Chapter 26 Separation of the Two Lungs
Double-Lumen Tubes, Endobronchial Blockers, and Endobronchial Single-Lumen Tubes
III Indications for Lung Separation
A Absolute Indications
Absolute indications for lung separation (Box 26-1) include protective isolation of the normal lung, establishment of adequate gas exchange when there is a change in pulmonary compliance or lung pathology, and unilateral bronchopulmonary lavage.
Box 26-1 Indications for Separation of the Two Lungs or One-Lung Ventilation
Absolute Indications
Several unilateral lung conditions lead to inadequate ventilation. A large bronchopleural or bronchocutaneous fistula can lead to little or no ventilation of the nonoperative, healthy lung. In this situation, the increased compliance of the diseased lung results in direction of most of the positive-pressure ventilation (PPV) toward the diseased lung, minimally ventilating the normal lung and producing inadequate gas exchange. Conversely, a relatively noncompliant transplanted lung cannot compete with the better compliance of the native lung, and as a result, the healthy transplanted lung can be severely underventilated. Another scenario involves a lung with bullous or cystic disease or a lung with tracheobronchial disruption.1 Tension pneumothorax or tension mediastinum could result during these scenarios from elevated airway pressures that are often observed with SLV in the lateral decubitus position.
Patients with alveolar proteinosis may require unilateral bronchopulmonary lavage, which involves multiple instillations of large fluid volumes into the target lung with subsequent drainage of the effluent fluid.2–4 Lung separation is mandatory to avoid lung cross-contamination and drowning caused by the large volume of fluid required to perform the lavage.
B Relative Indications
Relative indications for lung isolation involve facilitating surgical exposure, avoiding lung trauma, and improving gas exchange. Operations such as repair of thoracic aneurysms, pneumonectomy, pulmonary lobectomies (especially of the upper lobe), video-assisted thoracoscopic surgery, esophageal surgery, and anterior spinal surgery all benefit from the optimized surgical exposure afforded by SLV (see Box 26-1). Lung isolation further improves recovery by minimizing lung instrumentation and trauma to the nonventilated, nondependent lung. In cases of unilateral lung trauma, oxygenation and recovery may be optimized with SLV by improving 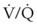 matching.
matching.
IV Techniques
A Double-Lumen Tubes
1 Anatomy
DLTs are essentially two tubes bonded together with a design that allows each tube to ventilate a specified lung. DLTs are right-sided or left-sided devices. Left-sided DLTs have a bronchial port that extends into the left main stem bronchus and a tracheal port that is designed to sit above the carina. In right-sided DLTs, the bronchial port extends into the right main stem bronchus, and the tracheal port sits above the carina. The cuff of the right-sided DLT may be at an oblique angle to facilitate ventilation of the right upper lobe bronchus at the Murphy eye (Fig. 26-1).
The original DLTs were reusable, red rubber tubes with high-pressure cuffs that became stiff and brittle over time, making placement more difficult and traumatic. Newer DLTs are made of nontoxic plastic (the Z-79 marking) and are disposable. As the plastic warms up from the surrounding body temperature, the DLT conforms to the anatomy of the patient. This increased malleability, however, makes it more difficult to reposition the same tube. Current DLTs employ high-volume, low-pressure, color-coded cuffs. The bronchial cuff and its pilot balloon/connector are blue. The tracheal cuff and its pilot balloon/connector are clear or white. Cuff inflation pressure requires a balance between preserving an adequate seal and maintaining mucosal perfusion. Measured cuff pressures between 15 and 30 mm Hg achieve these goals.5–8 In cases involving the use of nitrous oxide, the cuff pressures should be checked periodically.
DLTs come in various French (F) sizes: 28, 32, 35, 37, 39, and 41, and 1 F equals an approximately 0.33-mm measurement of the outer diameter (OD). In most adult men, a 39-F DLT fits well, having adequate length and appropriate diameter, while providing the capability of suctioning or fiberoptic bronchoscopy (FOB), and a 37-F DLT fits most adult women. A radiopaque line may be seen at the end of each lumen to allow for radiographic positioning. A Y-adapter for the proximal end allows ventilation of both lumens through a single circuit. The cross section of the DLT is designed as one round bronchial lumen and one crescent-shaped tracheal lumen. Left- and right-sided DLTs are curved at the distal end to enable advancement into the respective main stem bronchus. DLTs from different manufacturers have their own characteristic feel and slight modifications to the basic design described. The depth required for insertion of the DLT correlates with the height of the patient. For any adult 170 to 180 cm tall, the average depth for a left-sided DLT is 29 cm. For every 10-cm increase or decrease in height, the DLT is advanced or withdrawn 1.0 cm.9
3 Disadvantages
The most significant disadvantages of the DLT are related to its size. Intubation with a DLT is often more difficult than with an SLT.6 Intubation is even more complex in patients with difficult airway anatomy.10 In cases of a distorted or compressed tracheobronchial tree, placement of a DLT can be impossible due to its size and rigidity. DLT size can contribute to airway damage during placement and when the device is left in place for a long period. Because of some difficulty managing DLTs in the ICU with regard to weaning and pulmonary toilet, they are often exchanged for SLTs. The process of exchanging a DLT for an SLT can be dangerous, especially after procedures in which airway edema has occurred. Although DLT lumens are relatively large, FOB may be cumbersome due to the extended length of each tube and the narrowed crescent shape of the tracheal lumen. Multiple ports and connections further require a good working knowledge of the DLT anatomy to prevent errors in ventilation and management.
4 Selection
a Right-Sided Versus Left-Sided Double-Lumen Tubes
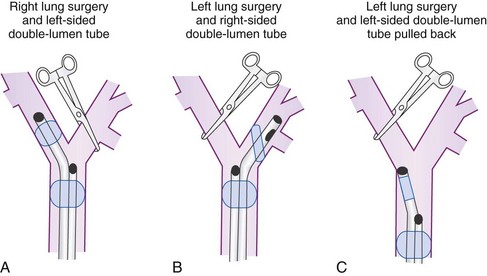
To minimize tube displacement, it may be recommended that the nonoperated bronchus be intubated (i.e., using a left-sided DLT for a patient undergoing right lung surgery) (Fig. 26-2).11 However, controversy exists in regard to left lung procedures because of the anatomic variability of the right upper lobe. The bronchial port of a right-sided tube may be difficult to position for adequate lung isolation and ventilation of the right upper lobe bronchus. This can result in difficulties during surgery, including severe hypoxia during isolated right lung ventilation.12
Many anesthesiologists prefer to use left-sided DLTs for all lung surgeries (see Fig. 26-2); however, there are insufficient data to support actual increased safety, as opposed to the perception of safety when intraoperative hypoxia, hypercapnia, and high airway pressures are used as criteria.13,14 If manipulation of the left main stem bronchus is required, the left-sided DLT is withdrawn and repositioned with the bronchial port above the carina. For operations on the left main stem, including sleeve resection, it may be preferable to use a right-sided DLT (Fig. 26-3).
Contraindications for use of a DLT include anatomic barriers that make positioning improbable or dangerous, such as carinal or bronchial lesions, strictures, vascular compression by aortic aneurysm, and aberrant bronchus.6,15,16 Right-sided DLTs may be indicated in cases with tortuosity and compression of the trachea or left main stem bronchus, which can make placement of a left-sided DLT impossible. Newly designed DLTs may be applicable for patients with special conditions including those with unusual anatomic variability.17
b Double-Lumen Tube Size
The ideal size of a DLT is one that results in a near-complete seal of the bronchial lumen without inflation of the cuff. The high inflation pressures of a small tube can cause as much mucosal damage as forcing too large a tube into a small bronchus.18 Even when height- and weight-based size estimates are used, it is impossible to choose the correct size of tube every time.15,19 Commonly, a 39-F or 41-F DLT is selected for men and a 37-F or 39-F DLT for women of average height and build. The intentional use of smaller DLTs has not had significant clinical benefits.20 A flexible fiberoptic bronchoscope (FFB) may be passed through the bronchial lumen to assess appropriate diameter and length of the DLT during placement (Table 26-1).
TABLE 26-1 Relation of Flexible Fiberoptic Bronchoscope Size to Double-Lumen Tube Size
| FFB OD Size (mm) | DLT Size (F) | Fit of FFB inside DLT |
|---|---|---|
| 5.6 | All sizes | Does not fit |
| 41 | Easy passage | |
| 39 | Moderately easy passage | |
| 4.9 | 37 | Tight fit, needs lubricant,* hand push |
| 35 | Does not fit | |
| 3.6-4.2 | All sizes | Easy passage |
| Approximately 2.0 | All sizes | Most operating rooms need special arrangements to obtain this size FFB |
DLT, Double-lumen tube; FFB, flexible fiberoptic bronchoscope; OD, outer diameter.
* Lubricant recommended is a silicon-based fluid made by the American Cystoscope Co.
5 Positioning
Malposition of the DLT can lead to life-threatening consequences. Ventilation can be severely impaired, leading to hypoxia, gas trapping, tension pneumothorax, cross-contamination of lung contents, and interference with surgical procedures. Multiple studies have shown that DLTs are often malpositioned.15,19 On the basis of these studies, indirect visualization with a FFB may be used routinely for confirmation of positioning. Various techniques using a FFB are discussed in the following sections.
a Placement of the Double-Lumen Tube
DLTs are placed similar to SLTs but with some additional maneuvers and considerations. DLTs are larger in diameter and longer than SLTs, making them more difficult to place. It is important never to force a DLT into position. For laryngoscopy, the shoulder of a Macintosh blade provides better tongue displacement and more space through which to insert the tube. The use of a video laryngoscope has also been described as effective and potentially time-saving.21 The bronchial tip of the DLT is placed through the cords, and the stylet is then removed to prevent trauma. After the bronchial portion has passed the cords, the DLT must be rotated 90 degrees toward the selected side of the bronchial lumen to sit properly. If resistance is encountered on rotating or advancing the tube, the use of a smaller tube needs to be considered. The average depth of insertion is 29 cm for a 170-cm individual. For each 10-cm increase or decrease in height, the tube depth is increased or decreased by 1 cm, respectively.15 When the tube depth is reached, the tracheal cuff is inflated, and the patient is connected to the ventilator. Care must be taken not to tear or puncture the tracheal cuff during intubation. For example, covering the teeth with an unopened alcohol swab can minimize cuff damage.
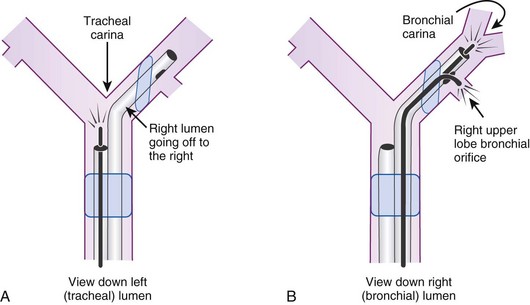
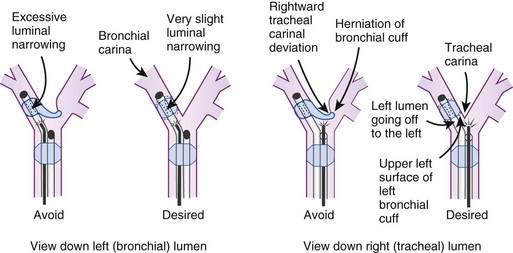
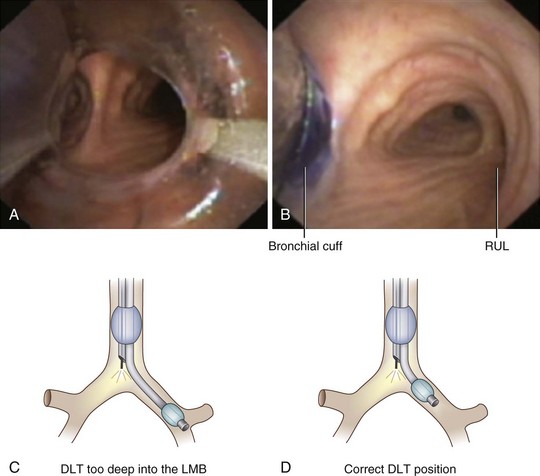
After confirmation of CO2 return and initiation of ventilation, an FFB is placed through the tracheal lumen (Fig. 26-4). The FFB is advanced, and the carina is identified. The bronchial lumen of the tube must be visualized entering the appropriate main stem bronchus (i.e., the bronchial lumen should be in the left main stem bronchus for a left-sided DLT). The balloon of the bronchial lumen should be inflated under direct vision and should lie just distal to the carina. The tube may have to be repositioned to visualize the balloon. Direct visualization of the balloon inflation helps to confirm tube position and size. Some DLTs have an indicator line just proximal to the bronchial cuff that should sit at the level of the carina. Direct visualization may be necessary to ensure that the bronchial balloon does not herniate over the carina or that the tracheal portion of the DLT does not encroach on the carina (Fig. 26-5).
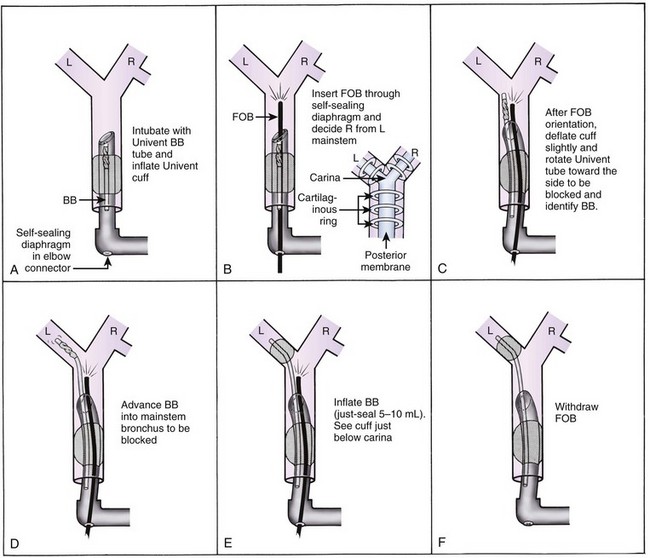
An alternative method of DLT placement is to use the FFB as an intubating stylet to guide the bronchial tip of the DLT directly into the correct main stem bronchus (Fig. 26-6). This is accomplished by inserting the FFB through the bronchial port of the DLT after the bronchial tip has passed the vocal cords. The FFB is then advanced down the trachea while identifying the anterior-posterior and right-left orientation. The FFB is advanced further to identifying the carina and the right and left main stem bronchi, then advanced into the appropriate bronchus. The DLT is advanced over the FFB. It is then necessary to remove the FFB after confirming that the bronchial tip is not obstructed and is proximal to the secondary bronchial branches. The FFB is then placed in the tracheal lumen to confirm the position of the bronchial cuff and to ensure that the tracheal lumen is not encroaching on the carina. This technique allows for assessment of the carina and tracheal rings and allows placement in tortuous airways. However, it takes more time to perform, and in some patients with poor pulmonary reserve, this extra time can lead to desaturation.
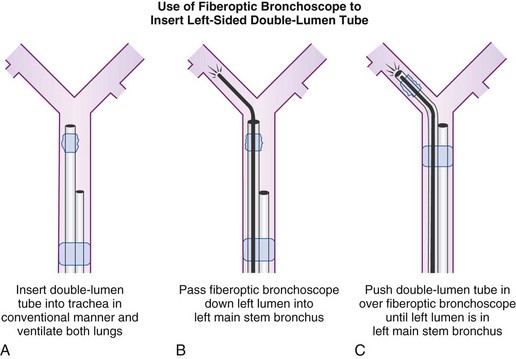
Figure 26-6 A double-lumen tube (DLT) can be put into the trachea in a conventional manner, and both lungs can be ventilated by both lumens (A). A flexible fiberoptic bronchoscope (FFB) may be inserted into the left lumen of the DLT through a self-sealing diaphragm in the elbow connector to the left lumen; this allows continued positive-pressure ventilation of both lungs through the right lumen without creating a leak. After the FFB has been passed into the left main stem bronchus (B), it is used as a stylet for the left lumen (C); the FFB is then withdrawn. Final precise positioning of the DLT is performed with the FFB in the right lumen (see Figs. 26-19 and 26-20).
(From Benumof JL: Anesthesia for thoracic surgery, Philadelphia, 1987, Saunders.)
b Confirmation of Proper Placement
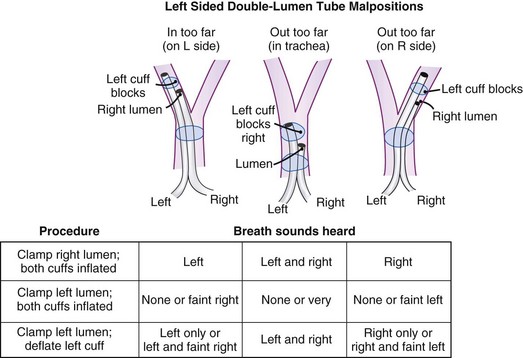
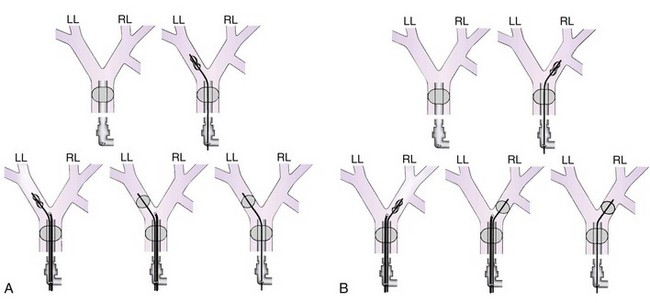
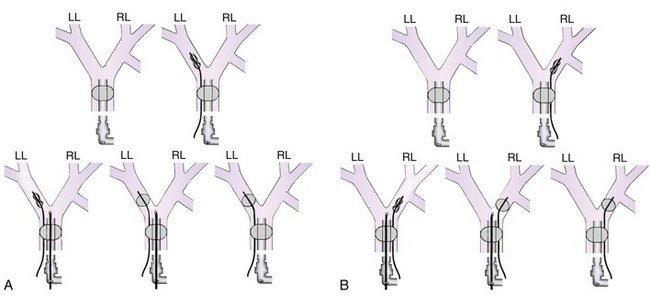
Many maneuvers have been described to assess the proper position of a DLT,22,23 including visualization of chest excursion while alternately clamping and unclamping the tracheal and bronchial ports, auscultation of lung fields while alternately clamping and unclamping the tracheal and bronchial ports (Fig. 26-7), and radiographic confirmation.24,25
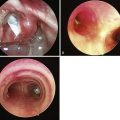
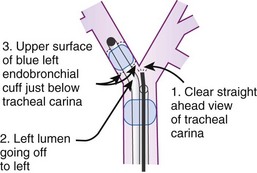
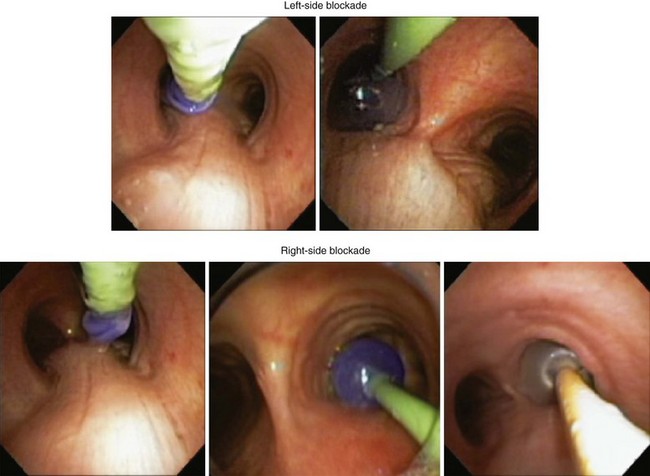
The most important advance in checking for the proper position of a DLT is the FFB (Fig. 26-8). Smith and colleagues26 demonstrated that when the disposable DLT was thought to be in the correct position by auscultation and physical examination, subsequent FOB showed that 48% of the tubes were malpositioned. Malpositions may not be clinically significant and may be missed by the clinician. When using a left-sided DLT, the bronchoscope is usually first introduced through the tracheal lumen to visualize the carina and to ensure that the bronchial cuff is not herniated. The upper surface of the blue endobronchial cuff should be just below the tracheal carina; the blue bronchial cuff of the disposable DLT is easily visualized. The bronchoscope is then passed through the bronchial lumen to identify the left upper lobe bronchial orifice. When a right-sided DLT is used, the carina should be visualized through the tracheal lumen, and the orifice of the right upper lobe bronchus should be identified when the bronchoscope is passed through the right upper lobe ventilating slot of the DLT.
Pediatric FOBs are available in several standard sizes: 5.6-, 4.9-, and 3.6-mm OD. The 4.9-mm OD bronchoscope can be passed through DLTs that are 37 F or larger. A 3.6-mm or smaller diameter bronchoscope is easily passed through all sizes of DLTs.27–29
DLT placement must be rechecked when the patient is repositioned, because movement of the tube is common. Training in the use of FFBs may be achieved during evaluation of any SLT-intubated patient and through the use of airway simulation and mannequins.30
6 Malpositioning and Complications
Use of a DLT is associated with a number of problems, the most important of which is malpositioning (see Fig. 26-7).26,31 The tube can be malpositioned in several ways.
A right-sided DLT may occlude the right upper lobe orifice. The mean distance from the carina to the right upper lobe orifice is 2.3 ± 0.7 cm in men and 2.1 ± 0.7 cm in women. With right-sided DLTs, the ventilatory slot in the side of the bronchial catheter must overlie the right upper lobe orifice to permit ventilation of this lobe. However, the margin of safety is extremely small and varies from 1 to 8 mm.32 It is difficult to ensure proper ventilation to the right upper lobe and avoid dislocation of the DLT during surgical manipulation.
The left upper lobe orifice may be obstructed by a left-sided DLT. Traditionally, the take-off of the left upper lobe bronchus was thought to be at a safe distance from the carina and that it would not be obstructed by a left-sided DLT. However, the mean distance between the left upper lobe orifice and the carina is 5.4 ± 0.7 cm in men and 5.0 ± 0.7 cm in women.33 The average distance between the openings of the right and left lumens on the left-sided disposable tubes is 6.9 cm. An obstruction of the left upper lobe bronchus is possible while the tracheal lumen is still above the carina. There is also a 20% variation in the location of the blue endobronchial cuff on the disposable tubes because this cuff is attached to the tube at the end of the manufacturing process.
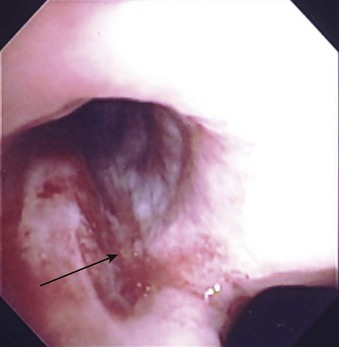
A rare complication with DLTs is tracheal laceration or rupture from the stiff tip of the bronchial lumen (Fig. 26-9). Overinflation of the bronchial cuff, inappropriate positioning, and trauma due to intraoperative dislocation that resulted in bronchial rupture have been associated with use of the Robertshaw tube and the disposable DLT.34 The pressure in the bronchial cuff should be assessed and decreased if the cuff is overinflated. If lung isolation is unnecessary, the bronchial cuff should be deflated and then reinflated slowly to avoid excessive pressure on the bronchial walls. The bronchial cuff should also be deflated during any repositioning of the patient unless lung separation is absolutely required during this time.
In a prospective trial, 60 patients were randomly assigned to two groups. SLV was achieved with an endobronchial blocker or a DLT. Postoperative hoarseness and sore throat were assessed at 24, 48, and 72 hours after surgery. Bronchial injuries and vocal cord lesions were examined by bronchoscopy immediately after surgery. Postoperative hoarseness occurred significantly more frequently in the double-lumen group than the blocker group (44% versus 17%). Similar findings were observed for vocal cord lesions (44% versus 17%). The incidence of bronchial injuries was comparable between groups.35
The most common minor complication is sore throat and temporary hoarseness. Other complications include laryngeal and bronchial injury, tracheobronchial tree disruption,36 inadvertent suturing of the DLT to thoracic structures, and direct vocal cord injury.37 Although most of the complications, except malpositioning, have been reported in the older-style DLTs (e.g., Carlens, Robertshaw), the newer designs can also pose risks.38–40
B Single-Lumen Tubes
In some situations, lung isolation is required, but the use of a DLT is not practical. In these instances, the use of a modified SLT with integrated blockers (i.e., Univent tube [Fig. 26-10]) or the use of blockers in conjunction with a standard SLT is appropriate.
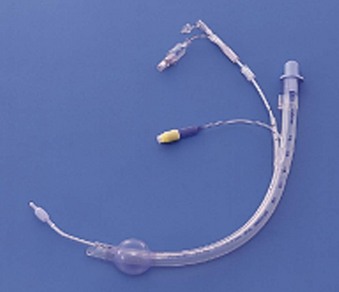
Figure 26-10 Univent single-lumen tube and bronchial blocker system.
(Courtesy LMA North America, Inc., San Diego, CA.)
1 Indications
a Difficult Airways
Patients presenting with difficult airway anatomy can be a particular challenge for lung isolation, because the placement of a conventional DLT can be impossible in such patients.41 Use of an SLT or a Univent tube (Fuji Systems, Tokyo, Japan) is advised, because these devices may be more easily placed than DLTs.42
b Segmental Lobe Isolation
Selective blockade of lung segments is sometimes desired. In these cases, a bronchial blocker device can be advanced into the desired lung segment.43 For example, a patient with a left lung pneumonia and right lower lobe and right middle lobe bronchocutaneous fistulas would likely fail this method because of profound desaturation from inability to ventilate the healthy right upper lobe. Selective lobar blockade is considered an advantageous technique to avoid total lung collapse and improve oxygenation.44 An SLT with a bronchial blocker isolating the right lower lobe and right middle lobe allows adequate saturation while the patient recovers from the pneumonia and the sealing of the bronchocutaneous fistula. It is very difficult to isolate lung segments with a DLT; combined use of a DLT and bronchial blocker devices would likely be required.
2 Disadvantages
c Bronchial Mucosal Damage
Some practitioners advocate techniques of “just-seal” bronchial cuff inflation volumes to minimize mucosal ischemia and damage.5,8 These techniques may be of little or no value, however, because most of those described are cumbersome and require specialized connectors between the ETT, the blocking device, and the circuit. The cuff pressure required for the bronchial blocker can change because of compliance changes of the ventilated lung.
C Univent Tubes
1 Anatomy
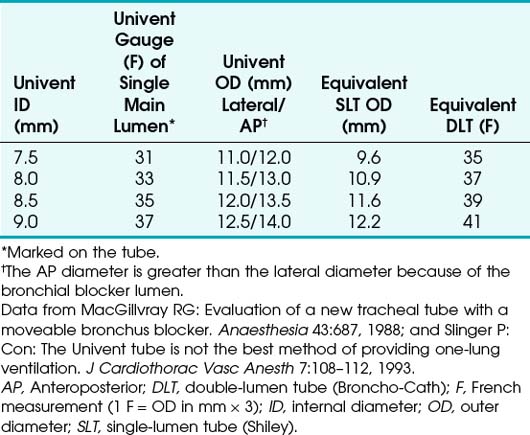
The Univent tube is a Silastic SLT with a built-in chamber that allows advancement of the integrated blocker (see Fig. 26-10).41,45,46 The integrated blocker has a small lumen along its entire length that facilitates lung deflation and allows very limited suctioning. At the distal tip of the blocker is a small balloon, and the proximal end of the blocker section contains a lumen cap. This cap needs to be engaged when the blocker balloon is deflated during full lung ventilation. Failure to engage the cap when the blocker balloon is deflated results in a circuit leak. Univent tubes are available in many sizes (Table 26-2), all designated by internal lumen size. Because of the thickness of the tube wall and the integrated blocker chamber, the outer size of these devices is much larger than that of a similarly designated SLT. For example, a 7.5-mm Univent tube has an 11.2-mm OD, compared with the 10.2-mm OD of a 7.5-mm SLT. Despite the larger size, these tubes are very useful.
2 Positioning
Before the Univent tube is placed, it must be prepared. Preparation involves removal of the distal and proximal tension wires that help keep the Univent’s shape during storage. The cuffs of the bronchial blocker and the main tube should be checked and deflated. After the tip of the bronchial blocker is bent to the shape of a hockey stick, it is retracted into the blocker chamber so that its distal tip is flush with the main tube. The tube is then inserted into the trachea with the tracheal balloon just distal to the cords. The main tube cuff is then inflated and secured at a distance of at least 2 to 3 cm between the distal tip of the main tube and the carina (Fig. 26-11) so that the curved shape of the blocker can be maintained and manipulated as it is advanced beyond the tip of the main tube. Failure to provide this adequate distance between the tip of the main tube and the carina makes directional control of the bronchial blocker very difficult.
Because the material of the Univent tube makes passage of a nonlubricated FFB difficult, the scope should be well lubricated before it is inserted into the main segment of the tube. It is often helpful to attach some type of self-sealing diaphragm device between the Univent tube and the elbow connection of the circuit. This allows continuous ventilation while the blocker is being positioned with the FFB. The FFB is advanced beyond the distal tip of the tube, and the anterior tracheal rings are identified, allowing proper orientation of the right and left main stem bronchi. The blocker portion of the tube is then visually advanced while the main tube position is maintained (see Fig. 26-11).
Placement of the blocker into the desired main stem bronchus is achieved by advancing the blocker with a clockwise or a counterclockwise rotation. If this maneuver is not sufficient to align the blocker into the desired bronchus, the entire Univent tube may be rotated in the desired direction to assist blocker orientation. The blocker is advanced into the correct location, and the locking cuff at the proximal end of the blocker is secured. It is useful to note the distance marker on the blocker. If lung isolation is desired, the blocker cuff is inflated (best performed under direct FFB visualization), and the proximal cap of the blocker may be disengaged to enhance lung deflation (see Fig. 26-11). Blind placement of the blocker is often unsuccessful. Blind placement may result in trauma to the tracheobronchial tree, resulting in complications including bleeding or even tension pneumothorax. Limitations of the Univent tube shown in Table 26-3. The solutions related to Univent blocker issues may be applicable to other types of bronchial blockers.
TABLE 26-3 Univent Bronchial Blocker Tube: Limitations and Solutions
| Limitation | Solution |
|---|---|
| Slow inflation time | Deflate the bronchial blocker cuff, and administer a positive-pressure breath through the main single lumen. Then carefully administer one short, high-pressure (20-30 psi) jet ventilation. |
| Slow deflation time | Deflate the bronchial blocker cuff, and compress and evacuate the lung through the main single lumen. Then apply suction to the bronchial blocker lumen. |
| Blockage of bronchial blocker lumen by blood or pus | Suction, use a wire stylet, and then suction. |
| High-pressure cuff | Use a just-seal volume of air. |
| Intraoperative leak in bronchial blocker cuff | Ensure the bronchial blocker cuff is positioned below the carina. Increase the inflation volume, and rearrange the surgical field. |
D Endobronchial Blockers
In modern thoracic anesthesia, lung separation can be achieved with a reusable bronchial blocker.47 Inflation of the cuff at the distal end of the blocker blocks ventilation to that lung. All modern blockers have a lumen that permits suctioning of the airway distal to the catheter tip, and depending on the clinical circumstance, oxygen can be insufflated through the catheter lumen. The main advantage of these blockers is that they can be placed through a conventional SLT. When a blocker is placed in the right main bronchus, it usually is positioned close to the carina to block the right upper lobe. Because the blocker balloon requires a high distending pressure, it easily slips out of the bronchus into the trachea because of changes in position or surgical manipulation. That movement can result in obstructing ventilation and losing the seal between the two lungs. The loss of lung separation can be a life-threatening situation if it was performed to prevent spillage of pus, blood, or fluid from bronchopulmonary lavage. Bronchial blockers are rarely used for these types of cases in which lung isolation is required.
1 Indications
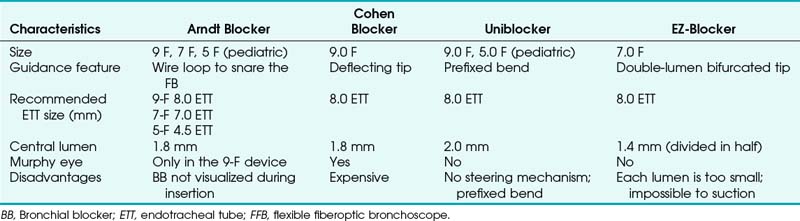
Indications for the use of a bronchial blocker are shown in Box 26-2. Because the blocker is placed through an SLT, it avoids the use of a DLT in a patient with a difficult airway. The use of a bronchial blocker also eliminates the need to change a DLT to an SLT at the conclusion of the procedure. This is important, because the airway at the conclusion of the procedure may be different from that in the initial period due to secretions and edema. In the past, Fogarty vascular embolectomy catheters were used for lung separation, but there is no indication for their use in the current practice of thoracic anesthesia. The balloon of the Fogarty is high pressure and low volume, and there is no lumen to allow egress of gas from the lung to facilitate deflation. The characteristics of bronchial blockers are summarized in Table 26-4.
2 Coaxial Stand-Alone Endotracheal Blockers
a Arndt Endobronchial Blocker
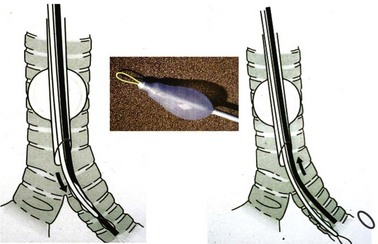
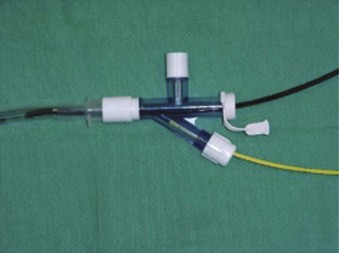
A snare-guided bronchial blocker, the Arndt Endobronchial Blocker (Cook Critical Care), was introduced to address the previously described problems. It is a wire-guided catheter with a loop snare (Fig. 26-12). A fiberscope is passed through the loop of the bronchial blocker and then guided into the desired bronchus. The blocker is then slid distally over the fiberscope and into the selected bronchus. Bronchoscopic visualization confirms blocker placement and bronchial occlusion (Fig. 26-13).
This balloon-tipped catheter has a hollow lumen of 1.6 mm, which allows suction to facilitate the collapse of the lung and insufflation of oxygen to the nondependent lung. The balloon is available in a spherical or elliptic shape. The set contains a multiport adapter (Fig. 26-14) that allows uninterrupted ventilation during positioning of the blocker. The wire may then be removed, and a 1.6-mm lumen may be used as a suction port or for oxygen insufflation. In the first generation of this device, it was not possible to reinsert the string after it had been pulled out, losing the ability to redirect the bronchial blocker if necessary. External reinforcement of the wire allows its reintroduction through the lumen. The OD necessitates a large SLT (at least 8.0 mm) to accommodate the bronchial blocker. The Arndt blocker is available in a 7-F size for adults and in a 5-F pediatric size. A disadvantage of the Arndt blocker is that it is advanced blindly over the FFB into the desired main bronchus. In some cases, the tip of the blocker may get caught at the main carina or at the Murphy eye of the SLT.
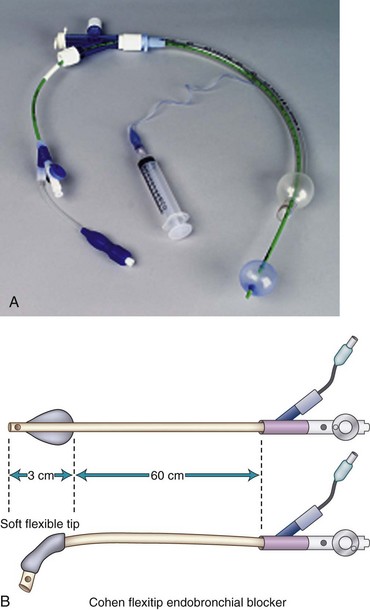
b Cohen Flexitip Endobronchial Blocker
The Cohen Flexitip Endobronchial Blocker (Cook Critical Care) is designed for use through an SLT with the aid of a small-diameter (4.0-mm) FOB (Fig. 26-15).48 The blocker has a rotating wheel that deflects the soft tip by more than 90 degrees and easily directs it into the desired bronchus. The blocker cuff is a high-volume, low-pressure balloon inflated through a 0.4-mm lumen inside the wall of the blocker. Its pear shape provides an adequate seal of the bronchus. It takes 6 to 8 mL of air to seal the bronchus with the cuff. The distinctive blue cuff is easily recognizable by FOB. It is best to inflate the cuff under direct vision by FOB, which is particularly important during right-sided blockade. In this case, the cuff is inflated near the carina, and proper position and cuff inflation are critical. The 9-F blocker has a central main lumen (1.6 mm) that allows limited suctioning of secretions and insufflation of oxygen to the collapsed lung.
c Uniblocker
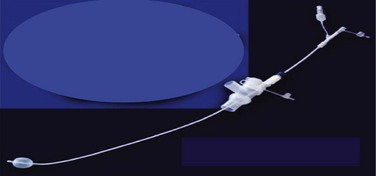
Fuji Systems introduced a 9-F, balloon-tipped, angled blocker with a multiple-port adapter that is essentially the same design as the Univent tube blocker, but it can be used as an independent blocker passed by means of a special connector through a standard ETT (Fig. 26-16). It has a prefixed bend to facilitate insertion into the desired bronchus. This blocker is also available in a 5.0-F size for the pediatric population.
d EZ-Blocker
The latest addition to the endobronchial blocker design is the EZ-Blocker (IQ Medical Ventures, Rotterdam, The Netherlands). It is a 7-F, four-lumen, 75-cm, disposable endobronchial blocker used to facilitate selective lung ventilation (Fig. 26-17). It has a symmetrical, Y-shaped bifurcation, and both branches have an inflatable cuff on each arm and a central lumen. The bifurcation resembles the bifurcation of the trachea. During insertion through a standard ETT, each of the two distal ends is placed into a main stem bronchus. The selected lung is isolated by inflating the blocker’s balloon to the least volume necessary to occlude the main stem under bronchoscopic visualization. This device should offer an advantage during bilateral procedures, because each lung can be deflated without the need for repositioning the blocker. The clinical experience with this device is limited.
The effectiveness of lung isolation with three devices—the left-sided Broncho-Cath DLT, the Univent torque-control blocker, and the wire-guided Arndt—has been compared in a prospective, randomized trial. There was no significant difference in tube malpositions for the three devices, but it took longer to position the Arndt blocker (86 versus 56 seconds) than the left-sided DLT and the Univent. Excluding the time for tube placement, the Arndt group also took longer for the lung to collapse (26 minutes), compared with the DLT group (18 minutes) or the Univent group (19.5 minutes). Unlike the other two groups, most of the Arndt patients required suction to achieve lung collapse. After lung isolation was achieved, overall surgical exposure was rated excellent for the three groups (Fig. 26-18). One minute longer to position a bronchial blocker or 6 minutes longer to collapse the lung with the bronchial blocker is insignificant considering the length of the thoracic procedure. The risk-benefit ratio and the patient safety profile for each patient and the clinical experience of the anesthesiologist should be considered when choosing the method for lung isolation.49,50
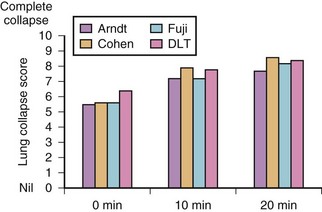
Figure 26-18 Comparison of lung collapse times for three bronchial blockers and a double-lumen tube.
Another study evaluated the use of the Cohen blocker, the Arndt blocker, the Uniblocker, and a DLT in four groups of 26 patients in each group. The investigators found no differences among the groups in the time taken to insert these lung isolation devices or in the quality of the lung collapse.51 The grading was done by the operating surgeons, who were blinded to which device was used. The number of cuff dislocations was higher among the bronchial blocker groups, which was highest with the Arndt blocker, possibly because the study protocol used the elliptical cuff. Regardless of the type of bronchial blocker or DLT selected to provide SLV, the choice of technique depends on the clinical circumstances and the physician’s experience and comfort with a particular device. However, the clinician should not limit his or her practice to the use of only one device but rather be versatile and comfortable in the use of several.
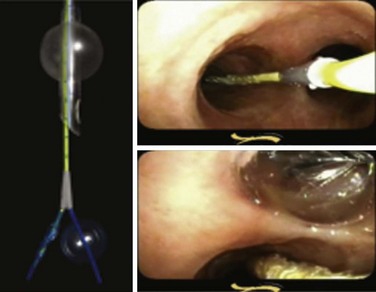
It is possible to perform lung isolation without the use of specialized tubes. In these cases, a bronchial blocking device is inserted through the lumen of an SLT (Fig. 26-19) or outside the SLT between the tracheal cuff and the trachea (Fig. 26-20). Placement is confirmed by FOB.
Any device that has a balloon-tipped catheter can be used as a bronchial blocker (see Fig. 26-19).52 The most common devices used are Fogarty embolectomy catheters and the Cook bronchial blockers.53
3 Para-axial Endotracheal Blockers
The advantage of placing the bronchial blocker device outside the SLT is that this method allows blocker placement with smaller ETTs because the blocker does not share the ETT lumen (see Fig. 26-20). It is often easier to position the bronchial blocker with this method, because the blocker and the FFB do not become caught up with each other within the ETT lumen. Disadvantages of this para-tube technique include the need to perform laryngoscopy to place the bronchial blocker into the trachea, the need to deflate the tracheal cuff while positioning the bronchial blocker, and the potential of rupturing the ETT cuff while manipulating the bronchial blocker. Because of these disadvantages, a coaxial placement may be advised, provided ETT lumen size is not an issue.
V Pediatric Lung Isolation
A Ventilation-Perfusion During Thoracic Surgery
In adults with unilateral lung disease, oxygenation is optimal when the patient is placed in the lateral decubitus position with the healthy lung dependent and the diseased lung nondependent.54 Presumably, this is related to an increase in blood flow to the dependent, healthy lung and a decrease in blood flow to the nondependent, diseased lung resulting from the hydrostatic pressure (or gravitational) gradient between the two lungs. This phenomenon promotes 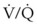 matching in the adult patient who is undergoing thoracic surgery in the lateral decubitus position.
matching in the adult patient who is undergoing thoracic surgery in the lateral decubitus position.
In infants with unilateral lung disease, on the other hand, oxygenation is improved when the healthy lung is nondependent.55 Several factors account for this discrepancy between adults and infants. Infants have a soft, easily compressible rib cage that cannot fully protect the underlying lung. The FRC is closer to the residual volume, making airway closure likely to occur in the dependent lung even during tidal breathing.56 When the adult is placed in the lateral decubitus position, the dependent diaphragm has a mechanical advantage because it is “loaded” by the abdominal hydrostatic pressure gradient. This pressure gradient is reduced in infants, reducing the functional advantage of the dependent diaphragm. The infant’s small size also results in a reduced hydrostatic pressure gradient between the nondependent and dependent lungs. Consequently, the favorable increase in perfusion to the dependent, ventilated lung is reduced in infants.
The infant’s increased O2 requirement, coupled with a small FRC, predisposes to hypoxemia. Infants normally consume 6 to 8 mL of O2/kg/min, compared with a normal O2 consumption in adults of 2 to 3 mL/kg/min.57 For these reasons, infants are at increased risk for significant O2 desaturation during surgery in the lateral decubitus position.
B Indications and Techniques for Single-Lung Ventilation in Infants and Children
Before 1995, almost all thoracic surgery in children was performed by thoracotomy. In most cases, anesthesiologists ventilated both lungs with a conventional ETT, and surgeons retracted the operative lung to gain exposure to the surgical field. During the past decade, the use of video-assisted thoracoscopic surgery (VATS) has dramatically increased in both adults and children. Reported advantages of VATS include smaller chest incisions, reduced postoperative pain, and more rapid postoperative recovery compared with thoracotomy.58–60 Advances in surgical technique as well as technology, including high-resolution microchip cameras and smaller endoscopic instruments, have facilitated the application of VATS in smaller patients.
VATS can be performed while both lungs are being ventilated with the use of CO2 insufflation and placement of a retractor to displace lung tissue in the operative field. However, SLV is extremely desirable during VATS because lung deflation improves visualization of thoracic contents and may reduce lung injury caused by the use of retractors.11 There are several different techniques that can be used for SLV in children.
C Single-Lumen Tube
The simplest means of providing SLV is to intubate the ipsilateral main stem bronchus with a conventional SLT.61 When the left bronchus is to be intubated, the bevel of the ETT is rotated 180 degrees and the head is turned to the right.62 The ETT is advanced into the bronchus until breath sounds on the operative side disappear. An FFB may be passed through, or alongside, the ETT to confirm or guide placement. When a cuffed ETT is used, the distance from the tip of the tube to the distal cuff must be shorter than the length of the bronchus, so that the cuff is not entirely in the bronchus.63 This technique is simple and requires no special equipment other than an FFB. This may be the preferred technique of SLV in emergency situations such as airway hemorrhage or contralateral tension pneumothorax.
Variations of this technique have been described, including intubation of both bronchi independently with small ETTs.64–67 One main stem bronchus is initially intubated with an ETT, after which another ETT is advanced over an FFB into the opposite bronchus.
D Balloon-Tipped Bronchial Blockers
A Fogarty embolectomy catheter or an end-hole, balloon wedge catheter may be used for bronchial blockade to provide SLV.52,68–70 Placement of a Fogarty catheter is facilitated by bending the tip of its stylet toward the bronchus on the operative side. An FFB may be used to reposition the catheter and confirm appropriate placement. When an end-hole catheter is placed outside the ETT, the bronchus on the operative side is initially intubated with an ETT. A guidewire is then advanced into that bronchus through the ETT. The ETT is removed, and the bronchial blocker is advanced over the guidewire into the bronchus. An ETT is then reinserted into the trachea alongside the blocker catheter. The catheter balloon is positioned in the proximal main stem bronchus under fiberoptic visual guidance. With an inflated bronchial blocker balloon, the airway is completely sealed, providing more predictable lung collapse and better operating conditions than with an ETT in the bronchus.
A potential problem with this technique is dislodgment of the bronchial blocker balloon into the trachea. The inflated balloon blocks ventilation to both lungs and prevents collapse of the operated lung. The balloons of most catheters currently used for bronchial blockade have low-volume, high-pressure properties, and overdistention can damage or even rupture the airway.71 One study, however, reported that bronchial blocker cuffs produced lower ratios of cuff to tracheal pressure than DLTs do.72 When closed-tip bronchial blockers are used, the operative lung cannot be suctioned, and CPAP cannot be provided to the operative lung if needed.
Adapters have been used that facilitate ventilation during placement of a bronchial blocker through an indwelling ETT.73,74 A 5-F endobronchial blocker that is suitable for use in children with a multiport adapter and FFB has been described (Cook Critical Care).75 The risk of hypoxemia during blocker placement is diminished, and repositioning of the blocker may be performed with FFB guidance during surgery. Even with an FFB with a 2.2-mm OD, the indwelling ETT must have an ID of 5.0 mm or larger to allow passage of the catheter and FFB. This technique typically is limited to children older than 18 months to 2 years.
E Univent Tube
The Univent tube is a conventional ETT with a second lumen containing a small tube that can be advanced into a bronchus.45,76,77 A balloon located at the distal end of this small tube serves as a blocker. Univent tubes require an FFB for successful placement. Univent tubes are available in sizes as small as 3.5- and 4.5-mm ID for use in children older than 6 years of age.78 Because the blocker tube is firmly attached to the main ETT, displacement of the Univent blocker balloon is less likely than when other bronchial blocker techniques are used. The blocker tube has a small lumen, which allows egress of gas and can be used to insufflate O2 or suction the operated lung.
A disadvantage of the Univent tube is the large amount of cross-sectional area occupied by the blocker channel, especially in the smaller tubes. Smaller Univent tubes have a disproportionately high resistance to gas flow.79 The Univent tube’s blocker balloon has low-volume, high-pressure characteristics, and mucosal injury can occur during normal inflation.80,81
F Double-Lumen Tubes
All DLTs are essentially two tubes of unequal length molded together. The shorter tube ends in the trachea and the longer tube in the bronchus. Marraro described a DLT for infants that consists of two separate uncuffed ETTs of different length attached longitudinally.82 This tube is not available in the United States. DLTs for older children and adults have cuffs located on the tracheal and bronchial lumens. The tracheal cuff, when inflated, allows PPV. The inflated bronchial cuff allows ventilation to be diverted to either or both lungs and protects each lung from contamination from the contralateral side.
DLTs are inserted in children by the same technique as is used in adults.83 The tip of the tube is inserted just beyond the vocal cords, and the stylet is withdrawn. The DLT is rotated 90 degrees to the appropriate side and then advanced into the bronchus. In the adult population, the depth of insertion is directly related to the height of the patient.84 No equivalent measurements are yet available in children. If FOB is to be used to confirm tube placement, an FFB with a small diameter and sufficient length must be available.85
A DLT offers the advantage of ease of insertion as well as the ability to suction and oxygenate the operative lung with CPAP. Left-sided DLTs are preferred to right-sided instruments because of the shorter length of the right main bronchus.19 Right-sided DLTs are more difficult to position accurately because of the greater risk of right upper lobe obstruction.
VII Clinical Pearls
• Lung isolation should be performed by well-trained and experienced practitioners who should understand the significant risks and benefits to such techniques.
• Absolute indications for lung separation include isolation of infection, contamination, or hemorrhage; control of ventilation distribution; and unilateral bronchopulmonary lavage.
• Relative indications for lung separation include surgical exposure and severe hypoxemia related to unilateral lung disease.
• Double-lumen tubes (DLTs) come in sizes 28, 32, 35, 37, 39, and 41 F, and the appropriate length is based on height, build, and airway examination. Positioning should be confirmed by flexible fiberoptic bronchoscopic (FFB) visualization, selective ventilation auscultation, or radiographic studies.
• DLTs are advantageous because they provide the capability of independent bilateral lung ventilation, bronchial suctioning, lung deflation, and stability, but they have the disadvantage of large size, rigidity, and difficulty in management for prolonged ventilation.
• For patients with difficult airways, anatomic anomalies, tracheal obstructions, tracheal-vascular distortions, or need for segmental lung isolation, the use of single-lumen tube (SLT) isolation devices should be strongly considered.
• Disadvantages to SLT isolation techniques include difficulty with lung deflation, difficulty suctioning the isolated lung, difficulty with stability and positioning, and inability to independently ventilate the isolated lung portion.
• Univent tubes are SLTs with an external bronchial blocker attachment; they can be used as an effective device for lung isolation, including large segmental-lobar isolation.
• Bronchial blockers come as stand-alone devices and can be used with SLTs or DLTs that have a low-volume, high-pressure cuff system; they may be used para-axially or axially for whole or segmental lung isolation.
• Pediatric lung isolation techniques are similar, if not identical, to those used for adults, although SLT techniques are often used; however, pediatric anatomy and physiology differs from that of adults in regard to positioning and ventilation.
All references can be found online at expertconsult.com.
5 Brodsky JB, Adkins MO, Gaba DM. Bronchial cuff pressures of double-lumen tubes. Anesth Analg. 1989;69:608–610.
11 Benumof J. Anesthesia for thoracic surgery, ed 2. Philadelphia: Saunders; 1995.
14 Ehrenfeld JM, Walsh JL, Sandberg WS. Right- and left-sided Mallinckrodt double-lumen tubes have identical clinical performance. Anesth Analg. 2008;106:1847–1852.
19 Benumof JL, Partridge BL, Salvatierra C, et al. Margin of safety in positioning modern double-lumen endotracheal tubes. Anesthesiology. 1987;67:729–738.
22 Brodsky JB, Shulman MS, Mark JB. Malposition of left-sided double-lumen endobronchial tubes. Anesthesiology. 1985;62:667–669.
24 Benumof JL. The position of a double-lumen tube should be routinely determined by fiberoptic bronchoscopy. J Cardiothorac Vasc Anesth. 1993;7:513–514.
27 Hurford WE, Alfille PH. A quality improvement study of the placement and complications of double-lumen endobronchial tubes. J Cardiothorac Vasc Anesth. 1993;7:517–520.
45 Kamaya H, Krishna PR. New endotracheal tube (Univent tube) for selective blockade of one lung. Anesthesiology. 1985;63:342–343.
52 Ginsberg RJ. New technique for one-lung anesthesia using an endobronchial blocker. J Thorac Cardiovasc Surg. 1981;82:542–546.
68 Hammer GB, Manos SJ, Smith BM, et al. Single-lung ventilation in pediatric patients. Anesthesiology. 1996;84:1503–1506.
1 Kvetan V, Carlon GC, Howland WS. Acute pulmonary failure in asymmetric lung disease: Approach to management. Crit Care Med. 1982;10:114–118.
2 Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest. 2009;136:1678–1681.
3 Nandkumar S, Desai M, Butani M, et al. Pulmonary alveolar proteinosis with respiratory failure-anaesthetic management of whole lung lavage. Indian J Anaesth. 2009;53:362–366.
4 Webb ST, Evans AJ, Varley AJ, et al. Anaesthesia for serial whole-lung lavage in a patient with severe pulmonary alveolar proteinosis: A case report. J Med Case Rep. 2008;2:360.
5 Brodsky JB, Adkins MO, Gaba DM. Bronchial cuff pressures of double-lumen tubes. Anesth Analg. 1989;69:608–610.
6 Cohen JA, Denisco RA, Richards TS, et al. Hazardous placement of a Robertshaw-type endobronchial tube. Anesth Analg. 1986;65:100–101.
7 Magee PT. Endobronchial cuff pressures of double-lumen tubes. Anesth Analg. 1991;72:265–266.
8 Neto PP. Bronchial cuff pressure of endobronchial double-lumen tubes. Anesth Analg. 1990;71:209.
9 Chow MY, Go MH, Ti LK. Predicting the depth of insertion of left-sided double-lumen endobronchial tubes. J Cardiothorac Vasc Anesth. 2002;16:456.
10 Saito S, Dohi S, Tajima K. Failure of double-lumen endobronchial tube placement: Congenital tracheal stenosis in an adult. Anesthesiology. 1987;66:83–85.
11 Benumof J. Anesthesia for thoracic surgery, ed 2. Philadelphia: Saunders; 1995.
12 Maguire DP, Spiro AW. Bronchial obstruction and hypoxia during one-lung ventilation. Anesthesiology. 1987;66:830–831.
13 Ehrenfeld JM, Mulvoy W, Sandberg WS. Performance comparison of right- and left-sided double-lumen tubes among infrequent users. J Cardiothorac Vasc Anesth. 2010;24:598–601.
14 Ehrenfeld JM, Walsh JL, Sandberg WS. Right- and left-sided Mallinckrodt double-lumen tubes have identical clinical performance. Anesth Analg. 2008;106:1847–1852.
15 Brodsky JB, Benumof JL, Ehrenwerth J, et al. Depth of placement of left double-lumen endobronchial tubes. Anesth Analg. 1991;73:570–572.
16 Iwamoto T, Takasugi Y, Hiramatsu K, et al. Three-dimensional CT image analysis of a tracheal bronchus in a patient undergoing cardiac surgery with one-lung ventilation. J Anesth. 2009;23:260–265.
17 Hagihira S, Takashina M, Mashimo T. Application of a newly designed right-sided, double-lumen endobronchial tube in patients with a very short right mainstem bronchus. Anesthesiology. 2008;109:565–568.
18 Hansen TB, Watson CB. Tracheobronchial trauma secondary to a Carlens tube. Presented at the Society of Cardiovascular Anesthesiologists 5th Annual Meeting, San Diego, CA. 1983.
19 Benumof JL, Partridge BL, Salvatierra C, et al. Margin of safety in positioning modern double-lumen endotracheal tubes. Anesthesiology. 1987;67:729–738.
20 Amar D, Desiderio DP, Heerdt PM, et al. Practice patterns in choice of left double-lumen tube size for thoracic surgery. Anesth Analg. 2008;106:379–383.
21 Bensghir M, Alaoui H, Azendour H, et al. [Faster double-lumen tube intubation with the videolaryngoscope than with a standard laryngoscope]. Can J Anaesth. 2010;57:980–984.
22 Brodsky JB, Shulman MS, Mark JB. Malposition of left-sided double-lumen endobronchial tubes. Anesthesiology. 1985;62:667–669.
23 Burk WJ, 3rd. Should a fiberoptic bronchoscope be routinely used to position a double-lumen tube? Anesthesiology. 1988;68:826.
24 Benumof JL. The position of a double-lumen tube should be routinely determined by fiberoptic bronchoscopy. J Cardiothorac Vasc Anesth. 1993;7:513–514.
25 Cohen E, Neustein SM, Goldofsky S, et al. Incidence of malposition of polyvinylchloride and red rubber left-sided double-lumen tubes and clinical sequelae. J Cardiothorac Vasc Anesth. 1995;9:122–127.
26 Alliaume B, Coddens J, Deloof T. Reliability of auscultation in positioning of double-lumen endobronchial tubes. Can J Anaesth. 1992;39:687–690.
27 Hurford WE, Alfille PH. A quality improvement study of the placement and complications of double-lumen endobronchial tubes. J Cardiothorac Vasc Anesth. 1993;7:517–520.
28 Lewis JW, Jr., Serwin JP, Gabriel FS, et al. The utility of a double-lumen tube for one-lung ventilation in a variety of noncardiac thoracic surgical procedures. J Cardiothorac Vasc Anesth. 1992;6:705–710.
29 Campos JH, Hallam EA, Ueda K. Training in placement of the left-sided double-lumen tube among non-thoracic anaesthesiologists: Intubation model simulator versus computer-based digital video disc, a randomised controlled trial. Eur J Anaesth. 2011;28:169–174.
30 McKenna MJ, Wilson RS, Botelho RJ. Right upper lobe obstruction with right-sided double-lumen endobronchial tubes: A comparison of two tube types. J Cardiothorac Anesth. 1988;2:734–740.
31 Smith GB, Hirsch NP, Ehrenwerth J. Placement of double-lumen endobronchial tubes: Correlation between clinical impressions and bronchoscopic findings. Br J Anaesth. 1986;58:1317–1320.
32 Benumof JL, Partridge BL, Salvatierra C, et al. Margin of safety in positioning modern double-lumen endotracheal tubes. Anesthesiology. 1987;67:729.
33 Thomas V, Neustein SM. Tracheal laceration after the use of an airway exchange catheter for double-lumen tube placement. J Cardiothorac Vasc Anesth. 2007;21:718.
34 Wagner DL, Gammage GW, Wong ML. Tracheal rupture following the insertion of a disposable double-lumen endotracheal tube. Anesthesiology. 1985;63:698.
35 Heike Knoll H, Stephan Ziegeler S, Jan-Uwe Schreiber JU, et al. Airway injuries after one-lung ventilation: A comparison between double-lumen tube and endobronchial blocker: A randomized, prospective, controlled trial. Anesthesiology. 2006;105:471.
36 Zinga E, Dangoisse M, Lechat JP. Tracheal perforation following double-lumen intubation: A case report. Acta Anaesthesiol Belg. 2010;61:71–74.
37 Read RC, Friday CD, Eason CN. Prospective study of the Robertshaw endobronchial catheter in thoracic surgery. Ann Thorac Surg. 1977;24:156–161.
38 Dryden GE. Circulatory collapse after pneumonectomy (an unusual complication from the use of a Carlens catheter): Case report. Anesth Analg. 1977;56:451–452.
39 Guernelli N, Bragaglia RB, Briccoli A, et al. Tracheobronchial ruptures due to cuffed Carlens tubes. Ann Thorac Surg. 1979;28:66–67.
40 Newman RW, Finer GE, Downs JE. Routine use of the Carlens double-lumen endobronchial catheter: An experimental and clinical study. J Thorac Cardiovasc Surg. 1961;42:327–339.
41 Inoue H, Shohtsu A, Ogawa J, et al. New device for one-lung anesthesia: Endotracheal tube with movable blocker. J Thorac Cardiovasc Surg. 1982;83:940–941.
42 Ho AM, Ng SK, Tsang KH, et al. A technique that may improve the reliability of endobronchial blocker positioning during adult one-lung anaesthesia. Anaesth Intensive Care. 2009;37:1012–1016.
43 Murakawa T, Ito N, Fukami T, et al. Application of lobe-selective bronchial blockade against airway bleeding. Asian Cardiovasc Thorac Ann. 2010;18:483–485.
44 Campos JH. Update on selective lobar blockade during pulmonary resections. Curr Opin Anaesthesiol. 2009;22:18–22.
45 Kamaya H, Krishna PR. New endotracheal tube (Univent tube) for selective blockade of one lung. Anesthesiology. 1985;63:342–343.
46 MacGillivray RG. Evaluation of a new tracheal tube with a movable bronchus blocker. Anaesthesia. 1988;43:687–689.
47 Neustein SM. The use of bronchial blockers for providing one-lung ventilation. J Cardiothorac Vasc Anesth. 2009;23:860–868.
48 Cohen E. The Cohen Flextip Endobronchial Blocker: an alternative to a double lumen tube. Anesth Analg. 2005;101:1877–1879.
49 Campos JH, Kernstine KH. A comparison of a left-sided Broncho-Cath with the torque control blocker Univent and the wire-guided blocker. Anesth Analg. 2003;96:283–289.
50 Campos JH. Progress in lung separation. Thorac Surg Clin. 2005;15:71–83.
51 Narayanaswamy M, McRae K, Slinger P, et al. Choosing a lung isolation device for thoracic surgery: a randomized trial of three bronchial blockers versus double-lumen tubes. Anesth Analg. 2009;108:1097–1101.
52 Ginsberg RJ. New technique for one-lung anesthesia using an endobronchial blocker. J Thorac Cardiovasc Surg. 1981;82:542–546.
53 Vale R. Selective bronchial blocking in a small child: Case report. Br J Anaesth. 1969;41:453–454.
54 Remolina C, Khan AU, Santiago TV, et al. Positional hypoxemia in unilateral lung disease. N Engl J Med. 1981;304:523–525.
55 Heaf DP, Helms P, Gordon I, et al. Postural effects on gas exchange in infants. N Engl J Med. 1983;308:1505–1508.
56 Mansell A, Bryan C, Levison H. Airway closure in children. J Appl Physiol. 1972;33:711–714.
57 Dawes GS. Foetal and neonatal physiology: A comparative study of the changes at birth. Chicago: Year Book Medical Publishers; 1968.
58 Angelillo Mackinlay TA, Lyons GA, et al. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg. 1996;61:1626–1630.
59 Mouroux J, Clary-Meinesz C, Padovani B, et al. Efficacy and safety of videothoracoscopic lung biopsy in the diagnosis of interstitial lung disease. Eur J Cardiothorac Surg. 1997;11:22–24. 25–26
60 Weatherford DA, Stephenson JE, Taylor SM, et al. Thoracoscopy versus thoracotomy: Indications and advantages. Am Surg. 1995;61:83–86.
61 Rowe R, Andropoulos D, Heard M, et al. Anesthetic management of pediatric patients undergoing thoracoscopy. J Cardiothorac Vasc Anesth. 1994;8:563–566.
62 Kubota H, Kubota Y, Toyoda Y, et al. Selective blind endobronchial intubation in children and adults. Anesthesiology. 1987;67:587–589.
63 Lammers CR, Hammer GB, Brodsky JB, et al. Failure to separate and isolate the lungs with an endotracheal tube positioned in the bronchus. Anesth Analg. 1997;85:946–947.
64 Cullum AR, English IC, Branthwaite MA. Endobronchial intubation in infancy. Anaesthesia. 1973;28:66–70.
65 McLellan I. Endobronchial intubation in children. Anaesthesia. 1974;29:757–758.
66 Watson CB, Bowe EA, Burk W. One-lung anesthesia for pediatric thoracic surgery: A new use for the fiberoptic bronchoscope. Anesthesiology. 1982;56:314–315.
67 Yeh TF, Pildes RS, Salem MR. Treatment of persistent tension pneumothorax in a neonate by selective bronchial intubation. Anesthesiology. 1978;49:37–38.
68 Hammer GB, Manos SJ, Smith BM, et al. Single-lung ventilation in pediatric patients. Anesthesiology. 1996;84:1503–1506.
69 Lin YC, Hackel A. Paediatric selective bronchial blocker. Paediatr Anaesth. 1994;4:391–392.
70 Turner MW, Buchanan CC, Brown SW. Paediatric one lung ventilation in the prone position. Paediatr Anaesth. 1997;7:427–429.
71 Borchardt RA, LaQuaglia MP, McDowall RH, et al. Bronchial injury during lung isolation in a pediatric patient. Anesth Analg. 1998;87:324–325.
72 Guyton DC, Besselievre TR, Devidas M, et al. A comparison of two different bronchial cuff designs and four different bronchial cuff inflation methods. J Cardiothorac Vasc Anesth. 1997;11:599–603.
73 Arndt GA, DeLessio ST, Kranner PW, et al. One-lung ventilation when intubation is difficult: Presentation of a new endobronchial blocker. Acta Anaesthesiol Scand. 1999;43:356–358.
74 Takahashi M, Horinouchi T, Kato M, et al. Double-access-port endotracheal tube for selective lung ventilation in pediatric patients. Anesthesiology. 2000;93:308–309.
75 Hammer GB, Harrison TK, Vricella LA, et al. Single lung ventilation in children using a new paediatric bronchial blocker. Paediatr Anaesth. 2002;12:69–72.
76 Gayes JM. Pro: One-lung ventilation is best accomplished with the Univent endotracheal tube. J Cardiothorac Vasc Anesth. 1993;7:103–107.
77 Karwande SV. A new tube for single lung ventilation. Chest. 1987;92:761–763.
78 Hammer GB, Brodsky JB, Redpath JH, et al. The Univent tube for single-lung ventilation in paediatric patients. Paediatr Anaesth. 1998;8:55–57.
79 Slinger PD, Lesiuk L. Flow resistances of disposable double-lumen, single-lumen, and Univent tubes. J Cardiothorac Vasc Anesth. 1998;12:142–144.
80 Benumof JL, Gaughan SD, Ozaki G. The relationship among bronchial blocker cuff inflation volume, proximal airway pressure, and seal of the bronchial blocker cuff. J Cardiothorac Vasc Anesth. 1992;6:404–408.
81 Kelley JG, Gaba DM, Brodsky JB. Bronchial cuff pressures of two tubes used in thoracic surgery. J Cardiothorac Vasc Anesth. 1992;6:190–192.
82 Marraro G. Paediatric selective bronchial blocker. Paediatr Anaesth. 1995;5:273.
83 Brodsky JB, Mark JB. A simple technique for accurate placement of double-lumen endobronchial tubes. Anesth Rev. 1983;10:26–30.
84 Brodsky JB, Macario A, Mark JB. Tracheal diameter predicts double-lumen tube size: A method for selecting left double-lumen tubes. Anesth Analg. 1996;82:861–864.
85 Slinger PD. Fiberoptic bronchoscopic positioning of double-lumen tubes. J Cardiothorac Anesth. 1989;3:486–496.

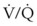 ) matching may occur. Excess positive end-expiratory pressure (PEEP), airway pressures, hypoxia, hypercapnia, and hypovolemia may contribute to an increase in PVR of the dependent lung, thereby increasing the shunt fraction. Improvement of the shunt fraction can be accomplished by decreasing blood flow or supplying O2 to the nondependent lung.
) matching may occur. Excess positive end-expiratory pressure (PEEP), airway pressures, hypoxia, hypercapnia, and hypovolemia may contribute to an increase in PVR of the dependent lung, thereby increasing the shunt fraction. Improvement of the shunt fraction can be accomplished by decreasing blood flow or supplying O2 to the nondependent lung.
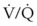 mismatch. General anesthesia, neuromuscular blockade, and mechanical ventilation cause a decrease in functional residual capacity (FRC) of both lungs. Compression of the dependent lung in the lateral decubitus position can cause atelectasis. Surgical retraction or SLV, or both, can result in collapse of the operative lung. Hypoxic pulmonary vasoconstriction, which acts to divert blood flow away from the underventilated lung, thereby minimizing the
mismatch. General anesthesia, neuromuscular blockade, and mechanical ventilation cause a decrease in functional residual capacity (FRC) of both lungs. Compression of the dependent lung in the lateral decubitus position can cause atelectasis. Surgical retraction or SLV, or both, can result in collapse of the operative lung. Hypoxic pulmonary vasoconstriction, which acts to divert blood flow away from the underventilated lung, thereby minimizing the 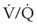 mismatch, may be diminished by inhalational anesthetic agents and other vasodilating drugs. These factors apply to infants, children, and adults. The overall effect of the lateral decubitus position on
mismatch, may be diminished by inhalational anesthetic agents and other vasodilating drugs. These factors apply to infants, children, and adults. The overall effect of the lateral decubitus position on 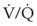 mismatching is different in infants than in older children and adults.
mismatching is different in infants than in older children and adults.