CHAPTER 77 Septic Arthritis
GENERAL CONSIDERATIONS
An infection can be defined as the clinical manifestation of a host response to a given inoculum. Aspects of the inoculum include the amount of bacteria, the type of entry, and the nature or virulence of the pathogen. Host factors can be classified as congenital or acquired. Congenital immunoincompetence syndromes are associated with deficiencies of the humeral or bursal immunologic systems and have been well described in standard medical textbooks. Acquired failures or alteration of immunocompetence can be either generalized or localized. Generalized processes include diabetes mellitus, corticosteroid therapy, cancer with or without im-munosuppressive therapy, human immunodeficiency virus (HIV) infection, and alcohol or other chemical addiction or abuse. Local processes that alter normal host resistance include anatomic site of involvement, scar formation from previous surgery, burns, radiation, or prior infection. In applying these observations specifically to the elbow, it should be noted that this is a subcutaneous joint. Hence, the elbow is vulnerable to direct inoculation of a pathogen, particularly because host resistance is compromised. In fact, a recent analytic assessment of a 10-year study of infections in England documented an 11% incidence of death with joint sepsis. Of great interest is that a multivariant analysis documented risk factors for mortality to be confusion at presentation, age greater than 65 years of age, multiple joint involvement, and interestingly, involvement of the elbow joint.78
DISEASE-MODIFYING ANTIRHEUMATIC DRUGS
Infection and DMARDS are well known to have dramatically changed the life and prognosis of patients suffering from rheumatoid arthritis. Yet, the orthopedic surgeon is very well aware of the potential adverse effects, both with regard to increased risk in surgical infection, as well as the potential for managing patients with spontaneous sepsis related to this type of medication. In spite of the opinion or perception of the orthopedic community that these agents are responsible for an increased infection rate, the literature is somewhat controversial in this regard.7 One can find as many references that fail to support the relationship between DMARDs and infection as exists to demonstrate that this relationship does exist.7,14,18,19,25 One recent study does reveal that treatment with the tumor necrosis factor (TNF) inhibitors does significantly increase the risk of infection in orthopedic surgery (<0.04).
Our review of the current literature leaves us to conclude that without any question, DMARDs do increase the potential for infection about the elbow. Although this may not be demonstrable for the hip, a fivefold increased risk for elbow infection has been documented.18 Interestingly it has also been noticed that the skin and soft tissues are also potentially at greatest risk.19 Thus, increased problems with wound healing around the elbow supports the increased incidence of infection.
The biggest question that remains unanswered is whether or not withholding the medication in advance subsequently has an effect on this phenomenon. There is evidence that methotrexate need not be withheld,29 whereas others have demonstrated that there is merit in withholding methotrexate therapy in the perioperative period.14
EXPERIENCE AT THE MAYO CLINIC
We are currently in the process of analyzing our experience with infected total elbow arthroplasty and the use of DMARDs. The difficulty of our and all such studies is knowing whether medications behave the same in different patients. To what extent is the drugs effect dose dependent, as appears to be the case in some instances? A study from the Mayo Clinic did demonstrate that the dose itself can be a very significant variable that influences the development of an infection with TNF antibody therapy.7
AUTHOR’S RECOMMENDATION
Based on our review of the literature and our clinical experience, there is little question in our mind that the anti-TNF agents are associated with increased orthopedic infections. For this reason, we do withhold treatment for at least a month before and a month after our surgical intervention. Although we believe that the increased incidence of sepsis is becoming well documented, what is less well known is whether or not all agents have the same adverse impact and whether withholding these agents is of value. What is the “safe period” both before and after surgery? It is hoped that this issue will be resolved over the next several years, but as mentioned earlier, because of the numerous variables that pertain to the issue, it is likely to be debated for many years to come.
OSTEOMYELITIS
Bone infection occurs (1) hematogenously, (2) by direct inoculation after surgery or open fracture, or (3) by contiguous spread from a local process.77
HEMATOGENOUS INFECTION
Hematogenous infection is the most common type of osteomyelitis and has been reported to occur at the elbow in about 4% of such cases.77 In the growing child, the end-arterial loop of the metaphyseal bone causes sluggish bone blood flow. The lack of phagocytic activity in these loops allows maturation of a septic thrombus at the arterial site.37 The abscess spreads through the haversian system into the subperiosteal space. If the metaphysis is intra-articular, as at the hip or shoulder, a septic joint will then result,75 but this is not the case at the elbow. As at other sites, acute hematogenous osteomyelitis is most common in children younger than 3 and older than 7 years of age. The first group is vulnerable owing to the lack of acquired host immunity; the second age group corresponds to the time of rapid growth.51 Our recent study reported 85% septic joints in children were of the knee, hip and ankle; only 8% involved the elbow.11
Presentation and Diagnosis
The clinical presentation is typical and includes local pain, warmth, and swelling. The patient may be afebrile but is not necessarily systemically ill. In children, a predisposing, traumatic event is common,50,83 but identity of a remote focus is less frequent. The elbow is held in flexion and pseudoparalysis, or reflex inhibition may be present. The physical examination includes the following:
Except in the very acute stages, an elevated leukocyte count is variably present,15,53 and the differential count tends to demonstrate a shift to the left in two thirds of the cases. The erythrocyte sedimentation rate has been the most sensitive blood test; it is elevated in the early stages of infection in about 80 percent of persons.15,52 Therefore, it is very sensitive, but not specific. Interestingly, the rate is statistically higher in joint infections than in bone infections and is a valuable means of following the treatment and resolution of the infection.15,41,50 Early radiographs are not helpful. After 7 to 14 days, osteoporosis may be present, followed by periosteal elevation or erosions.
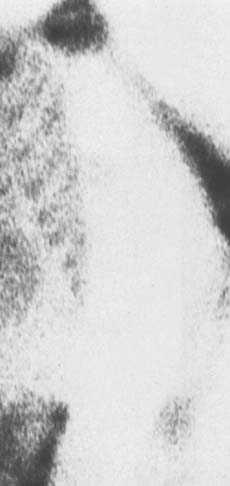
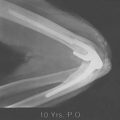
The classic appearance of osteomyelitis by bone scan is a well-defined focal area of increased uptake at the site of active infection (Fig. 77-1). The 99mTc scan may be positive as early as 24 hours after the onset of symptoms in a patient with osteomyelitis.24 The increased activity of equal intensity on both sides of the joint indicates joint disease, arthritis, or synovitis but is not a reliable indicator in the neonate. Nonspecific inflammatory arthritides of rheumatoid arthritis or gout limits the value of the Ga scan.34 False-negative results are also common.72 At the present time, we recommend first a 99mTc scan as a low-cost screening test to provide immediate results and localization. The 111In-labeled leukocyte scan may be useful primarily for more acute suppurative infection. More recently, Erdman states that magnetic resonance imaging (MRI) has 100% sensitivity and 0% specificity in a mixed group of acute and chronic osteomyelitis.17
With the continuing advances being made with imaging techniques, it is currently believed that the MRI and nuclear medicine are the most sensitive and specific modalities for detection of the septic process. MRI is most helpful to determine the extent of damage, and ultrasound is an effective, noninvasive method to assess soft tissue involvement. Computed tomography (CT) helps document sequestra. The newer positron emission tomography (PET) and single photon emission computed tomography (SPECT) are emerging as useful to evaluate chronic conditions.59,71 The T1-weighted images of the MRI are also very useful to detect concurrent osteomyelitis in the presence of the septic joint.38 Ongoing efforts to refine short time inversion recovery (STIR) and T1 spin echo (T1 SE) imaging techniques reveal enhanced sensitivity, specifically for osteomyelitis.45 Joint aspiration remains the most simple and reliable means of making the diagnosis of elbow joint sepsis, but even this is positive in only about 85% of cases.78
SPREAD FROM A CONTIGUOUS FOCUS
Infection by spread from a contiguous focus occurs at the elbow from a septic joint or from an infected olecranon bursa. A recent survey of a 10-year experience with septic bursae from Spain documented that three of 69 patients (7.7%) developed osteomyelitis.21 These circumstances are most commonly noted in a patient with rheumatoid arthritis, and the management is the same as that for a septic joint. Because a sympathetic sterile effusion may occur with metaphyseal osteomyelitis, even though the metaphysis is extra-articular, the joint should be aspirated to exclude septic arthritis in the presence of an effusion.
MICROORGANISMS
The most common pathogen causing acute hematogenous osteomyelitis is Staphylococcus aureus. Opportunistic organisms are isolated in the debilitated patient, and Pseudomonas aeruginosa is most commonly associated with drug addiction or chronic, draining wounds. Organisms not generally considered pathogenic must be viewed as such when isolated on several occasions, particularly in patients with compromised resistance. In one report, two of the three cases of diphtheroid osteomyelitis involved the elbow, and both patients had altered local immunocompetency.52 Serratia infection of open fractures has also been reported in the humerus and forearm in about one third of such cases.40 The infected prosthesis is discussed in Chapter 62.
TREATMENT
For the elbow, there are no unique management features of acute hematogenous osteomyelitis, except that prolonged immobilization is to be strictly avoided. In cases that are diagnosed early, antibiotics alone may be adequate.6,53 Surgery is indicated when there is no response to parenteral antibiotic therapy after 36 to 48 hours. It has been our experience that acute hematogenous osteomyelitis in which symptoms have been present for less than 10 days may be treated with specific intravenous antibiotics for 2 to 3 weeks. If symptoms have been present for more than 10 days, a discrete soft tissue or bone abscess may have developed and must be surgically decompressed. Subperiosteal needle aspiration is sometimes helpful in accurately defining metaphyseal infection. Today we rely on the MRI to assist in the early diagnosis of bone involvement.20
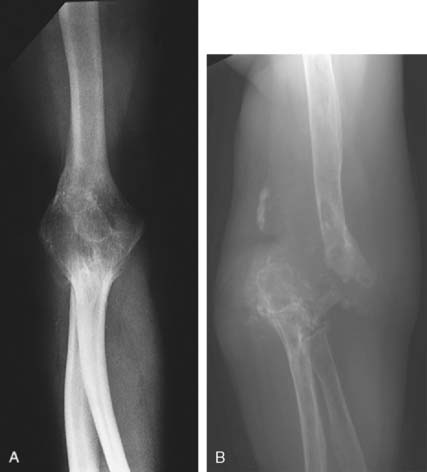
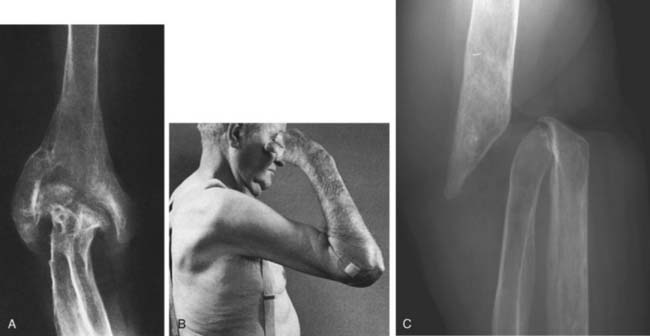
Intravenous antibiotics should be continued for about 3 weeks. Oral agents can be used for an additional 4 weeks, but bactericidal levels should be attained and monitored.43 Infection involving bone may become subacute or chronic, and in this instance, incision and drainage with limited bone débridement and secondary soft tissue healing may be necessary. An extensive débridement of bone severely impairs function because the joint becomes dysfunctionally unstable and is difficult to fuse (Fig. 77-2). Burkhalter and colleagues60 have discussed the upper extremity restrictions of function created by elbow fusion and the common complications of failure of union and secondary fracture (see Chapter 70). They recommended a posterior compression plate and pointed out that often the hardware is exposed during the process of healing of the fusion. They also accurately emphasize the limited indications for and the occurrence of forearm motion loss after arthrodesis.60 Occasionally, spontaneous arthrodesis occurs after débridement, but extensive removal of bone causes gross instability (Fig. 77-3). If soft tissue coverage is a problem, the flexor carpi ulnaris muscle pedicle flap may be used for coverage of the proximal ulna and elbow. Fourteen centimeters of muscle is available from the pedicle entering 5 cm distal to the elbow joint line. Such a flap brings improved blood supply as well as soft tissue coverage.49
In the acute phase of treatment, the joint should be splinted, elevated, and put to rest. As the process resolves, gentle active motion is encouraged as soon as possible. Continuous passive motion may prove to be beneficial in some patients to avoid ankylosis or adhesions.64
RESULTS
In the preantibiotic area, Wilensky80 reported that three of 18 patients developed spontaneous ankylosed elbows owing to immobilization for osteomyelitis of the elbow region. Other long-term residual effects include sinus tract formation, recurrent infection, pathologic fracture, growth disturbances, and development of chronic osteomyelitis. West and associates79 observed that chronic osteomyelitis of the humerus appears to have a better prognosis than chronic infection of the tibia or femur. The duration of the septic process is the most important prognostic feature.53,57 Today, it is anticipated that better function will be realized with better techniques for early detection.
SEPTIC ARTHRITIS OF THE ELBOW
Septic arthritis after joint replacement51 is discussed in Chapter 62. In the experience at the Mayo Clinic, septic arthritis of the elbow occurred in 6% of adults39 and 3% of children diagnosed with septic joints.50 Other investigators report an incidence of 9% to 13%,3,26,78 noting that the ages at risk were those “at the extremes of life.”3 The frequent association of septic arthritis with rheumatoid arthritis is well recognized.30,31,42,61,68 This association is believed to be even more likely in those treated with DMARDs. Kellgren and colleagues42 reported that 33% of septic joints in patients with rheumatoid arthritis involved the elbow. Also noted were the multiple sites of involvement and the high incidence of Staphylococcus aureus organisms (83%).
DIAGNOSIS
An incorrect or delayed initial diagnosis is common.39 The clinical examination and an index of suspicion are crucial for a prompt and correct diagnosis. The maximum capacity of the joint is at about 80 degrees of flexion,55 so the patient will present with the joint held in this position. Pain and swelling about the elbow are invariably present, and gentle, passive motion is painful.
Differential diagnosis of an infected elbow in the child includes juvenile rheumatoid arthritis, unrecognized trauma, acute rheumatic fever (which affects the elbow in about 15% of cases), and transient synovitis. In the adult, 5% of patients with gout or pseudogout will have involvement of the elbow. The occurrence of elbow sepsis in those with immunodeficiency is also becoming recognized.10,22,60 Differentiation from osteoarthritis or post-traumatic arthritis should not be a difficult problem. An additional differential diagnosis is nonsuppurative infectious arthritis; such as that associated with Lyme disease and hepatitis or from mycobacterium.87 Gonococcal arthritis is suspected with appropriate sexual history. Rheumatoid arthritis with a secondary infection is, of course, a difficult clinical diagnosis that is usually resolved only with aspiration.
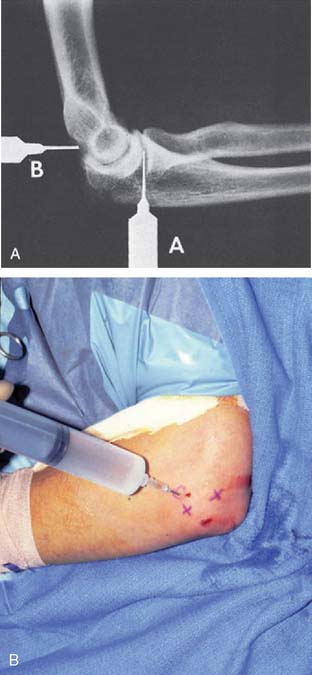
Systemic symptoms are variably present. A leukocytosis is often present early, but the shift to the left of the differential leukocyte count is more reliable. The sedimentation rate is invariably increased, but this does not distinguish the patient with active rheumatoid arthritis.15 Even the infected joint replacement shows elevated sedimentation rates, but the effect of the surgery itself is sometimes a source of confusion.66 As with any joint infection, the key to the diagnosis is joint aspiration. The distended joint is easy to enter either from the lateral “triangle” or at the posterior olecranon fossa (Fig. 77-4). Joint aspiration must be done under sterile conditions, because inoculation of the sterile joint is possible with aspiration and has been reported.3
In addition to joint aspiration for culture and cell count, gas-liquid chromatography may be helpful in differentiating a bacterial from a nonseptic inflammatory process.8
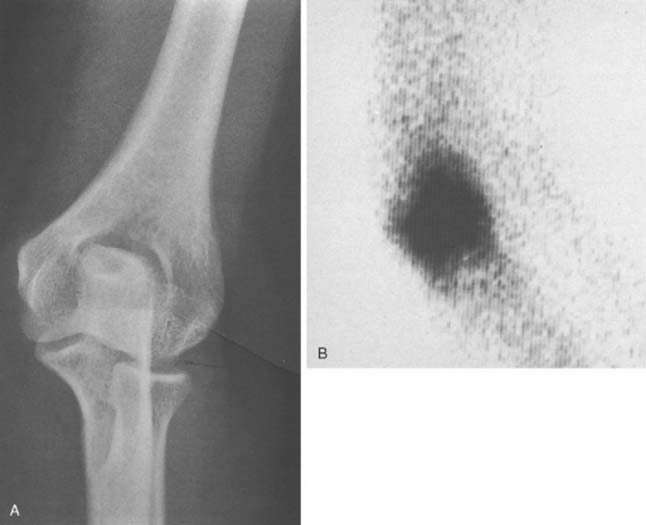
The radiographic assessment is not helpful in the early diagnosis of elbow infection, but an increased amount of synovial fluid may show an anterior or posterior fat pad sign.9,46 In this setting, the 99mTc scan is invariably positive (Fig. 77-5). Gallium or labeled white cell scans may be more specific than technetium scans, but they have the disadvantage of a 24- to 72-hour delay in diagnosis and have fallen into disfavor in recent years. An MRI lacks the specificity of distinguishing septic from nonseptic fluid20 but can assist in the diagnosis of a contiguous osteomyelitis. Later, osteopenia and subtle bone erosion at the synovial attachment occur, progressing to uniform thinning of the articular cartilage and then more extensive erosions and subchondral disruption. Additional laboratory investigations include blood cultures, which are positive in about 40% to 70% of patients3,28,53 and in up to 90% if multiple joints are involved. We have observed that the limited use of oral antibiotics will tend to result in a negative blood culture, but the joint aspirate will still reveal the organism.53
Staphylococcus aureus is isolated in about two thirds of cases of bacterial arthritis in adults39 and in at least half of those in children.50 In the neonatal age group, coliform and gram-positive organisms are not uncommon.54 At ages 3 months to 3 years, the child is at risk for Haemophilus influenzae, but in children older than 3 years, S. aureus is the predominant organism.50
TREATMENT
Initial specific antibiotic treatment is first based on the Gram stain. If infection is suspected and no organism is isolated, initial antibiotic treatment should be based on age and presentation. This should include penicillin in the young healthy patient, Staphylococcus and gram-negative coverage in the older patient with underlying disease, and drugs for H. influenzae in the young child. Mayo Clinic treatment consists of 3 to 4 weeks of intravenous administration of antibiotics, but this may be somewhat conservative, and the duration should be tailored to the clinical setting. Others have begun an appropriate oral antibiotic approximately 1 week after serum bactericidal levels have been obtained.43 Serial monitoring of the serum antibiotic levels is continued on an outpatient basis.
In addition to systemic antibiotics, the treatment, as in any diarthrodial joint, requires removal of accumulated cellular debris and pus. Cartilage is destroyed by digestion from enzymes elaborated from neutrophils, synovium, and bacteria.16 When there is capsular distension, aspiration is easily accomplished (see Fig. 77-5). Rather than simple aspiration for diagnosis, we strongly recommend that the joint be lavaged with sterile saline at the time of aspiration. Lavage has been clearly shown to be effective in preventing collagen loss in the rabbit.15 Hence, we perform a saline lavage at the time of the initial operation and inject 0.5 to 1 g of cefazolin sodium after lavage. Successively decreasing cell counts from the joint aspirate may also be used as an indicator of recovery.26,27 Intermittent joint distension-suction through percutaneous catheters35 has given way to arthroscopic débridement in those not responding immediately to the initial aspiration, lavage and anti-biotic deposition.
It should be noted that intra-articular administration of antibiotics is controversial in the treatment of septic arthritis. Adequate levels of antibiotics occur in the synovial fluid from parenteral treatment, and postinfection synovitis lasting up to 8 weeks in as many as 40% of the cases has been related to the intra-articular use of penicillin.3 Yet experimental evidence indicates that the joint can be sterilized more rapidly by intra-articular injection of an antibiotic.5 Lacking evidence that the chemical synovitis is harmful to the joint, if there has been a delay in diagnosis of 4 to 5 days, we may inject 0.5 g of cefazolin sodium diluted in 10 mL of sterile water, particularly if a gram-positive organism is suspected.
Arthroscopy of the elbow allows inspection, clearance of loculations and adhesions, thorough irrigation, and synovial tissue culture as well as insertion of drainage catheters. Hence, this has emerged as the treatment of choice for most cases that do not respond to aspiration or in those patients requiring synovectomy.74 Aggressive synovectomy and débridement is an important concept in treating the infected prosthesis65 (see Chapter 38).
If extra-articular involvement is present, arthrotomy may be necessary and early motion is begun. Early active motion after incision and drainage was recommended as early as 1919 by Willems,81 who along with others4 reported good results with both knee and elbow infections. The basis of the beneficial effect of early motion has been carefully studied by Salter and associates,64 who found protection of articular cartilage and concluded that this technique (1) prevents adhesions and pannus, (2) improves nutrition of cartilage, (3) enhances clearance of exudate including lysosomal enzymes, and (4) stimulates the living chondrocytes.
RESULTS
Delay in diagnosis and treatment is probably the most important factor affecting prognosis.27,42,50 A normal joint is unlikely if treatment is delayed for more than 1 week after the onset of symptoms.50,57 The degeneration of articular cartilage and the development of fibrous adhesions50 are responsible for the poor results after an infection. Virulence of the organism is also an important prognostic variable.26 In one series, complete recovery occurred in 90% of those infected with Streptococcus, 60% of those infected with Staphylococcus, and less than one third of patients infected with a gram-negative organism.27 Gram-negative infection has a poor prognosis and is often associated with compromised host resistance. Gonococcal arthritis also involves the elbow in about 10% of cases,48 and treatment offers predictably good results. Nongonococcal Neisseria infections have also been reported to involve the elbow, again often with compromised host resistance or in association with a crystalline-induced arthritis.17
Loss of function, not recurrence, is the most common sequela of this infection (Fig. 77-6). In the Mayo series of 103 septic joints, acute infection was eradicated in all but one, with only four recurrences. Argen and associates3 found no evidence of reinfection or chronic osteomyelitis in any of the elbow infections, and no secondary procedures were necessary.50 The ultimate result depends on the state of the joint before the infection.
NONBACTERIAL INFECTIONS
The elbow joint is also somewhat prone to nonbacterial infection and is involved in approximately 10% of all skeletal infections from tuberculosis.47 Unlike suppurative arthritis, the adjacent bone may also be involved. A tuberculous infection of the elbow is diagnosed by aspiration in 25% to 75% of instances, but most consistently (95%), it results from biopsy of the synovial tissue. Pulmonary tuberculosis is present in only about half of the cases.85 Atypical Mycobacterium infection, for example, M. kansasii,77 may also occur, both from direct inoculation and from lung involvement, and may be slowly progressive over many years.
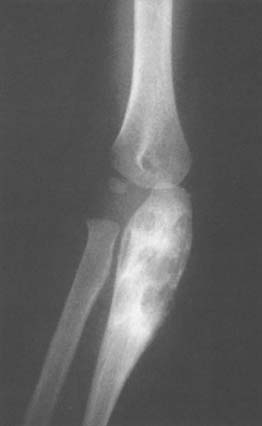
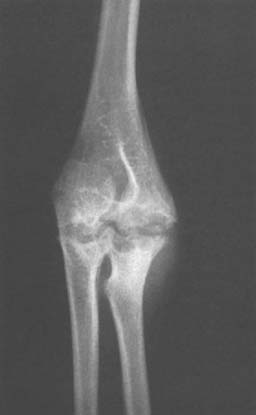
The articular cartilage is preserved well into the course of the disease. The radiographic stages seen in the elbow include: soft tissue involvement, localized cystic lesions in the bone (Fig. 77-7), or resorption with cystic subchondral changes (Fig. 77-8). Wilson pointed out that coronoid lesions are associated with joint space loss, and, as expected, massive lesions of the joint have a poor prognosis for function despite synovectomy.82
In a study of 29 joints involved with tuberculosis, 28 attained a useful joint after 12 months of treatment with chemotherapy alone.47 At present, chemotherapy with early motion is the treatment of choice, with surgery being used only to make the diagnosis. For residual dysfunction, a synovectomy and radial head resection might be considered, as well as excisional arthroplasty. Arthrodesis may be difficult2 and is recognized as a poor salvage procedure that should be used only if the infection cannot be controlled.
Coccidioidomycosis caused by Coccidioides immitis has been reported in the elbow in three of 25 joint infections.84 The treatment recommended is synovectomy with intravenous amphotericin B for the disseminated disease. Kumar44 recently reported a case of elbow sepsis occurring from Chryseobacterium meningosepticum.
SEPTIC OLECRANON BURSITIS
Olecranon bursitis has been the topic of several reports10,21,58,62,69,86 and is discussed in Chapter 84. The superficial location of the extensor surface of the elbow places the olecranon bursa at increased risk of trauma,21,63 especially in certain occupations.10 A pre-existing local infection or associated conditions such as diabetes mellitus or alcoholism have also been noted. Infection of the olecranon bursa does not necessarily indicate elbow joint infection, because normally the two structures do not communicate. However, the bursa may communicate with the elbow in rheumatoid arthritis76 and after total elbow replacement. The association with immunoincompetent states, including HIV infection, is also recognized.10 Elbow joint aspiration and sinography may be necessary to determine this association. Of those who do develop a septic olecranon bursa, about one third give a history of a previous noninfected olecranon bursitis.1 Septic olecranon bursitis is rare in children, because this bursa develops around ages 7 to 10 years.
The presentation varies widely from acute onset of cellulitis (Fig. 77-9) to a low-grade subacute process of 2 or more weeks’ duration. Importantly, pain and erythema are suggestive of a septic rather than a mechanical process. The range of motion is restricted at the extremes only, aiding the differentiation of bursal from elbow joint sepsis. The differential diagnosis includes septic, traumatic, or inflammatory bursitis, and in patients with rheumatoid arthritis, septic elbow.10,12,23,32,63 The relationship with septic arthritis in the patient with rheumatoid arthritis is particularly important. The coexistence of the two is well established, and septic arthritis may occasionally present as an infected olecranon bursitis.76 Fluid aspirate yields S. aureus in 90% of cases.13,33 Other less common organisms include group A Streptococcus31 and anaerobic,73 tuberculous,67 and even parasitic and yeast organisms.1,56 In the series of Pien and colleagues58 that evaluated 34 cases, 41% of septic olecranon bursitis presented as associated cellulitis and lymphadenitis.
Sterile hemodialysis olecranon bursitis is now a well-known entity (see Chapter 84).36 If the bursa becomes secondarily infected, usually with S. aureus, the condition may appear to be indistinguishable from noninfected bursitis and the diagnosis must be confirmed by aspiration.
Examination for crystals should be done because gout may coexist with or even predispose to bursal sepsis (see Chapter 84).23 A key point is that aspiration with cell count, crystal determination, and Gram stain probably should be done before corticosteroid injection into the bursa in instances in which underlying diagnosis is uncertain. Rheumatoid and gouty fluid may appear purulent, and infective fluid may be only slightly turbid. Crystal examination with a polarizing microscope should confirm the diagnosis of gout and pseudogout, but the birefringent crystals of a long-acting steroid may confuse the diagnosis. Cell counts below 1000/mm3 are not worrisome, but those above this level are at least suspicious. White cell counts are generally less in the bursa than in joint infection, so a low white count should be cautiously interpreted.
TREATMENT
The length of treatment with antibiotics is based on the clinical response and the duration of symptoms. A sterile aspiration serves as an appropriate end point, but usually the clinical course is an adequate indicator. Other factors that may influence the duration of treatment include the underlying disease, the completeness of bursal drainage, the appropriateness of antibiotic treatment, and most important, the duration of infection before the initiation of treatment. Ho and Su32 recommend continued treatment for 5 days after a sterile aspirate has been obtained. Roschman and Bell62 found in immunocompromised patients with olecranon bursitis a mean of 11 days of antibiotic therapy before bursal fluid cultures were negative.
Surgical drainage is performed for (1) failed aspiration and antibiotic treatment, (2) chronic, recurrent infection, (3) an infected bursa that has been altered by a prior inflammatory process or surgery, (4) infection with a resistant organism not responding to treatment, and (5) exploration for extent of infection or foreign body including accessory bursae about the triceps.33 However, surgical drainage may result in a chronic fistula. Hence, arthroscopic débridement, as recently suggested, may emerge as a viable or even preferred treatment option.
Indications for excision of the olecranon bursa include prolonged drainage after surgical incision or rupture, recurrent septic bursitis, and chronic bursitis with contiguous osteomyelitis. Ablation is difficult, especially in the patient with rheumatoid arthritis. We have found that meticulous dissection under magnification with preliminary staining of the bursal wall using methylene blue and hydrogen peroxide is helpful.70 The incision should be lateral to the midline, not over the center of the bursa. In order to decrease the likelihood of recurrence, we lavage with a dilute solution of doxycycline, known as tissue-sclerosing agent. This approach seems to be effective in lessening the likelihood of a recurrence.
1 Ahbel D.E., Alexander A.H., Kleine M.L., Lichtman D.M. Protothecal olecranon bursitis. J. Bone Joint Surg. 1980;62A:835.
2 Arafiles R. A new technique of fusion for THERABAND arthritis of the elbow. J. Bone Joint Surg. 1981;63A:1396.
3 Argen R.J., Wilson C.H., Wood P. Suppurative arthritis. Arch. Intern. Med. 1966;117:661.
4 Ballard A., Burkhalter W.E., Mayfield G.W., Dehne E., Brown P.W. The functional treatment of pyogenic arthritis of the adult knee. J. Bone Joint Surg. 1975;57A:1119.
5 Bardenheier J.A., Morgan H.C., Stamp W.G. Treatment and sequelae of experimentally produced septic arthritis. Surg. Gynecol. Obstet. 1966;122:249.
6 Blockey N.J., McAllister T.H. Antibiotics in acute osteomyelitis in children. J. Bone Joint Surg. 1972;54B:299.
7 Bongartz T., Sutton A.J., Sweeting M.J., Buchan I., Matteson E.L., Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies. Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J. A. M. A. 2006;295:2275.
8 Brook I., Reza M., Bricknell K.S., Bluestone R., Finegold S.M. Abnormalities in synovial fluid of patients with septic arthritis detected by gas-liquid chromatography. Ann. Rheum. Dis. 1980;39:168.
9 Brower A.C. Septic arthritis. Radiol. Clin. North Am. 1996;34:293.
10 Buskila D., Tenenbaum J. Septic bursitis in human immunodeficiency virus infection. J. Rheumatol. 1989;16:1374.
11 Caksen H., Ozturk M.K., Uzum K., Yuksel S., Ustunba H.B., Per H. Septic arthritis in childhood. Pediatr Int. 2000;42:534.
12 Canoso J.J. Idiopathic or traumatic olecranon bursitis. Arthritis Rheum. 1977;20:1213.
13 Canoso J.J., Sheckman P.R. Septic subcutaneous bursitis: report of sixteen cases. J. Rheumatol. 1979;6:1.
14 Carpenter M.T., West S.G., Vogelgesang S.A., Casey Jones D.E. Postoperative joint infections in rheumatoid arthritis patients on methotrexate therapy. Orthopedics. 1996;19:207.
15 Covey D.C., Albright J.A. Clinical significance of the erythrocyte sedimentation rate in orthopaedic surgery. J. Bone Joint Surg. 1987;69A:148.
16 Daniel D., Akeson W., Amiel D., Ryder M., Boyer J. Lavage of septic joints in rabbits: effects of chondrolysis. J. Bone Joint Surg. 1976;58A:393.
17 Degan T.J., Rand J.A., Morrey B.F. Musculoskeletal infection with nongonococcal Neisseria species not associated with meningitis. Clin. Orthop.. 1983;176:206.
18 den Broeder A.A., Creemers M.C.W., Fransen J., de Jong E., de Rooij D.-J.R., Wymenga A., de Waal-Malefijt M., van den Hoogen F.H.J. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: A large retrospective study. J. Rheumatol. 2007;34:689.
19 Dixon W.G., Watson K., Lunt M., Hyrich K.L., Silman A.J., Symmons D.P. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368.
20 Erdman W.A., Tamburro F., Jayson H.T., Weatherall P.T., Ferry K.B., Peshock R.M. Osteomyelitis: characteristics and pitfalls of diagnosis with MR imaging. Radiology. 1991;180:533.
21 Garcia-Porrua C., Gonzalez-Gay M.A., Ibanez D., Garcia-Pais M.J. The clinical spectrum of severe septic bursitis in northwestern Spain: A 10 year study. J. Rheum. 1999;26:663.
22 Gardner G.C., Weisman M.H. Pyarthrosis in patients with rheumatoid arthritis: a report of 13 cases and a review of the literature from the past 40 years. Am. J. Med. 1990;88:503.
23 Gerster J.C., Lagier R., Boivin G. Olecranon bursitis related to calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 1982;25:989.
24 Gilday D.L., Eng B., Paul D.J., Paterson J. Diagnosis of osteomyelitis in children by combined blood pool and bone imaging. Radiology. 1975;117:331.
25 Giles J.T., Bartlett S.J., Gelberg A.C., Nanda S., Fontaine K., Ruffing V., Bathon J.M. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55:333.
26 Goldenberg D.L., Brandt K.D., Cohen A.S., Cathcart E.S. Treatment of septic arthritis. Arthritis Rheum. 1975;18:83.
27 Goldenberg D.L., Cohen A.S. Acute infectious arthritis. Am. J. Med. 1976;60:369.
28 Goldenberg D.L., Cohen A.S. Synovial membrane histopathology in differential diagnosis of arthritis. Medicine. 1978;57:239.
29 Grennan D.M., Gray J., Loudon J., Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann. Rheum. Dis. 2001;60:214.
30 Gristina H. Spontaneous septic arthritis in rheumatoid arthritics. J. Bone Joint Surg. 1974;56A:1180.
31 Ho G. Bacterial arthritis. Curr. Opin. Rheum. 1992;4:509.
32 Ho G., Su E.Y. Antibiotic therapy of septic bursitis. Arthritis Rheum. 1981;24:905.
33 Ho G., Tice A.D., Kaplan S.R. Septic bursitis in the prepatellar and olecranon bursae. Ann. Intern. Med. 1978;89:21.
34 Hughes S. Radionuclides in orthopedic surgery. J. Bone Joint Surg. 1980;62B:141.
35 Jackson R.W., Parsons C.J. Distension-irrigation treatment of major joint sepsis. Clin. Orthop. 1973;96:160.
36 Jain V.K., Cestero R.V.M., Baum J. Septic and aseptic olecranon bursitis in patients on maintenance dialysis. Clin. Exp. Dialysis Apheresis. 1981;5:4.
37 Kahn D.S., Pritzker K. The pathophysiology of bone infection. Clin. Orthop. 1973;96:12.
38 Karchevsky M., Schweitzer M.E., Morrison W.B., Parellada J.A. MRI findings of septic arthritis and associated osteomyelitis in adults. Am. J. Radiol. 2004;182:119.
39 Kelley P.J., Martin W.J., Coventry M.B. Bacterial (suppurative) arthritis in the adult. J. Bone Joint Surg. 1970;52A:1595.
40 Kelley P.J. Musculoskeletal infections due to Serratia.. Clin. Orthop.. 1973;96:76.
41 Kelley P.J. Bacterial arthritis in the adult. Orthop. Clin. North Am. 1975;6:973.
42 Kellgren J.H., Ball J., Fairbrother R.W., Barns K.L. Suppurative arthritis complicating rheumatoid arthritis. B.M.J. 1958;1:1193.
43 Kolyvas E., Ahroneim G., Marks M.I., Gledhill R., Owen H., Rosenthal L. Oral antibiotic therapy of skeletal infections in children. Pediatrics. 1980;65:867.
44 Kumar R., Stephens J.L. Septic arthritis caused by Chryseobacterium meningosepticum in an elbow joint prosthesis. South. Med. J.. 2004;97:74.
45 Mahnken A.H., Bucker A., Adam G., Gunther R.W. MRI of osteomyelitis: Sensitivity and specificity of STIR sequences in comparison with contrast-enhanced T1 spin echo sequences. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2000;172:1016.
46 Markowitz R.I., Davidson R.S., Harty P.M., Bellah R.D., Hubbard A.M., Rosenberg H.K. Sonography of the elbow in infants and children. Am. J. Rheum. 1992;159:829.
47 Martini M., Gottesman H. Results of conservative treatment of TB of the elbow. Int. Orthop. 1980;4:83.
48 Masi A.T., Eisenstein B.I. Disseminated gonococcal infection and gonococcal arthritis. Semin. Arthritis Rheum. 1981;10:173.
49 Meals R.A. The use of flexor carpi ulnaris muscle flap in treatment of infected non-union of the proximal ulna: A case report. Clin. Orthop. 1989;240:168.
50 Morrey B.F., Bianco A.J. Septic arthritis in children. Orthop. Clin. North Am. 1975;6:923.
51 Morrey B.F., Bryan R.S. Infection after total elbow arthroplasty. J. Bone Joint Surg. 1983;65A:330.
52 Morrey B.F., Fitzgerald R.H., Kelly P.J., Dobyns J.H., Washington J.A.III. Diphtheroid osteomyelitis. J. Bone Joint Surg. 1977;59A:527.
53 Morrey B.F., Peterson H.A. Hemotogenous pyogenic osteomyelitis in children. Orthop. Clin. North Am. 1975;6:935.
54 Nelson J.D. The bacterial etiology and antibiotic management of septic arthritis in infants and children. Pediatrics. 1972;50:437.
55 O’Driscoll S.W., Morrey B.F., An K.N. Intraarticular pressure and capacity of the elbow. Arthroscopy. 1990;6:100.
56 Ornvold K., Paepke J. Aspergillus terreus as a cause of septic olecranon bursitis. Am. J. Clin. Pathol.. 1992;97:114.
57 Peterson S., Knudsen F.U., Andersen E.A., Egebald M. Acute hematogenous osteomyelitis and septic arthritis in children. Acta Orthop. Scand. 1980;51:451.
58 Pien F.D., Ching D., Kim E. Septic bursitis: Experience in a community practice. J. Orthop. 1991;14:981.
59 Pineda C., Vargas A., Rodriguez A.V. Imaging of osteomyelitis: Current concepts. Infect. Dis. Clin. N. A. 2006;20:789.
60 Rashkoff E., Burkhalter W.E. Arthrodesis of the salvage elbow. Orthopedics. 1986;9:733.
61 Rimoin D.L., Wennberg J.F. Acute septic arthritis complicating chronic rheumatoid arthritis. J. A. M. A. 1966;196:109.
62 Roschmann R.A., Bell C.L. Septic bursitis in immunocompromised patients. Am. J. Med. 1987;83:661.
63 Saini M., Canoso J.J. Traumatic olecranon bursitis. Acta Radiol. Diagn. 1982;23:255.
64 Salter R.B., Bell R.S., Kelley F.W. The protective effect of continuous passive motion on living articular cartilage in acute septic arthritis. Clin. Orthop. 1981;159:223.
65 Schoifet S.D., Morrey B.F. Treatment of infection after total knee arthroplasty by debridement with retention of components. J. Bone Joint Surg. 1990;72A:1383.
66 Schulak D.J., Rayhack J.M., Lippert F.G.III, Convery F.R. The erythrocyte sedimentation rate in orthopaedic patients. Clin. Orthop. 1982;167:197.
67 Sharma S.V., Varma B.P., Khanna S. Dystrophic calcification in tubercular lesions of bursae. Acta Orthop. Scand. 1978;49:445.
68 Shulman G., Waugh T.R. Acute bacterial arthritis in the adult. Orthop. Rev. 1988;17:955.
69 Soderquist B., Hedstom S.A. Predisposing factors, bacteriology and antibiotic therapy in thirty-five cases of septic bursitis. Scand. J. Infect. Dis. 1986;18:305.
70 Stewart N.J., Manzanares J.B., Morrey B.F. Surgical treatment of aseptic olecranon bursitis. J. Shoulder Elbow Surg. 1997;6:49.
71 Stumpe K.D.M., Strobel K. 18F FDG-PET imaging in musculoskeletal infection. The Q. J. Nucl. Med. Mol. Imaging. 2006;50:131.
72 Sullivan D.C., Rosenfield N.S., Ogden J., Gottschalk A. Problems in the scintigraphic detection of osteomyelitis in children. Radiology. 1980;135:731.
73 Tollerud A. Anaerobic septic bursitis [obletter]. Ann. Intern. Med. 1979;91:494.
74 Törholm C., Hedström S.A., Sundén G., Lidgren L. Synovectomy in bacterial arthritis. Acta Orthop. Scand. 1983;54:748.
75 Trueta J. Three types of acute hematogenous osteomyelitis. J. Bone Joint Surg. 1959;41B:671.
76 Viggiano D.A. Septic arthritis presenting as olecranon bursitis in patients with rheumatoid arthritis. J. Bone Joint Surg. 1980;62A:1011.
77 Waldvogel F.A., Medoff G., Swartz M.D. Osteomyelitis: A review of clinical features, therapeutic considerations and unusual aspects. N. Engl. J. Med. 1970;282:198. 260, 316
78 Weston V.C., Jones A.C., Bradbury N., Fawthrop F., Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann. Rheum. Dis. 1999;58:214.
79 West W.F., Kelley P.J., Martin W.J. Chronic osteomyelitis. J. A. M. A. 1970;213:1837.
80 Wilensky A.O. Osteomyelitis. Its Pathogenesis, Sym-ptomatology, and Treatment. New York: Macmillan, 1934.
81 Willems C. Treatment of purulent arthritis by wide arthrotomy followed by immediate active mobilization. Surg. Gynecol. Obstet. 1919;28:546.
82 Wilson J.N. Tuberculosis of the elbow. J. Bone Joint Surg. 1953;35B:558.
83 Winroth G., Hedström S.A., Lidgren L. Posttraumatic bacterial arthritis with luxation of the elbow: A case report. Arch Orthop. Trauma Surg. 1984;103:227.
84 Winter W.G., Larson R.K., Honeggar M.M., Jacobsen D.T., Pappagianis D., Huntington R.W. Coccidioidal arthritis and its treatment. J. Bone Joint Surg. 1975;57A:1152.
85 Wolfgang G.L. Tuberculous joint infection. Clin. Orthop. 1978;136:257.
86 Zimmermann B.III, Mikolich D.C., Ho G. Septic bursitis. Semin. Arthritis Rheum. 1995;24:391.
87 Zretina J.R., Foster J., Reyes C.V. Mycobacterium kansasii infection of the elbow joint. J. Bone Joint Surg.. 1979;61A:1099.