Chapter 67 Severe head injury
Despite improvements in resuscitation and vital organ support, the management of patients with traumatic brain injury in the intensive care unit (ICU) presents a challenge to all members of the critical care team. As head injury is associated with a high mortality and morbidity, the benefits of intensive treatment and care may not become apparent until months or years later during rehabilitation after injury.
EPIDEMIOLOGY
AETIOLOGY
Vehicular trauma, industrial accidents, falls and assaults account for the majority of head injury, with marked variations in patterns of injury across the world. For example, in Australasia, the incidence of head injury due to vehicular trauma is decreasing due to the success of preventive strategies such as restraint devices, speed control and stricter drink-driving legislation.1
PATHOPHYSIOLOGY
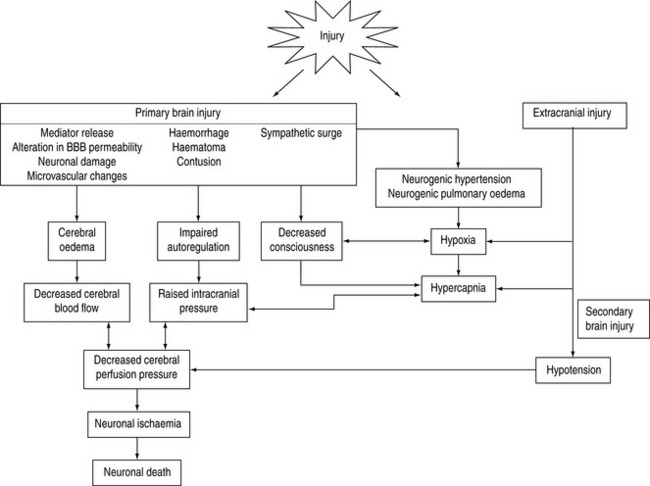
Brain injury is a heterogenous pathophysiological process. It encompasses a spectrum of injury that includes the degree of brain damage at the time of injury (primary injury) in addition to insults that occur during the post-injury phase (secondary injury). These processes are depicted in Figure 67.1.
SECONDARY BRAIN INJURY
Secondary brain insults are characterised by a reduction in cerebral substrate utilisation, particularly oxygen (Table 67.1). Of these insults, hypotension (defined as a systolic blood pressure of < 90 mmHg), hypoxia (oxygen saturation < 90% or PaO2 < 50 mmHg), hypoglycaemia, hyperpyrexia (temperature > 39°C) and prolonged hypocapnia (PaCO2 < 30 mmHg) have been shown to independently worsen survival following traumatic brain injury.
Table 67.1 Secondary brain insults following traumatic brain injury that are associated with increased morbidity and mortality
| Systemic | Intracranial |
|---|---|
| Hypoxia | Seizure |
| Hypotension | Delayed haematoma |
| Hypocapnia | Subarachnoid haemorrhage |
| Hypercapnia | Vasospasm |
| Hyperthermia | Hydrocephalus |
| Hypoglycaemia | Neuroinfection |
| Hyperglycaemia | |
| Hyponatraemia | |
| Hypernatraemia | |
| Hyperosmolality | |
| Infection |
INTRACRANIAL INFLAMMATION
Traumatic brain injury invokes an inflammatory response characterised by the release of cytokines, freeradicals, excitatory amino acids and other mediators. The consequence of this response is disruption and alteration in the permeability of the blood–brain barrier, glial swelling and alterations in regional and global cerebral blood flow. The extent of this inflammatory process is an important determinant of intracranial pressure that may persist for some time following injury. Furthermore, alteration in blood–brain permeability may render the cerebral circulation susceptible to the effects of drugs that do not normally cross, such as osmotic diuretics and exogenous catecholamines.
CEREBRAL BLOOD FLOW AND AUTOREGULATION
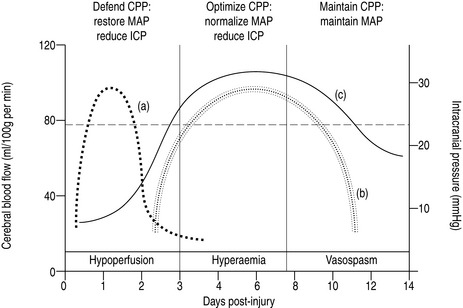
Normally, cerebral blood flow is maintained at a constant rate in the presence of changing perfusion pressures by myogenic and metabolic autoregulation. These homeostatic mechanisms are impaired following head injury due to neuronal damage and intracranial inflammation. Distinct patterns of cerebral blood flow have been described following head injury that have direct clinical relevance with regard to management2 (Figure 67.2).
THE HYPOPERFUSION PHASE
In order to maintain cerebral perfusion, systemic blood pressure must be maintained during this phase so that cerebral perfusion pressure (defined as the difference between mean arterial pressure and intracranial pressure) is maintained between 60 and 70 mmHg.3
THE HYPERAEMIC PHASE
This hyperaemic phase may persist for up to 7–10 days post injury and occurs in 25–30% patients. As there is restoration or increased cerebral blood flow during this phase, a range of cerebral perfusion pressure is recommended (50–70 mmHg).4
THE VASOSPASTIC PHASE
In a small cohort of patients (10–15%), particularly those with severe primary and secondary injuries or those with significant traumatic subarachnoid haemorrhage, a vasospastic phase characterised by typical cerebral blood flow patterns may persist. This phase represents a complex of cerebral hypoperfusion due to arterial vasospasm, posttraumatic hypometabolism and impaired autoregulation.5
RESUSCITATION
INITIAL ASSESSMENT
The resuscitation of head-injured patients should follow the principles outlined in the Advanced Trauma Life Support (ATLS®) guidelines for the early management of severe trauma.6
With respect to head-injured patients, the following principles in the initial assessment apply.7,8
BREATHING (= VENTILATION)
Empirical hyperventilation during initial resuscitation is not indicated until adequate oxygenation, haemodynamic stability and urgent computed tomography have been achieved.9
CIRCULATION (= CONTROL OF SHOCK)
Prompt restoration of circulating blood volume and restoration of a euvolaemic state is critical.10
An emerging body of evidence recommends the use of crystalloids, specifically normal saline, for fluid resuscitation of patients with traumatic brain injury. The use of albumin for fluid resuscitation is associated with increased mortality and should be avoided.11 Hypertonic saline may have a role as a small-volume resuscitation fluid that is useful in expanding intravascular volume, with additional beneficial effects on cerebral blood flow and reduction of cerebral oedema,12 although there is no evidence of reduced mortality when used in the prehospital period.13
Inotropes, such as adrenaline (epinephrine) or noradrenaline (norepinephrine), or vasopressors such as phenylephrine or metaraminol, may be used to defend blood pressure once correction of hypovolaemia is underway or achieved.4 This may be necessary if sedatives or narcotics are coadministered.
The use of military antishock trousers (MAST suit) in traumatic brain injury is not recommended.
DISABILITY (= NEUROLOGICAL ASSESSMENT)
Level of consciousness
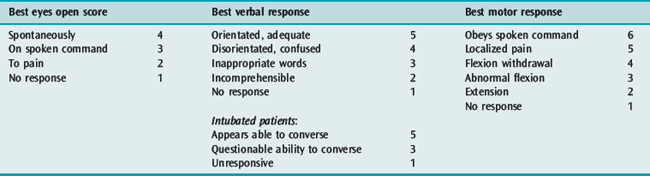
The Glasgow Coma Scale (GCS) has an established place in the management of traumatic brain injury and is the most widely accepted and understood scale.14 Whilst originally described as a prognostic index, it provides an overall assessment of neurological function, derived from three parameters: eye opening, verbal response and motor response (Table 67.2).
Table 67.2 The Glasgow Coma Scale.14 The best response following non-surgical resuscitation is scored
Pupillary responses
In the absence of traumatic mydriasis, abnormalities of pupil size and reactivity may indicate compression of the third cranial nerve, suggesting raised intracranial pressure or impending herniation, particularly when associated with lateralising motor signs and depressed consciousness
Papilloedema is uncommon in the acute phase of head injuries.
SECONDARY SURVEY
Once the initial assessment is complete and resuscitation underway, a thorough secondary survey adopting a ‘top-to-toe’ approach is mandatory. This is outlined in the ATLS® approach to the traumatised patient.6
BRAIN-SPECIFIC RESUSCITATION
HYPERVENTILATION
Ventilation-induced reductions in PaCO2 result in marked reductions in cerebral blood flow and consequently in intracranial pressure. However, as cerebral blood flow may be reduced during the initial period following injury, further reductions in cerebral perfusion will result if hyperventilation is used during this phase (Figure 67.2).
However, hyperventilation remains a potent non-surgical clinical tool of reducing intracranial pressure. In the resuscitated head-injured patient with unequivocal clinical signs of raised intracranial pressure or impending tentorial herniation (pupillary dilatation, lateralising signs or a witnessed neurological deterioration), hyperventilation is an option.Reductions of PaCO2 to levels ≤30 mmHg (4 kPa) may be therefore considered prior to urgent imaging or surgery for evacuation of a mass lesion.9
OSMOTHERAPY
Given the high risk with minimal benefit during resuscitation, the routine use of mannitol is not recommended in the absence of raised intracranial pressure and in patients where cerebral blood flow is compromised.15
Hypertonic saline (3% solution) exerts similar osmotic plasma expanding effects to mannitol. These solutions do not exert an osmolal gap so that serum sodium reflects serum osmolality allowing easier titration. These solutions have been advocated as ‘small-volume resuscitation fluids’ that may be very effective in restoring systemic and cerebral perfusion in the acute phase following injury. In addition to reducing intracranial pressure, these solutions would appear to be superior to mannitol for resuscitation.16
IMAGING
X-RAYS
All head-injured patients receive the routine ‘trauma series’ of X-rays, namely chest, pelvis and cervical spine (lateral, anteroposterior and peg views), although the last may be superseded by high resolution CT scan of the entire cervical spine.17
COMPUTED TOMOGRAPHY (CT SCAN)
The following patients should undergo CT head scan following traumatic brain injury:
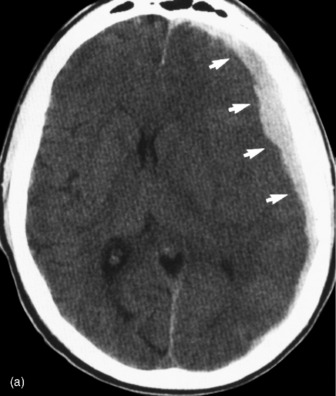
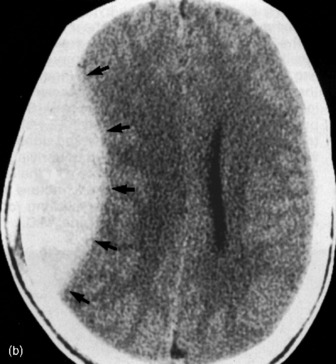
Technological advances in imaging now enable quick, high resolution digital images of the brain parenchyma and bony compartments. The most important role of CT scanning is prompt detection of mass lesion such as extradural or subdural haematomas. Thereafter, the degree of brain injury may be quantified by radiological criteria (Table 67.3 and Figure 67.3a).18
Table 67.3 Classification of CT scan appearance following traumatic brain injury.16 Examples are shown in Figure 67.3a
| Category | Definition |
|---|---|
| Diffuse injury (DI) I | No visible intracranial pathology seen on CT scan |
| DI II (diffuse injury) | Cisterns are present with midline shift 0–5 mm and/or Lesion densities present No high or mixed density > 25 mm May include bony fragments and foreign bodies |
| DI III (swelling) | Cisterns are compressed or absent with midline shift 0–5 mm No high or mixed density > 25 mm |
| DI IV (shift) | Midline shift > 5 mm No high or mixed density > 25 mm |
| Evacuated mass lesion | Any lesion surgically evacuated |
| Non-evacuated mass lesion | High or mixed density lesion > 25 mm, not surgically evacuated |
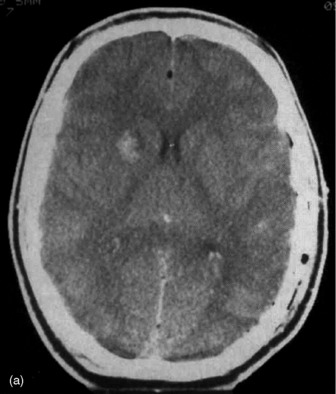
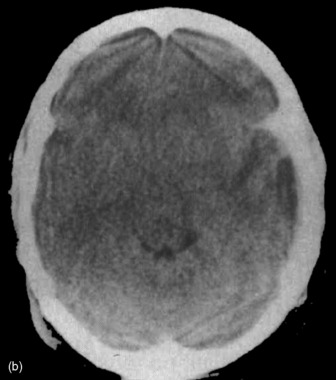
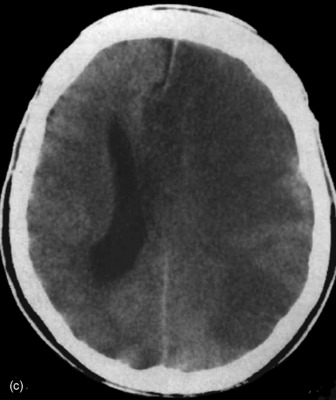
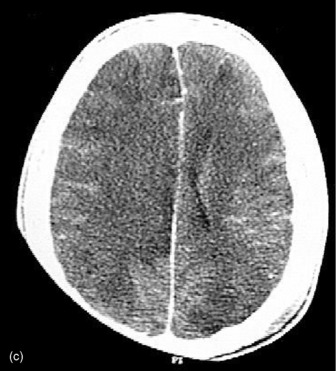
Figure 67.3a Computed tomographic classification of diffuse axonal injury (Table 67.3).33 Panel (a) Diffuse injury II; Panel (b) Diffuse injury III; Panel (c) Diffuse injury IV.
These criteria are important for:
These criteria should be recorded following each CT scan, particularly when patients are transferred to secondary or tertiary centres. Examples of typical injuries appear in Figure 67.3a and 67.3b.
The presence of traumatic subarachnoid haemorrhage should be recorded. This is an important index of severity of injury and is relevant for prognostication.19
INTERHOSPITAL TRANSFER
All severely head-injured patients should be managed in a specialised neurotrauma centre in close collaboration with intensive care physicians and neurosurgeons.20 This may involve intra- or interhospital transportation. This is a potentially hazardous exercise and has the potential to adversely affect outcome by causing secondary insults. Consequently, appropriately skilled and equipped personnel should only do this once resuscitation, stabilisation and initial imaging are completed.
INTENSIVE CARE MANAGEMENT
There is no standard or uniform method of managing traumatic brain injury in the ICU. Most practices are determined by local preferences and experience, caseload and resources. The Brain Trauma Foundation of the American Association of Neurological Surgeons21 and the European Brain Injury Consortium22 have published evidence-based management guidelines for the management of severe brain injury. These publications provide very few standards by which to direct therapy and the majority of issues addressed are presented either as management guidelines or options.
SUPPORTIVE THERAPY
HAEMODYNAMIC MANAGEMENT
Monitoring
Accurate measurement of systemic blood pressure is essential and should be measured via an arterial catheter referenced to the aortic root. A large artery such as the femoral artery should be considered in haemodynamically unstable patients as radial or dorsalis pedis arterial catheters may underestimate true systemic pressure in shocked patients. Given the importance of maintaining adequate systemic pressures, non-invasive measurement of bloodpressure is not recommended during the acute phase of monitoring.10
Inotropic therapy
Inotropes such as adrenaline, noradrenaline or dopamine are frequently used to augment mean arterial pressure to attain an adequate cerebral perfusion pressure. These should only be commenced once volume resuscitation is actively underway or complete. The early use of inotropes is increasingly being advocated during resuscitation as an important strategy during the hypoperfusion phase.4
There are no conclusive trials to recommend one inotrope over another or combination of inotropes. Adrenaline, noradrenaline and dopamine are equally effective in augmenting cerebral perfusion pressure. The degree by which these agents directly affect the cerebral circulation following head injury is unknown, although there is some evidence suggesting that dopamine has both direct cerebrovascular and adverse neuroendocrine effects.23,24
Adrenaline is widely used as an initial inotropic agent in doses titrated to achieve a desired mean or cerebral perfusion pressure. Whilst effective, it may be associated with metabolic side-effects such as hyperlactataemia and hyperglycaemia, which may complicate metabolic management. For this reason, noradrenaline is currently regarded by many as the initial agent of choice for patients with traumatic brain injury.25
RESPIRATORY THERAPY
Ventilation
Trials of extubation should be carefully considered so that subsequent hypoxic episodes do not occur, as these are potent secondary insults.
Neurogenic pulmonary oedema
The process is usually self-limiting and treatment is primarily supportive, aimed at ensuring adequate oxygenation and ventilation. This usually requires endotracheal intubation and mechanical ventilation with the administration of PEEP. Ablation of sympathetic overactivity is effectively done with adequate sedation; β-blockade is usually unnecessary. Diuretics are effective but must be titrated against the volume status of the patient so that cerebral perfusion pressure is not compromised.26
Nosocomial pneumonia
Head-injured patients who require prolonged ventilation are at increased risk of nosocomial pneumonia. This is associated with secondary brain injury and an increased mortality.27 Risk factors include barbiturate and hypothermia therapy. Diagnosis and treatment are discussed elsewhere.
SEDATION, ANALGESIA AND MUSCLE RELAXANTS
The use of propofol as a sole sedating agent has become popular.28 It provides deep levels of sedation, which is effective in controlling systemic sympathetic swings and rises in intracranial pressure. It is rapidly reversible on cessation allowing prompt assessment of neurological status and does not accumulate. In addition, pupillary responses are not directly affected. Propofol should be used with caution in haemodynamically unstable patients, as it is a potent negative inotrope. The prolonged use of propofol is associated with tachyphylaxis and significant caloric loading from the lipid vector. Concerns have been raised about myocardial depression and sudden cardiac death, particularly if large doses are administered.29
BODY POSITION
Patients should be nursed at 30–45° head elevation to facilitate ventilation, improve oxygenation, and reduce the risk of aspiration. The head should be kept in a neutral position.30
METABOLIC MANAGEMENT
Hyperglycaemia is common following severe head injury and is usually centrally mediated and transient. Blood sugar levels should be maintained within normal limits with insulin infusions. The role of ‘tight’ glycaemic control (i.e. 4.0–6.0 mmol/l) in patients with traumaticbrain injury has not been determined and as hypoglycaemia is a recognised secondary insult, normal blood sugar levels (i.e. 8.0–10.0 mmol/l) are recommended.
NUTRITION
The caloric needs of head-injured patients must be addressed as soon as possible following resuscitation. Early enteral feeding is recommended.31
THROMBOPROPHYLAXIS
Head-injured patients, particularly those requiring prolonged ventilation and sedation, or with extracranial injuries, are at increased risk for developing thromboembolism. The use of anticoagulants such as fractionated or low molecular weight heparins is contraindicated in patients with intracranial haemorrhage. Consequently, the role of thromboprophylactic agents in head injury is difficult and there are no standards for their use.32
ANTIBIOTICS
These should be used sparingly and in accordance with accepted microbiological principles. Prophylactic antibiotics should only be prescribed to cover insertion of intracranial pressure monitors and are not recommended for basal skull fractures.33 Frequent cultures of leaking or draining cerebrospinal fluid should be taken and infection treated specifically.
BRAIN-SPECIFIC MONITORING
INTRACRANIAL ELASTANCE
Intracranial pressure monitoring
The Brain Trauma Foundation guidelines recommend intracranial pressure monitoring in patients with traumatic coma (severe head injury: GCS = 8 following non-surgical resuscitation)34 with either:
Coagulopathy is a contraindication to intracranial pressure monitoring. Measurement of intracranial pressure35 with an intraventricular catheter is the most accurate and clinically useful method. It has the advantages of zero calibration, cerebrospinal fluid drainage for raised intracranial pressure and allows dynamic testing of pressure–volume relationships. Disadvantages include technical difficulty with insertion, particularly in patients with cerebral oedema and compression of the lateral ventricles, and an increased incidence of infection.
CEREBRAL BLOOD FLOW
A number of qualitative measurement techniques are available that provide an indirect assessment of cerebral blood flow that may be useful, particularly when used in conjunction with other modalities such as intracranial pressure monitoring and CT scanning. Interpretation of these measurements must be taken within the clinical context, particularly the time course of the underlying injury (Figure 67.2).
Jugular bulb oximetry
Measurement of oxygen saturation in the jugular bulb by the retrograde placement of a fibreoptic catheter provides an indirect assessment of cerebral perfusion. Low jugular venous saturations (< 55%) may be indicative of cerebral hypoperfusion due to systemic hypotension or hypocapnia. High levels of jugular venous saturation (> 85%) may be indicative of cerebral hyperaemia or inadequate neuronal metabolism, such as occurs during the hyperaemic phase or during the evolution of brain death. Both low and high jugular venous saturation are associated with adverse outcomes.36
Transcranial Doppler
Transcranial Doppler ultrasonography with a 2 MHz pulsed Doppler probe allows non-invasive, intermittent or continuous assessment of the velocity of blood flow through large cerebral vessels. Insonation through a naturally occurring acoustic window such as the transtemporal approach allows insonation of the anterior, middle and posterior cerebral arteries, terminal internal carotid artery and anterior and posterior communicating arteries.37
CEREBRAL FUNCTION AND METABOLISM
Electroencephalography
Electroencephalography has been used for many years to assess seizure activity that may be masked by sedatives or muscle relaxants and to provide an objective estimate of the degree of electrical neuronal depression with barbiturate therapy. Whilst seizures may not be clinically apparent in a proportion of patients, and may constitute an important secondary insult, the accuracy and reliability of electroencephalography in ICUs is questionable due to outside electrical interference from monitors and ventilators.38
The development of bispectral index (BIS monitoring) as a measurement of depth of anaesthesia has led to the suggestion that this may be an alternative to the use of electroencephalography with barbiturate or sedative therapy in traumatic brain injury. Bispectral index has not been validated in this context and cannot be recommended as a titration end-point.39
Evoked potentials
Measurement of evoked potentials, assessing the integrity of sensory and motor pathways, may provide diagnostic and prognostic information; however, because of the complexity of the technique, it is not recommended for general use.40 The reliance of one variable, such as evoked potentials, to predict outcome from traumatic brain injury is not recommended.
Neuronal function
Cerebral microdialysis is a technique that measures brain extracellular fluid metabolites. Dialysate is obtained through a microdialysis catheter inserted through the same burr hole as an intracranial pressure monitor to measure concentration and fluxes of markers of intracranial inflammation (e.g. lactate, pyruvate and purines).41
BRAIN INJURY SURVEILLANCE
CT scans should be assessed and scored according to the classification outlined in Table 67.3. The progression or resolution of axonal injury, cerebral oedema, contusion and haemorrhages should be recorded. Other parameters such as intracranial pressure and jugular venous saturation should be interpreted in accordance with the CT scan appearance.
BRAIN-SPECIFIC THERAPY
Treatment options directed at ameliorating brain injury are limited. Despite intensive research into defining the pathobiological processes in primary injury, studies analysing therapies designed to modulate intracranial inflammation have not been successful. These include aminosteroids, calcium channel blockade and N-methyl-D-aspartic acid (NMDA) antagonists.42
Brain-specific or ‘targeted’ therapy is directed at maintaining cerebral perfusion pressure and minimising intracranial pressure. Whilst there is an inherent relationship between these two principles, priorities are different depending on the time course of the underlying injury. This is important as strategies directed at one may have adverse effects in the other (Figure 67.2).
DEFENCE OF CEREBRAL PERFUSION PRESSURE
The pathophysiological principles outlined on p. 765 are important in defining strategies to optimise cerebral perfusion pressure following injury. Whilst the Brain Trauma Foundation guidelines recommend a cerebral perfusion pressure of 60–70 mmHg, this figure may not be applicable over the entire time course following injury.3
Hypoperfusion phase (0–72 hours)
During this phase, medical therapies directed at raised intracranial pressure such as osmotherapy or hyperventilation should only be used if cerebral perfusion pressure is maintained at an appropriate level and the patient is adequately monitored.
Hyperaemic phase (3–7 days)
Approximately 25–30% of patients will develop clinical signs of cerebral hyperaemia, characterised by raised intracranial pressure, persistent cerebral oedema on CT scan and possibly confirmed by high jugular venous saturations.43 This may be due to vasogenic cerebral oedema caused by intracranial inflammation or by catecholamine infusions often used to augment cerebral perfusion pressure.
REDUCTION OF INTRACRANIAL PRESSURE
A number of medical strategies directed at reducing intracranial pressure have been used for many years. Despite widespread use and firmly held beliefs, there is little evidence to support the routine use of these therapies.44
Intracranial pressure should be maintained at < 25 cmH2O.45 Trends of intracranial pressure are equally as important and should be assessed within the context of cerebral perfusion pressure and the methods used to defend it.
Decompressive craniectomy
Wide, bilateral, frontoparietal craniectomies are increasingly being advocated to reduce intracranial pressure in patients with refractory intracranial hypertension. Whilst there are numerous case series demonstrating dramatic improvements, there are no conclusive outcome-based trials to make firm recommendations.46
Recent experience in paediatric patients is encouraging and the role of pre-emptive decompressive craniectomy in high-risk patients is yet to be determined.47
Osmotherapy
There is no evidence that osmotherapy or induced dehydration improves outcome or is more effective in cytotoxic than vasogenic cerebral oedema.15
Hyperventilation
Current evidence-based guidelines do not recommend the use of routine hyperventilation.9
Hypothermia
Induced hypothermia has been used for many years to reduce cerebral metabolism and thereby raised intracranial pressure. A sound theoretical benefit exists in experimental models where hypothermia is induced immediatelyfollowing injury with prompt reduction in raised intracranial pressure. However, these benefits have not translated into improved outcomes in a number of clinical trials.48
Barbiturate coma
Barbiturates are a ‘second tier’ option in patients with refractory intracranial hypertension and not recommended on current evidence.49
Steroids
Steroids have been advocated for many years to ameliorate intracranial inflammation, thereby reducing intracranial pressure. A large international study has provided conclusive evidence that steroids may be associated with adverse outcomes in traumatic brain injury, and therefore have no place in management.50,51
Cerebral blood volume regulation
Following resuscitation and stabilisation, a strategy reducing cerebral perfusion pressure to > 50 mmHg using colloids, β-blockade, clonidine and dihydroergotamine may be effective in minimising secondary cerebral hyperaemia and vasogenic oedema. This strategy requires a measurement of cerebral blood flow to demonstrate adequate cerebral perfusion.52
CEREBRAL VASOSPASM
Treatment options remain limited. Calcium antagonists, such as nimodipine, have not been shown to be effective in traumatic subarachnoid haemorrhage. Strategies that have been used in aneurysmal subarachnoid haemorrhage such as ‘triple H’ therapy (induced hypertension, hypervolaemia and haemodilution) or chemical angioplasty have not been evaluated in traumatic subarachnoid haemorrhage and are not recommended.5
SEIZURE PROPHYLAXIS
Seizures are infrequent following traumatic brain injury and usually present at the time of injury. These should be treated with anticonvulsants (e.g. diazepam, midazolam) when they occur. Subsequent prophylaxis with phenytoin is recommended only in patients with destructive parenchymal lesions on CT scan and continued for 10 days following injury. Short-term seizure prophylaxis does not prevent late onset posttraumatic epilepsy.53
OUTCOME AND PROGNOSIS
Patient factors that determine outcome from traumatic brain injury include severity of primary and secondary injuries, low GCS on presentation,54 advanced age (> 60 years)55 and comorbidities. Prediction of outcome is difficult and must be made with circumspection as significant functional improvements, particularly in young patients, may occur over time.
1 Myburgh JA, Cooper DJ, Finfer SR, et al. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J Trauma. 2008;64:854-862.
2 Martin NA, Patwardhan RV, Alexander MJ, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9-19.
3 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Guidelines for cerebral perfusion pressure. J Neurotrauma. 2000;17:497-506.
4 Myburgh JA. Driving cerebral perfusion pressure with pressors: how, which, when? Crit Care Resusc. 2005;7:200-205.
5 Oertel M, Boscardin WJ, Obrist WD, et al. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg. 2005;103:812-824.
6 American College of Surgeons Committee on Trauma. Advanced Trauma Life Support for Doctors. Chicago IL: American College of Surgeons, 1997.
7 BTF Guidelines. The Brain Trauma Foundation. The American Association of Neurological Surgeons. Initial management. J Neurotrauma. 2000;17:463-470.
8 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Resuscitation of blood pressure and oxygenation. J Neurotrauma. 2000;17:471-478.
9 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Hyperventilation. J Neurotrauma. 2000;17:513-520.
10 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Hypotension. J Neurotrauma. 2000;17:591-595.
11 SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
12 Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28:3301-3313.
13 Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350-1357.
14 Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84.
15 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Mannitol. J Neurotrauma. 2000;17:521-526.
16 Doyle JA, Davis DP, Hoyt DB. The use of hypertonic saline in the treatment of traumatic brain injury. J Trauma. 2001;50:367-383.
17 Cooper DJ, Ackland HM. Clearing the cervical spine in the unconscious head injured patient. Crit Care Resusc. 2005;7:181-184.
18 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Computed tomography scan features. J Neurotrauma. 2000;17:597-627.
19 Servadei F, Murray GD, Teasdale GM, et al. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002;50:261-267.
20 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Trauma systems. J Neurotrauma. 2000;17:457-462.
21 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Management and prognosis of severe traumatic brain injury. J Neurotrauma. 2000;17:449-554.
22 Maas AI, Dearden M, Teasdale GM, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien). 1997;139:286-294.
23 Myburgh JA, Upton RN, Grant C, et al. A comparison of the effects of norepinephrine, epinephrine, and dopamine on cerebral blood flow and oxygen utilisation. Acta Neurochir Suppl (Wien). 1998;71:19-21.
24 Van den Berghe G, de Zegher F. Anterior pituitary function during critical illness and dopamine treatment. Crit Care Med. 1996;24:1580-1590.
25 Ract C, Vigue B. Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients. Intensive Care Med. 2001;27:101-106.
26 McManis P, Lee C, Morgan M, et al. Neurogenic pulmonary oedema. Aust N Z J Med. 2000;30:514.
27 Ewig S, Torres A, El Ebiary M, et al. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999;159:188-198.
28 Kelly DF, Goodale DB, Williams J, et al. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. J Neurosurg. 1999;90:1042-1052.
29 Cremer OL, Moons KGM, Bouman ACB, et al. Long-term propofol infusion and cardiac failure in adult head-injured patients. Lancet. 2001;357:117-118.
30 Winkelman C. Effect of backrest position on intracranial and cerebral perfusion pressures in traumatically brain-injured adults. Am J Crit Care. 2000;9:373-380.
31 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Nutrition. J Neurotrauma. 2000;17:539-548.
32 Hammond FM, Meighen MJ. Venous thromboembolism in the patient with acute traumatic brain injury: screening, diagnosis, prophylaxis, and treatment issues. J Head Trauma Rehabil. 1998;13:36-50.
33 Jacobs DG, Westerband A. Antibiotic prophylaxis for intracranial pressure monitors. J Natl Med Assoc. 1998;90:417-423.
34 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Indications for intracranial pressure monitoring. J Neurotrauma. 2000;17:479-492.
35 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Recommendations for intracranial pressure monitoring technology. J Neurotrauma. 2000;17:497-506.
36 Macmillan CS, Andrews PJ, Easton VJ. Increased jugular bulb saturation is associated with poor outcome in traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;70:101-104.
37 Castillo MA. Monitoring neurologic patients in intensive care. Curr Opin Crit Care. 2001;7:49-60.
38 Procaccio F, Polo A, Lanteri P, et al. Electrophysiologic monitoring in neurointensive care. Curr Opin Crit Care. 2001;7:74-80.
39 Myles PS, Cairo S. Artifact in the bispectral index in a patient with severe ischemic brain injury. Anesth Analg. 2004;98:706-707.
40 Carter BG, Butt W. Review of the use of somatosensory evoked potentials in the prediction of outcome after severe brain injury. Crit Care Med. 2001;29:178-186.
41 Peerdeman SM, Girbes AR, Vandertop WP. Cerebral microdialysis as a new tool for neurometabolic monitoring. Intensive Care Med. 2000;26:662-669.
42 Maas AI, Steyerberg EW, Murray GD, et al. Why have recent trials of neuroprotective agents in head injury failed to show convincing efficacy? A pragmatic analysis and theoretical considerations. Neurosurgery. 1999;44:1286-1298.
43 Marmarou A, Fatouros PP, Barzo P, et al. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J Neurosurg. 2000;93:183-193.
44 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Critical pathway for the treatment of established intracranial hypertension. J Neurotrauma. 2000;17:537-538.
45 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Intracranial pressure treatment threshold. J Neurotrauma. 2000;17:493-495.
46 Munch E, Horn P, Schurer L, et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315-322.
47 Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154-162.
48 Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
49 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Use of barbiturates in the control of intracranial hypertension. J Neurotrauma. 2000;17:527-530.
50 Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury – outcomes at 6 months. Lancet. 2005;365:1957-1959.
51 Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321-1328.
52 Eker C, Asgeirsson B, Grande PO, et al. Improved outcome after severe head injury with a new therapy based on principles for brain volume regulation and preserved microcirculation. Crit Care Med. 1998;26:1881-1886.
53 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Role of antiseizure prophylaxis following head injury. J Neurotrauma. 2000;17:549-554.
54 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Glasgow Coma Scale Score. J Neurotrauma. 2000;17:563-572.
55 BTF Guidelines. Brain Trauma Foundation. American Association of Neurological Surgeons. Age. J Neurotrauma. 2000;17:573-582.