Objectives
![]()
Be sure to check out the bonus material, including free self-assessment exercises, on the Evolve web site at http://evolve.elsevier.com/Urden/priorities/.
Patients in critical care units include an increasing number of older adults. According to a U.S. Department of Health and Human Services report, the United States population older than 65 years reached 35.6 million, accounting for 12.3% of the overall population. Those in the 65- to 74-year age group numbered 18.3 million, 75- to 84-year-olds accounted for 12.7 million, and those in the 85-year or older age group numbered 6 million. This latter group is expected to reach 9.6 million by 2030.1 In 2001 a 65-year-old woman had a life expectancy of 19.4 more years, whereas men could expect to live another 16.4 years.1
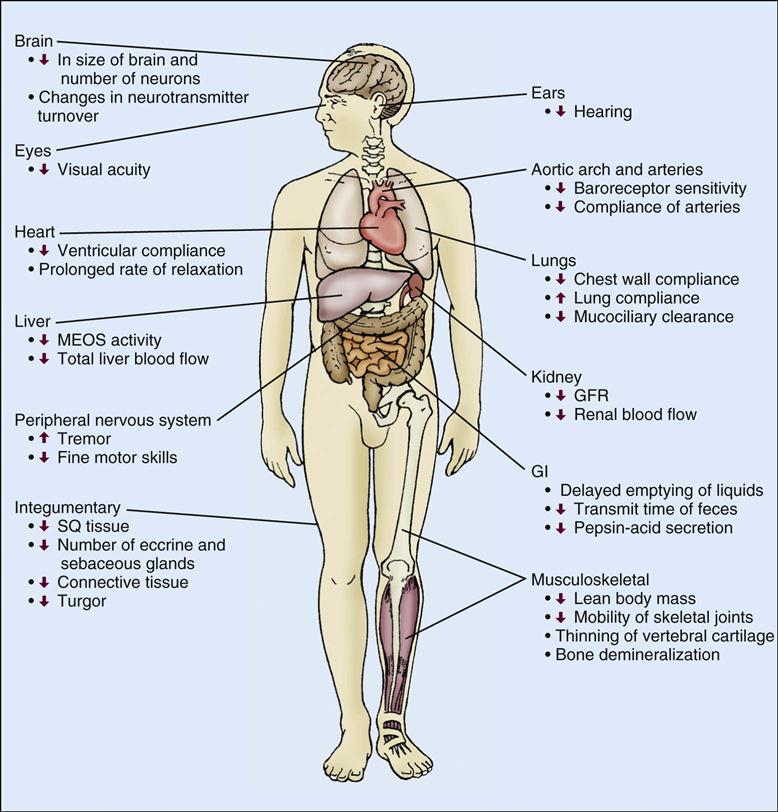
The process of senescence (growing old) is characterized by tissue and organ changes. This, in combination with the prevalence of chronic conditions in the older adult, contributes to increased morbidity and mortality in the critical care unit. Aging is accompanied by physiological changes in the cardiovascular, respiratory, renal, gastrointestinal (GI), hepatic, integumentary, immune, and central nervous systems. With advancing age the incidence of disease increases, with cardiovascular and neoplastic diseases being the most common causes of death.2 However, although physiological decline and disease processes influence each other, physiological decline occurs independently of disease and is responsible for the development of symptoms at an earlier stage of disease in older adults than in their younger counterparts.2 Therefore changes in physiological function are important to consider when caring for the older adult patient (Table 7-1, Figure 7-1, Box 7-1).
TABLE 7-1
SUMMARY OF AGE-RELATED PHYSIOLOGICAL CHANGES AND RELATED CLINICAL CONSIDERATIONS
| AGE-RELATED EFFECT | CLINICAL CONSIDERATIONS |
| Cardiovascular System | |
| ↓ Inotropic and chronotropic response of myocardium to catecholamine stimulation | The increase in cardiac output during stress or exercise is achieved by an increase in diastolic filling (increased dependence on Starling’s law of the heart) |
| ↑ Myocardial collagen content | Leads to a decrease in the compliance of the ventricle (higher filling pressures are needed to maintain stroke volume) |
| ↓ Baroreceptor sensitivity | ↑ Tendency for orthostatic hypotension after prolonged bed rest or if patient is taking antihypertensive medication or has systolic hypertension |
| Prolonged rate of relaxation | May predispose the elderly patient to hemodynamic derangements in the presence of tachydysrhythmias, hypertension, or ischemic heart disease |
| ↓ Compliance of blood vessels | ↑ Peripheral vascular resistance and blood pressure |
| Respiratory System | |
| ↓ Strength of the respiratory muscles, recoil of lungs, chest wall compliance, and efficiency and number of cilia in airways | ↑ Susceptibility to aspiration, atelectasis, and pulmonary infection Patient may require more frequent deep breathing, coughing, and position change |
| ↓ Pao2 level | ↓ Ventilatory response to hypoxia and hypercapnia ↑ Sensitivity to narcotics |
| Renal System | |
| ↓ Glomerular filtration rate | Careful observation of patient when administering aminoglycosides, antibiotics, and contrast dyes |
| ↓ Ability to concentrate and conserve water | May predispose patient to development of dehydration and hypernatremia, especially if patient is fluid-restricted and insensible losses are high (e.g., during mechanical ventilation or fever) |
| ↓ Ability to excrete salt and water loads, as well as urea, ammonia, and drugs | Observe for clinical manifestations of fluid overload and drug reactions |
| ↓ Response to an acid load | After an acid load (i.e., metabolic acidosis), the older patient may be in a state of uncompensated metabolic acidosis for a longer period |
| Liver | |
| ↓ Total liver blood flow | Adverse drug reactions, especially with polypharmacy |
| Gastrointestinal System | |
| Diminished ability to swallow | May predispose older patient to aspiration pneumonia |
| Impaired esophageal motility | Assess for proper fit of dentures and ability to chew |
| Delayed emptying of liquids | Flex head forward 45 degrees Develop awareness for complaints of food or medications “sticking in throat” Assess for complaints of heartburn or epigastric discomfort Avoid prolonged supine position |
| ↓ Stool weight and transit time | Examine abdomen for distention Investigate complaints of anorexia Obtain thorough bowel history and note routine use of laxative Increase intake of dietary fiber and assess for fecal incontinence and impaction |
| Neurological System | |
| ↑ Cranial dead space | Older persons may sustain a significant amount of hemorrhage before symptoms are apparent |
| ↓ Number of neurons and dendrites and length of dendrite spines | Delayed or impaired processing of sensory and motor information |
| Delay in the rate of synaptogenesis Changes in neurotransmitter turnover |
May cause desynchronization of neurotransmission |
CO, Cardiac output; GFR, glomerular filtration rate.
Modified from Rebenson-Piano M: The physiologic changes that occur with aging. Crit Care Q 12(1):1, 1989.
Cardiovascular System
Advancing age has many effects on the cardiovascular system. With advancing age both the myocardium and the vascular system undergo a multitude of anatomic and cellular changes that alter the function of both the myocardium and peripheral vascular system.3 These changes in cardiovascular function significantly impact critical illness in the older adult because of the age-related effects on cardiovascular structure and function. In addition, because age is a major risk factor for cardiovascular disease in the older adult, this high-risk population will encounter more cardiovascular events when admitted for noncardiac problems to the critical care unit.4
Age-Related Changes in Myocardial Structure and Function
Myocardial collagen content increases with age.5,6 Collagen is the principal noncontractile protein occupying the cardiac interstitium.7 Increased myocardial collagen content renders the myocardium less compliant; therefore a decrease in myocardial compliance can adversely affect diastolic filling (through decreased distensibility and dilation) and myocardial relaxation. Consequently, the left ventricle must develop a higher filling pressure for a given increase in ventricular volume. Decreased left ventricular compliance may be evident in the older adult by the presence of an S4 heart sound.8
The functional consequence of these changes could be an increase in myocardial oxygen consumption. Under normal physiological conditions, an increase in myocardial oxygen demand is met with a corresponding increase in coronary artery blood flow. However, in the presence of coronary artery disease, coronary artery blood flow can be limited because of atherosclerotic-mediated narrowing of the coronary arteries. Hence the older patient is at risk for developing myocardial ischemia and/or infarction. Clinical manifestations of myocardial ischemia include electrocardiographic (ECG) changes and chest pain. However, the sensation of chest pain may be altered in the older adult. Atypical symptoms, such as dyspnea, confusion, and failure to thrive are frequently the only symptoms associated with myocardial infarction in this high-risk population.9
The aging heart also undergoes a modest degree of hypertrophy that is similar to pressure overload-induced hypertrophy. Such hypertrophy entails a thickening of the left ventricular wall without appreciable changes in left ventricular cavity size.10 However, increases in left ventricular cavity size associated with aging occur only in men.9 The increase in left ventricular wall thickness is a result primarily of an increase in muscle cell size. In older individuals the myocardial hypertrophy may be caused by corresponding increases in aortic impedance and systemic vascular resistance.11
Myocardial contractility depends on numerous factors. However, the most important determinants of myocardial contraction are the intracellular level of free calcium and the sensitivity of the contractile proteins for calcium.11,12 Because peak contractile force in the senescent myocardium is unaltered, this suggests that neither the amount of intracellular free calcium during systole nor the sensitivity of the contractile proteins for calcium is altered. The prolonged duration of contraction (systole) is caused in part by a slowed or delayed rate of myocardial relaxation, which may be an adaptive mechanism to preserve contractile function compromised by age-related increases in afterload.3,12
Age-Associated Changes in Hemodynamics and the Electrocardiogram
Resting (supine) heart rate decreases with age.13,14 Cinelli et al13 reported a decrease in the resting heart rate from 78.8 beats/min in young adults to 62.3 beats/min in older adults. Heart rate is an important determinant of cardiac output (CO), and the normal resting heart beats approximately 70 times a minute. At rest or with minimal activity, the older adult probably will not experience any untoward cardiovascular effect (i.e., a decrease in CO) with a heart rate of 62 beats/min. However, if the heart rate response is attenuated during exercise, the older person’s capacity for exercise may be limited.
Resting CO and stroke volume (SV) are not changed with advancing age. At rest, left ventricular end-diastolic volume (LVEDV, preload), end-systolic volume, and the ejection fraction are not affected by age.15 In the older human myocardium, the early diastolic filling period and isovolumic phase of myocardial relaxation are prolonged.15–17 However, these changes, although suggestive of diastolic dysfunction, do not translate into decreases in end-diastolic volume or stroke volume.16,17 Finally, aging is associated with a moderate increase in pulmonary artery pressure.18
Advancing age produces changes in the ECG. R-wave and S-wave amplitude significantly decrease in persons older than 49 years, whereas QT duration increases19 (Table 7-2). The incidence of asymptomatic cardiac dysrhythmias increases in older patients.20 The most common dysrhythmia occurring in older individuals is the premature ventricular contraction (PVC). Carom et a121 and Fleg and Kennedy22 report that 70% to 80% of all patients older than 60 years experience PVCs. Other common types of dysrhythmias are sinus node dysfunction (atrial fibrillation, atrial flutter, or paroxysmal supraventricular tachycardia) and atrioventricular conduction disturbances.15,19,20 Because the majority of patients are asymptomatic, the use of antidysrhythmics is generally not recommended. The side effects and toxic effects of antidysrhythmics impose more of a risk, as compared with the risk of mortality or morbidity related to the dysrhythmia.20,23 In contrast, for patients who are symptomatic and have malignant ventricular dysrhythmias (sustained ventricular tachycardia and/or fibrillation), pharmacological therapy is warranted.20,23
TABLE 7-2
AGE-RELATED CHANGES IN ELECTROCARDIOGRAPHIC VARIABLES
| AGE (YEARS) | ||||
| ECG VARIABLE | YOUNGER THAN 30 | 30-39 | 40-49 | OLDER THAN 49 |
| R wave amplitude (mm) | 10.43 | 10.53 | 9.01 | 9.25 |
| S wave amplitude (mm) | 15.21 | 14.21 | 12.22 | 12.42 |
| Frontal plane axis (degrees) | 48.93 | 48.13 | 36.50 | 38.83 |
| PR duration (ms) | 15.89 | 16.23 | 16.04 | 16.25 |
| QRS duration (ms) | 7.64 | 7.51 | 7.36 | 8.00 |
| QT duration (ms) | 37.83 | 37.50 | 37.99 | 39.58 |
| T-wave amplitude (ms) | 5.21 | 4.57 | 4.31 | 4.42 |
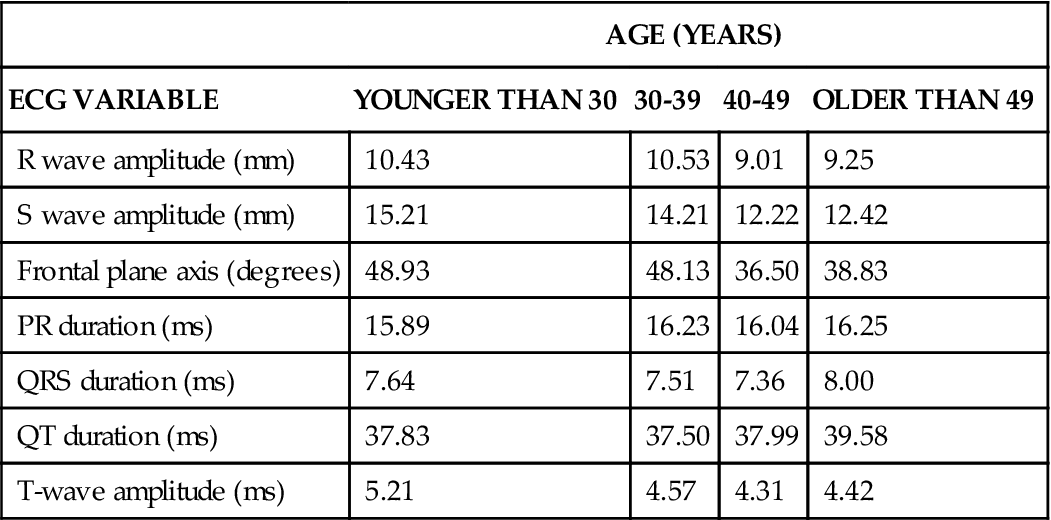
Data from Bachman S, Sparrow D, Smith LK: Effect of aging on the electrocardiogram. Am J Cardiol 48(3):513, 1981.
Age-Related Changes in Baroreceptor Function
Baroreceptor reflex function is altered with aging.24 Baroreceptors, located at the bifurcation of the common carotid artery and aortic arch, are mechanoreceptors that respond to stretch and other changes in the blood vessel wall.25 Impulses arising in the baroreceptor region project to the vasomotor center (nucleus of tractus solitarius) in the medulla. Abrupt changes in blood pressure caused by increases in peripheral resistance, CO, or blood volume are sensed by the baroreceptors, resulting in an increase in the impulse frequency to the vasomotor center within the medulla. This increase inhibits vasoconstrictor impulses arising from the vasoconstrictor region within the medulla.25 The result is a decrease in heart rate (HR) and peripheral vasodilation; both these effects return the blood pressure to within normal limits.
Postural hypotension was once thought to occur more frequently in older persons and to be related to age. However, recent studies have shown that the prevalence of postural hypotension is quite low in older persons.26,27 The prevalence of orthostatic hypotension is greater in institutionalized older patients who are receiving antihypertensive medications.28
Left Ventricular Function
In most individuals, aging is associated with a decline in exercise performance. The thickening of the left ventricular wall along with stiffening of the aortic and mitral valves makes the aging heart less able to provide adequate contractile strength.29 With advancing age the maximal HR achieved during exercise is attenuated; however, the decreased HR response is accompanied by an increase in LVEDV and SV. This augmentation in LVEDV and SV offsets the attenuated HR response and maintains CO in exercise.
Healthy older persons have no age-associated decline in CO during exercise, but other factors (e.g., neural functioning, skeletal/joint functioning, pulmonary function) may limit an older individual’s ability to exercise.
Peripheral Vascular System
The effects of aging on the peripheral vascular system are reflected in the gradual but linear rise in systolic blood pressure.30,31 Diastolic blood pressure is less affected by age and generally remains the same or decreases.31
Important determinants of systolic blood pressure include the compliance of the vasculature and the blood volume within the vascular system. Similar to the heart, the compliance of the vasculature is determined by its cell type and tissue composition. With advancing age the intimal layer thickens, principally because of an increase in smooth muscle cells that have migrated from the medial layer, and the amount of connective tissue (collagen, elastic tissue) increases.30 These changes occur in the intima of the large and distal arteries. This gradual decrease in arterial compliance, or “stiffening of the arteries,” is known as arteriosclerosis. Arteriosclerotic and atherosclerotic processes cause the arteries to become progressively less distensible, altering the vascular pressure-volume relationship. These changes are clinically significant because small changes in intravascular volume are accompanied by disproportionate increases in systolic blood pressure. The decrease in arterial compliance and disproportionate increase in systolic blood pressure may lead to an increase in afterload and the development of concentric (pressure-induced) ventricular hypertrophy in the elderly patient.32
Arterial pressure is also governed by the amount of blood volume, which in turn is regulated by plasma levels of sodium and water and the activity of the renin-angiotensin system.33 Plasma renin activity declines with age, and aging per se has no appreciable effect on sodium and water homeostasis.34,35 As noted later, however, age-related changes occur in renal tubular function, and the glomerular filtration rate (GFR) decreases, both of which can affect overall sodium and water homeostasis. Circulating levels of sodium-regulating hormones, such as natriuretic hormone, aldosterone, and antidiuretic hormone (ADH), are not appreciably altered by advancing age.35,36 However, a delayed natriuretic response after sodium loading and plasma volume expansion and a diminished renal response to ADH secretion have been reported in older persons.36
Pulmonary System
Many of the changes in the pulmonary system that occur with aging are reflected in pulmonary function tests and include changes in thoracic wall expansion and respiratory muscle strength, morphology of alveolar parenchyma, and decreases in arterial oxygen tension (PaO2)37 (Table 7-3). These changes occur progressively as age advances and should not alter the older person’s ability to breathe effortlessly. However, factors such as repeated exposure to environmental pollutants, cigarette smoking, and frequent pulmonary infections can accelerate age-related changes, thereby making it difficult to identify the age-associated changes in pulmonary function.
TABLE 7-3
PROGRESSIVE CHANGES IN ARTERIAL OXYGEN TENSION (PaO2) AND CARBON DIOXIDE TENSION (PaCO2)
| AGE-GROUP (YEARS) | PaO2 (MM HG) | PaCO2 (MM HG) |
| ≤30 | 94 | 39 |
| 31-40 | 87 | 38 |
| 41-50 | 84 | 40 |
| 51-60 | 81 | 39 |
| >60 | 74 | 40 |
Modified from Sorbini CA, et al: Arterial oxygen tension in relation to age in healthy subjects. Respiration 25(1):3, 1968.
Thoracic Wall and Respiratory Muscles
Upper airway changes include weakening support of upper and lower cartilage, predisposing older persons to obstructive changes. Submucosal glands decrease production of mucus, leading to dryness and thickened secretions.38 With advancing age the chest wall (thoracic skeleton) and vertebrae undergo a small degree of osteoporosis, and at the same time the costal cartilages that connect the rib cage together become calcified and stiff. These changes may produce kyphosis and reduce chest wall compliance, respectively.37,39,40 The functional effect is a decrease in thoracic wall excursion. Other factors, such as an increase in abdominal girth and change in posture, also decrease thoracic excursion. These anatomic changes are reflected by an increase in residual volume and decrease in vital capacity.
The strength of the respiratory muscles (diaphragm, external/internal intercostal muscles) gradually decreases. Respiratory muscle weakness begins as early as age 55.41 During aging, skeletal muscle progressively atrophies and its energy metabolism decreases, which may partially explain the declining strength of the respiratory muscles.42,43 In addition, an age-associated decrease occurs in the effectiveness of the cough reflex, possibly caused by a decrease in ciliary responsiveness and motion.44
Alveolar Parenchyma
With advancing age a diminished recoil (or increased compliance) of the lung occurs.45 The reduced recoil results from the increase in the ratio of elastin to collagen content that occurs with advancing age.46 Collagen, elastin, and reticulin are the primary connective tissue proteins of the lung tissue.47,48 They are responsible for the elasticity and performance of the airways of the lung. Whereas total lung collagen remains unaltered, the amount of elastin increases with age in the interlobular septa and pleura and possibly within the bronchi and their vessels. These anatomic changes are reflected by an increase in residual volume and a decrease in forced expiratory volume. With changes in cartilage the trachea and bronchi become stiffer and less compliant.38 Also, the size of the alveolar ducts increases after age 40.37 The bronchial enlargement displaces inhaled air volume away from the alveoli that line the alveolar ducts (Figure 7-2).
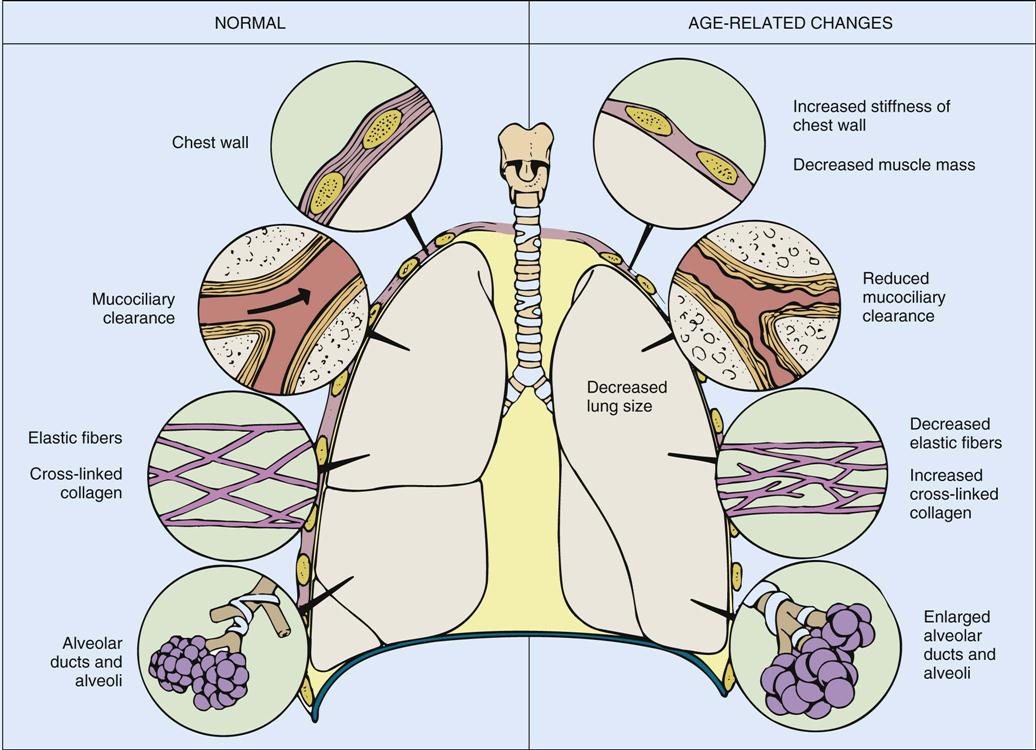
With advancing age, compliance of the chest wall and lung tissue changes, with reduced clearance of mucus by cilia that line the pulmonary tree and enlargement of alveolar ducts and alveoli. (From Urden LD, Stacy KM, Lough ME: Critical care nursing: Diagnosis and management, ed 6, St. Louis, 2010, Mosby.)
Ventilation and oxygen/carbon dioxide exchange (diffusion) depend on numerous factors, including the surface area available for diffusion. A displacement of inhaled air volume away from the alveoli limits the surface area available for gas exchange. This may partly explain the progressive and linear decrease in the pulmonary diffusion capacity, which depends on both surface area and capillary blood volume. Capillary blood volume and surface area have been reported to decrease with advancing age.49
Pulmonary Gas Exchange
The arterial oxygen tension (PaO2) decreases with age, such that the median PaO2 for healthy persons older than 60 years is 74.3 mm Hg, as compared with 94 mm Hg for younger adults.50 In contrast, arterial carbon dioxide (PaCO2) does not change with advancing age (see Table 7-2).50 The decrease in Pao2 may be the result of an increase of air trapping as the result of a ventilation/perfusion mismatch.51,52 Consequently, dependent lung zones may be ventilated intermittently, leading to regional differences in ventilation. It is possible that alterations in blood volume and vascular resistance within the pulmonary circulation may also contribute to ventilation/perfusion (V/Q) mismatching. Other factors, such as smoking and pulmonary disease, also have an impact on the level of arterial oxygenation.
Lung Volumes and Capacities
With advancing age, total lung capacity and tidal volume do not change.39 Residual volume (RV) increases with age, paralleling the decrease in chest wall compliance and reduced strength of the respiratory muscles. The increase in RV may also add to the diminished strength of the inspiratory muscles by stretching the diaphragm and altering the tension-length relationship.
Renal System
Aging produces changes in renal structure and function, many of which begin at approximately 30 to 40 years of age.53,54 One of the prominent changes is a decrease in the number and size of the nephrons, which begins in the cortical regions and progresses toward the medullary portions of the kidney.55 The decrease in the number of nephrons corresponds to a 20% decrease in the weight of the kidney between 40 and 80 years of age.55 Initially this loss of nephrons does not appreciably alter renal function because of the large renal reserve: the kidney contains approximately 2 million to 3 million nephrons, all of which are not needed to maintain adequate fluid and acid-base homeostasis. However, with time the geriatric patient also loses this renal reserve.55 Nephron loss is caused by a gradual reduction in blood flow to the glomerular capillary tuft.56 Total renal blood flow declines after the fourth decade of life53 because of hyaline arteriosclerosis.56,57 The etiology of this vascular lesion within the glomerular tuft is unknown. By the eighth decade of life, 50% of the glomeruli are lost as a result of this arteriolar hyalinization.55
Fluid Filtration
GFR decreases with advancing age.58,59 In older persons the decrease in GFR is most likely caused by the decrease in nephron number as well as decreased renal blood flow.58
Even though the remaining nephrons adapt to the loss of nephrons by glomerular hyperfiltration and increased solute load per nephron, the reduced GFR predisposes the elderly patient to adverse drug reactions and drug-induced renal failure. Some drugs are excreted unchanged in the urine, whereas other drugs have active or nephrotoxic metabolites that are excreted in the urine. In addition, the senescent kidney is more susceptible to injury by hypotensive episodes because of the age-related decrease in renal blood flow and reduced pressure gradient across the afferent arteriole.60
Age-related changes also occur in tubular function. The age-related changes in tubular function become apparent when extreme changes occur in the body fluid composition or acid-base balance. For example, with systemic acidosis the rate and amount of total acid excretion (bicarbonate, titratable acid, ammonium) are reduced.58,60 This predisposes the older patient to metabolic acidosis, volume depletion, and hyperchloremia. At a normal pH level, however, the kidney of an older person can maintain acid-base homeostasis.
The senescent kidney has diminished capability to excrete a free water load, conserve water during periods of dehydration, and conserve sodium during periods of low salt intake.58 Older persons are at high risk for dehydration because of these renal changes, along with decreased overall total body water, decreased concentrating ability, and decreased thirst perception.61 Age-related changes also occur in extrarenal mechanisms, such as the decreased activity and responsiveness of the senescent kidney to the sympathetic nervous system and renin-angiotensin-aldosterone system, which are important in integrating overall fluid homeostasis and maintaining blood pressure in response to changes in body position.34
Gastrointestinal System
Age-related gastrointestinal changes occur in the processes of swallowing, motility, and absorption.62,63 Swallowing may be difficult for the older person because of incomplete mastication of food.63 Deteriorating dentition, diminished lubrication (secondary to salivary dysfunction), and poorly fitting dentures result in insufficient mastication of food within the oral cavity, predisposing the older patient to aspiration.62 In addition, the number and velocity of the peristaltic contractions of the older person’s esophagus decrease, and the number of nonperistaltic contractions increases.63
These changes in esophageal motility are referred to as presbyesophagus. These changes may predispose the patient to erosion of the esophageal wall (recurrent esophagitis) because food remains in the esophagus longer. In addition, bed rest and reclining in a supine position for a prolonged period can cause esophageal reflux, which also can lead to esophagitis.
The aging process produces thinning of the smooth muscle within the gastric mucosa.64 The epithelial layer of the gastric mucosa, which contains the chief and parietal cells, undergoes a modest degree of atrophy, resulting in the hyposecretion of pepsin and acid, respectively.65
Mucin secretion from the mucous cells decreases, thereby altering the protective function of the gastric mucosal (bicarbonate) barrier. Because of this, the stomach wall is more susceptible to acid injury, thus increasing the incidence of gastric ulcerations.66 Aging does not appreciably alter gastric emptying of solid foods. Alterations within the small intestine include a decrease in intestinal weight after age 50 and a flattening and shortening of jejunal villi.67 Age produces no change in the small intestine’s absorption of fats and proteins; however, decreased carbohydrate absorption has been reported.68,69 There is essentially no change in vitamin or mineral absorption, except for a decrease in calcium absorption from the aged duodenum.63
Liver
With advancing age, both hepatocyte number and liver weight decrease.70 Total liver blood flow decreases by 50% between 25 and 65 years of age.70–72 The liver has many complex functions, including carbohydrate storage, ketone body formation, reduction/conjugation of adrenal and gonadal steroid hormones, synthesis of plasma proteins, deamination of amino acids, storage of cholesterol, urea formation, and detoxification of toxins and drugs. Despite changes in hepatocyte number and blood flow, however, liver function is not appreciably altered.72 Several liver function tests, including serum bilirubin, alkaline phosphatase, and aspartate aminotransferase (AST) levels, are not altered with advancing age. However, because of the decrease in total liver blood flow, first-pass clearance of drugs is somewhat reduced. The most important age-related change in liver function is the decrease in the liver’s capacity to metabolize drugs.73,74 Although liver function tests do not reflect this change in metabolism, it is well recognized that drug side effects and toxic effects occur more frequently in older adults than in young adults.74
Central Nervous System
Cognitive Functioning
Cognitive functioning involves the process of transforming, synthesizing, storing, and retrieving sensory input. Additional components include perception, attention, thinking, memory, and problem solving. For the aging individual, cognition is altered by the speed at which information is processed and retrieved.75 Performance on timed tests declines slowly after age 20. Intelligence remains fairly stable after age 30 until the mid-80s. Although the rate at which complex tasks are completed may be diminished, these age-related changes are not synonymous with cognitive impairment.
Marked deterioration of any component of cognitive functioning is not a normal expectation of the aging process.76 Cognitive impairment in older adults more often results from acute and chronic etiologies. Acute problems such as infection, electrolyte imbalance, and pharmacological toxicity are generally reversible once identified. Long-term chronic impairment develops from more organic causations, such as multi-infarct dementia or Alzheimer’s disease.77
Changes in Structure and Morphology
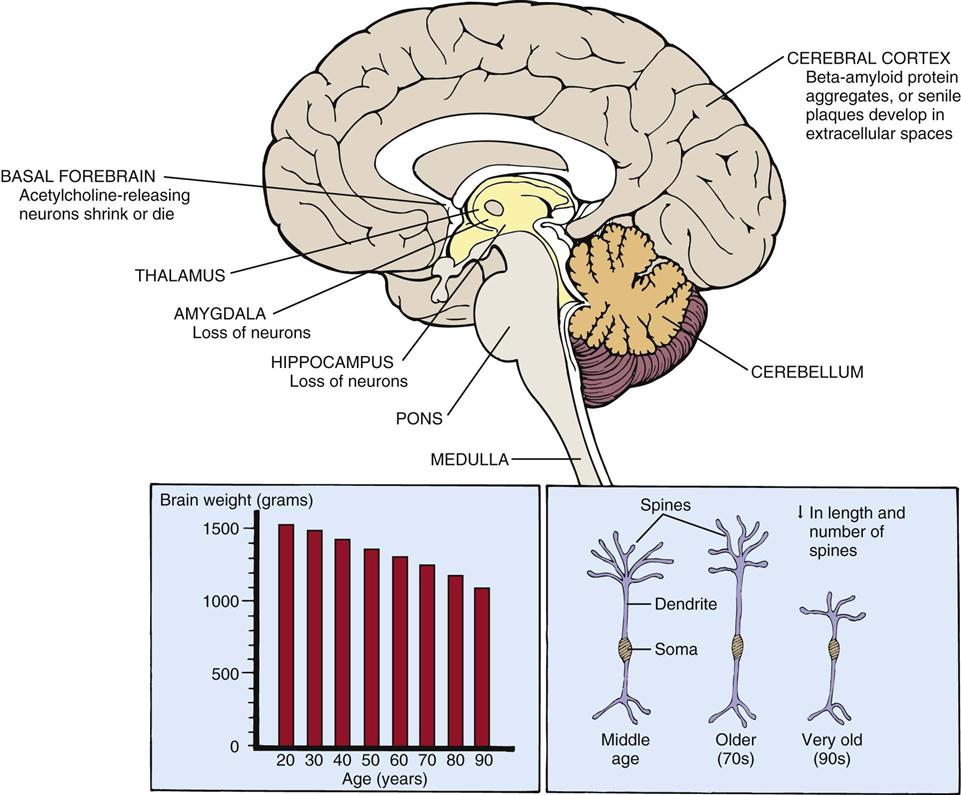
The brain decreases approximately 20% in size between 25 and 95 years of age (Figure 7-3).78 The reduced brain weight may be related in part to the overall decrease in the number of neurons that occurs with advancing age. Neurons are lost from the hippocampus, amygdala, and cerebellum and from areas of the brainstem such as the locus ceruleus, dorsal motor nucleus of vagus nerve, and substantia nigra.75 In contrast, very few neurons disappear with advancing age in areas such as the hypothalamus.77 In addition, portions of the cerebral cortex atrophy, principally the frontal (superior frontal gyrus) and temporal (superior temporal gyrus) cortical association areas.78
The cerebral ventricles enlarge and develop an asymmetric appearance. Cerebrospinal fluid (CSF) also accumulates in the ventricles, although total brain CSF is not increased.79 Accompanying the loss of neurons are changes in the ultrastructure and intracellular structures of the neuron.80 Also, neuron shrinkage and degenerative changes in the cell bodies and axons of certain acetylcholine-secreting neurons have been reported. There are also increases in norepinephrine and dopamine synthesis.81 These changes may explain alterations in processing and receiving information.77
In the senescent brain, synaptogenesis (synaptic regeneration) still occurs after partial nerve degeneration. After a nerve fiber is damaged, neighboring undamaged neurons often sprout new fibers and form new connections. However, synaptogenesis occurs at a slower rate in the older brain.80
Cerebral Metabolism and Blood Flow
Cerebral blood flow decreases with advancing age. This decrease parallels the decrease in brain weight and is most likely caused by the reduction in neuron number and metabolic needs of the cerebral tissue.82
Immune System
Several changes in immune function render the older adult more susceptible to infections.83–88 Infections in the geriatric population are associated with higher rates of mortality.88 Common infections in the older adult include bacterial pneumonia, urinary tract infection, intraabdominal infections, gram-negative bacteremia, and decubitus ulcers.88 The reasons for the increased susceptibility are multifactorial and include changes in cell- and humoral-mediated immunity; breakdown in physical barriers, such as the skin and oral mucosa; and changes in nutrition.
Cell-Mediated and Humoral-Mediated Immunity
Immune system function depends on many cell types with distinct functions. T cells are the primary effector of cell-mediated immunity, whereas bone marrow-derived B cells produce antibodies that are the effector cells of humoral-mediated immunity.83,85,87 With aging, cell-mediated immunity declines. Even though the total number of T cells remains unchanged with advancing age, T-cell function decreases.85,87 For example, there is a decrease in T-cell production of interleukin-2 (IL-2) and in differentiation of T cells into effector cells. IL-2 is essential for activating B cells, which eventually differentiate into antibody-secreting cells. Subsets of T cells mature into cytotoxic cells, whereas other T cells activate B cells and stimulate B-cell proliferation. Changes in B-lymphocyte function are not as well understood, even though with age the ability of B cells to produce antibodies into new antigens declines.83,85
Furthermore, inadequate emptying of urine secondary to bed rest, obstruction, or side effects from anticholinergic medications can result in stagnation of urine and recurrent urinary tract infections. Long-term placement of urinary catheters is a significant source of bacteriuria. However, treatment with antibiotic therapy is not indicated unless the patient becomes symptomatic with anorexia or cognitive impairment or has a history of a chronic illness such as diabetes or chronic obstructive pulmonary disease.83,88
Integumentary and Musculoskeletal Systems
The loss of elastic and connective tissue causes the skin to wrinkle; both skin wrinkles and sagging may be found over many areas of the body. Underlying structures, such as the veins and muscles, are more visible because of the transparency of the skin.
Multiple ecchymotic areas may result from decreased protective subcutaneous tissue layers, increased capillary fragility, and flattening of the capillary bed, all of which predispose elderly persons to developing ecchymosis.89–92 In conjunction with frequent aspirin use, these physiological factors result in increased bleeding tendencies and the appearance of ecchymotic areas. However, areas of unexplained ecchymosis may also indicate elder abuse.
Changes that occur in the musculoskeletal system are a decrease in lean body mass, compression of the spinal column resulting from the thinning of cartilage between vertebra, and a decrease in the mobility of skeletal joints.93 Muscle rigidity increases, especially in the neck, shoulders, hips, and knees,94 possibly causing changes in range of motion.
Bone demineralization affects both men and women as they age but occurs four times more often in women than men. Bone demineralization refers to an increase in osteoblast and osteoclast activity, which decreases calcium absorption into the bone.93 Osteoporosis produces bones that are more “porous” or fragile. With extensive bone demineralization, an older patient may sustain multiple fractures.
Changes in Pharmacokinetics and Pharmacodynamics
The many benefits of modern advancements in pharmacological therapy are frequently counterbalanced by adverse drug effects, medication interactions, and therapeutic failure.84 Adverse drug effects and medication interactions are related to pharmacokinetics and pharmacodynamics. There are many age-related changes in drug pharmacokinetics, which is the manner in which the body absorbs, distributes, metabolizes, and excretes a drug.95,96 The aging process is associated with changes in gastric acid secretion, which can alter the ionization or solubility of a drug and hence its absorption95,96 (Table 7-4).
TABLE 7-4
AGE-RELATED CHANGES IN PHARMACOKINETICS
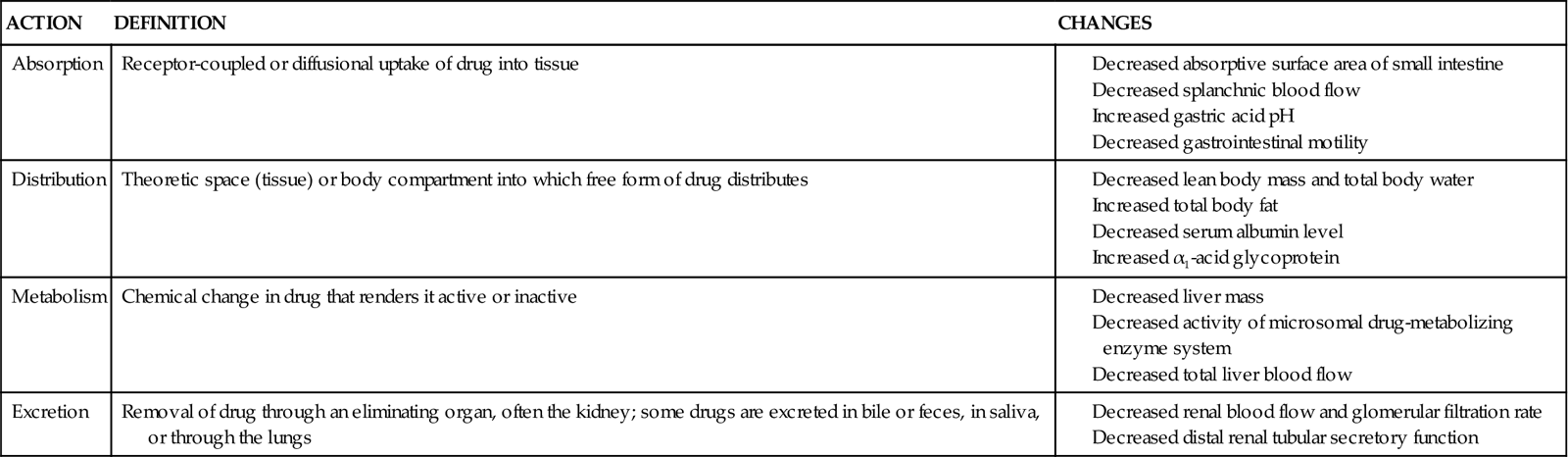
Data from Gilman AG, et al, editors: Goodman and Gilman’s the pharmacological basis of therapeutics, ed 8, London, 1990, Pergamon; and Vestal RE, Cusack BJ: In Schneider EL, Rowe JW, editors: Handbook of the biology of aging, San Diego, 1990, Academic Press.
Drug distribution depends on body composition, as well as the physiochemical properties of the drug. With advancing age, fat content increases, lean body mass decreases, and total body water decreases, which can alter the drug disposition.96 For example, because of the increase in the ratio of body fat content to body weight, lipophilic drugs have a greater volume of distribution per body weight in older adults as compared with younger adults. Other age-related factors95,97 affecting drug disposition are listed in Table 7-4.
As noted, the senescent liver and kidneys are less able to metabolize and excrete drugs, which also affects clinical outcomes. For example, the rate of absorption, time to peak plasma concentration, and clearance of loop diuretics is reduced in older adults, which may necessitate high dosing regimens in order to facilitate diuresis.98,99 This poses an increased risk of metabolic acidosis, because the higher diuretic dose increases competition for the organic acid transport pathway at the proximal tubule. Using the example of diuretics, bioavailability between agents may also be variable. For instance, bumetanide has a fairly consistent bioavailability in advanced age, whereas that of furosemide varies from 20% to 80%.99
Similarly, other drugs associated with management of common disorders seen in critically ill patients—such as digoxin, angiotensin II-converting enzyme (ACE) inhibitors, and angiotensin II-receptor blockers (ARBs)97—have delayed excretion, increased serum concentration, and more prolonged duration of action because their excretion parallels GFR (which decreases with age).97 See Table 7-4 for age-related changes in drug pharmacokinetics.
Age-related changes in pharmacodynamics have also been reported. Pharmacodynamics refers to the pharmacological or physiological response to a drug that occurs after the drug interacts with its receptor on the plasma membrane. The chronotropic and inotropic effects of b-adrenergic agonists reportedly decrease in older patients.98,99 There also are reports that age produces no change in heparin-stimulated increases in partial thromboplastin time, whereas the effects of warfarin (Coumadin) are very susceptible to medication interactions.
The use of multiple medications in the presence of multiple comorbidities has been associated with an increase in adverse drug reactions. Although this is not always avoidable, it is important to avoid choosing an agent for its side effect profile (e.g., diphenhydramine for sedative effects) and monitor the effects of the chosen agent. A major cause of therapeutic failure is the underuse or inappropriate use of drug therapy that is indicated for the treatment of a particular problem. It is not uncommon for delirium not associated with a withdrawal syndrome to be treated with benzodiazepines in critical care. However, this frequently makes agitation worse, once the sedative effects are gone, in comparison to a low-dose antipsychotic agent.100,101.