CHAPTER 242 Secondary Procedures for Brachial Plexus Injuries
So-called secondary operations are performed in situations in which additional function can be augmented or provided by performing muscle, tendon, bone, or other soft tissue reconstruction. These procedures may be performed in patients in whom there has been a delay between development of the lesion and initial consultation, when nerve reconstruction was deemed too late to expect a reasonable functional outcome, or in those who may have undergone previous procedures such as neurorrhaphy, nerve grafting, or nerve transfer and recovery has been less than satisfactory. Unlike primary operations dealing with nerve and muscle end-organs, which are time sensitive for recovery, secondary procedures can be performed at any time after an injury, assuming that the joints are supple. Because of the magnitude of brachial plexus injury, oftentimes only a combination of nerve repair and secondary procedures is used to maximize the patient’s functional use of a paralyzed limb. A 26% rate of secondary procedures has been reported in a series of 362 brachial plexus patients.1,2
Secondary procedures may address form, function, and pain. They are undertaken to achieve the following major goals: (1) active control of the shoulder, (2) reestablishment of useful elbow flexion, (3) stabilization of the wrist, and (4) improvement in hand function.2,3 When planning secondary operations, the fundamental question is which function the patient needs most in activities of daily living. The possibilities and potential use of secondary procedures should be discussed with the patient and realistic goals set forth. The most important factor in producing a successful result from a secondary operation is a cooperative and well-informed patient who understands the goals of the operation or operations and will work hard during rehabilitation to obtain the best result possible.
Tendon Transfer
General Principles
Other Considerations
In addition, there are special considerations for tendon transfer in patients with brachial plexus injury. Many patients complain of severe pain after brachial plexus lesions, and this pain needs to be addressed before tendon transfer. Even the best attempts to restore muscle balance in a painful limb may not succeed in reconstructing a functional limb.4 When choosing donor motors, reneurotized motor units should generally be excluded because regeneration is often incomplete and thus muscle strength is insufficient; furthermore, these muscles frequently do not have good independent control. Synergistic transfer may not be possible because of the limited availability of donor motors. For this reason, one transfer is sometimes designed to achieve two functions as long as they are not opposing actions. This is done by transferring the muscle across two joints and passing the motor around a pulley for strengthening.3,5 It should be noted that a transferred muscle-tendon unit that crosses multiple joints will always have its maximal action on the most proximal joint.
Tendon Transfer for Shoulder Function
The shoulder is a complex joint with many muscles required for full function. Its major functions are abduction, external rotation, internal rotation, and adduction. Shoulder function also depends on the stability of the scapula with the rhomboid and serratus anterior muscles. Tendon transfers around the shoulder to achieve functional control can be futile unless multiple transfers are attempted to duplicate the normal interaction of opposing and synergistic groups of muscles.6 If part of the deltoid is functional, the entire muscle can be detached subperiosteally and rotated anteriorly so that the posterior functioning portion will occupy the position of the middle portion. A paralyzed deltoid can be supplemented with a latissimus dorsi transposed in a bipolar manner on top of the shoulder.7,8 A more common procedure is a trapezius transfer9,10; the trapezius, an extraplexal muscle, is spared in the majority of complete brachial plexus injuries and can be transferred (although not as effectively) after distal spinal accessory nerve transfer to the suprascapular nerve. The levator scapulae is another remaining muscle for restoration of shoulder function. Transfer of this muscle elongated with fascia lata or tendon allograft onto the supraspinatus is efficient in regaining some extent of abduction.8 The long head of the triceps can be brought to the acromion posteriorly and the short head or long head of the biceps anteriorly.11 The external rotators can be reinforced by posterolateral transfer of the latissimus dorsi and teres major.12,13 Such an extensive approach is not always possible as a result of the extent of the injury and limited functioning muscle motors.
Transfer of the Trapezius
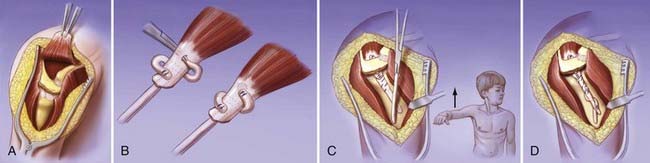
A “U”-shaped skin incision begins above the clavicle over the trapezius insertion, traverses the lateral aspect of the clavicle, and crosses around the acromion and along the spine of the scapula. The upper part of the trapezius is dissected from the clavicle and scapular spine to 2 cm from the vertebral border of the scapula. Its attachment at the acromion can be addressed either by preserving the attachment with a bone segment from the acromion or by completely detaching and prolonging it with a tendon graft. The neurovascular bundle of the spinal accessory nerve is protected and mobilized to facilitate transposition of the trapezius muscle. A vertical incision is made along the mid-deltoid. The deltoid is detached from the acromion and split along its fiber orientation to expose the proximal end of the humerus. If a bone segment of the acromion is used, the humeral shaft is roughened with an osteotome to facilitate growth of bone to the detached acromion-tendon unit. If the trapezius is prolonged with a tendon graft, holes are drilled in the humerus and the tendon graft is woven into the humerus. With the arm abducted 90 degrees, the acromion bone segment with the attached trapezius muscle is brought to the proximal end of the humerus as close to the tuberosity as possible and fixed with cortical lag screws. Similarly, if the trapezius is prolonged with a tendon, the arm is abducted and the tendon graft inserted underneath the acromion, placed through the drill holes, tensioned appropriately, and sewn back to itself. The deltoid is sutured over the new trapezius insertion and the wound closed in layers. Postoperatively, the shoulder is immobilized with a spica bandage in 90 degrees of abduction for 6 weeks to allow union of bone between the humerus and the acromion segment. The arm should be supported with a series of abduction splints and gradually lowered to adduction to prevent overstretching of the trapezius while muscle-strengthening exercises begin (Fig. 242-1).
The outcome of this transfer varies in different cases. Generally, better recovery of abduction is seen in shoulders with additional tendon transfers or shoulders with some remnant muscle function. In a series of 6 patients who underwent trapezius transfer after brachial plexus injuries, shoulder abduction improved from an average of 13 degrees to 76 degrees, whereas flexion increased from 18 to 78 degrees.10 Abolishment of shoulder subluxation plus improvement in abduction from a preoperative average of 3.5 degrees to a postoperative average of 45.4 degrees was seen in another series of 27 patients.9 Even when functional recovery is not adequate, the trapezius transfer is strong enough to keep the shoulder stable and correct subluxation of the glenohumeral joint, which allows some active abduction.
Latissimus Dorsi and Teres Major Transfer
Combined latissimus dorsi and teres major transfer for external rotation of the shoulder is carried out for the treatment of sequelae of obstetric brachial plexus palsy.12,14–16 This procedure increases the range of external rotation and abduction and provides considerable improvement in shoulder function. Theoretically, the transfer is equally effective in children and adults, provided that the latissimus has full strength. In brachial plexus injury in adults, however, this is not seen frequently. Poor results were observed after latissimus dorsi and teres major transfer in adult patients.17 Surgical techniques for this procedure are described elsewhere.18
Tendon Transfer for Elbow Function
There are various flexorplasty procedures.19,20 Selection of motors depends on the type and extent of the plexus injury, the degree of nerve recovery, and the muscles available. The latissimus dorsi is the most powerful, followed by pectoralis major. If these muscles are not available, the Steindler procedure can provide good flexion but is not as strong and in our experience not as consistent; it also leaves the patient with an elbow flexion contracture. Triceps transfer can reliably restore elbow flexion when the triceps is strong. It is especially advantageous in patients with biceps/triceps co-contraction.21–23 However, it is not suitable for patients who need triceps function to use assistive devices such as crutches. Transfer of the long head of the triceps has been reported to achieve MRC grade 4− and 85 degrees of elbow flexion with preserved elbow extension at reduced muscle strength24; we have no experience using this transfer technique with a portion of the triceps. Other options include sternocleidomastoid transfer. The muscle is extended with a fascial graft and woven into the biceps tendon. The procedure can produce appropriate elbow flexion but causes an unsightly prominence at the lateral aspect of the neck, a cosmetic appearance that is not acceptable to most patients.25
Steindler’s Procedure
The original transfer of the flexor-pronator mass from the medial epicondyle of the humerus proximally to enhance elbow flexion was described by Steindler in 1918.26 The pronator, flexor carpi radialis, palmaris longus, flexor carpi ulnaris, and flexor digitorum superficialis are detached from the medial epicondyle, advanced 5 to 7 cm proximally, and sutured to the medial intermuscular septum. To reduce pronation and flexion contracture side effects (i.e., the Steindler effect), modifications have been adopted.27–29 The flexor-pronator mass is detached along with a bone segment from the medial epicondyle. The muscles are mobilized, while protecting both the median and ulnar nerves, to enable proximal translocation of the muscle pedicle and bone up to 4 cm above the elbow and then attached to the anterior cortex of the humerus. However, this transfer results in just minor improvement in elbow flexion strength. Therefore, the Steindler flexorplasty technique should be used mainly as an augmentative procedure when the elbow flexors have retained some strength or exhibit partial recovery.
Pectoralis Major Transfer
Pectoralis muscle transfer can be done when the pectoralis innervation is not compromised in the plexopathy. Such can be the case with an upper trunk injury or combined upper and middle trunk injury. In these situations, the sternocostal portion of the pectoralis major is functioning because its innervating medial pectoral nerve contains fibers from C8-T1. The lower sternocostal portion is ideal for transfer with respect to its orientation and length. Since Clark’s description of pectoralis major flexorplasty,30 multiple modifications have been reported.31–33 Transfer of both the origin and insertion of the pectoralis major has been advocated. This bipolar transfer involves rotation of the mobilized muscle on its neurovascular pedicle. The insertion of the pectoralis major is sutured to the coracoid process and its origin to the biceps tendon. The added benefit of this procedure is that the bipolar transfer that originates from the acromion may provide a sufficient anterior glenohumeral buttress to stabilize the shoulder.34,35 Pectoralis major transfer is not recommended in female patients because the resulting donor site can be disfiguring.
Latissimus Dorsi Transfer
Latissimus dorsi myocutaneous flap transfer can be used not only for restoration of elbow flexion but also for soft tissue coverage and restoration of arm contour when significant trauma to the upper limb has occurred, which sometimes accompanies brachial plexus injury. When used for flexorplasty alone, the muscle and its neurovascular pedicle are mobilized. The transfer can be unipolar, with retention of its humeral origin,36 or it can be bipolar, in which case both the humeral and thoracic origins are detached and the muscle insertions repositioned.37 A longitudinal incision is made from the posterior axillary fold to the iliac crest. A skin island is created along the incision to reduce skin tension after insetting the flap and for monitoring muscle flap circulation. The latissimus dorsi is elevated from the origin to its insertion. The thoracodorsal nerve and artery are identified at the deep surface of the muscle and preserved. A curved incision is made along the anterior aspect of the arm. The muscle is passed through a subcutaneous tunnel created in the axilla. Care must be taken to avoid kinking and twisting of the neurovascular bundle. The distal pole of the muscle is sutured to the biceps tendon with the elbow flexed at 90 degrees and the forearm maintained in supination. In bipolar transfer, the proximal pole of the latissimus dorsi is sutured to the coracoid and coracoacromial ligament while the elbow is held flexed at 120 degrees. Only a limited number of procedures involving latissimus dorsi transfer have been reported.38,39 Most brachial plexus injuries that result in complete biceps (C5-6) paralysis are usually severe enough to negate the latissimus dorsi (C5-7) as a valid motor. In addition, if restoration of shoulder stability and external rotation by latissimus dorsi transfer is part of the algorithm for reconstruction, this muscle should not be considered for elbow flexorplasty.
Tendon Transfer for Wrist Stabilization
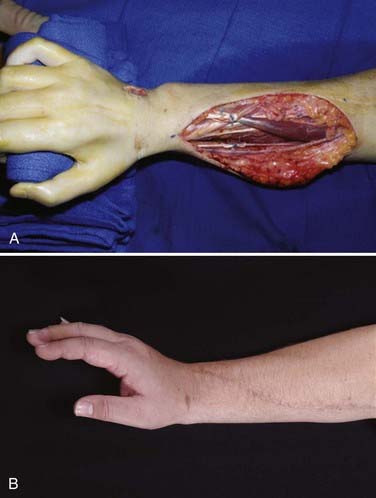
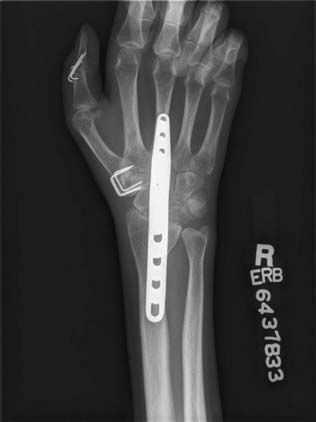
Mobility of the wrist should be maintained whenever possible. Only if it cannot be achieved because of the absolute shortage of available motors should the wrist be fused. Tendon transfer for wrist extension is usually satisfactory. In a series of 109 tendon transfers for secondary reconstruction of brachial plexus injuries, 21 were performed for wrist extension (Fig. 242-2). The flexor carpi ulnaris, flexor carpi radialis, pronator teres, and flexor digitorum superficialis have been used. Twenty of the 21 transfers yielded good or improved function.20 When muscles from forearm donors are not available, the brachialis muscle can be used instead; in three patients transfer of the brachialis muscle to the extensor carpi radialis brevis resulted in recovery of 20 degrees of active wrist extension against resistence.40
Tendon Transfer for the Hand
After brachial plexus injury, the goals for hand function are key pinch, active finger flexion and finger extension (Fig. 242-2), and thumb opposition. Partial brachial plexus lesions can be treated as though individual peripheral nerves are involved.41–44 Careful evaluation of functioning muscles plus selection of relatively spareable donors is key to the procedure. All other principles of tendon transfer should be followed. With total brachial plexus lesions, no forearm muscle is available for transfer, and an FFMT can be considered in selected cases.
Functioning Free Muscle Transfer
FFMTs are attempted for restoration of elbow flexion, shoulder abduction, elbow extension, finger flexion, and finger extension. Muscles used have included the gracilis, rectus femoris, latissimus dorsi, pectoralis major, tensor fasciae latae, and adductor longus.39,44–52 We have a large experience with using the gracilis transfer performed in a single stage and neurotized by intercostal nerves or the spinal accessory nerve; most commonly this has been performed by our group for elbow flexion alone or combined elbow flexion and finger flexion.48 It can be performed with functioning extraplexal or intraplexal motor nerves or even a fascicle of one.53 Other groups have proposed a two-stage procedure when the nerve pedicle is short and motor outflow is distant. In the first stage, the nerve ends are coapted, and in the second stage when an advancing Tinel sign approaches the distal end of the nerve, the free muscle is harvested and then revascularized and neurotized.
Selection of the muscle involves several considerations: strength, excursion, anatomy of the neurovascular pedicle, and quality of the tendon insertions.48 The gracilis and rectus femoris muscles are most commonly used. We prefer using the gracilis. The proximal location of its obturator nerve branch allows proximal and direct nerve coaptation for rapid reinnervation of the muscle transplant. The length of the gracilis muscle makes it possible to span the shoulder, elbow, and wrist for augmentation of multiple joints. This is especially advantageous in patients with complete brachial plexus injury when both the proximal and distal muscles are paralyzed. The rectus femoris muscle has the best fit for muscle strength and a type I vascular anatomy with a single vascular pedicle. However, it has a short excursion.
In recent years, increasing numbers of FFMTs have been performed in an attempt to restore prehensile function51 based on the knowledge that the results of nerve grafting or nerve transfers for hand function are much less favorable even when done in a timely fashion.
Functioning Free Muscle Transfer for Elbow Function
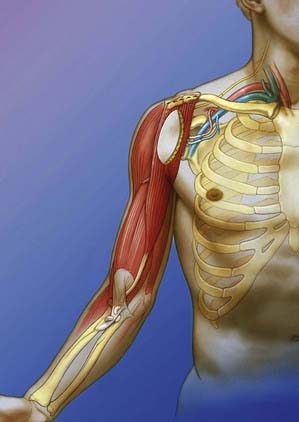
The gracilis is harvested after preparation of the recipient site. The muscle is detached from its origin and insertion at the pubic symphysis and pes anserinus after dissection of the obturator nerve and the muscle branch from the profunda femoris artery to gain maximal neurovascular pedicle length. The proximal pole of the gracilis muscle is anchored to the clavicle. Revascularization is achieved by anastomosis of its vessels to the thoracoacromial vessels. The muscle is neurotized with the spinal accessory or intercostal nerves. The distal pole of the muscle is then secured to the biceps tendon while the arm is held at 30 degrees of flexion and the muscle at its normal resting length (Fig. 242-3).
Excellent results have been reported when a single gracilis muscle is transferred for elbow flexion reconstruction.49,52,54,55 Use of the spinal accessory and intercostal nerves provided equivalent results in one series,49 better results with the intercostal nerves in another series,54 and stronger recovery of muscles reinnervated by the spinal accessory nerve in yet another series.56
Functioning Free Muscle Transfer for Prehensile Function
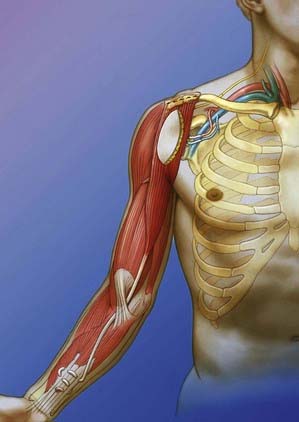
Because of the extensive length of the gracilis muscle, a free functioning gracilis transfer can also be performed to allow finger flexion. The muscle is anchored to the second rib at its proximal pole. The distal pole is tunneled from the arm to the forearm underneath the pronator teres to create a pulley effect and then woven into the flexor digitorum profundus and flexor pollicis longus tendons. The muscle is innervated by intercostal nerves and supplied by thoracodorsal vessels. The graft is tensioned to allow the fingers to extend with elbow flexion and to permit the thumb and fingers to close during elbow extension (Fig. 242-4).
Double Functioning Free Muscle Transfer
Doi and colleagues introduced and popularized double gracilis transfer in the early management of patients with four or five root avulsions to achieve elbow flexion, controlled wrist function, and hand prehension.50,57,58 By combining nerve grafts when there is one functioning root left, nerve transfers, and two free muscle transfers, shoulder stability, elbow flexion and extension, wrist extension, and hand prehension and protective sensation can be achieved if all components of the reconstruction are successful. This procedure is done in two stages. In the first stage, nerve reconstruction is carried out to restore shoulder function. A free muscle transfer is performed to restore elbow flexion and wrist or finger extension. At a second stage, two intercostal nerves are used to neurotize a second gracilis transfer to the finger flexors. Another two intercostals power the triceps. The sensory intercostals are sutured to the lateral cord contribution of the median nerve for hand sensation.
With double gracilis transfer, Doi and associates were able to restore good to excellent elbow flexion in 96% of their patients.50 More than 30 degrees of active finger motion was seen in 65% of their patients. The significance of elbow control and shoulder stability has been emphasized to ensure good prehensile function after double muscle transfer.59,60 In a study by our group, a gracilis transfer for combined elbow flexion and wrist/finger extension diminished the extent of M4 elbow flexion from 79% to 63%.49 The grasping resulting from the second gracilis transfer proved less reliable.
Arthrodesis
In earlier years when opinions about functional restoration after brachial plexus injury were still pessimistic, shoulder arthrodesis was carried out along with amputation and fitting of a prosthesis. In the modern era of brachial plexus reconstruction, shoulder arthrodesis still has its place, particularly in patients with severe palsy involving the shoulder and a painful subluxated shoulder.61–65 Although many surgeons consider shoulder arthrodesis the last operation to be considered when neural reconstruction has failed, some surgeons advocate shoulder arthrodesis early to spare the valuable and limited donor nerves for more distal nerve reconstruction.
The optimal position for fusion remains controversial. Many choose to fuse the glenohumeral joint at 30 degrees of abduction, forward flexion, and internal rotation. A practical measurement is one that enables patients to get their hand to their mouth while maintaining an abducted position. Internal fixation with a dynamic compression plate or pelvic reconstruction plate seems to be the standard of care.66 A bone graft is usually unnecessary as long as there is good contact between the humeral head and the glenoid fossa.
When performed well, shoulder arthrodesis results in fairly predictable outcomes. It provides stability, eliminates or decreases subluxation pain, and increases abduction, forward flexion, and internal rotation. Not much external rotation is gained. Range of motion depends closely on scapulothoracic muscle strength, especially strength of the serratus anterior muscle. In cases in which elbow flexion is restored, shoulder fusion can improve function by placing the limb in a better position and making elbow flexion more useful. One study has shown that glenohumeral arthrodesis improves function in patients who have recovered active elbow flexion after brachial plexus palsy, even when the hand remains paralyzed.65
Arthrodesis of the wrist is indicated when transferable muscles for restoring active extension are lacking (Fig. 242-5). Generally, wrist arthrodesis weakens power grip. A splint or orthosis can be used to stabilize the wrist. This application can also help in assessing the effect on function before arthrodesis if it so be chosen. Caution should be taken to avoid massive scarring of the dorsal finger extensor gliding bed.67 Arthrodesis of the wrist may further allow use of a functioning wrist extensor or flexor tendon.
Tenodesis
Tenodesis is not frequently performed because it tends to relax secondarily and its function can be lost. The most common tenodesis is that of the wrist to position and stabilize it in a functional position. In cases in which muscles, such as wrist flexors, are not strong enough to be used for tendon transfer, tenodesis of antagonistic muscles, such as the finger extensors, can result in opening the fingers while actively flexing the wrist because of the dynamic tenodesis effect.68
Corrective Osteotomy
In cases in which the supraspinatus and long head of the biceps have recovered but active external rotation is absent, function may be improved by rotation osteotomy of the humerus. The arm of these patients hangs down in an inwardly rotated position, and elbow flexion is hindered by the lower part of the arm striking against the thorax. A transverse osteotomy can be done in the midthird of the humerus and the distal part of the humerus rotated outward for 30 to 60 degrees. A dynamic compression plate is used for osteosynthesis. As a result of the osteotomy, external rotation is improved so that patients are able to move their hands to their faces without the lower part of the arm striking against the chest during elbow flexion.69 External rotation osteotomy of the humerus is performed more frequently for the treatment of shoulder sequelae secondary to obstetric brachial plexus palsy. Similarly, osteotomy of the forearm may also be an effective means of correcting fixed forearm rotational deformities in children.
Amputation/Prostheses
Careful consideration is essential before making a decision to amputate. Oftentimes patients will request amputation, particularly after they see or hear of new developments in prosthetics related to targeted reinnervation performed by some groups as means of primary reconstruction.70 The specific indication for amputation is a failed reconstruction in which the upper extremity has become a nuisance or mechanically painful. An arm that “gets in the way” or poses a potential hazard for injury may benefit from amputation. Mechanical pain from shoulder subluxation can also be addressed by amputation of a failed reconstruction.
Rehabilitation
To maintain muscle activity during the time of reinnervation, different strategies have been used. Electrical muscle stimulation, administered transcutaneously, is used as part of the rehabilitation program after nerve reconstruction in some centers. However, external stimulation leads to only poor to moderate success in maintaining muscle size and function because of limited stimulation levels, inadequate frequency of stimulation, and poor patient compliance.71 We do not routinely apply muscle stimulation in our brachial plexus practice.
Functional electrical stimulation neuroprostheses can be used to replace lost motor function in persons with paralysis of upper motoneuron origin.72 This technology has subsequently been shown to be effective and safe in restoring hand function in adults with spinal cord injury.
When dealing with a nonfunctioning limb after brachial plexus lesions, one should take into account the individual neuromuscular defect, passive joint function, and bony deformities. Splints may be protective or corrective. Because the major problem of stiffness that develops in a paralyzed arm occurs at the shoulder and in the hand, prophylactic splintage is applied to address these joints. In the case of a paralyzed shoulder joint seen after an upper trunk injury or total avulsion, a flail arm sling should be worn whenever the patient is up and about to prevent inferior subluxation of the joint and progressive joint damage. In C8-T1 injuries, a static opposition splint will help maintain the first web space and opposition when full abduction and opposition of the thumb are lost. When such a splint is extended above the wrist in extension, the finger metacarpophalangeal joints drop into flexion and prevent the commonly seen contractures in extension. Different procedures such as muscle transpositions, arthrodeses, and corrective osteotomies can then be performed to improve function of the upper extremity. Each form of operative treatment presents patients with certain benefits, and all should be integrated into a total treatment plan for the affected extremity.43 When such procedures are exhausted or patients opt to not undergo further operations, functional splintage can be used. One such example is the intrinsic minus splint, which prevents full metacarpophalangeal joint extension, allows the intrinsic finger extensors to extend the proximal phalangeal joints in C8-T1 injuries, and preserves some long extensor function.
Pain Management
An interdisciplinary pain clinic is necessary to address the pain that afflicts many patients with brachial plexus injuries. Some patients may benefit from medications and a tincture of time—not to mention neural interventions performed at primary procedures. Other patients, however, do not improve. We have no experience with late nerve transfers performed for relief of pain from preganglionic injury.73 For patients with refractory pain, other procedures, including dorsal root entry zone (DREZ) lesions, may be performed with thermocoagulation to target the deafferentated dorsal horn in those with root avulsion. After DREZ lesions, outcomes have ranged from success rates of 66% to 90%,74 although long-term data reflect some gradual decrease to 50%.75 Some groups have also proposed the use of motor cortex stimulation for these patients.76
, Bishop AT. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005;21:91-102.
, Merle M, Foucher G, Dap F, et al. Tendon transfers for treatment of the paralyzed hand following brachial plexus injury. Hand Clin. 1989;5:33-41.
, Narakas AO. Muscle transpositions in the shoulder and upper arm for sequelae of brachial plexus palsy. Clin Neurol Neurosurg. 1993;95(suppl):S89-S91.
, Pearle AD, Voos JE, Kelly BT, et al. Surgical technique and anatomic study of latissimus dorsi and teres major transfers. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2)):284-296.
, Rühmann O, Schmolke S, Bohnsack M, et al. Reconstructive operations for the upper limb after brachial plexus palsy. Am J Orthop. 2004;33:351-362.
, Smith RJ. Tendon transfers for brachial plexus injuries. In: Smith RJ, editor. Tendon Transfers of the Hand and Forearm. Boston: Little, Brown; 1987:151-175.
, Wahegaonkar AL, Doi K, Hattori Y, et al. Surgical technique of pedicled bipolar pectoralis major transfer for reconstruction of elbow flexion in brachial plexus palsy. Tech Hand Up Extrem Surg. 2008;12:12-19.
1 Berger A, Brenner P. Secondary surgery following brachial plexus injuries. Microsurgery. 1995;16:43-47.
2 Berger A, Becker MH. Brachial plexus surgery: our concept of the last twelve years. Microsurgery. 1994;15:760-767.
3 Berger A, Schaller E, Mailänder P. Brachial plexus injuries: an integrated treatment concept. Ann Plast Surg. 1991;26:70-76.
4 Smith RJ. Tendon transfers for brachial plexus injuries. In: Smith RJ, editor. Tendon Transfers of the Hand and Forearm. Boston: Little, Brown; 1987:151-175.
5 Berger A, Schaller E, Becker MH. Pulley for strengthening a muscle replacement operation across two joints in brachial plexus lesion: description of the surgical technique. Handchir Mikrochir Plast Chir. 1994;26:51-54.
6 Harmon PH. Surgical reconstruction of the paralytic shoulder by multiple muscle transplantations. J Bone Joint Surg Am. 1950;32:583-595.
7 Itoh Y, Sasaki T, Ishiguro T, et al. Transfer of latissimus dorsi to replace a paralysed anterior deltoid. A new technique using an inverted pedicled graft. J Bone Joint Surg Br. 1987;69:647-651.
8 Narakas AO. Muscle transpositions in the shoulder and upper arm for sequelae of brachial plexus palsy. Clin Neurol Neurosurg. 1993;95(suppl):S89-S91.
9 Aziz W, Singer RM, Wolff TW. Transfer of the trapezius for flail shoulder after brachial plexus injury. J Bone Joint Surg Br. 1990;72:701-704.
10 Mir-Bullo X, Hinarejos P, Mir-Batlle P, et al. Trapezius transfer for shoulder paralysis. 6 patients with brachial plexus injuries followed for 1 year. Acta Orthop Scand. 1998;69:69-72.
11 Tang CY, Mak AF, Hung LK, et al. Stability of reconstructed paralyzed shoulders using a reflected long head biceps technique. J Biomech Eng. 2001;123:227-233.
12 Freund RK, Terzis JK, Jordan L, et al. Modified latissimus dorsi and teres major transfer for external rotation deficit of the shoulder. Orthopedics. 1986;9:505-506.
13 Chen L, Gu YD, Hu SN. Functional reconstruction of the irreparable upper trunk defect of the brachial plexus—a case report. Hand Surg. 2004;9:125-129.
14 Vallejo GI, Toh S, Arai H, et al. Results of the latissimus dorsi and teres major tendon transfer on to the rotator cuff for brachial plexus palsy at birth. Scand J Plast Reconstr Surg Hand Surg. 2002;36:207-211.
15 Edwards TB, Baghian S, Faust DC, et al. Results of latissimus dorsi and teres major transfer to the rotator cuff in the treatment of Erb’s palsy. J Pediatr Orthop. 2000;20:375-379.
16 Hoffer MM, Phipps GJ. Closed reduction and tendon transfer for treatment of dislocation of the glenohumeral joint secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80:997-1001.
17 Sedel L. Palliative treatment of a series of 103 cases of paralysis from elongation of the brachial plexus. Spontaneous changes and results. Rev Chir Orthop Reparatrice Appar Mot. 1977;63:651-665.
18 Pearle AD, Voos JE, Kelly BT, et al. Surgical technique and anatomic study of latissimus dorsi and teres major transfers. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2)):284-296.
19 Stern PJ, Caudle RJ. Tendon transfers for elbow flexion. Hand Clin. 1988;4:297-307.
20 Leffert RD, Pess GM. Tendon transfers for brachial plexus injury. Hand Clin. 1988;4:273-288.
21 Rühmann O, Wirth CJ, Gossé F. Triceps to biceps transfer to restore elbow flexion in three patients with brachial plexus palsy. Scand J Plast Reconstr Surg Hand Surg. 2000;34:355-362.
22 Hoang PH, Mills C, Burke FD. Triceps to biceps transfer for established brachial plexus palsy. J Bone Joint Surg Br. 1989;71:268-271.
23 Rühmann O, Schmolke S, Gossé F, et al. Transposition of local muscles to restore elbow flexion in brachial plexus palsy. Injury. 2002;33:597-609.
24 Haninec P, Szeder V. Reconstruction of elbow flexion by transposition of pedicled long head of triceps brachii muscle. Acta Chir Plast. 1999;41:82-86.
25 Carroll RE. Restoration of elbow flexion by transplantation of sternocleidomastoid muscle. J Bone Joint Surg Am. 1962;44:1039.
26 Steindler A. Orthopaedic reconstruction work on hand and forearm. N Y Med J. 1918;108:1117-1119.
27 Mayer L, Green W. Experiences with the Steindler flexorplasty at the elbow. J Bone Joint Surg Am. 1954;36:775-789.
28 Brunelli GA, Vigasio A, Brunelli GR. Modified Steindler procedure for elbow flexion restoration. J Hand Surg Am. 1995;20:743-746.
29 Chen WS. Restoration of elbow flexion by modified Steindler flexorplasty. Int Orthop. 2000;24:43-46.
30 Clark JMP. Reconstruction of the biceps brachii by pectoral muscle transplantation. Br J Surg. 1946;34:180.
31 Brooks DM, Seddon HJ. Pectoral transplantation for paralysis of the flexors of the elbow; a new technique. J Bone Joint Surg Br. 1959;41:36-43.
32 Schottstaedt ER, Larsen LJ, Bost FC. Complete muscle transposition. J Bone Joint Surg Am. 1955;37:897-918.
33 Wahegaonkar AL, Doi K, Hattori Y, et al. Surgical technique of pedicled bipolar pectoralis major transfer for reconstruction of elbow flexion in brachial plexus palsy. Tech Hand Up Extrem Surg. 2008;12:12-19.
34 Carroll RE, Kleinman WB. Pectoralis major transplantation to restore elbow flexion to the paralytic limb. J Hand Surg Am. 1979;4:501-507.
35 Beaton DE, Dumont A, Mackay MB, et al. Steindler and pectoralis major flexorplasty: a comparative analysis. J Hand Surg Am. 1995;20:747-756.
36 Hovnanian AP. Latissimus dorsi transplantation for loss of flexion or extension at the elbow; a preliminary report on technic. Ann Surg. 1956;143:493-499.
37 Zancolli E, Mitre H. Latissimus dorsi transfer to restore elbow flexion. An appraisal of eight cases. J Bone Joint Surg Am. 1973;55:1265-1275.
38 Rivet D, Boileau R, Saiveau M, et al. Restoration of elbow flexion using the latissimus dorsi musculo-cutaneous flap. Ann Chir Main. 1989;8:110-123.
39 Terzis JK, Vekris MD, Soucacos PN. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast Reconstr Surg. 1999;104:1221-1240.
40 Bertelli JA, Ghizoni MF. Brachialis muscle transfer to reconstruct finger flexion or wrist extension in brachial plexus palsy. J Hand Surg Am. 2006;31:190-196.
41 Merle M, Foucher G, Dap F, et al. Tendon transfers for treatment of the paralyzed hand following brachial plexus injury. Hand Clin. 1989;5:33-41.
42 Bincaz LE, Cherifi H, Alnot JY. Palliative tendon transfer for reanimation of the wrist and finger extension lag. Report of 14 transfers for radial nerve palsies and ten transfers for brachial plexus lesions. Chir Main. 2002;21:13-22.
43 Haninec P, Dubový P, Sámal F. Reconstruction of elbow flexion, wrist and finger extension by transposition of pedicled latissimus dorsi muscle and flexor carpi ulnaris muscle. Acta Chir Plast. 2001;43:80-85.
44 Rühmann O, Schmolke S, Bohnsack M, et al. Reconstructive operations for the upper limb after brachial plexus palsy. Am J Orthop. 2004;33:351-362.
45 Chuang DC. Functioning free muscle transplantation for brachial plexus injury. Clin Orthop Relat Res. 1995;314:104-111.
46 Chuang DC. Neurotization and free muscle transfer for brachial plexus avulsion injury. Hand Clin. 2007;23:91-104.
47 Berger A, Flory PJ, Schaller E. Muscle transfers in brachial plexus lesions. J Reconstr Microsurg. 1990;6:113-116.
48 Bishop AT. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005;21:91-102.
49 Barrie KA, Steinmann SP, Shin AY, et al. Gracilis free muscle transfer for restoration of function after complete brachial plexus avulsion. Neurosurg Focus. 2004;16(5):E8.
50 Doi K, Muramatsu K, Hattori Y, et al. Restoration of prehension with the double free muscle technique following complete avulsion of the brachial plexus. Indications and long-term results. J Bone Joint Surg Am. 2000;82:652-666.
51 Doi K, Sakai K, Kuwata N, et al. Reconstruction of finger and elbow function after complete avulsion of the brachial plexus. J Hand Surg Am. 1991;16:796-803.
52 Akasaka Y, Hara T, Takahashi M. Free muscle transplantation combined with intercostal nerve crossing for reconstruction of elbow flexion and wrist extension in brachial plexus injuries. Microsurgery. 1991;12:346-351.
53 Sungpet A, Suphachatwong C, Kawinwonggowit V. Transfer of one fascicle of ulnar nerve to functioning free gracilis muscle transplantation for elbow flexion. Aust N Z J Surg. 2003;73:133-135.
54 Chuang DC, Carver N, Wei FC. Results of functioning free muscle transplantation for elbow flexion. J Hand Surg Am. 1996;21:1071-1077.
55 Doi K, Sakai K, Ihara K, et al. Reinnervated free muscle transplantation for extremity reconstruction. Plast Reconstr Surg. 1993;91:872-883.
56 Hattori Y, Doi K, Ikedu K, et al. Ultrasonographic evaluation of functioning free muscle transfer: comparison between spinal accessory and intercostal nerve reinnervation. J Reconstr Microsurg. 2006;22:423-427.
57 Doi K, Kuwata N, Muramatsu K, et al. Double muscle transfer for upper extremity reconstruction following complete avulsion of the brachial plexus. Hand Clin. 1999;15:757-767.
58 Doi K, Sakai K, Kuwata N, et al. Double free-muscle transfer to restore prehension following complete brachial plexus avulsion. J Hand Surg Am. 1995;20:408-414.
59 Doi K, Shigetomi M, Kaneko K, et al. Significance of elbow extension in reconstruction of prehension with reinnervated free-muscle transfer following complete brachial plexus avulsion. Plast Reconstr Surg. 1997;100:364-372.
60 Doi K, Hattori Y, Ikeda K, et al. Significance of shoulder function in the reconstruction of prehension with double free-muscle transfer after complete paralysis of the brachial plexus. Plast Reconstr Surg. 2003;112:1596-1603.
61 Chammas M, Meyer zu Reckendorf G, Allieu Y. Arthrodesis of the shoulder for post-traumatic palsy of the brachial plexus. Analysis of a series of 18 cases. Rev Chir Orthop Reparatrice Appar Mot. 1996;82:386-395.
62 Solomons M, Cvitanich M. A one-stage shoulder arthrodesis and Brooks Seddon pectoralis major to biceps tendon transfer for upper brachial plexus injuries. J Hand Surg Eur Vol. 2007;32:18-23.
63 Wong EL, Kwan MK, Loh WY, et al. Shoulder arthrodesis in brachial plexus injuries—a review of six cases. Med J Malaysia. 2005;60(suppl C):72-77.
64 Bedi A, Miller B, Jebson PJ. Combined glenohumeral arthrodesis and above-elbow amputation for the flail limb following a complete posttraumatic brachial plexus injury. Tech Hand Up Extrem Surg. 2005;9:113-119.
65 Chammas M, Goubier JN, Coulet B, et al. Glenohumeral arthrodesis in upper and total brachial plexus palsy. A comparison of functional results. J Bone Joint Surg Br. 2004;86:692-695.
66 Clare DJ, Wirth MA, Groh GI, et al. Shoulder arthrodesis. J Bone Joint Surg Am. 2001;83:593-600.
67 Addosooki A, Doi K, Hattori Y, et al. Wrist arthrodesis after double free-muscle transfer in traumatic total brachial plexus palsy. Tech Hand Up Extrem Surg. 2007;11:29-36.
68 Ochiai N, Nagano A, Yamamoto S, et al. Tenodesis of extensor digitorum in treatment of brachial plexus injuries involving C5, 6, 7 and 8 nerve roots. J Hand Surg Br. 1995;20:671-674.
69 Rühmann O, Gossé F, Schmolke S, et al. Osteotomy of the humerus to improve external rotation in nine patients with brachial plexus palsy. Scand J Plast Reconstr Surg Hand Surg. 2002;36:349-355.
70 O’Shaughnessy KD, Dumanian GA, Lipschutz RD, et al. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am. 2008;90:393-400.
71 Nicolaidis SC, Williams HB. Muscle preservation using an implantable electrical system after nerve injury and repair. Microsurgery. 2001;21:241-247.
72 Keith MW. Neuroprostheses for the upper extremity. Microsurgery. 2001;21:256-263.
73 Berman J, Anand P, Chen L, et al. Pain relief from preganglionic injury to the brachial plexus by late intercostal nerve transfer. J Bone Joint Surg Br. 1996;78:759-760.
74 Sindou MP, Blondet E, Emery E, et al. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. 2005;102:1018-1028.
75 Chen HJ, Tu YK. Long term follow-up results of dorsal root entry zone for intractable pain after brachial plexus avulsion injuries. Acta Neurochir Suppl. 2006;99:73-75.
76 Lazorthes Y, Sol JC, Fowo S, et al. Motor cortex stimulation for neuropathic pain. Acta Neurochir Suppl. 2008;97:37-44.