Chapter 92 Segment-oriented anatomic liver resections
Overview
Decreased intraoperative blood loss and preservation of parenchyma are key contributors in recent advances in liver surgery that have resulted in reduced morbidity and mortality in liver resection. The understanding of the segmental anatomy of the liver has been pivotal in the evolution of a safe liver surgery (Scheele et al, 1995). Segmental liver resection offers maximum preservation of liver parenchyma with minimal blood loss and without compromising oncologic safety (Agrawal & Belghiti, 2011; Billingsley et al, 1998; Bismuth et al, 1988; Machado et al, 2003; Polk et al, 1995). The ability to resect one or more segments, rather than the entire lobe, allows parenchymal preservation in patients with diseased parenchyma or in re-resection patients with limited residual volume. Segmental vascular inflow control facilitates the resection by precisely mapping the transection plane. In addition, anatomic resections that involve the removal of a hepatic segment confined by tumor-bearing portal tributaries for the eradication of intrahepatic metastases in the vicinity of the primary tumor represent an oncologic approach to liver resection for some malignant tumors (Agrawal & Belghiti, 2011; Liau et al, 2004).
Anatomy and Terminology
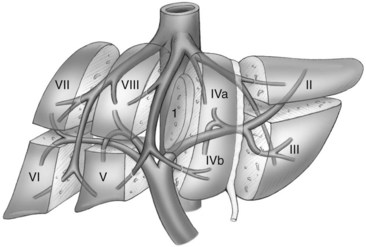
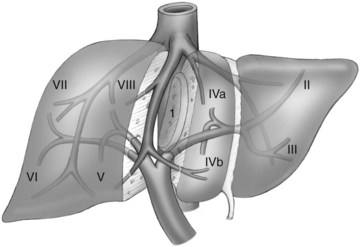
The current understanding of the segmental anatomy of the liver has come from the original 1952 descriptions of Claude Couinaud (1952a, 1952b, 1956). Based on his analysis of vascular and biliary casts of the liver, Couinaud determined that the human liver consists of eight segments, each with its own portal triad—vein, hepatic artery, and hepatic duct—and hepatic venous outflow. Subsequently it has been shown that each segment can be resected independently (Bismuth et al, 1982). These segments have evolved to become the standard for hepatic nomenclature. This so-called Brisbane terminology (Pang, 2002) eliminates confusing lobes and sectors used in the American, European, and Japanese descriptions of liver anatomy. The terms hemiliver (first-order division), section (second-order division), and segment (third-order division) are not interchangeable; these provide universal terminology for better communication among liver surgeons.
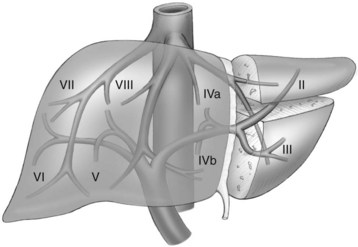
The first-order divisions are right liver (segments V through VIII) and left liver (segments I through IV), or hemiliver, the boundary of which lies along the Cantlie line marked by the path of the middle hepatic vein (MHV), from the middle of the gallbladder fossa to its termination in the inferior vena cava (IVC) (Fig. 92.1). The second-order division into liver sections is based upon hepatic arterial supply and biliary drainage. The sections are derived from the primary divisions of the major right and left portal triads. The right hemiliver is divided into sections known as the right anterior (segments V and VIII) and right posterior (segments VI and VII), separated by the right hepatic vein (RHV). The left hemiliver is divided into left lateral (segments II and III) and left medial (segments IVa and IVb) sections by the umbilical fissure and falciform ligament. Together segments II and III are often erroneously referred to as the left lateral segment.
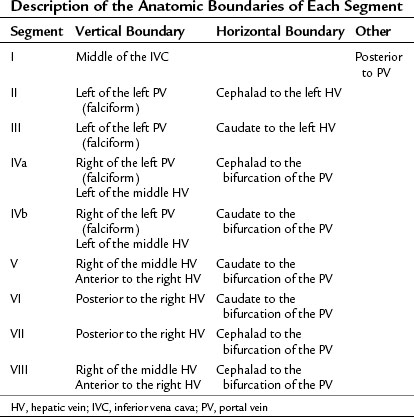
The third-order division, segments I through VIII, is defined by hepatic arterial supply and biliary drainage. The axial plane is at the level of the intersection of the hepatic veins and the axial plane of the bifurcation of the portal vein (Table 92.1 and Fig. 92.2). When considering a segmental resection, it is important to identify the common anomalies that may determine resectability. The biliary and arterial anomalies are the most common: about 30% of patients have a major arterial anomaly, and up to 50% have nonstandard biliary anatomy.
Patient Selection
A liver resection based on the segmental anatomy should be the first consideration for any liver resection. Excluding the fenestration of liver cysts or small “wedge excisions” of superficial lesions, an understanding of the patient’s segmental liver anatomy is essential to a safe, controlled resection. This technique is applicable for all types of livers and indications for resection (Hasegawa et al, 2005).
Achieving balance between parenchymal sparing and radical oncologic clearance is of the utmost importance in hepatocellular carcinoma (HCC) patients with cirrhosis. Segmental liver resection forms a bridge between major hepatectomy and nonanatomic resection to preserve maximum liver volume and prevent postoperative liver failure (Agrawal & Belghiti, 2011; Wakai et al, 2007). Replacement with nonanatomic resection should be done only when the anatomy is unsuitable, or when the residual liver would be at high risk for complications. Segmental liver resection is preferred in patients who have colorectal metastasis with multifocal lesions and are undergoing preoperative chemotherapy, which is frequently associated with steatosis and hepatic cirrhosis (DeMatteo et al, 2000).
General Operative Principles
Preoperative Assessment and Anesthesia
The preoperative assessment and preparation of the patient for a liver resection have been described in Chapter 2. Comorbidities should be identified and optimized. Careful assessment of liver function is important, particularly in those with intrinsic liver disease. Preoperative decompression of an obstructed biliary tree should be considered for improving postoperative liver function (Belghiti & Ogata, 2005). Volumetric analysis should be performed in patients with potentially marginal residual liver size and function. Preoperative portal vein embolization (PVE) may facilitate the safety of a complex or extended liver resection by inducing regeneration of the potential contralateral remnant liver segments (Abulkhir et al, 2008). Particular attention should be paid to the reduction of the central venous pressure during transection to reduce blood loss, and measures to prevent hypothermia are also important. The appropriate prophylaxis of infections and venous thromboembolism is required.
Intraoperative Assessment
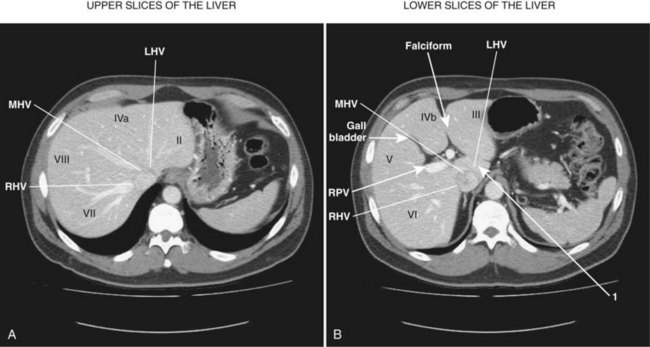
Intraoperative assessment of the liver requires correlation of the findings of inspection and palpation of the mobilized liver with those of the preoperative imaging. Intraoperative US has been advocated to localize the lesion and further stage the proposed remnant liver (Makuuchi et al, 1991); however, with the increased precision of preoperative imaging, intraoperative ultrasound (IOUS) seldom alters the procedure (Jarnagin et al, 2001). IOUS of the liver may be valuable to identify venous tumor thrombus and to assess the proposed plane of transection and its relationship with major hepatic veins and portal triads.
Recently, systematic segmentectomy and subsegmentectomy by IOUS-guided finger compression has been described (Torzilli et al, 2010). This technique can be applied in each segment of liver, as long as the thickness of the parenchyma and the anatomy of liver are suitable. IOUS-guided finger compression of the vascular pedicle feeding the tumor at the level closest to it results in a demarcation area, allowing oncologic resection.
Transection Techniques
There are at least two distinct philosophies of liver transection, which result in distinct surgical techniques and surgical styles. The first is that blood loss from the transected liver is minimized by speed, external compression, vascular occlusion (outflow and/or inflow) (Bismuth et al, 1989; Stephen et al, 1996), and the use of surgical interventions to stop bleeding using cautery, sutures, and tissue glues. The second is that blood loss is best minimized by prevention of injury to vascular structures, using transection techniques that dissect out structures from the surrounding parenchyma as understood and anticipated by preoperative imaging and planning. Use of the Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab, Boulder, CO), which reduces blood loss better than the clamp-crushing technique (Fan et al, 1996), has become the standard technique of liver transection even in cirrhotic liver (Takayama et al, 2001).
More recently, a third technique has been described that reflects a philosophy of prevention of bleeding that uses destructive hemostatic control of the parenchyma before transection (Ayav et al, 2007; Curro et al, 2008). We prefer the second approach, although clamp crushing, the conventional method of liver transection, is still used in some centers (Imamura et al, 2003; Jarnagin et al, 2002; Lin, 1974). Surgical techniques that facilitate a precise, controlled transection of liver parenchyma and allow the dissection of intrahepatic structures are the Helix Hydro-Jet dissector (ERBE USA, Marietta, GA) (Baer et al, 1991) and the CUSA dissector (Little & Hollands, 1991). Each provides selective destruction of liver parenchyma with relative sparing of denser fibrotic tissue, such as hepatic veins and portal triads. Inflow and outflow vascular occlusion may be added to these techniques for better hemostasis. Because no evidence clearly supports the superiority of any one technique (Clavien et al, 2003), the transection method for any particular operation should be dependent on local expertise.
Techniques that achieve destructive control of the parenchyma and any crossing structures before division include linear cutting staplers, in-line radiofrequency ablation (Habib; Angiodynamics, Latham, NY), and bipolar cautery (Gyrus, Gyrus ACMI, Southborough, MA; and LigaSure, Covidien, Boulder, CO). In-line radiofrequency ablation (RFA) allows surgeons to perform minor and major hepatectomies with minimal blood loss, low blood transfusion requirement, and reduced mortality and morbidity (Ayav et al, 2008); however, this device is seldom used in tertiary reference centers because of concerns about the preservation of venous drainage of the remnant liver and the risk of postoperative bile leak and necrosis (Kim et al, 2003; Lupo et al, 2007). The role of this technology is probably limited to segmental or wedge excision because of the potential risk of bile duct injury, when using this instrument near the liver hilum, and its inability to control bleeding from large venous branches.
Pretransection vascular control is used by many surgeons with oncologic, anatomic delimitation and hemostasis (Bismuth et al, 1989; Stephen et al, 1996). Early occlusion of the hepatic venous outflow of any resected segments may reduce the risk of venous tumor emboli. Occlusion of the hepatic artery (HA) and portal vein (PV) to those segments being resected facilitates the procedure by defining the line of division between ischemic segments to be removed and the well-perfused remnant liver for reduction of blood loss. Pretransection occlusion of the inflow of the segments to be resected before outflow results in better hemostasis. Occlusion of the inflow to sectors of the right hemiliver (VI and VII and V and VIII) or to the segments of the left lobe (II, III, IVa, and IVb) can be performed either by dissection and division of the artery and vein outside the liver, leaving the transection of the hepatic duct and remaining hilar plate for later in the procedure (Figueras et al, 2003), or by the glissonian technique (Launois & Jamieson, 1992), in which the sectoral or segmental pedicle is encircled, using either an anterior or posterior intrahepatic approach; this is done by incising the reflection of the Glisson capsule onto the portal structures to drop the hilar plate off the liver parenchyma, thereby encircling the pedicle. The duct, artery, and vein can be transected en masse either by suture or with a linear stapler.
Procedures
Monosegment and Bisegment Resections
Segment III or III Resection
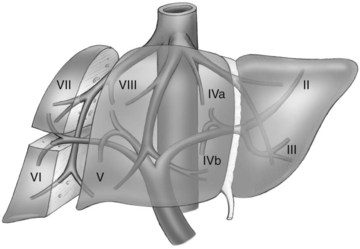
Given the discrepancy between portal venous supply and biliary drainage, and also given the difficulty of preserving left hepatic venous drainage, the combined segment II and III resection (left lateral sectionectomy; Fig. 92.3) is safer and more feasible than isolated resection of segments II or III. When either of these segmentectomies is being planned, the plane of resection is by either side of the LHV (left and posterior for segment II resection, right and anterior for segment III). The inflow is approached on the left side of the falciform ligament by incising the peritoneal reflection onto the liver and identifying either the segment II or III portal pedicles. Once the inflow is divided, the line of parenchymal transection becomes prominent between the ischemic segment to be resected and the normally perfused residual segment.
Combined Resection of Segments II and III: Left Lateral Sectionectomy
The left lateral section is mobilized by division of the falciform ligament and the left triangular ligament (Fig. 92.4). The extrahepatic portion of the left hepatic vein can be lengthened by dividing the fibrous tissue to the left of the IVC down to the level of the fissure for the ligamentum venosum, which is then ligated and divided as it enters the posterior aspect of the LHV; this allows encircling of this vein if the joining with the MHV is not intraparenchymal. Otherwise, the isolation of the LHV is delayed until completion of the parenchymal transection.
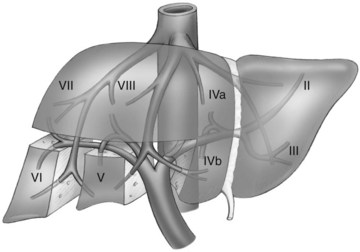
Segment VI or VII Resection
The right posterior sectoral pedicle can be identified in the incisura dextra of Ganz, which corresponds to the horizontal fissure lateral to the gallbladder fossa. The segment VI/VII branch of the right HA can usually be isolated extraparenchymally; however, the segment VI/VII portal branch, which corresponds to the posterior division of the right portal branch, is more difficult to isolate. Therefore the pedicle for an isolated resection of segment VI or VII is usually approached within the liver during parenchymal transection (Fig. 92.5). A resection of segment VI or VII requires complete mobilization of the right lobe of the liver with division of the segment VI and VII hepatic veins, when present, to the IVC.
Segment VI Resection
Isolated segment VI resection (Figs. 92.5 and 92.6) commences with an oblique transection line along the incisura dextra in the inferior surface of the liver and anteriorly halfway toward the IVC, following the posterior side of the RHV. The horizontal plane of resection is at the level of the PV bifurcation. The descending branches of the right posterior sectoral portal triad are encountered about two thirds of the way through the dissection. The resection is completed by taking a horizontal transection plane through the posterior surface of the liver lateral to the right hepatic vein.
Segment VII Resection
An isolated segment VII resection (see Fig. 92.5) commences with the horizontal transection line just above the bifurcation of the portal vein; this line is congruous with the horizontal resection line for a segment VI resection. The resection continues medially to the lateral margin of the RHV. During the horizontal resection, the ascending right posterior sectoral branches can be isolated and divided, which will allow for demarcation of the medial aspect of the resection. The vertical transection line runs obliquely through the liver lateral and posterior to the RHV.
Combined Resection of Segments VI and VII
This right posterior sectorectomy (see Fig. 92.5) is a procedure often considered as an alternative to a right hepatectomy, especially in patients with diseased parenchyma. Identification of the right posterior sectoral pedicle may be possible from outside the liver. Early division of the inflow facilitates the identification of the transection margins. Otherwise, the segment VI and VII HA and PV may be divided separately, leaving the transection of the VI and VII duct and plate for later, during the parenchymal transection. The plane of transection posterior to the RHV is coronal and results in a relatively large surface area of cut parenchyma, which requires specific attention for hemostasis and biliostasis.
Combined Resection of Segments V and VI
Resection of these two segments is very commonly performed. The vertical plane of transection is along the Cantlie line on the right side of the MHV from the edge of the liver to halfway up toward the IVC (see Fig. 92.6). The horizontal plane of transection is at the level of the PV. The portal pedicles to segments V and VI are usually divided during the parenchymal transection, which requires full mobilization of the right lobe of the liver from the diaphragm with division of segment VI and most, if not all, of the segment VII hepatic veins on the anterior surface of the IVC. The transection starts in the middle of the gallbladder fossa and extends along the Cantlie line up to the level of the right portal pedicle. Then with a dissection to the left, the horizontal plane of transection is developed from the top of the vertical plane. The segment V portal pedicle may be identified at the base and divided, followed by division of the segment VI pedicle posteriorly. From right to left, the horizontal dissection plane reaches the RHV.
Three or More Segmental Resections
Resection of Segments V Through VIII: Right Hepatectomy
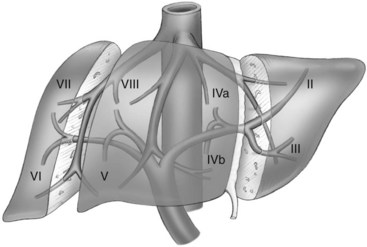
Right hepatectomy is a very well-standardized procedure, consisting of resection of liver parenchyma on the right side of the Cantlie line in the right side of the MHV (see Fig. 92.4). The inclusion of the MHV corresponds to an extended right hepatectomy. Inflow and outflow control prior to transection may be appropriate and can be accomplished in many ways, and parenchymal resection can be performed with or without an intermittent Pringle maneuver. Mobilization commences by dividing the falciform ligament as it triangulates onto the IVC. The right middle groove, between the right hepatic and middle hepatic veins, is developed taking care not to injure a small tributary that may empty directly into the IVC from segment VIII.
Resection of Segments II to IV: Left Hepatectomy
A left hepatectomy consists of resection of segments II, III, IVa, and IVb along the the Cantlie line to the left of the MHV (see Fig. 92.4). An extended left hepatectomy includes the MHV and/or segment I (caudate lobe). Inflow and outflow control prior to transection may be appropriate and can be accomplished in many ways, and parenchymal resection can be performed with or without an intermittent Pringle maneuver. Mobilization commences by dividing the falciform ligament as it triangulates onto the IVC. The plane between the LHV and MHV may be developed anterior to the IVC; however, the LHV and MHVs may join within the substance of the liver, and the left vein may not be able to be encircled outside the liver. To encircle the LHV, the ligamentum venosum is divided within its fissure to allow access to the posterior aspect of the LHV. Alternatively, the LHV may be divided at the end of the parenchymal transection, and control of vascular inflow may be appropriate. Using the glissonian approach, the left portal pedicle is encircled, and the pedicle may be transected en masse using an Endo GIA stapler. Alternatively, the branches of the left HA and the left PV may be divided outside the liver, leaving the left hepatic duct and plate to be divided late during the parenchymal transection.
Resection of Segments IV, V, and VIII: Mesohepatectomy
This resection is very uncommonly performed, and it is indicated primarily for large central tumors where a formal extended right or left hepatectomy will not leave enough residual liver. The left-side plane of transection is to the right of the falciform ligament and the umbilical fissure, and the right-side plane is to the left side of the RHV (Fig. 92.7). The coronal transection plane is above the hilum and anterior to the right posterior sectoral portal pedicle.
Abulkhir A, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57.
Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656-665.
Ayav A, et al. Bloodless liver resection using radiofrequency energy. Dig Surg. 2007;24:314-317.
Ayav A, et al. Liver resection with a new multiprobe bipolar radiofrequency device. Arch Surg. 2008;143:396-401.
Baer HU, et al. New water-jet dissector: initial experience in hepatic surgery. Br J Surg. 1991;78:502-503.
Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford). 2005;7:252-253.
Billingsley KG, et al. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998;187:471-481.
Bismuth H, et al. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13-19.
Bismuth H, et al. Segmental surgery of the liver. Ann Surg. 1988;20:291-310.
Bismuth H, Houssin D, Castaing D. Major and minor segmentectomies “reglees” in liver surgery. World J Surg. 1982;6(1):10-24.
Clavien PA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843-850.
Couinaud C. Segmental and lobar left hepatectomies. J Chir (Paris). 1952;68:821-839.
Couinaud C. Segmental and lobar left hepatectomies: studies on anatomical conditions. J Chir (Paris). 1952;68:697-715.
Couinaud C. Contribution of anatomical research to liver surgery. Fr Med. 1956;19:5-12.
Curro G, et al. Radiofrequency-assisted liver resection in patients with hepatocellular carcinoma and cirrhosis: preliminary results. Transplant Proc. 2008;40:3523-3525.
DeMatteo RP, et al. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178-184.
Fan ST, et al. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117-120.
Figueras J, et al. Hilar dissection versus the “glissonean” approach and stapling of the pedicle for major hepatectomies: a prospective, randomized trial. Ann Surg. 2003;238:111-119.
Hasegawa K, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259.
Imamura H, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206.
Jarnagin WR, et al. What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J Am Coll Surg. 2001;192:577-583.
Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406.
Kim J, et al. Increased biliary fistulas after liver resection with the Harmonic Scalpel. Am Surg. 2003;69:815-819.
Launois B, Jamieson GG. The importance of Glisson’s capsule and its sheaths in the intrahepatic approach to resection of the liver. Surg Gynecol Obstet. 1992;174:7-10.
Liau KH, et al. Segment-oriented approach to liver resection. Surg Clin North Am. 2004;84:543-561.
Lin TY. A simplified technique for hepatic resection: the crush method. Ann Surg. 1974;180:285-290.
Little JM, Hollands MJ. Impact of the CUSA and operative ultrasound on hepatic resection. HPB Surg. 1991;3:271-277.
Lupo L, et al. Randomized clinical trial of radiofrequency-assisted versus clamp-crushing liver resection. Br J Surg. 2007;94:287-291.
Machado MA, et al. A standardized technique for right segmental liver resections. Arch Surg. 2003;138:918-920.
Makuuchi M, et al. The value of ultrasonography for hepatic surgery. Hepatogastroenterology. 1991;38:64-70.
Polk W, et al. A technique for the use of cryosurgery to assist hepatic resection. J Am Coll Surg. 1995;180(2):171-176.
Scheele J, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59-71.
Stephen MS, et al. Hepatic resection with vascular isolation and routine supraceliac aortic clamping. Am J Surg. 1996;171:351-355.
Takayama T, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928.
Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2002;2:333-339. HPB (Oxford) 4:99-100 2000
Torzilli G, et al. Anatomical segmental and subsegmental resection of the liver for hepatocellular carcinoma: a new approach by means of ultrasound-guided vessel compression. Ann Surg. 2010;251:229-235.
Wakai T, et al. Anatomic resection independently improves long-term survival in patients with T1-T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1356-1365.