Serologic Diagnosis of Infectious Diseases
1. Define the two categories of human specific immune response, cell mediated and antibody mediated, including the definition of T cells and B cells and their role in the responses.
2. List the five classes of antibodies, define their roles in infectious disease, and explain the three antibody functions.
3. Explain the following serologic tests, giving consideration to their clinical applications: bacterial agglutination, particle agglutination, and flocculation tests.
4. Describe a cross reaction and explain why it occurs and how it may affect antibody testing.
5. In defining hemagglutination and neutralization assays, explain their similarity in testing, along with their disparities.
6. Explain how the difference in the size and structure of the IgM antibody is important to its activity and function.
7. Explain what the complement fixation test is and describe the two-step reaction.
8. Explain the principle of the Western blot assay and why it is used as a confirmatory test for many assays.
Immunochemical methods are used as diagnostic tools for serodiagnosis of infectious disease. An understanding of how these methods have been adapted for this purpose requires a basic working knowledge of the components and functions of the immune system. Immunology is the study of the components and functions of the immune system. The immune system is the body’s defense mechanism against invading “foreign” antigens. One of the functions of the immune system is distinguishing “self” from “nonself” (i.e., the proteins or antigens from foreign substances). (Chapter 3 presents a more in-depth discussion of the host’s response to foreign substances.) This chapter is intended to provide a brief overview and review of immunology. The complexity and detail required to fully understand immunology and serology are beyond the scope of this text.
Features of the Immune Response
Characteristics of Antibodies
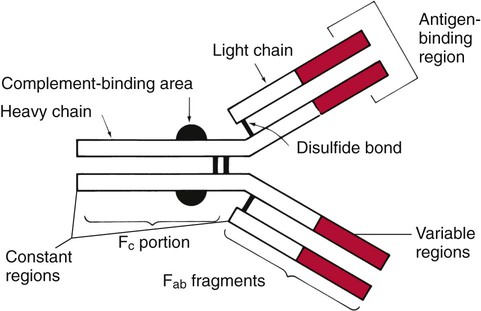
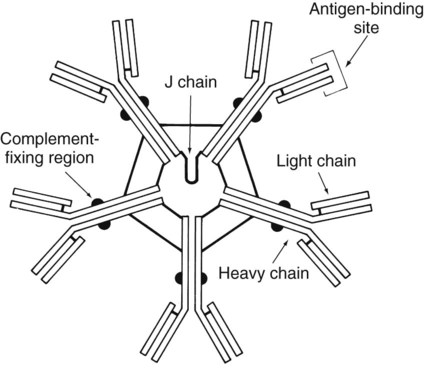
The basic structure of an antibody molecule comprises two mirror images, each composed of two identical protein chains (Figure 10-1). At the terminal ends are the antigen binding sites, or variable regions, which specifically attach to the antigen against which the antibody was produced. Depending on the specificity of the antibody, antigens of some similarity, but not total identity, to the inducing antigen may also be bound; this is called a cross reaction. The complement binding site is found in the center of the molecule in a structure similar for all antibodies of the same class and is referred to as the constant region. IgM is produced as a first response to many antigens, although the levels remain high transiently. Thus, the presence of IgM usually indicates recent or active exposure to an antigen or infection. IgG, on the other hand, may persist long after an infection has run its course.
The IgM antibody type (Figure 10-2) consists of five identical proteins (pentamer), with the basic antibody structures linked at the bases with 10 antigen binding sites on the molecule. IgG consists of one basic antibody molecule (monomer) that has two binding sites (see Figure 10-1). The differences in the size and conformation between these two classes of immunoglobulins result in differences in activities and functions.
Features of the Humoral Immune Response Useful in Diagnostic Testing
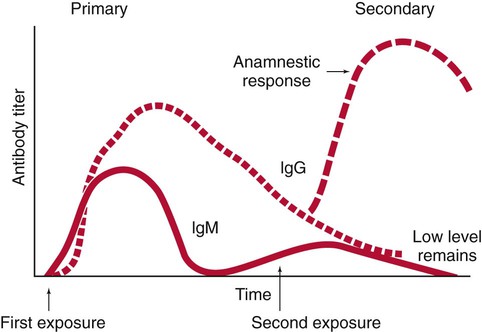
IgG is often more specific for the antigen (i.e., it has higher avidity). IgG has two antigen binding sites, but it can also bind complement. Complement is a complex series of serum proteins that is involved in modulating several functions of the immune system, including cytotoxic cell death, chemotaxis, and opsonization. When IgG is bound to an antigen, the base of the molecule (Fc portion) is exposed in the environment. Structures on this Fc portion attract and bind the cell membranes of phagocytes, increasing the chances of engulfment and destruction of the pathogen by the host cells. A second exposure to the same pathogen induces a faster and greater IgG response and a much lesser IgM response. Several B lymphocytes retain memory of the pathogen, allowing a more rapid response and a higher level of antibody production than the primary exposure or response. This enhanced response is called the anamnestic response. B-cell memory is not perfect. Occasional clones of memory cells can be stimulated through interaction with an antigen that is similar but not identical to the original antigen. Therefore, the anamnestic response may be polyclonal and nonspecific. For example, reinfection with cytomegalovirus may stimulate memory B cells to produce antibody against Epstein-Barr virus (another herpes family virus), which the host encountered previously, in addition to antibody against cytomegalovirus. The relative humoral responses are diagrammatically represented in Figure 10-3.
Serodiagnosis of Infectious Diseases
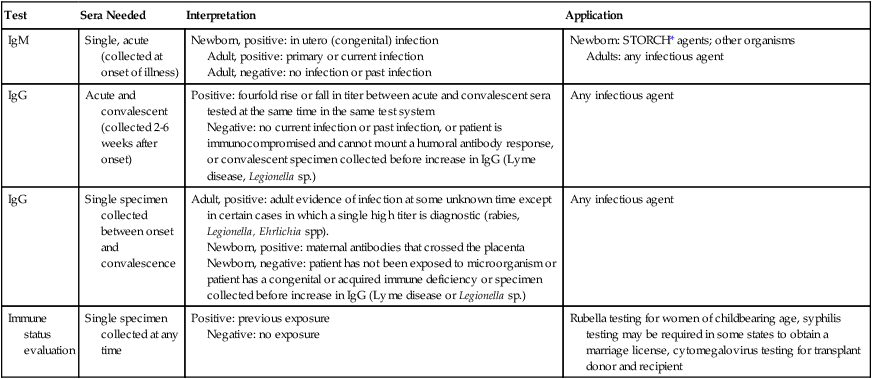
Table 10-1 provides a brief list of representative serologic tests available for immunodiagnosis of infectious diseases, the specimen required, interpretation of positive and negative test results, and examples of applications of each technique. Because serologic assays are rapidly evolving, this table is not intended to be all-inclusive.
TABLE 10-1
Noninclusive Overview of Tests Available for Serodiagnosis of Infectious Diseases
| Test | Sera Needed | Interpretation | Application |
| IgM | Single, acute (collected at onset of illness) | Newborn, positive: in utero (congenital) infection Adult, positive: primary or current infection Adult, negative: no infection or past infection |
Newborn: STORCH* agents; other organisms Adults: any infectious agent |
| IgG | Acute and convalescent (collected 2-6 weeks after onset) | Positive: fourfold rise or fall in titer between acute and convalescent sera tested at the same time in the same test system Negative: no current infection or past infection, or patient is immunocompromised and cannot mount a humoral antibody response, or convalescent specimen collected before increase in IgG (Lyme disease, Legionella sp.) |
Any infectious agent |
| IgG | Single specimen collected between onset and convalescence | Adult, positive: adult evidence of infection at some unknown time except in certain cases in which a single high titer is diagnostic (rabies, Legionella, Ehrlichia spp). Newborn, positive: maternal antibodies that crossed the placenta Newborn, negative: patient has not been exposed to microorganism or patient has a congenital or acquired immune deficiency or specimen collected before increase in IgG (Lyme disease or Legionella sp.) |
Any infectious agent |
| Immune status evaluation | Single specimen collected at any time | Positive: previous exposure Negative: no exposure |
Rubella testing for women of childbearing age, syphilis testing may be required in some states to obtain a marriage license, cytomegalovirus testing for transplant donor and recipient |
*STORCH, Syphilis, Toxoplasma, rubella virus, cytomegalovirus, herpes simplex virus.
Principles of Serologic Test Methods
Methods of Antibody Detection
Direct Whole Pathogen Agglutination Assays
Particle Agglutination Tests
Numerous serologic procedures have been developed to detect antibody via the agglutination of an artificial carrier particle with antigen bound to its surface. As noted in Chapter 9, similar systems using artificial carriers coated with antibodies are commonly used for detection of microbial antigens. Either artificial carriers (e.g., latex particles or treated red blood cells) or biologic carriers (e.g., whole bacterial cells) can carry an antigen on their surface capable of binding with antibody. The size of the carrier enhances the visibility of the agglutination reaction, and the artificial nature of the system allows the antigen bound to the surface to be extremely specific.
Flocculation Tests
The RPR test is commercially available as a complete system containing positive and negative controls, the reaction card, and the prepared antigen suspension. The antigen, cardiolipin-lecithin–coated cholesterol with choline chloride, also contains charcoal particles to allow for macroscopically visible flocculation. Sera can be tested without heating, and the reaction takes place on the surface of a specially treated cardboard card, which is then discarded (Figure 10-4). The RPR test is not recommended for testing of cerebrospinal fluid. All procedures are standardized and clearly described in product inserts, and these procedures should be strictly followed. Overall, the RPR appears to be a more specific screening test for syphilis than the VDRL, and it is not as technically complex. Several modifications have been made, such as the use of dyes to enhance visualization of results and the use of automated techniques.
Immunodiffusion Assays
The Ouchterlony double immunodiffusion assay, which closely resembles the precipitation test, is used to detect antibodies directed against fungal cell components (see Chapter 9). Whole-cell extracts or other antigens of the suspected fungus are placed in wells in an agarose plate, and the patient’s serum and a positive control serum are placed in adjoining wells. If the patient has produced specific antibody against the fungus, precipitin lines become visible in the agarose between the homologous (identical) antigen and antibody wells; the patient’s sample identity, with similar lines from the control serum, helps confirm the results. The type and thickness of the precipitin bands may have both prognostic and diagnostic value. Antibodies against the pathogenic fungi Histoplasma, Blastomyces, Coccidioides, and Paracoccidioides spp., as well as some opportunistic fungi, are routinely detected by immunodiffusion. The test usually requires at least 48 hours, but additional time may be required for the bands to become visible.
Complement Fixation Assays
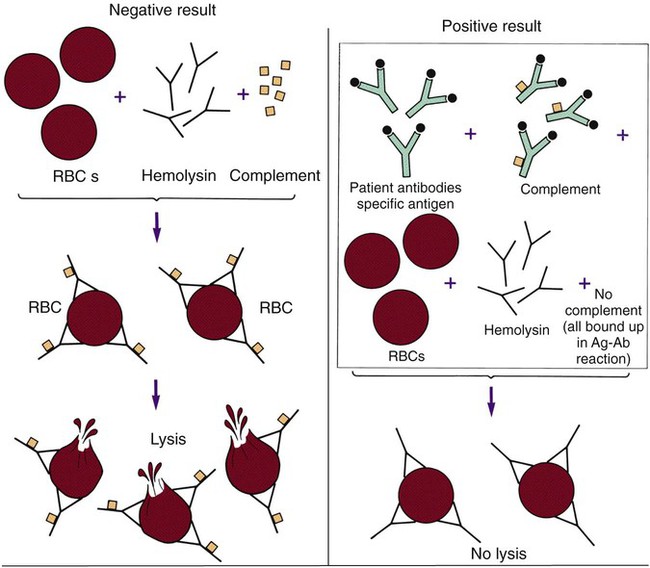
One of the classic methods of demonstrating the presence of antibody in a patient’s serum is the complement fixation (CF) test. This test consists of two separate systems. The first (the test system) consists of the antigen suspected of causing the patient’s disease and the patient’s serum. The second (the indicator system) consists of a combination of sheep red blood cells, complement-fixing antibody (IgG) raised against the sheep red blood cells in another animal, and an exogenous source of complement (usually guinea pig serum). When these three components are mixed together in optimum concentrations, the anti-sheep erythrocyte antibody binds to the surface of the red blood cells, and the complement then binds to the antigen-antibody complex, ultimately causing lysis (bursting) of the red blood cells. For this reason the anti-sheep red blood cell antibody is also called hemolysin. For the CF test, these two systems are tested in sequence (Figure 10-5). The patient’s serum is first added to the putative antigen; then the limiting amount of complement is added to the solution. If the patient’s serum contains antibody to the antigen, the resulting antigen-antibody complexes bind all the complement added. In the next step, the sheep red blood cells and the hemolysin (indicator system) are added. The patient’s complement is available to bind to the sheep cell–hemolysin complexes and cause lysis if the complement has not been bound by a complex formed with antibody from the patient’s serum. A positive result, meaning the patient has complement-fixing antibodies, is revealed by failure of the red blood cells to lyse in the final test system. Lysis of the indicator cells indicates lack of antibody and a negative CF test result.
Enzyme-Linked Immunosorbent Assays
ELISA tests available for the detection of antibodies to infectious agents are sensitive and specific. As described in depth in Chapter 9, the presence of a specific antibody is detected by the ability of a second antibody, conjugated to a colored or fluorescent marker, to bind to the target antibody, which is bound to its homologous antigen. (Various enzyme-substrate systems, including the use of avidin-biotin to bind marker substances, are also discussed in Chapter 9.) The antigen to which the antibodies bind, if antibodies are present in the patient’s sera, is either attached to the inside of the wells of a microtiter plate, adherent to a filter matrix, or bound to the surface of beads or plastic paddles. Advantages of ELISA tests include ease of performance on many serum samples at the same time and easy detection of the colored or fluorescent end products with appropriate instrumentation, removing the element of subjectivity inherent in so many serologic procedures. Disadvantages include the need for special equipment, the fairly long reaction times (often hours instead of minutes for particle agglutination tests), the relative end point of the test (which relies on measuring the amount of a visible end product that is not dependent on the original antigen-antibody reaction itself but on a second enzymatic reaction, compared to a directly quantitative result), and the requirement for batch processing to ensure cost effectiveness.
Indirect Fluorescent Antibody Tests and Other Immunomicroscopic Methods
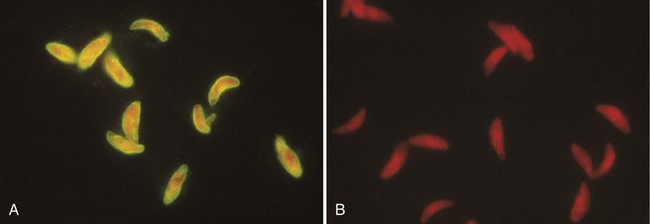
Indirect fluorescent antibody determination (IFA) is a widely applied method of detecting diverse antibodies (see Chapter 9). For these types of tests, the antigen against which the patient makes antibody (e.g., whole Toxoplasma organisms or virus-infected tissue culture cells) is affixed to the surface of a microscope slide. The patient’s serum is diluted and placed on the slide, covering the area in which antigen was placed. If present in the serum, antibody binds to the specific antigen. Unbound antibody is then removed by washing the slide. In the second stage of the procedure, a conjugate of antihuman globulin directed specifically against IgG or IgM and a fluorescent dye (e.g., fluorescein) is placed on the slide. This labeled marker for human antibody binds to the antibody already bound to the antigen on the slide and serves as a detector, indicating binding of the antibody to the antigen when viewed under a fluorescence microscope (Figure 10-6). Commercially available test kits include slides coated with the antigen, positive and negative control sera, diluent for the patients’ sera, and the properly diluted conjugate. As with other commercial products, IFA systems should be used as units, without modification of the manufacturer’s instructions. Commercially available IFA tests include those for antibodies to Legionella species, B. burgdorferi, T. gondii, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus capsid antigen, early antigen and nuclear antigen, herpes simplex viruses types 1 and 2, rubella virus, M. pneumoniae, T. pallidum (the fluorescent treponemal antibody absorption test [FTA-ABS]), and several rickettsiae. Most of these tests, if performed properly, give extremely specific and sensitive results. Proper interpretation of IFA tests requires experienced and technically competent technologists. These tests can be performed rapidly and are cost effective.
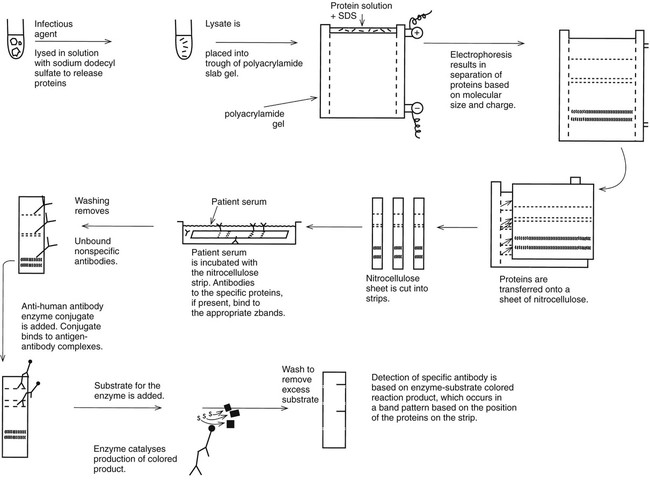
Western Blot Immunoassays
Requirements for the detection of very specific antibodies have driven the development of the Western blot immunoassay (Figure 10-7). The method is based on the electrophoretic separation of major proteins of an infectious agent in a two-dimensional agarose (first dimension) and acrylamide (second dimension) matrix. A suspension of the organism is mechanically or chemically disrupted, and the solubilized antigen suspension is placed at one end of a polyacrylamide (polymer) gel. Under the influence of an electrical current, the proteins migrate through the gel. Most bacteria or viruses contain several major proteins that can be recognized based on their position in the gel after electrophoresis. Smaller proteins travel faster and migrate farther in the lanes of the gel. The protein bands are transferred from the gel to a nitrocellulose or other type of thin membrane, and the membrane is treated to immobilize the proteins. The membrane is then cut into many thin strips, each carrying the pattern of protein bands. When patient serum is layered over the strip, antibodies bind to each of the protein components represented by a band on the strip. The pattern of antibodies present can be used to determine whether the patient has a current infection or is immune to the agent (Figure 10-8). Antibodies against microbes with numerous cross-reacting antibodies, such as T. pallidum, B. burgdorferi, herpes simplex virus types 1 and 2, and HIV, are identified more specifically using this technology than a single method that is used to identify a single antibody type. For example, the CDC defines an ELISA or immunofluorescence assay as a first-line test for Lyme disease antibody, but positive or equivocal results must be confirmed by a Western blot test.