluid is a subject of debate and depends on the situation.15,23–25 Fluid resuscitation with normal saline or with albumin produces similar outcomes regardless of baseline serum albumin level, and both are considered safe.26,27 Crystalloid solutions are inexpensive and effective. Advantages of colloids include faster restoration of intravascular volume and use of smaller amounts. Colloids are believed to stay in the intravascular space, unlike crystalloids, which readily leak into the extravascular space. Disadvantages include expense, allergic reactions, and difficulties in typing and cross-matching blood. Colloids also can leak out of damaged capillaries and cause a variety of additional problems, particularly in the lungs.
Blood should be considered to augment oxygen transport if the patient’s hemoglobin level is critically low, although controversy exists about what threshold value should be used.4,15 Transfusion of stored red blood cells does not substantially increase oxygen consumption and has been associated with immunosuppression, infection, impairment of microcirculatory flow, coagulopathy, and increased mortality. Restrictive transfusion practice has demonstrated lower mortality.4,16,28 Transfusion-related acute lung injury (TRALI) resulting from immune and nonimmune neutrophil activation has become the leading cause of transfusion-related death and may occur with transfusion of any plasma-containing blood or blood product.28–30
Vasoconstrictor agents are used to increase afterload by increasing the systemic vascular resistance (SVR) and improving the patient’s blood pressure level. Vasodilator agents are used to decrease preload or afterload, or both, by decreasing venous return and SVR. Positive inotropic agents are used to increase contractility. Antidysrhythmic agents are used to influence heart rate.31 Box 26-2 provides examples of each of these agents.
Sodium bicarbonate is not recommended in the treatment of shock-related lactic acidosis.16,32,33 No overall benefit has been found, and the risks associated with its use are significant. They include shifting of the oxyhemoglobin dissociation curve to the left, rebound increase in lactic acid production, development of hyperosmolar state, fluid overload resulting from excessive sodium, and rapid cellular electrolyte shifts.11,32,33
The patient should be started on nutritional support therapy as early as possible. The type of nutritional supplementation initiated varies according to the cause of shock, and it should be tailored to the individual patient’s needs, as indicated by the underlying condition and laboratory data. The enteral route is preferred over the parenteral, although parental nutrition should be considered when enteral feeding is contraindicated.25,34–37 Supplementation of enteral feeding with parenteral nutrition to increase caloric intake should be considered but has not been shown to improve patient outcomes.35–37
Glucose control is recommended for all critically ill patients.38,39 Benefits of glucose control in the critically ill include lower incidences of infection, renal failure, sepsis, polyneuropathy, need for blood transfusion, prolonged mechanical ventilation, and death.38–42
Nursing Management
The nursing management of a patient in shock is a complex and challenging responsibility. It requires an in-depth understanding of the pathophysiology of the disease and the anticipated effects of each intervention, as well as a solid understanding of the nursing process. Later sections discuss specific interventions for the patient in shock.
The psychosocial needs of the patient and family dealing with shock are extremely important. These needs are based on situational, familial, and patient-centered variables. Nursing priorities in managing the patient in shock are directed toward (1) providing information on patient status, (2) explaining procedures and routines, (3) supporting the family, (4) encouraging the expression of feelings, (5) facilitating problem solving and shared decision making, (6) individualizing visitation schedules, (7) involving the family in the patient’s care, and (8) establishing contacts with necessary resources.43 Patients and families should be given the option of family presence during invasive procedures and resuscitation.43–45 Collaborative management of the patient with shock is outlined in the Collaborative Management Box on Shock.
Hypovolemic Shock
Hypovolemic shock occurs from inadequate fluid volume in the intravascular space. The lack of adequate circulating volume leads to decreased tissue perfusion and initiation of the general shock response. Hypovolemic shock is the most commonly occurring form of shock.
Etiology
Hypovolemic shock can result from absolute or relative hypovolemia. Absolute hypovolemia occurs when there is a loss of fluid from the intravascular space. This can result from an external loss of fluid from the body or from internal shifting of fluid from the intravascular space to the extravascular space. Fluid shifts can result from a loss of intravascular integrity, increased capillary membrane permeability, or decreased colloidal osmotic pressure. Relative hypovolemia occurs when vasodilation produces an increase in vascular capacitance relative to circulating volume (Box 26-3).
Pathophysiology
Hypovolemia results in a loss of circulating fluid volume. A decrease in circulating volume leads to a decrease in venous return, which results in a decrease in end-diastolic volume or preload. Preload is a major determinant of stroke volume (SV) and CO. A decrease in preload results in a decrease in SV and CO. The decrease in CO leads to inadequate cellular oxygen supply and ineffective tissue perfusion (Figure 26-1).
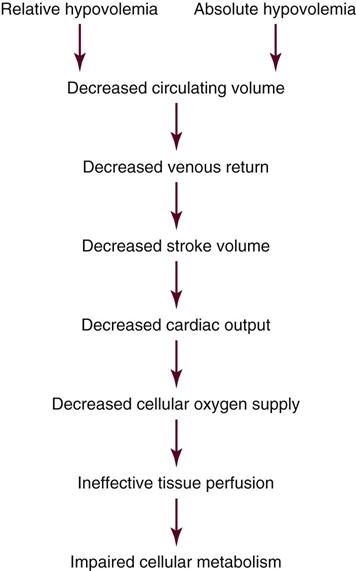
Assessment and Diagnosis
The clinical manifestations of hypovolemic shock depend on the severity of fluid loss and the patient’s ability to compensate for it. Clinical classes have been developed by the American College of Surgeons to describe the levels of severity of hypovolemic shock. Class I indicates a fluid volume loss up to 15% or an actual volume loss up to 750 mL. Compensatory mechanisms maintain CO, and the patient appears free of symptoms other than slight anxiety.4,46
Class II hypovolemia occurs with a fluid volume loss of 15% to 30% or an actual volume loss of 750 to 1500 mL. Falling CO activates more intense compensatory responses. The heart rate increases to more than 100 beats/minute in response to increased SNS stimulation unless blocked by preexisting beta-blocker therapy. The pulse pressure narrows as the diastolic blood pressure increases because of vasoconstriction. The respiratory rate increases to 20 to 30 breaths/minute, and respiratory depth increases in an attempt to improve oxygenation. ABG specimens drawn during this phase reveal respiratory alkalosis and hypoxemia, as evidenced by a low partial pressure of carbon dioxide (PaCO2) and a low partial pressure of oxygen (PaO2), respectively. Urine output starts to decline to 20 to 30 mL/hour as renal perfusion decreases. The urine sodium level decreases, whereas urinary osmolality and specific gravity increase as the kidneys start to conserve sodium and water. The patient’s skin becomes pale and cool with delayed capillary refill because of peripheral vasoconstriction. Jugular veins appear flat as a result of decreased venous return.4,46
Hypovolemic shock that is class III occurs with a fluid volume loss of 30% to 40% or an actual volume loss of 1500 to 2000 mL. This level of severity produces the progressive stage of shock as compensatory mechanisms become overwhelmed and ineffective tissue perfusion develops. Blood pressure decreases. The heart rate increases to more than 120 beats/minute, and dysrhythmias develop as myocardial ischemia ensues. Respiratory distress occurs as the pulmonary system deteriorates. ABG values during this phase reveal respiratory and metabolic acidosis and hypoxemia, as evidenced by a high PaCO2, low bicarbonate (HCO3–), and low PaO2, respectively. Decreased renal perfusion results in the development of oliguria. Blood urea nitrogen (BUN) and serum creatinine levels start to rise as the kidneys begin to fail. The patient’s skin becomes ashen, cold, and clammy, with marked delayed capillary refill. The patient appears confused as cerebral perfusion decreases and the level of consciousness deteriorates.4,46
Class IV hypovolemic shock is usually refractory in nature. It occurs with a fluid volume loss of greater than 40% or an actual volume loss of more than 2000 mL. The compensatory mechanisms of the body completely deteriorate, and organ failure occurs.5 Severe tachycardia and hypotension ensue. Peripheral pulses are absent, and because of marked peripheral vasoconstriction, capillary refill does not occur. The skin appears cyanotic, mottled, and extremely diaphoretic. Urine output ceases. The patient becomes lethargic and unresponsive, and various clinical manifestations associated with failure of the different body systems develop.4,46
Assessment of the hemodynamic parameters of a patient in hypovolemic shock varies by stage but commonly reveals a decreased CO and cardiac index (CI). Loss of circulating volume leads to a decrease in venous return to the heart, which results in a decrease in the preload of the right and left ventricles. This is evidenced by a decline in the central venous pressure (CVP) or right atrial pressure (RAP) and pulmonary artery occlusion pressure (PAOP). Vasoconstriction of the arterial system results in an increase in the afterload of the heart, as evidenced by an increase in the SVR. This vasoconstriction may produce a falsely elevated systolic blood pressure when measured by arterial catheter. MAP is more accurate in this low-flow state.5
Medical Management
The major goals of therapy for the patient in hypovolemic shock are to correct the cause of the hypovolemia, restore tissue perfusion, and prevent complications. This approach includes identifying and stopping the source of fluid loss and administering fluid to replace circulating volume. Fluid administration can be accomplished with use of a crystalloid solution, a colloid solution, blood products, or a combination of fluids. The type of solution used depends on the type of fluid lost, the degree of hypovolemia, the severity of hypoperfusion, and the cause of hypovolemia.
Aggressive fluid resuscitation in trauma and surgical patients is the subject of great debate. The benefit of limited or hypotensive (systolic blood pressure >80 mm Hg) volume resuscitation in patients with uncontrolled hemorrhage is postulated to lessen bleeding and improve survival.22,47–49 The type and amount of solutions used for fluid resuscitation and the rate of administration influence immune function, inflammatory mediator release, coagulation, and the incidence of cardiac, pulmonary, and gastrointestinal complications.23,24,50–52 Consensus on the optimal resuscitative strategy for hypovolemic shock is lacking.23,24,48
Nursing Management
Prevention of hypovolemic shock is one of the primary responsibilities of the nurse in the critical care area. Preventive measures include the identification of patients at risk and frequent assessment of the patient’s fluid balance. Accurate monitoring of intake and output and daily weights are essential components of preventive nursing care. Early identification and treatment result in decreased mortality.
Management of the patient in hypovolemic shock requires continuous evaluation of intravascular volume, tissue perfusion, and response to therapy. The patient in hypovolemic shock may have any number of nursing diagnoses, depending on the progression of the process (Nursing Diagnosis Priorities Box on Hypovolemic Shock). Nursing priorities are directed toward (1) minimizing fluid loss, (2) administering volume replacement, (3) providing comfort and emotional support, and (4) maintaining surveillance for complications.
Measures to minimize fluid loss include limiting blood sampling, observing lines for accidental disconnection, and applying direct pressure to bleeding sites. Measures to facilitate the administration of volume replacement include insertion of large-bore peripheral intravenous catheters, rapid administration of prescribed fluids, and positioning the patient with the legs elevated, trunk flat, and head and shoulders above the chest. Monitoring the patient for clinical manifestations of fluid overload or complications related to fluid and blood product administration is essential for preventing further problems.
Cardiogenic Shock
Cardiogenic shock is the result of failure of the heart to effectively pump blood forward. It can occur with dysfunction of the right or the left ventricle, or both. The lack of adequate pumping function leads to decreased tissue perfusion and circulatory failure. It occurs in approximately 5% to 8% of the patients with an ST-segment myocardial infarction (MI), and it is the leading cause of death of patients hospitalized with MI.53,54 The mortality rate for cardiogenic shock has decreased with the advent of early revascularization therapy and is currently about 47% to 60%.53–56
Etiology
Cardiogenic shock can result from primary ventricular ischemia, structural problems, and dysrhythmias.53,54 The most common cause is acute MI resulting in the loss of 40% or more of the functional myocardium. It can occur with ST-elevation or non-ST-elevation MI.54,57 The damage to the myocardium may occur after one massive MI (usually of the anterior wall), or it may be cumulative as a result of several smaller MIs or a small MI in a patient with preexisting ventricular dysfunction.53,57 End-stage cardiomyopathy may also cause cardiogenic shock as may structural problems of the cardiopulmonary system and dysrhythmias if they disrupt the forward motion of the blood through the heart (Box 26-4).53,54,57
Pathophysiology
Cardiogenic shock results from the impaired ability of the ventricle to pump blood forward, which leads to a decrease in SV and an increase in the blood left in the ventricle at the end of systole. The decrease in SV results in a decrease in CO, which leads to decreased cellular oxygen supply and ineffective tissue perfusion. Typically, myocardial performance spirals downward as compensatory vasoconstriction increases myocardial afterload and low blood pressure worsens myocardial ischemia. Evidence of SIRS has been observed in a substantial number of patients with cardiogenic shock.55,57–59 Activation of inflammatory cytokines induce systemic vasodilation, defective cellular oxygen use, and occasionally, normalization of the CO. Whether this process contributes to the genesis or the outcome of cardiogenic shock is uncertain, but it is thought to be activated by acute MI and to facilitate development of sepsis.55,57,58 As left ventricular contractility declines and ventricular compliance decreases, an increase in end-systolic volume results in blood backing up into the pulmonary system and the subsequent development of pulmonary edema. Pulmonary edema causes impaired gas exchange and decreased oxygenation of the arterial blood, which further impair tissue perfusion (Figure 26-2). Death due to cardiogenic shock may result from multiple organ failure or cardiopulmonary collapse.55,58
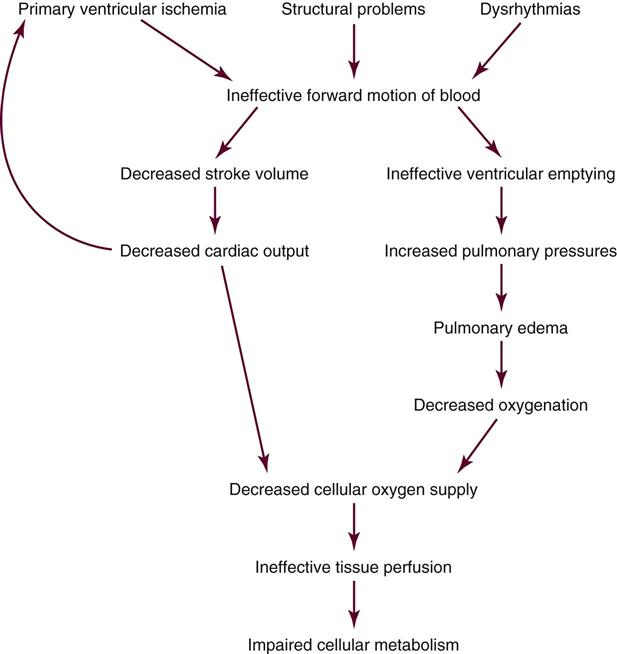
Assessment and Diagnosis
A variety of clinical manifestations occur in the patient in cardiogenic shock, depending on etiological factors in pump failure, the patient’s underlying medical status, and the severity of the shock state. Some clinical manifestations are caused by failure of the heart as a pump, whereas many are related to the overall shock response (Box 26-5).
Initially, clinical manifestations reflect the decline in CO. These signs and symptoms include systolic blood pressure less than 90 mm Hg or an acute drop in systolic or mean blood pressure of 30 mm Hg or more; decreased sensorium; cool, pale, moist skin; and urine output of less than 30 mL/hour.57,60 The patient also may complain of chest pain. Tachycardia develops to compensate for the decrease in CO. A weak, thready pulse develops, and diminished S1 and S2 heart sounds may occur as a result of the decreased contractility. The respiratory rate increases to improve oxygenation. ABG values at this point indicate respiratory alkalosis, as evidenced by a decrease in PaCO2. Urinalysis findings demonstrate a decrease in urine sodium level and an increase in urine osmolality and specific gravity as the kidneys start to conserve sodium and water. The patient also may experience a variety of dysrhythmias, depending on the underlying problem.54
As the left ventricle fails, auscultation of the lungs may disclose crackles and rhonchi, indicating the development of pulmonary edema. Hypoxemia occurs, as evidenced by a fall in PaO2 and SaO2 as measured by ABG values. Heart sounds may reveal an S3 and S4. Jugular venous distention is evident with right-sided failure.
Assessment of the hemodynamic parameters of a patient in cardiogenic shock reveals a decreased CO with a CI less than 2.2 L/minute/m2 in the presence of an elevated PAOP of more than 15 to 18 mm Hg.53,54,57,59 A proportional pulse pressure (systolic BP/pulse pressure) less than 25% is indicative of left ventricular failure and a CI less than 2.2 and may be useful when direct measurement of CI is unavailable.61 Increased filling pressures are necessary to rule out hypovolemia as the cause of circulatory failure. The increase in PAOP reflects an increase in the left ventricular end-diastolic pressure (LVEDP) and left ventricular end-diastolic volume (LVEDV) resulting from decreased SV. With right ventricular failure, the RAP also increases. Compensatory vasoconstriction results in an increase in the afterload of the heart, as evidenced by an increase in the SVR. Echocardiography confirms the diagnosis of cardiogenic shock and rules out other causes of circulatory failure.53,54,57
As compensatory mechanisms fail and ineffective tissue perfusion develops, other clinical manifestations appear. Myocardial ischemia progresses, as evidenced by continued increases in heart rate, dysrhythmias, and chest pain. Pulmonary function deteriorates, which leads to respiratory distress. ABG values during this phase reveal respiratory and metabolic acidosis and hypoxemia, as indicated by a high PaCO2, low HCO3–, and low PaO2, respectively. Renal failure occurs, as exhibited by the development of anuria and increases in BUN and serum creatinine levels. Cerebral hypoperfusion manifests as a decreasing level of consciousness.
Medical Management
Treatment of the patient in cardiogenic shock requires an aggressive approach. The major goals of therapy are to treat the underlying cause, enhance the effectiveness of the pump, and improve tissue perfusion. This approach includes identifying the etiological factors of pump failure and administering pharmacological agents to enhance CO. Inotropic agents are used to increase contractility and maintain adequate blood pressure and tissue perfusion. A vasopressor may be necessary to maintain blood pressure when hypotension is severe.57,60 Diuretics are used for preload reduction. After blood pressure has been stabilized, vasodilating agents are used for preload and afterload reduction. Antidysrhythmic agents should be used to suppress or control dysrhythmias that can affect CO.53 Intubation and mechanical ventilation may be necessary to support oxygenation.
Intraaortic balloon pump (IABP) support should be instituted if drug therapy does not quickly reverse the shock state.54–57 The IABP is a temporary measure to decrease myocardial workload by improving myocardial supply and decreasing myocardial demand. It achieves this goal by improving coronary artery perfusion and reducing left ventricular afterload. Chapter 13 provides more information about IAPB therapy.
After the cause of pump failure has been identified, measures should be taken to correct the problem if possible. If the problem is related to an acute MI, early revascularization by coronary angioplasty or coronary artery bypass surgery provides significant survival benefit.53–57,62 Thrombolytic agents may be used in select patients. When conventional therapies fail, a ventricular assist device (VAD) or extracorporeal life support with a membrane oxygenator may be used to support the patient in acute cardiogenic shock.54,57,63,64 These mechanical circulatory assist devices provide an external means to sustain effective organ perfusion, allowing time for the patient’s ventricle to heal or for cardiac transplantation to take place.
Nursing Management
Prevention of cardiogenic shock is one of the primary responsibilities of the nurse in the critical care area. Preventive measures include the identification of patients at risk, facilitation of early reperfusion therapy for acute MI, and frequent assessment and management of the patient’s cardiopulmonary status.
The patient in cardiogenic shock may have any number of nursing diagnoses, depending on the progression of the process (Nursing Diagnosis Priorities Box on Cardiogenic Shock). Nursing priorities are directed toward (1) limiting myocardial oxygen demand, (2) enhancing myocardial oxygen supply, (3) providing comfort and emotional support, and (4) maintaining surveillance for complications. Measures to limit myocardial oxygen demand include administering analgesics, sedatives, and agents to control afterload and dysrhythmias; positioning the patient for comfort; limiting activities; providing a calm and quiet environment and offering support to reduce anxiety; and teaching the patient about the condition. Measures to enhance myocardial oxygen supply include administering supplemental oxygen, monitoring the patient’s respiratory status, and administering prescribed medications.
Effective nursing management of cardiogenic shock requires precise monitoring and management of heart rate, preload, afterload, and contractility. This is accomplished through accurate measurement of hemodynamic variables and controlled administration of fluids and inotropic and vasoactive agents. Close assessment and management of respiratory function is also essential to maintain adequate oxygenation. Dysrhythmias are common and require immediate recognition and treatment.
Patients who require IABP therapy need to be observed frequently for complications. Complications include embolus formation, infection, rupture of the aorta, thrombocytopenia, improper balloon placement, bleeding, improper timing of the balloon, balloon rupture, and circulatory compromise of the cannulated extremity.
Anaphylactic Shock
Anaphylactic shock, a type of distributive shock, is the result of an immediate hypersensitivity reaction. It is a life-threatening event that requires prompt intervention. The severe antibody-antigen response leads to decreased tissue perfusion and initiation of the general shock response.65,66
Etiology
Anaphylactic shock is caused by an antibody-antigen response. Almost any substance can cause a hypersensitivity reaction. These substances, known as antigens, can be introduced by injection or ingestion or through the skin or respiratory tract. A number of antigens have been identified that can cause a reaction in a hypersensitive person. This list includes foods, food additives, diagnostic agents, biological agents, environmental agents, drugs, and venoms (Box 26-6).66–68 In the hospital environment, latex is an extremely problematic antigen for patients and health care providers (Patient Safety Priorities Box on Latex Allergy).
Anaphylactic reactions can be IgE-mediated or non-IgE-mediated responses. IgE is an antibody that is formed as part of the immune response. The first time an antigen enters the body, an antibody IgE, specific for the antigen, is formed. The antigen-specific IgE antibody is then stored by attachment to mast cells and basophils. This initial contact with the antigen is known as a primary immune response. The next time the antigen enters the body, the preformed IgE antibody reacts with it, and a secondary immune response occurs. This reaction triggers the release of biochemical mediators from the mast cells and basophils and initiates the cascade of events that precipitates anaphylactic shock.66,69,70
Some anaphylactic reactions are non-IgE-mediated responses in that they occur in the absence of activation of IgE antibodies. These responses occur as a result of direct activation of the mast cells to release biochemical mediators. Direct activation of mast cells can be triggered by humoral mediators, such as the complement system and the coagulation-fibrinolytic system. Biochemical mediators can be released as a direct or indirect response to many drugs. This type of reaction, formerly known as anaphylactoid reaction, is produced in persons not previously sensitized, and it can occur with the first exposure to an antigen.66,68,70
Pathophysiology
The antibody-antigen response (immunological stimulation) or the direct triggering (nonimmunological activation) of the mast cells results in the release of biochemical mediators. These mediators include histamine, eosinophil chemotactic factor of anaphylaxis (ECF-A), neutrophil chemotactic factor of anaphylaxis (NCF), platelet-activating factor (PAF), proteinases, heparin, serotonin, leukotrienes (also known as slow-reacting substance of anaphylaxis), and prostaglandins. The activation of the biochemical mediators causes vasodilation; increased capillary permeability; laryngeal edema; bronchoconstriction; excessive mucus secretion; coronary vasoconstriction; inflammation; cutaneous reactions; and constriction of the smooth muscle in the intestinal wall, bladder, and uterus. Coronary vasoconstriction causes severe myocardial depression. Cutaneous reactions cause stimulation of nerve endings, followed by itching and pain.65,66,69
ECF-A promotes chemotaxis of eosinophils, facilitating the movement of eosinophils into the area. During allergic reactions, eosinophils phagocytose the antibody-antigen complex and other inflammatory debris and release enzymes that inhibit vasoactive mediators, such as histamine and leukotrienes. Secondary mediators such as bradykinin and plasmin are produced that enhance or inhibit the already released biochemical mediators. Peripheral vasodilation results in relative hypovolemia and decreased venous return. Increased capillary membrane permeability results in the loss of intravascular volume, worsening the hypovolemic state. Decreased venous return results in decreased end-diastolic volume and SV. The decline in SV leads to decreased CO and ineffective tissue perfusion. Death may result from airway obstruction or cardiovascular collapse, or both (Figure 26-3).65,66,69,70
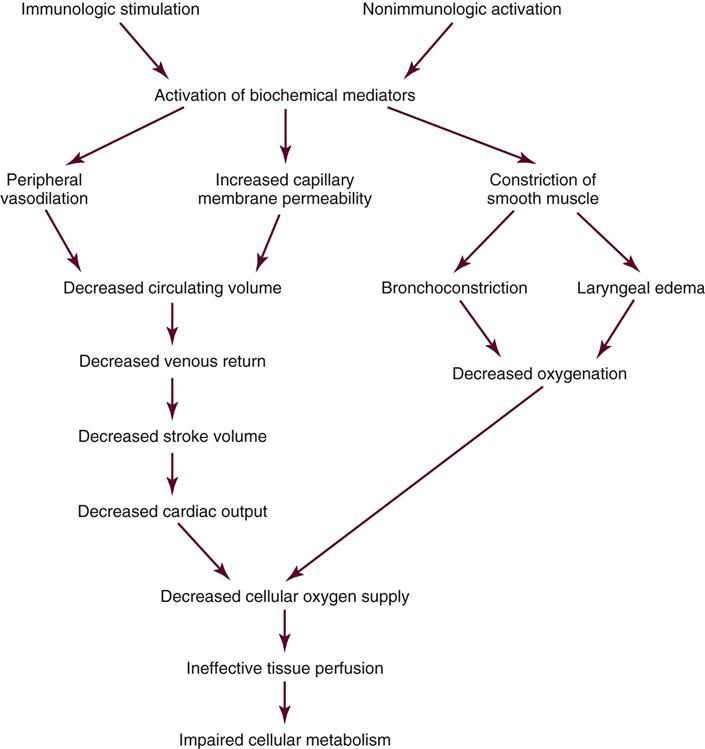
Assessment and Diagnosis
Anaphylactic shock is a severe systemic reaction that can affect multiple organ systems. A variety of clinical manifestations occur in the patient in anaphylactic shock, depending on the extent of multisystem involvement. The symptoms usually start to appear within minutes of exposure to the antigen, but they may not occur for up to 1 hour (Box 26-7).65 Symptoms may also reappear after a 1- to 72-hour window of resolution. These late-phase reactions may be similar to the initial anaphylactic response, milder, or more severe.65,71
The cutaneous effects may appear first and include pruritus, generalized erythema, urticaria, and angioedema. Commonly seen on the face and in the oral cavity and lower pharynx, angioedema develops as a result of fluid leaking into the interstitial space. The patient may appear restless, uneasy, apprehensive, and anxious and may complain of being warm. Respiratory effects include the development of laryngeal edema, bronchoconstriction, and mucous plugs. Clinical manifestations of laryngeal edema include inspiratory stridor, hoarseness, a sensation of fullness or a lump in the throat, and dysphagia. Bronchoconstriction causes dyspnea, wheezing, and chest tightness.65,66,69 Gastrointestinal and genitourinary manifestations, which may develop as a result of smooth muscle contraction, include vomiting, diarrhea, cramping, and abdominal pain.
As the anaphylactic reaction progresses, hypotension and reflex tachycardia develop. This occurs in response to massive vasodilation and loss of circulating volume. Jugular veins appear flat as right ventricular end-diastolic volume is decreased. The eventual outcome is circulatory failure and ineffective tissue perfusion.65,66,69 The patient’s level of consciousness may deteriorate to unresponsiveness.
Assessment of the hemodynamic parameters of a patient in anaphylactic shock reveals a decreased CO and CI. Venous vasodilation and massive volume loss lead to a decrease in preload, which results in a decline in the RAP and PAOP. Vasodilation of the arterial system results in a decrease in the afterload of the heart, as evidenced by a decrease in the SVR. Box 26-8 outlines the clinical criteria for diagnosing anaphylaxis.
Medical Management
Treatment of anaphylactic shock requires an immediate and direct approach. The goals of therapy are to remove the offending antigen, reverse the effects of the biochemical mediators, and promote adequate tissue perfusion. When the hypersensitivity reaction occurs as a result of administration of medications, dye, blood, or blood products, the infusion should be immediately discontinued. Often, it is not possible to remove the antigen because it is unknown or has already entered the patient’s system.
Reversal of the effects of the biochemical mediators involves the preservation and support of the patient’s airway, ventilation, and circulation. This is accomplished through oxygen therapy, intubation, mechanical ventilation, and administration of drugs and fluids.
Epinephrine is the first-line treatment of choice for anaphylaxis. It promotes bronchodilation and vasoconstriction and inhibits further release of biochemical mediators. In mild cases of anaphylaxis, 0.3 to 0.5 mg (0.3 to 0.5 mL) of a 1 : 1000 dilution of epinephrine is administered by intramuscular injection into the anterolateral thigh and repeated every 5 to 15 minutes until anaphylaxis is resolved.65,66,70–72 Subcutaneous injection is no longer recommended.71 For anaphylactic shock with hypotension, epinephrine is administered intravenously. The intravenous dose is 0.1 (1 mL) of a 1 : 10,000 dilution administered over 5 minutes. If hypotension persists, a continuous infusion of epinephrine is recommended, administered at 1 to 4 mcg/min with titration up to 10 mcg/min as needed.66,67,69,73 Patients receiving beta-blockers may have a limited response to epinephrine. Intravenous glucagon administered as a 20 to 30 mcg/kg bolus over 5 minutes followed by continuous infusion at 5 to 15 mcg/minute is recommended for inotropic and vasoactive support for these patients.65,66,71,73
Diphenhydramine (Benadryl), given 1 to 2 mg/kg (25 to 50 mg) by a slow intravenous route every 4 to 8 hours, is a second-line agent used to block the histamine response.65,66,69,71,73 Corticosteroids also may be given with the goal of preventing a delayed reaction and stabilizing capillary membranes.65,66,71 Fluid replacement is accomplished by use of a crystalloid or colloid solution. Positive inotropic agents and vasoconstrictor agents may be necessary to reverse the effects of myocardial depression and vasodilation.65,66,69,71
Nursing Management
Prevention of anaphylactic shock is one of the primary responsibilities of the nurse in the critical care area. Preventive measures include the identification of patients at risk and cautious assessment of the patient’s response to the administration of drugs, blood, and blood products. A complete and accurate history of the patient’s allergies is an essential component of preventive nursing care. In addition to a list of the allergies, a detailed description of the type of response for each one should be obtained.
The patient in anaphylactic shock may have any number of nursing diagnoses, depending on the progression of the process (Nursing Diagnosis Priorities Box on Anaphylactic Shock). Nursing priorities are directed toward (1) facilitating ventilation, (2) administering volume replacement, (3) providing comfort and emotional support, and (4) maintaining surveillance for complications.
Measures to facilitate ventilation include positioning the patient to assist with breathing and instructing the patient to breathe slowly and deeply. Airway protection through prompt administration of prescribed medications is essential. Measures to facilitate the administration of volume replacement include inserting large-bore peripheral intravenous catheters; rapidly administering prescribed fluids; and positioning the patient with the legs elevated, trunk flat, and head and shoulders above the chest. Measures to promote comfort include administering medications to relieve itching, applying warm soaks to skin, and if necessary, covering the patient’s hands to discourage scratching. Observing the patient for clinical manifestations of a delayed reaction is critical. Patient education about how to avoid the precipitating allergen is essential for preventing future episodes of anaphylaxis.
Neurogenic Shock
Neurogenic shock, another type of distributive shock, is the result of the loss or suppression of sympathetic tone. The lack of sympathetic tone leads to decreased tissue perfusion and initiation of the general shock response. Neurogenic shock is the most uncommon form of shock.
Etiology
Neurogenic shock can be caused by anything that disrupts the SNS. The problem can occur as the result of interrupted impulse transmission or blockage of sympathetic outflow from the vasomotor center in the brain.74–76 The most common cause is spinal cord injury. Neurogenic shock may mistakenly be referred to as spinal shock. The latter condition refers to loss of neurological activity below the level of spinal cord injury, but it does not necessarily involve ineffective tissue perfusion.77–79
Pathophysiology
Loss of sympathetic tone results in massive peripheral vasodilation, inhibition of the baroreceptor response, and impaired thermoregulation. Arterial vasodilation leads to a decrease in SVR and a fall in blood pressure. Venous vasodilation leads to relative hypovolemia and pooling of blood in the venous circuit. The decreased venous return results in a decrease in end-diastolic volume or preload, causing a decrease in SV and CO. The fall in blood pressure and CO leads to inadequate or ineffective tissue perfusion. Loss of sympathetic tone and inhibition of the baroreceptor response result in bradycardia.74–76,78 The slow heart rate worsens CO, which further compromises tissue perfusion. Impaired thermoregulation occurs because of loss of vasomotor tone in the cutaneous blood vessels that dilate and constrict to maintain body temperature. The patient becomes poikilothermic, or dependent on the environment for temperature regulation (Figure 26-4).

Assessment and Diagnosis
The patient in neurogenic shock characteristically presents with hypotension, bradycardia, and warm, dry skin.74–76 The decreased blood pressure results from massive peripheral vasodilation. The decreased heart rate is caused by inhibition of the baroreceptor response and unopposed parasympathetic control of the heart.79 Hypothermia develops from uncontrolled peripheral heat loss. The warm, dry skin occurs as a consequence of pooling of blood in the extremities and loss of vasomotor control in surface vessels of the skin that control heat loss.
Assessment of the hemodynamic parameters of a patient in neurogenic shock reveals a decreased CO and CI. Venous vasodilation leads to a decrease in preload, which results in a decline in the RAP and PAOP. Vasodilation of the arterial system causes a decrease in the afterload of the heart, as evidenced by a decrease in the SVR.76
Medical Management
Treatment of neurogenic shock requires a careful approach. The goals of therapy are to treat or remove the cause, prevent cardiovascular instability, and promote optimal tissue perfusion. Cardiovascular instability can result from hypovolemia, bradycardia, and hypothermia. Specific treatments are aimed at preventing or correcting these problems as they occur.
Hypovolemia is treated with careful fluid resuscitation. The minimal amount of fluid is administered to ensure adequate tissue perfusion. Volume replacement is initiated for systolic blood pressure lower than 90 mm Hg, urine output of less than 30 mL/hour, or changes in mental status that indicate decreased cerebral tissue perfusion. The patient is carefully observed for evidence of fluid overload. Vasopressors are used as necessary to maintain blood pressure and organ perfusion.74,76,78 The bradycardia associated with neurogenic shock rarely requires specific treatment, but atropine or electrical pacing can be used when necessary.69,74 Hypothermia is treated with warming measures and environmental temperature regulation.
Nursing Management
Prevention of neurogenic shock is one of the primary responsibilities of the nurse in the critical care area. This includes the identification of patients at risk and constant assessment of the neurological status. Vigilant immobilization of spinal cord injuries and slight elevation of the head of the patient’s bed after spinal anesthesia are essential components of preventive nursing care. Early identification allows for early treatment and decreased mortality.
The patient in neurogenic shock may have any number of nursing diagnoses, depending on the progression of the process (Nursing Diagnosis Priorities Box on Neurogenic Shock). Nursing priorities are directed toward (1) treating hypovolemia, (2) maintaining normothermia, (3) monitoring for dysrhythmias, (4) providing comfort and emotional support, and (5) maintaining surveillance for complications.
Venous pooling in the lower extremities promotes the formation of deep vein thrombosis (DVT), which can result in a pulmonary embolism. All patients at risk for DVT should be started on prophylaxis therapy. DVT-prophylactic measures include monitoring of calf and thigh measurements, passive range-of-motion exercises, application of sequential pneumatic stockings, and administration of prescribed anticoagulation therapy.
Severe Sepsis and Septic Shock
Sepsis occurs when microorganisms invade the body and initiate a systemic inflammatory response. This host response often results in perfusion abnormalities with organ dysfunction (severe sepsis) and eventually hypotension (septic shock). The primary mechanism of this type of shock is the maldistribution of blood flow to the tissues.3 Severe sepsis is estimated to occur in more than 750,000 patients annually in the United States, with an estimated mortality rate of 30% to 50%.80 It is the leading cause of death in noncoronary critical care units.15
Specific terms are used to describe the continuum of conditions that the patient with an infection may experience. In 1991 at the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) Consensus Conference, definitions were developed to describe and differentiate these conditions (Box 26-9).81 These definitions were clarified and reinforced in subsequent conferences in 2001, 2004, and 2008.15,16,82 This discussion focuses on severe sepsis and septic shock.
Etiology
Sepsis is caused by a wide variety of microorganisms, including gram-negative and gram-positive aerobes, anaerobes, fungi, and viruses. The source of these microorganisms varies. Exogenous sources include the hospital environment and members of the health care team. Endogenous sources include the patient’s skin, gastrointestinal tract, respiratory tract, and genitourinary tract. In recent years, the incidence of chest-related infections has risen dramatically, and the lungs have replaced the intraabdominal organs as the most common site of infection producing severe sepsis and septic shock.83,84 Gram-positive bacteria are responsible for more than one half of the cases of sepsis.85 Sepsis and septic shock are associated with a wide variety of intrinsic and extrinsic precipitating factors (Box 26-10). All of these factors interfere directly or indirectly with the body’s anatomic and physiological defense mechanisms. Several of the intrinsic factors are not modifiable or are very difficult to control. Several of the extrinsic factors may be required for diagnosis and management. All critically ill patients are therefore at risk for septic shock.80
Pathophysiology
The syndrome encompassing severe sepsis and septic shock is a complex systemic response that is initiated when a microorganism enters the body and stimulates the inflammatory/immune system. Shed protein fragments and the release of toxins and other substances from the microorganism activate the plasma enzyme cascades (complement, kinin/kallikrein, coagulation, and fibrinolytic factors), as well as platelets, neutrophils, monocytes, and macrophages. On activation, these systems and cells release a variety of mediators, or cytokines, that initiate a chain of complex interactions leading to a maladaptive SIRS.86–91
After the mediators are activated, a variety of physiological and pathophysiological events occur that affect clotting, the distribution of blood flow to the tissues and organs, capillary membrane permeability, and the metabolic state of the body. Subsequently, a systemic imbalance between cellular oxygen supply and demand develops that results in cellular hypoxia, damage, hibernation, and death (Figure 26-5).4,88,90,91
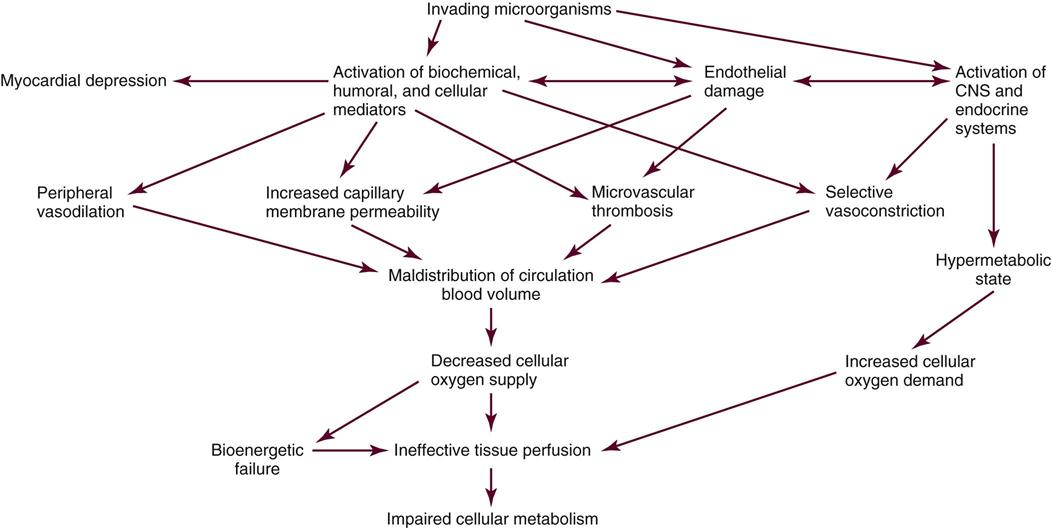
Hallmarks of severe sepsis are endothelial damage and coagulation dysfunction.87,88,92,93 Tissue factor is released from endothelial cells and monocytes in response to stimulation by the inflammatory cytokines.87,88 Release of tissue factor initiates the coagulation cascade, producing widespread microvascular thrombosis and further stimulation of the systemic inflammatory pathways.88 Diffuse endothelial damage impairs endogenous anticlotting mechanisms.88 Mediator-induced suppression of fibrinolysis slows clot breakdown. The result is DIC with eventual consumption of coagulation factors, bleeding, and hemorrhage.92,94
Significant alterations in cardiovascular hemodynamics are caused by the activation of inflammatory cytokines and endothelial damage.32,88,93 Massive peripheral vasodilation results in the development of relative hypovolemia. Increased capillary permeability produces a loss of intravascular volume to the interstitium, which accentuates the reduction in preload and CO. These changes, coupled with the microvascular thrombosis, produce maldistribution of circulating blood volume, decreased tissue perfusion, and inadequate oxygen delivery to the cells. Microcirculatory shunting is a key feature of this distributive shock.88,95,96 Impaired ventricular contractility results from cytokine activity.4,88
Activation of the central nervous and endocrine systems also occurs as part of the response to invading microorganisms. This activation leads to stimulation of the SNS and the release of ACTH. These events trigger the release of epinephrine, norepinephrine, glucocorticoids, aldosterone, glucagon, renin, and growth hormone resulting in the development of a hypermetabolic state and contributing to vasoconstriction of the renal, pulmonary, and splanchnic beds. Selective vasoconstriction in the splanchnic bed may contribute to hypoperfusion of the gastric mucosa. The resulting gut injury propagates the inflammatory response.2,97
Several metabolic alterations occur as a result of CNS, endocrine system, and cytokine activation. The hypermetabolic state increases energy expenditure and oxygen demand, and it contributes to cellular hypoxia. Lactic acid is produced as a result of increased metabolic lactate production and hypoxic anaerobic metabolism. Glucocorticoids, ACTH, epinephrine, glucagon, and growth hormone are all catabolic hormones that are released as part of this response. In conjunction with the inflammatory cytokines, these hormones stimulate catabolism of protein stores in the visceral organs and skeletal muscles to fuel glucose production in the liver, hyperglycemia, and insulin resistance.8 The cytokines also stimulate the use of fats for energy production (lipolysis).8,91,98
Metabolic derangements in severe sepsis and septic shock include an inability of the cells to use oxygen even if blood flow is adequate. Mitochondrial dysfunction is thought to be the underlying mechanism.88,91,98 This bioenergetic failure plays an important role in the development of multiple organ dysfunction.88,91,98 The exaggerated inflammatory response in severe sepsis results in apoptosis, a programmed cell death or cellular suicide affecting endothelial and immune cells in particular.88,91,93
These complex and interrelated pathophysiological changes associated with severe sepsis and septic shock produce a pathological imbalance between cellular oxygen demand and cellular oxygen supply and consumption. If unabated, this situation ultimately results in tissue ischemia, MODS, and death.
Assessment and Diagnosis
Effective treatment of severe sepsis and septic shock depends on timely recognition. The diagnosis of severe sepsis is based on the identification of three conditions: known or suspected infection, two or more of the clinical indications of the systemic inflammatory response, and evidence of at least one organ dysfunction. Clinical indications of systemic inflammatory response and sepsis were included in the original ACCP/SCCM consensus definitions and are listed in Box 26-9. The second consensus conference expanded this list to facilitate prompt clinical recognition (Box 26-11).82
Signs of individual organ dysfunction are discussed later in the chapter. The two most common organs to demonstrate dysfunction in severe sepsis are the cardiovascular system and the lungs. The patient with persistent hypotension requiring vasopressor therapy despite adequate volume resuscitation is demonstrating cardiovascular dysfunction. Pulmonary dysfunction is manifested by a PaO2/FiO2 (fraction of oxygen in inspired air) ratio of less than 300, indicating ALI.87 Signs indicating septic shock are hypotension despite adequate fluid resuscitation and the presence of perfusion abnormalities such as lactic acidosis, oliguria, or acute change in mentation.
The patient in severe sepsis or septic shock may present with a variety of clinical manifestations that may change dynamically as the condition progresses (Box 26-12). During the initial stage, massive vasodilation occurs in the venous and arterial beds. Dilation of the venous system leads to a decrease in venous return to the heart, which results in a decrease in the preload of the right and left ventricles. This is evidenced by a decline in the RAP and PAOP. Dilation of the arterial system results in a decrease in the afterload of the heart, as evidenced by a decrease in the SVR. The patient’s skin becomes pink, warm, and flushed as a result of the massive vasodilation. Myocardial contractility is decreased, as evidenced by a decline in the left ventricular stroke work index (LVSWI).
The heart rate rises in response to increased SNS, metabolic, and adrenal gland stimulation. If circulating volume and preload are adequate, this results in a normal-to-high CO and CI despite impaired contractility. The pulse pressure widens as the diastolic blood pressure decreases because of the vasodilation, and the systolic blood pressure increases because of the elevated CO. A full, bounding pulse develops. The net result of these changes is a relatively normal blood pressure in severe sepsis. However, as the reduction in preload and afterload becomes overwhelming and contractility fails, hypotension ensues, resulting in septic shock.
In the lungs, ventilation/perfusion mismatching develops as a result of pulmonary vasoconstriction and the formation of pulmonary microemboli. Hypoxemia occurs, and the respiratory rate increases to compensate for the lack of oxygen. Crackles develop as increased pulmonary capillary membrane permeability leads to pulmonary edema.79
The level of consciousness starts to change as a result of decreased cerebral perfusion, immune mediator activation, hyperthermia, and lactic acidosis. This septic encephalopathy is demonstrated by acute onset of impaired cognitive functioning, or delirium, which may fluctuate during its course.80 The patient may appear disoriented, confused, combative, or lethargic.
ABG values initially reveal hypocarbia, hypoxemia, and metabolic acidosis. This is demonstrated by a low PaO2, low PaCO2, and low HCO3– level, respectively. The respiratory alkalosis is caused by the patient’s increased respiratory rate. As pathological pulmonary changes progress and the patient becomes fatigued, the effectiveness of respirations decreases and the PaCO2 increases, resulting in respiratory acidosis. The metabolic acidosis is the result of a lack of oxygen to the cells and the development of lactic acidemia. Serum lactate levels increase above 2 mmol/L because of anaerobic metabolism. The mixed venous oxygen saturation (SvO2) may increase because of microcirculatory shunting or decrease because of inadequate oxygen delivery.88,96 The white blood cell (WBC) count is elevated as part of the immune response to the invading microorganisms. The WBC differential count reveals an increase in immature neutrophils (shift to the left). This occurs because the body has to mobilize increasing numbers of WBCs to fight the infection. An elevated procalcitonin level is a valuable indicator of significant infection.88,99 Serum glucose levels increase as part of the hypermetabolic response and the development of insulin resistance. The patient’s temperature is elevated in response to pyrogens released from the invading microorganisms, immune mediator activation, and increased metabolic activity. Urine output declines because of decreased perfusion of the kidneys. As impaired tissue perfusion develops, a variety of other clinical manifestations appear that indicate the development of MODS.
Medical Management
Treatment of the patient in severe sepsis or septic shock requires a multifaceted approach. The goals of treatment are to reverse the pathophysiological responses, control the infection, and promote metabolic support. This approach includes supporting the cardiovascular system and enhancing tissue perfusion, identifying and treating the infection, limiting the systemic inflammatory response, restoring metabolic balance, and initiating nutritional therapy. Dysfunction of the individual organ systems must be prevented. Early treatment reduces mortality.16,90,100,101 Guidelines for the management of severe sepsis and septic shock have been developed and updated under the auspices of the Surviving Sepsis Campaign (SSC), an international effort of more than 11 organizations to improve patient outcomes.15,16 From these guidelines, a group (“bundle”) of selected interventions was identified as having the most impact on patient outcomes (Box 26-13). The sepsis resuscitation bundle should be implemented within the first 6 hours, and the sepsis management bundle should be implemented within the first 24 hours. More information regarding these interventions is available at the SSC website (www.survivingsepsis.org).
The patient in severe sepsis or septic shock requires immediate resuscitation of the hypoperfused state. Specific interventions are aimed at increasing cellular oxygen supply and decreasing cellular oxygen demand. These treatments include administration of fluids, vasopressors, and positive inotropic agents. Early goal-directed therapy during the first 6 hours of resuscitation improves survival100 and is recommended in the SSC guidelines.15,16 This therapy includes aggressive fluid resuscitation to augment intravascular volume and increase preload until a CVP of 8 to 12 mm Hg (12 to 15 mm Hg in mechanically ventilated patients) is achieved. Crystalloids or colloids may be used. A fluid challenge for hypovolemia should be initiated with at least 1000 mL of crystalloids or 300 to 500 mL of colloids over 30 minutes. Vasopressors (norepinephrine or dopamine as first-choice agents) should be administered as necessary to maintain a MAP of at least 65 mm Hg. These agents reverse the massive peripheral vasodilation and increase SVR. Epinephrine is recommended as an alternative agent if response to norepinephrine or dopamine is poor.16 Arterial line placement is recommended for any patient requiring vasopressor therapy. Intermittent or continuous monitoring of central venous or mixed venous oxygen saturation (SCvO2 or SvO2) allows evaluation of the effectiveness of oxygen delivery. If the SCvO2 is less than 70% or the SvO2 is less than 65% after the CVP goal is achieved, administration of packed red cells to achieve a hematocrit of at least 30%16 or inotropic stimulation with dobutamine (administered to a maximum of 20 mcg/kg/minute) to counteract myocardial depression and maintain adequate CO is recommended to obtain this goal.16,100 The dobutamine infusion should be reduced or discontinued if a tachycardia greater than 120 beats/minute develops.100
Intubation and mechanical ventilatory support are usually required to optimize oxygenation and ventilation for the patient in severe sepsis or septic shock. Ventilation with lower than traditional tidal volumes (6 versus 12 mL/kg) in patients with ALI and acute respiratory distress syndrome (ARDS) decreases mortality.102 SSC guidelines recommend the goals of 6 mL/kg of predicted body weight and plateau pressures no more than 30 cm H2O for patients with severe sepsis or septic shock with ALI or ARDS.16 Increased PaCO2 may result from this therapy and is acceptable if tolerated as evidenced by hemodynamic stability. Ventilator settings should include positive end-expiratory pressure and be adjusted to provide the patient with a PaO2 greater than 70 mm Hg. Patients receiving mechanical ventilation should be maintained in a semirecumbent position with the head of the bed raised to 45 degrees to decrease the incidence of ventilator-associated pneumonia.16 Prone positioning should be considered in the septic patient with ARDS requiring high levels of oxygen.16 Sedation protocols using intermittent bolus or continuous infusion using a standardized sedation scale and specific goals are recommended for all patients requiring mechanical ventilation. Daily interruption of sedative infusions to allow wakefulness and reevaluation of sedation needs reduces duration of mechanical ventilation and is recommended.16 Neuromuscular blocking agents should be avoided, if possible, to prevent prolonged blockade after discontinuation.16
A key measure in the treatment of septic shock is finding and eradicating the cause of the infection. At least two blood cultures plus urine, sputum, and wound cultures should be obtained to find the location of the infection before antibiotic therapy is initiated.16 Antibiotic therapy should be started within 1 hour of recognition of severe sepsis without delay for cultures.16 Each hour of delay is associated with a substantial drop in the survival rate.101 If the microorganism is unknown, antiinfective therapy with one or more agents known to be effective against likely pathogens should be initiated, with daily reassessment of the regimen. Combination therapy is recommended for known or suspected Pseudomonas infection and for neutropenic patients but should be limited to less than 3 to 5 days.16 A specific source of infection should be established within 6 hours of presentation.16 Surgical intervention to débride infected or necrotic tissue or to drain abscesses may be necessary to facilitate removal of the septic source.16 Intravascular devices that may be the source of the infection should be removed after establishment of alternative vascular access.
Intravenous corticosteroids reduce mortality in catecholamine-dependent septic shock patients with relative adrenal insufficiency.107 Intravenous hydrocortisone is recommended only for the patient in septic shock who is poorly responsive to fluid resuscitation and vasopressor therapy.16 Doses greater than 300 mg/day may be harmful and should not be used and steroid therapy should be weaned when vasopressors are no longer required.16
Continuous infusion of insulin and glucose to maintain a blood glucose level of 150 mg/dL or less improves outcomes42 and is recommended by SSC guidelines after initial stabilization.16 Glucose levels should be monitored every 1 to 2 hours until stable and then every 4 hours. Low glucose levels measured by capillary testing may be inaccurate in this population.16 Platelets should be administered when counts are less than 5000/mm3 and red blood cell transfusions are recommended when the hemoglobin level is less than 7.0 g/dL to obtain a target value of 7 to 9 g/dL.16 Stress ulcer prophylaxis using histamine2 (H2) blockers or proton-pump inhibitors and DVT prophylaxis are recommended for all patients with severe sepsis or septic shock. The SCCM guidelines recommend against the use of sodium bicarbonate for lactic acidemia if the pH is equal to or greater than 7.15.16 Low-dose dopamine infusion for renal protection is not beneficial and should not be used.16
The initiation of nutritional therapy is critical in the management of the patient in severe sepsis or septic shock. The goal is to improve the patient’s overall nutritional status, enhance immune function, and promote wound healing. A daily caloric intake of 25 to 30 kcal/kg of usual body weight is recommended. The enteral route is preferred. The ideal nutritional supplement for the patient in septic shock should be high in protein because of the metabolic derangements that develop in the hypermetabolic state. The amount of protein calories given depends on the patient’s nitrogen balance. In early sepsis, the mix of nonprotein calories may be divided evenly between carbohydrates and fats. In the later stages, significant alterations in fat metabolism occur, and the lipid content should be limited to 10% to 15% of the total nonprotein calories. Specific nutritional therapies to reduce the inflammatory and hypermetabolic responses associated with sepsis, such as antioxidant supplementation and feeding with long-chain n-3 polyunsaturated fatty acids, are the source of much debate and are being evaluated.8,108–111 Glutamine is considered by some to be an essential amino acid in critically ill patients and has the most empirical support.88,108,109,111 Arginine has produced negative outcomes and is not recommended.110
Nursing Management
Prevention of severe sepsis and septic shock is one of the primary responsibilities of the nurse in the critical care area. These measures include the identification of patients at risk and reduction of their exposure to invading microorganisms. Hand washing, aseptic technique, and an understanding of how microorganisms can invade the body are essential components of preventive nursing care. Early identification allows for early treatment and decreases mortality.80 Box 26-14 depicts a simple screening tool for identifying patients with severe sepsis.
The patient in septic shock may have any number of nursing diagnoses, depending on the progression of the process (Nursing Diagnosis Priorities Box on Septic Shock). Nursing priorities are directed toward (1) early identification of sepsis syndrome, (2) administering prescribed fluids and medications, (3) providing comfort and emotional support, and (4) maintaining surveillance for complications. Continual observation to detect subtle changes that indicate the progression of the septic process is also very important.
Evidence-based guidelines for the management of the patient with severe sepsis or septic shock are listed in the Evidence-Based Practice box on Severe Sepsis and Septic Shock Management Guidelines.
Multiple Organ Dysfunction Syndrome
MODS results from progressive physiological failure of two or more separate organ systems. It is defined as the “presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention.”81 Dysfunction of one organ may amplify dysfunction in another. Lack of consensus regarding definitions for organ dysfunction, the number of organs involved, and the duration of organ dysfunction have hampered an accurate account of organ dysfunction in critically ill patients. Despite some variations in how previous researchers have defined organ dysfunction, mortality has been closely linked to the number of organ systems involved. Impairment of two or more organs is associated with an estimated mortality rate of 45% to 55%. This may increase to 80% with three or more organ systems and to 100% if three or more organ systems are severely compromised for longer than 4 days.112
Although various patient populations are at risk for organ dysfunction, trauma patients are particularly vulnerable because they often experience ischemia-reperfusion events resulting from hemorrhage, blunt trauma, or sympathetic nervous system-induced vasoconstriction.113 Other high-risk patients include those who have experienced infection, a shock episode, various ischemia-reperfusion events, acute pancreatitis, sepsis, burns, aspiration, multiple blood transfusions, or surgical complications. Patients age 65 years or older are at increased risk because of their decreased organ reserve and comorbidities.114
Etiology
Organ dysfunction may be a direct consequence of the insult (primary MODS) or can manifest latently and involve organs not directly affected in the initial insult (secondary MODS). Patients can experience both primary and secondary MODS (Figure 26-6).
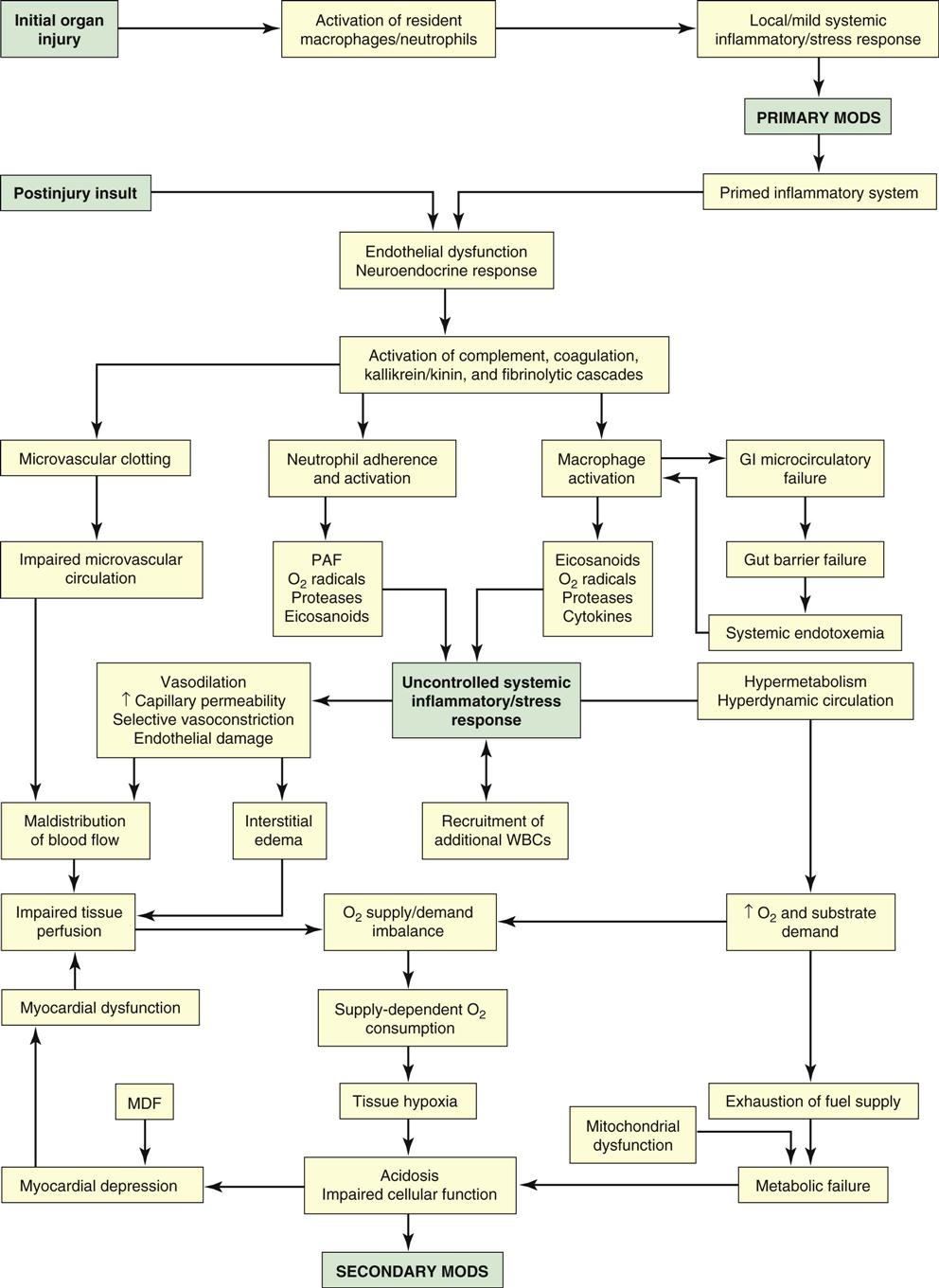
GI, gastrointestinal; MDF, myocardial depressant factor; MODS, multiple organ dysfunction syndrome; PAF, platelet activating factor; WBCs, white blood cells. (From Cheek DJ, et al: Shock, multiple organ dysfunction syndrome, and burns in adults. In McCance KL, Huether SE, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.)
Primary MODS “directly results from a well-defined insult in which organ dysfunction occurs early and is directly attributed to the insult itself”3 and accounts for only a small fraction of MODS cases. Direct insults initially cause localized inflammatory responses. Examples of primary MODS include the immediate consequences of posttraumatic pulmonary failure, thermal injuries, acute tubular necrosis, or invasive infections.115 These cellular or microcirculatory events may lead to a loss of critical organ function induced by failure of delivery of oxygen and substrates, coupled with the inability to remove end-products of metabolism.112,115,116 The inflammatory response in primary MODS has a less apparent presentation and may resolve without long-term implications. This primary dysfunction is thought to set the system up for a more observable inflammatory response leading to secondary MODS.112
Secondary MODS is a consequence of widespread systemic inflammation that results in dysfunction of organs not involved in the initial insult.81,82 Secondary MODS develops latently after an initial insult. The early impairment of organs normally involved in immunoregulatory function, such as the liver and the GI tract, intensifies the host response to the insult. It is postulated that the initial insult “primes” the inflammatory system in such a way that a mild second insult may perpetuate a hyperinflammatory response.117
Systemic inflammatory response syndrome (SIRS) or sepsis is a common initiating event in the development of secondary MODS. The systemic inflammatory response is an abnormal host response characterized by generalized inflammation in organs remote from the initial insult. SIRS is widespread inflammation or clinical responses to inflammation that occurs in patients suffering a variety of insults. Clinical conditions and manifestations associated with SIRS are listed in Box 26-15. These insults produce similar or identical systemic inflammatory responses, even in the absence of infection. SIRS is diagnosed when at least two of four clinical manifestations occur in the high-risk patient. Manifestations of SIRS must represent an acute alteration from the patient’s normal baseline and must not be related to other causes (e.g., neutropenia from chemotherapy). Organ dysfunction or failure, such as acute lung injury (ALI), acute renal failure, and MODS, is a complication of SIRS.81,82,112,115 In epidemiological studies, SIRS was found to occur in one third of all hospitalized patients, in 50% to 93% of all patients in critical care units, and in about 80% of all patients in surgical critical care units.118,119
When SIRS is a result of infection, the term sepsis is used. Severe sepsis is sepsis with hypoperfusion or systemic manifestations of hypoperfusion. Septic shock is sepsis-induced hypotension despite fluid resuscitation. SIRS, sepsis, severe sepsis, and septic shock represent a hierarchical continuum of the inflammatory response to infection.16 Although infection and shock remain the most common precipitating factors, any disease that can induce a major inflammatory response is capable of initiating the events that lead to MODS.115
When SIRS is not contained locally, several consequences occur that lead to organ dysfunction, including intense, uncontrolled activation of inflammatory cells; direct damage of vascular endothelium; disruption of immune cell function; persistent hypermetabolism; and maldistribution of circulatory volume to organ systems.112,115 Inflammation becomes a systemic, self-perpetuating process that is inadequately controlled and results in organ dysfunction.115,120 During hypermetabolism, changes occur in cellular anabolic and catabolic function, resulting in autocatabolism. Autocatabolism manifests as a severe decrease in lean body mass, severe weight loss, anergy, and increased cardiac output and VO2 resulting from profound alterations in carbohydrate, protein, and fat metabolism.112,121 Concurrently, GI, hepatic, and immunological dysfunction may occur, which intensifies the SIRS.122 Clinical consequences may affect gut function, wound healing, muscles wasting, host response, respiratory function, and continued promotion of the hypermetabolic response.121
Not all patients develop MODS from SIRS. The development of MODS appears to be associated with failure to control the source of inflammation or infection, persistent hypoperfusion, flow-dependent oxygen consumption (VO2), or the continued presence of necrotic tissue.113,115
Pathophysiology
Secondary MODS results from altered regulation of the patient’s acute immune and inflammatory responses. Dysregulation, or failure to control the host inflammatory response, leads to the excessive production of inflammatory cells and biochemical mediators that cause widespread damage to vascular endothelium and organ damage.112,115,120 The critically ill patient’s compromised immune state also fosters an environment conducive to organ failure.
The definitive clinical course of secondary MODS has not been completely identified. One theory suggests that organ dysfunction may occur in a sequential or progressive pattern. This pattern begins with the lungs, the most commonly affected major organ, and then goes on to involve the liver, gut, and kidneys. A late component is cardiac and, sometimes, bone marrow dysfunction. Neurological and autonomic system impairment may occur and propagate the progression of organ failure and is associated with illness severity and mortality.123 Organs may fail simultaneously; for example, renal dysfunction may take place concurrently with hepatic dysfunction. After the initial insult and resuscitation, patients develop persistent hypermetabolism, a metabolic consequence of sustained systemic inflammation and physiological stress, followed closely by pulmonary dysfunction, manifested as ALI.
Certain cellular and biochemical activity evoke the inflammatory and immune responses implicated in SIRS and MODS. The mediators associated with SIRS and MODS can be classified as inflammatory cells, biochemical mediators, or plasma protein systems (Box 26-16). Activation of one mediator often leads to activation of another. The biological activity of inflammatory cells, biochemical mediators, and plasma protein systems and how they work in concert to cause SIRS and MODS have not been totally determined.124
Assessment and Diagnosis
Secondary MODS is a systemic disease with organ-specific manifestations. Organ dysfunction is influenced by numerous factors, including organ host defense function, response time to the injury, metabolic requirements, organ vasculature response to vasoactive drugs, organ sensitivity to damage, and physiological reserve. The responses of the gastrointestinal, hepatobiliary, cardiovascular, pulmonary, renal, and hematological systems are discussed in the following paragraphs. Clinical manifestations of organ dysfunction are outlined in Box 26-17.
Gastrointestinal Dysfunction
The gastrointestinal tract plays an important role in MODS. Gastrointestinal organs normally have immunoregulatory functions, and the gastrointestinal tract contains about 70% to 80% of the immunological tissue of the entire body. A normally functioning gastrointestinal tract prevents bacteria from entering the systemic circulation.112 Normal gut flora and gut environment are altered in patients with severe SIRS.122 With microcirculatory failure to the gastrointestinal tract, the gut’s barrier function may be lost. Consequently, gastrointestinal dysfunction amplifies SIRS and gut damage, which may lead to bacterial translocation and endogenous endotoxemia.112,120
Three specific mechanisms link the gastrointestinal tract and latent organ dysfunction. First, hypoperfusion and shock-like states damage the normal gastrointestinal mucosa barrier by decreasing mesenteric blood flow, leading to hypoperfusion of the villi, mucosal edema, ischemic necrosis, sloughing of the mucosa, and malabsorption. The gastrointestinal tract is extremely vulnerable to oxygen metabolite-induced reperfusion injury. Endothelial injury and gastrointestinal lesions occur in response to mediator-induced tissue damage. Ischemic events and the absence of feedings can disrupt the normal metabolism of the gastric or intestinal lumen and the normal protective function of the gut barrier.125,126
Second, the translocation of normal gastrointestinal bacteria through a “leaky gut” into the systemic circulation initiates and perpetuates an inflammatory focus in the critically ill patient.126 The gastrointestinal tract harbors organisms that present an inflammatory focus when translocated from the gut into the portal circulation and inadequately cleared by the liver. Healthy probiotics (e.g., Bifidobacterium, Lactobacillus) are decreased in a SIRS state, and pathogenic organisms (e.g., Staphylococcus, Pseudomonas) proliferate.122 Hepatic macrophages respond to the presence of enteric organisms by producing tissue-damaging amounts of tumor necrosis factor (TNF), which further propagates the inflammatory mechanisms. The primary mechanism of bacterial translocation has been associated with intestinal bacterial overgrowth.122
The third mechanism linking the gastrointestinal tract and organ dysfunction is colonization. The oropharynx of the critically ill patient becomes colonized with potentially pathogenic organisms from the gastrointestinal tract. Pulmonary aspiration of colonized secretions presents an inflammatory focus that can contribute to concomitant pulmonary dysfunction.127
Hepatobiliary Dysfunction
The liver plays a vital role in host homeostasis related to the acute inflammatory response. The liver responds to SIRS by selectively changing carbohydrate, fat, and protein metabolism. Consequently, hepatic dysfunction after a critical insult threatens the patient’s survival.128
The liver normally controls the inflammatory response by several mechanisms. Kupffer cells, which are hepatic macrophages, detoxify substances that may normally induce systemic inflammation and vasoactive substances that cause hemodynamic instability. Failure to detoxify gram-negative bacteria translocated from the gastrointestinal tract causes endotoxemia, perpetuates SIRS, and may lead to MODS. The liver also produces proteins and antiproteases to control the inflammatory response; however, hepatic dysfunction limits this response.128
The liver and gallbladder are extremely vulnerable to ischemic injury. Ischemic hepatitis occurs after a prolonged period of physiological shock and is associated with centrilobular hepatocellular necrosis.129 The degree of hepatic damage is related directly to the severity and duration of the shock episode. Terms such as shock liver and posttraumatic hepatic insufficiency have been used to describe ischemic hepatitis. Anoxic and reperfusion injuries damage hepatocytes and the vascular endothelium.130 Patients at high risk for ischemic hepatitis after a hypotensive event include those with a history of cardiac failure or cardiac dysrhythmias. Clinical manifestations of hepatic insufficiency are evident 1 to 2 days after the insult. Jaundice and transient elevations in serum transaminase and bilirubin levels occur. Hyperbilirubinemia, possibly the most reliable measure of hepatic dysfunction, results from hepatocyte anoxic injury and an increased production of bilirubin from hemoglobin catabolism.129 Ischemic hepatitis may resolve spontaneously or progress to acute liver failure. Although ischemic hepatitis is not a life-threatening complication, it can contribute to morbidity and mortality as a component of MODS.130 Researchers have proposed that serum bilirubin is a valid indicator of hepatic dysfunction in MODS because it significantly differentiates MODS survivors from nonsurvivors.129 Acute liver failure is discussed further in Chapter 22.
Acalculous cholecystitis manifests 3 to 4 weeks after an insult. Its pathogenesis is unclear, but it may be related to ischemic reperfusion injury, positive end-expiratory pressure (PEEP) greater than 5 cm H2O, volume depletion, total parenteral nutrition, narcotics, and cystic duct obstruction as a result of hyperviscous bile.131 Visceral hypotension and vasoactive medication use may decrease perfusion of the gallbladder mucosa contributing to ischemia. Bacterial invasion may stimulate activation of factor XII and initiate the coagulation pathway.131 Clinical manifestations of acalculous cholecystitis may mimic acute cholecystitis with gallstones. However, patients may demonstrate vague symptoms, including right upper quadrant pain and tenderness. Critical to the detection of acalculous cholecystitis is the recognition of abdominal distention, unexplained fever, loss of bowel sounds, and a sudden deterioration in the patient’s condition. About 50% of patients with acalculous cholecystitis have gallbladder gangrene, and 10% have gallbladder perforation, requiring a cholecystectomy.131
Pulmonary Dysfunction
The lungs, which are frequent and early target organs for mediator-induced injury, are usually the first organs affected in the progression of SIRS to MODS.124 Acute lung injury (ALI) is the pulmonary manifestation of the systemic condition of MODS. Patients who develop MODS usually have pulmonary symptoms; however, not all patients with ALI develop secondary MODS. ALI patients who develop SIRS or sepsis concurrently with acute respiratory failure are at the greatest risk for MODS.132
ALI associated with MODS usually occurs 24 to 72 hours after the initial insult. Patients initially exhibit a low-grade fever, tachycardia, dyspnea, and mental confusion. As dyspnea, hypoxemia, and the work of breathing increase, intubation and mechanical ventilation are required. Acute pulmonary dysfunction results in refractory hypoxemia caused by intrapulmonary shunting, decreased pulmonary compliance, and altered airway mechanics; there usually is radiographic evidence of noncardiogenic pulmonary edema.133
Mediators associated with ALI include inflammatory cells such as polymorphonuclear cells, macrophages, monocytes, endothelial cells, and mast cells; and biochemical mediators such as AA metabolites, toxic oxygen metabolites, proteases, TNF, plate activating factor (PAF), and interleukins. Intense mediator activity damages the pulmonary vascular endothelium and the alveolar epithelium, resulting in surfactant deficiency, mild pulmonary hypertension, and increased pulmonary capillary permeability leading to increased lung water (noncardiogenic pulmonary edema).133 ALI is discussed further in Chapter 15.
Renal Dysfunction
Acute kidney failure is a common manifestation of MODS. The kidney is highly vulnerable to reperfusion injury. Consequently, renal ischemic-reperfusion injury may be a major cause of renal dysfunction in MODS. The patient may demonstrate oliguria or anuria resulting from decreased renal perfusion and relative hypovolemia. Early oliguria is likely caused by decreases in renal perfusion related to shock-like states; late oliguria is typically a sign of evolving renal injury and ischemia. The condition may become refractory to diuretics, fluid challenges, and dopamine. Prerenal oliguria may progress to acute tubular necrosis, necessitating hemodialysis or other renal therapies.121,134 The frequent use of nephrotoxic drugs during critical illness also intensifies the risk of progressive renal impairment. An elevated serum creatinine level is usually a late sign, but it is typically accepted as the index for renal dysfunction.134 Researchers have proposed that the serum creatinine level is a valid indicator of renal function because it significantly differentiates MODS survivors from nonsurvivors.135 Additional signs of renal impairment may include decreased erythropoietin-induced anemia, vitamin D malabsorption, and altered fluid and electrolyte balance. Acute renal failure is discussed further in Chapter 20.
Cardiovascular and Hematological System Dysfunction
The initial cardiovascular response in SIRS or sepsis is myocardial depression; decreased right atrial pressure and systemic vascular resistance (SVR); and increased venous capacitance, VO2, cardiac output (CO), and heart rate. Despite an increased CO, myocardial depression occurs and is accompanied by decreased SVR, increased heart rate, and ventricular dilation. These compensatory mechanisms help maintain CO during the early phase of SIRS or sepsis. An inability to increase CO in response to a low SVR may indicate myocardial failure or inadequate fluid resuscitation, and it is associated with increased mortality. Oxygen consumption may be twice that of normal and may be flow dependent.136
As MODS progresses, cardiac failure develops. Cardiac dysfunction is characterized by ventricular dilation, decreased diastolic compliance, and decreased systolic contractile function. Cardiovascular function becomes vasopressor dependent. Cardiac failure may be caused by immune mediators, TNF, acidosis, or myocardial depressant factor, a substance secreted by the pancreas. TNF has a myocardial-depressant effect and is associated with myocardial depression during septic shock. Myocardial depression is exacerbated by myocardial hypoperfusion from a low CO state and persistent lactic acidosis. Cardiogenic shock and biventricular failure occur and lead to death.136 Cardiac failure is discussed further in Chapter 12, and more information on cardiogenic shock can be found earlier in this chapter.
The most common manifestations of hematological dysfunction in sepsis or MODS are thrombocytopenia, coagulation abnormalities, and anemia.137 The most severe is coagulation system dysfunction manifesting as DIC. DIC is a complex, consumptive coagulopathy that occurs in patients with a variety of disorders, including sepsis, tissue injury, and shock; it is overstimulation of the normal coagulation process. DIC results simultaneously in microvascular clotting and hemorrhage in organ systems, leading to thrombosis and fibrinolysis in life-threatening proportions. Clotting factor derangement leads to further inflammation and further thrombosis. Microvascular damage leads to further organ injury. Cell injury and damage to the endothelium activate the intrinsic or extrinsic coagulation pathways.94 Low platelet counts and elevated D-dimer concentrations and fibrinogen degradation products are clinical indicators of DIC. DIC is discussed further in Chapter 27.
Medical Management
The patient with MODS requires multidisciplinary collaboration in clinical management, including fluid resuscitation and hemodynamic support (when appropriate), prevention and treatment of infection, maintenance of tissue oxygenation, nutritional and metabolic support, comfort and emotional support, and support for individual organ function.81,124 The use of investigational therapies may be part of the patient’s clinical management. Collaborative management of the patient with MODS is outlined in the Collaborative Management Box on Multiple Organ Dysfunction Syndrome.
Identification and Treatment of Infection
Identification and treatment of the underlying source of inflammation or infection are the most important ways to reduce mortality. Medical and surgical intervention to remove sources of infection or contamination may limit the inflammatory response and improve chances of recovery.124 Surgical procedures such as early fracture stabilization, removal of infected organs or tissue, and burn excision are helpful. Appropriate antibiotics are needed if the cause cannot be removed by surgical débridement or incision and draining.124 Other timely interventions, such as prevention of skin ulceration and early nutritional support, can improve outcomes.138 Regardless of the identification of potential risk factors, clinical markers, bacterial contaminants, and investigative approaches for detection and prevention, treatment remains largely supportive and little improvement in the mortality rate has been appreciated.139
Maintenance of Tissue Oxygenation
Normally, under steady state conditions, VO2 is relatively constant and independent of oxygen delivery (DO2) unless delivery becomes severely impaired. The relationship is called supply-independent oxygen consumption. Consequently, a percentage of oxygen is not used (physiological reserve). Patients with SIRS/MODS often develop supply-dependent oxygen consumption in which VO2 becomes dependent on DO2, rather than demand, at a normal or high DO2. When VO2 does not equal demand, a tissue oxygen debt develops, subjecting organs to failure.124
Hypoperfusion and resultant organ hypoxemia often occur in patients at high risk for MODS, subjecting essential organs to failure. Effective fluid resuscitation and early recognition of flow-dependent VO2 is essential, and patients at risk for MODS require hemodynamic monitoring, frequent measurements or surrogate measurements of DO2 and VO2, and serum lactate levels to guide therapy. Serum lactate levels provide information regarding the severity of impaired perfusion and the presence of lactic acidosis and differ significantly in MODS survivors and nonsurvivors.16,121 Failure to maintain adequate oxygenation to vital organs results in organ dysfunction. Despite adequate DO2, VO2 may not meet the needs of the body duringMODS.
Patients with ALI, ARDS, MODS, or sepsis frequently manifest supply-dependent oxygen consumption and are unable to use oxygen appropriately despite normal delivery.124 Interventions that decrease oxygen demand and increase oxygen delivery are essential. Sedation, mechanical ventilation, rest, and temperature and pain control may be able to decrease oxygen demand.123 Oxygen delivery may be increased by maintaining normal hematocrit and PaO2 levels, using PEEP, increasing preload or myocardial contractility to enhance CO, or reducing afterload to increase CO. Various methods of kinetic or prone therapies are available and may enhance alveolar recruitment, improve oxygenation delivery, and decrease other potential complications.
Nutritional and Metabolic Support
Hypermetabolism in SIRS or MODS results in profound weight loss, cachexia, and loss of organ function. The goal of nutritional support is the preservation of organ structure and function. Although nutritional support may not alter the course of organ dysfunction, it prevents generalized nutritional deficiencies and preserves gut integrity. The enteral route is preferable to parenteral support.35 Enteral feedings are given distal to the pylorus to reduce the risk of pulmonary aspiration. Enteral feedings may limit bacterial translocation. In addition to early nutritional support, the pharmacological properties of enteral feeding formulas may limit SIRS for selected critical care populations. Supplementation of enteral feedings with glutamine may be beneficial;111,140 however, arginine, a precursor to nitric oxide, should be avoided in critically ill patients.110 Enteral feedings with omega-3 fatty acids may lessen the development of SIRS and improve outcomes.110 Nutritional support is discussed further in Chapter 8.
Nursing Management
Preventive measures include a multitude of assessment strategies to detect early organ manifestations of this syndrome. Patients who continue to experience sites of inflammation, septic foci, and inadequate tissue perfusion may be at higher risk. Hand hygiene, aseptic technique, and an understanding of how microorganisms can invade the body are essential components of preventive nursing care.
Nursing management of the patient with MODS incorporates a variety of nursing diagnoses (Nursing Diagnosis Priorities Box on Multiple Organ Dysfunction Syndrome). Nursing priorities are directed toward (1) preventing development of infection, (2) facilitating oxygen delivery and limiting tissue oxygen demand, (3) facilitating nutritional support, (4) providing comfort and emotional support, and (5) maintaining surveillance for complications.
Patients are assessed closely for inflammation and infection. Subtle expressions of infection warrant investigation. Nursing measures include strict adherence to standards of practice to prevent infection. Practices related to infection control with invasive hemodynamic monitoring, urinary catheters, endotracheal tubes, intracranial pressure monitoring devices, total parenteral nutrition (TPN), and wound care must be stringent to prevent further infection. Prevention of a concomitant ventilator-associated pneumonia or aspiration pneumonia is a priority.16,141
Measures to limit tissue oxygen consumption include (1) administering analgesics and sedatives, (2) positioning the patient for comfort, (3) limiting activities, (4) offering support to reduce anxiety, (5) providing a calm and quiet environment, and (6) teaching the patient about the condition. Measures to enhance tissue oxygen supply include administering supplemental oxygen, monitoring the patient’s respiratory status, and administering prescribed fluids and medications.