Chapter 8. Scans
More recently, the availability of high quality magnetic resonance imaging (MRI) has increased, and the detail possible with this technique may be invaluable in the assessment of infants with congenital neurological abnormalities or those who have suffered an episode of perinatal asphyxia.
This chapter contains ultrasound, CT and MRI scans along with relevant clinical histories. As with x-rays, interpretation is required as well as proposed management in some. In others you will be asked to comment upon the possible long-term significance of the abnormality that has been detected. There are many textbooks available for reference. 123 and 4
QUESTIONS
2. The same infant had the following chest x-ray taken.
a. Describe the chest x-ray.
b. What is the diagnosis from the chest x-ray?
c. How is this image linked with the MRI scan?
 |
| Figure 8.2. |
3. A term baby is born following a difficult vaginal delivery. No resuscitation is required and he is returned to his mother. Paediatricians are asked to review him at 18 hours of age as it is thought that he may have made some abnormal movements. He appears well on examination and an MRI scan is performed.
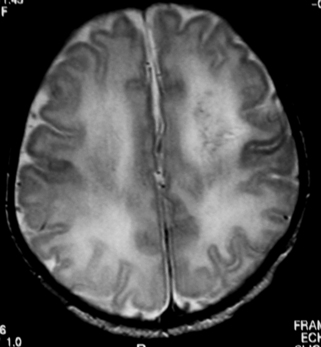 |
| Figure 8.3. |
4. A preterm baby, born at 28 weeks’ gestation has a follow-up scan carried out at 34 weeks corrected gestational age.
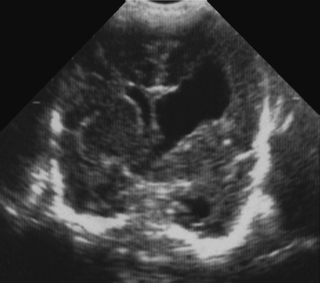 |
| Figure 8.4. |
a. What type of scan is this?
b. What does it show?
c. Why has this happened?
d. What is the outlook for this baby?
5. This scan was carried out on a 25 week gestation infant, who is now 72 hours old. He had a very stormy first two days, requiring maximal inotropic support and high ventilatory pressures. He is now requiring no blood pressure support and is on minimal ventilation.
 |
| Figure 8.5. |
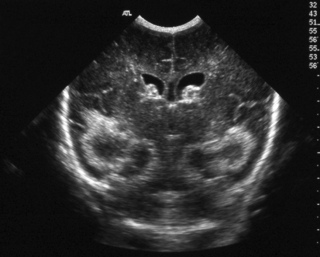
6. A 27 week gestation baby has a routine head scan performed at 2 days of age.
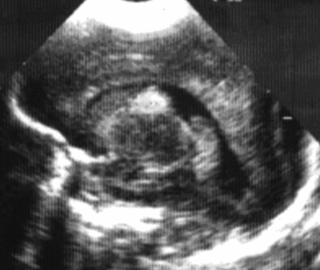 |
| Figure 8.6. |
a. Describe the scan.
b. Will this affect the baby’s long-term outcome?
7. A routine head scan is performed in a 29 week gestation infant. Parents want to know what it shows and whether it means that their baby is going to be normal or not.
 |
| Figure 8.7. |
a. Describe the scan.
b. What is the likely outcome for this baby?
9. This scan was carried out on day 7 in an ex-26 week gestation infant.
a. Describe the scan.
b. What is the most likely cause?
c. What is your management?
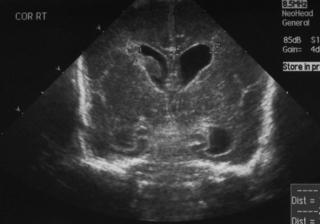 |
| Figure 8.9. |
11. This is a routine scan carried out on a baby born at 30 weeks’ gestation who requires no ventilatory support and is tolerating full milk feeds by 5 days of life.
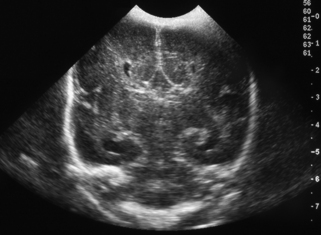 |
| Figure 8.12. |
a. Describe the scan.
b. What is the diagnosis?
c. What is the outcome for this baby?
13. A 29 week infant has a routine cranial ultrasound scan performed.
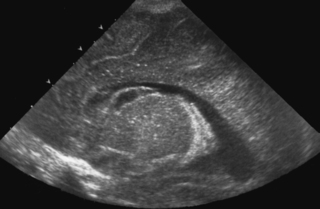 |
| Figure 8.14. |
a. Describe the scan.
b. Why has this happened?
c. What is the outlook for this baby?
14. A baby is born at term through meconium-stained liquor and requires full resuscitation. She has seizures noted at 1 hour of age and is given a loading dose of phenobarbitone. She is ventilated for the first 12 hours and then copes without respiratory support. She has established feeding by day 7 and is discharged at day 10. An MRI is carried out the day before discharge.
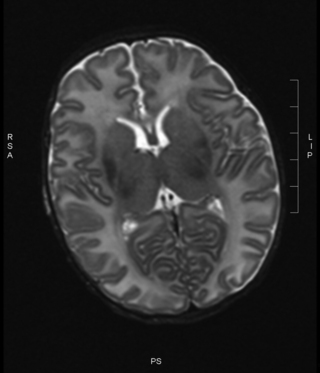 |
| Figure 8.15. |
15. A baby girl is delivered by caesarean section for failure to progress. She has a large abdomen at birth with masses palpable bilaterally.
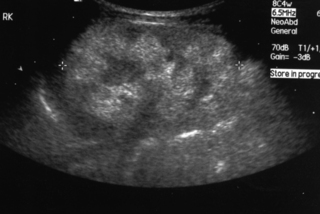 |
| Figure 8.16. |
a. Describe the scan.
b. What is the likely diagnosis?
c. What problems might the baby have?
d. What are the known associations?
16. A routine cranial ultrasound scan is carried out on a 31 week gestation infant.
 |
| Figure 8.17. |
a. Describe the scan.
b. What is the diagnosis?
c. What is the outlook for this baby?
17. A baby born at 36 weeks gestation has severe RDS with a history of prolonged rupture of membranes for 5 days. He is treated with antibiotics and is transferred for high frequency oscillation ventilation. He was initially hypotensive requiring maximal inotropic support. A scan is carried out on day 3 after transfer.
a. Describe the scan.
 |
| Figure 8.18a. |
A few days later, a further scan was obtained.
b. Describe the changes.
 |
| Figure 8.18b. |
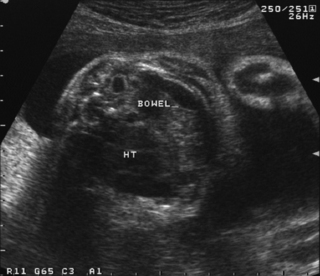
19. A woman is scanned antenatally at 30 weeks.
 |
| Figure 8.20. |
a. What does the scan show?
b. What will be your management of the baby at birth?
c. What are the baby’s chances of survival?
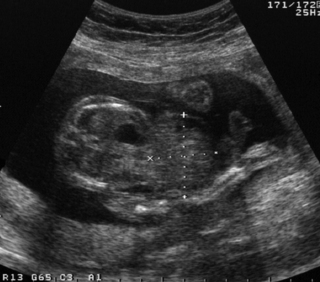
21. A woman is referred for a detailed fetal scan and has been found to have a raised alphafetoprotein.
a. What is the abnormality on this scan?
b. What other investigations would you carry out postnatally?
c. What conditions is this anomaly associated with?
 |
| Figure 8.22. |
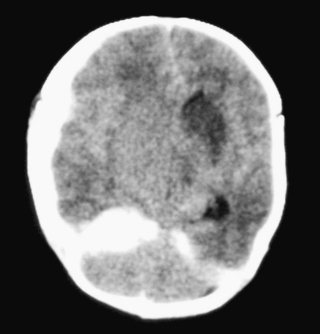
22. A term infant is born vaginally following a rapid delivery. At 24 hours of age she is noted to be irritable and feeding is poor. A cranial ultrasound is performed and raises some concerns. Another investigation is performed.
 |
| Figure 8.23. |
23. An infant is noted on postnatal examination to have a large head circumference, with a bulging fontanelle. He is asymptomatic and feeding well by bottle. An MRI scan is performed.
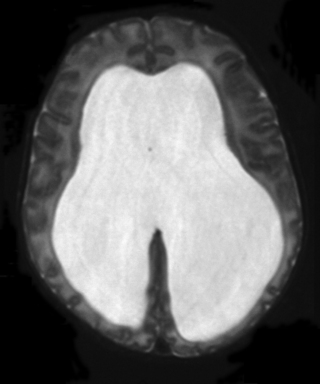 |
| Figure 8.24. |
a. Is this a T1 or T2 weighted MRI?
b. What does it show?
25. An MRI scan is carried out on a six month old infant.
a. Describe two abnormalities with this scan.
b. What is the likely cause?
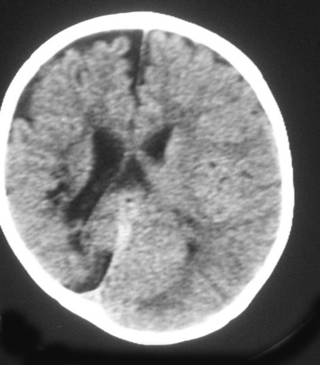 |
| Figure 8.26. |
27. A cranial ultrasound scan is carried out in a 25 week infant. She has been transferred in for intensive care from a level 2 unit. She seemed to be relatively stable for the first 24 hours after birth but at the age of 26 hours becomes acidotic and pale and required fluid resuscitation and increasing ventilation. A scan is carried out and is shown. Describe the scan.
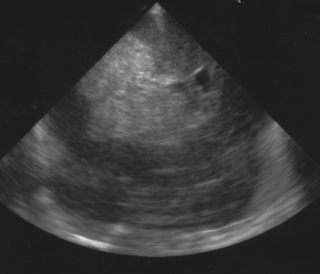 |
| Figure 8.28. |
ANSWERS
1.
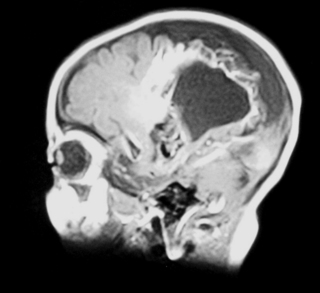
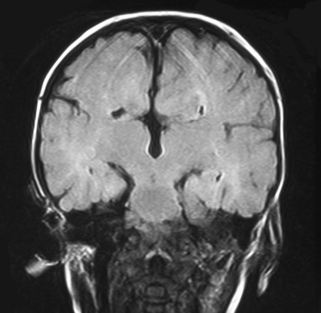
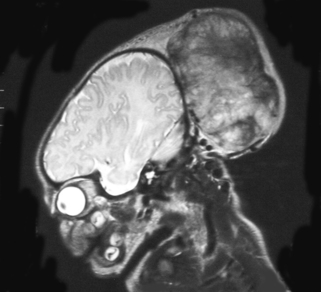
a. This is an MRI scan of the brain.
b. It shows a loss of cortex in the centre of the brain with an area filled with fluid. There is also increased extra-axial fluid around the brain. Blood vessels are tortuous and highlighted on the scan above the area filled with fluid.
2.
a. The chest x-ray shows a grossly enlarged heart with very little lung tissue visible.
b. The chest x-ray would be consistent with cardiac failure or possibly a cardiomyopathy.
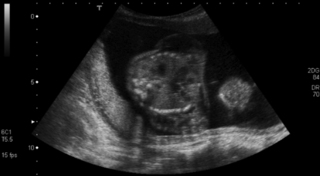
c. The two images are linked. The MRI shows a large AV malformation and the chest x-ray shows cardiac failure. Arteriovenous malformation is a rare cause of neonatal intracranial haemorrhage. The vein of Galen is most commonly involved, but a malformation here rarely presents as an intracranial haemorrhage. Approximately half of all cases present with cardiac manifestations, especially heart failure due to high cardiac output. Other clinical presentations, depending on the site of the AVM, may be seizures, hydrocephalus due to increased venous pressure or obstruction of CSF drainage, or opisthotonus with other brain stem signs in association with malformations originating in the posterior fossa.
3.
b. It is likely to be due to an infarction of the middle cerebral artery. The left middle cerebral artery is more commonly affected than the right, up to 3 or 4 times so.
c. Infants with focal infarction do not usually have encephalopathy. The most common presentation is seizures, starting unilaterally but then becoming more generalised. Abnormalities in tone can also be present. Abnormal signs in the neonatal period are not predictive of later outcome. 5 The prognosis for neurodevelopmental outcome is variable. Normal outcome has been reported in between one-third and one-half of all affected infants but some may develop signs of a hemiplegia. 5 and 6
d. In most cases of arterial infarction, the aetiology is unknown. Where a cause can be defined it is usually due to either thrombus, embolus or ischaemia. Of these, embolic phenomena appear most common and there is an association with twin-to-twin transfusion and congenital heart disease including the presence of a PDA. Other causes that have been reported include maternal cocaine abuse, polycythaemia, and elevated maternal cardiolipin and anti-phospholipid antibodies. Activated protein C deficiency in association with factor V Leiden deficiency is becoming increasingly recognised. Adverse perinatal events are reported in nearly 50% of affected infants. 7
e. It would be useful to carry out an EEG as this may have prognostic value. If the background activity on the EEG is abnormal, there is likely to be an abnormal motor outcome. 7
4.
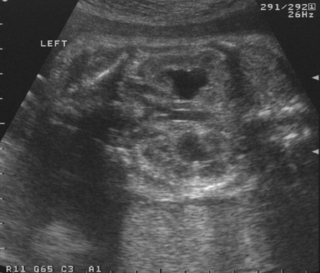
a. This is a coronal section on a cranial ultrasound scan.
b. It shows a very large single cavity cyst which is in communication with the left lateral ventricle. The right lateral ventricle appears unaffected. This is a porencephalic cyst.
c. Porencephalic cysts are the end results of a destructive process in the brain, such as intracranial haemorrhage, infection or surgery. The parenchyma is replaced by fluid, hence the cyst formation. Communication with the ventricles, as in this case, may or may not be present.
d. The prognosis depends on the size and whether unilateral or bilateral. Anecdotally every neonatologist knows of one or more infants who had such a lesion and were normal on long-term follow-up. There is no doubt that with extensive infarction, particularly bilaterally, outcome is likely to be poor. 8 However, for infants with unilateral and relatively small lesions a substantial number of infants may be normal at follow-up. 9
5.
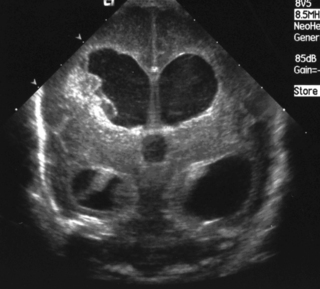
a. This is a cranial ultrasound scan, coronal view. It shows midline shift, with complete obliteration of the right lateral ventricle with marked echodensity through much of the hemisphere. The left lateral ventricle can be seen but is displaced and distorted. There is pressure on the right side of the cavum of the septum pellucidum.
b. There has been an intraventricular haemorrhage in the right lateral ventricle with secondary haemorrhage into the parenchyma. It is difficult to say from the scan whether this is solely extensive haemorrhage but it is likely that there is venous infarction occurring as well as this is thought to be the major contributory factor for parenchymal involvement after intraventricular bleeding. Following the bleed on the lateral wall and floor of the ventricle, pressure is exerted on a venous plexus that drains the periventricular cerebral cortex. Ischaemia develops in this region resulting in haemorrhagic infarction.
c. Outlook is difficult to define in the early period. The long-term outlook will be influenced by the size and position of the lesion, and the degree of cystic degeneration that results. Sources quoting neurodevelopmental outcome have widely differing estimates ranging from an incidence of significant neurological deficit from as low as 20% to in excess of 90%. There are also widely varying estimates of mortality with figures as high as 60% quoted. As the likely final extent of damage is uncertain early on, sequential scans are essential.
6.
a. This is a parasagittal view of a cranial ultrasound showing the lateral ventricle. It shows an area of echo density in the floor of the lateral ventricle. The diagnosis is a subependymal haemorrhage or germinal matrix haemorrhage. There is no intraventricular haemorrhage seen. Using a grading system, this is a grade I GMH-IVH.
b. Previously it was thought that babies with a subependymal haemorrhage would have no long-term complications. However, a recent study looking at infants with grade I and II intraventricular haemorrhages, i.e. germinal matrix bleeds with or without blood in the ventricles with no dilatation or parenchymal involvements, has demonstrated an increased risk of neurodevelopmental problems. When compared with infants with normal cranial ultrasounds, those with grade I–II IVH at 20 months corrected age had a significantly lower mean Mental Development Index (MDI) score than infants with a normal scan (74±16 compared to 79±14, p=0.006). They were more likely to have an MDI<70 (45% compared to 25%; OR, 2.00; 95% CI, 1.20–3.30; p=0.008), major neurological abnormality (13% compared to 5%; OR, 2.60; 95% CI, 1.06–6.36; p=0.036), and neurodevelopmental impairment (47% v 28%; OR, 1.83; 95% CI, 1.11–3.03; p=0.018) at 20 months corrected age, even when adjusting for confounding factors. Advanced imaging such as MRI may give further information about other injuries that are associated with grade I–II IVH, which could explain these outcomes. 10 It is important to appreciate that this implies an association between these grades of IVH and an abnormal outcome but does not demonstrate causation.
7.
a. This is a normal cranial ultrasound scan.
b. It is likely, but by no means certain, that this baby will do well in the long term. Severe abnormalities on cranial scans are important predictors of cerebral palsy and mental retardation, and a normal head scan commonly implies the absence of major impairment. However, a recent study followed infants with normal head scans and showed that up to 30% of ELBW infants had either cerebral palsy or a low score on the mental development index. Risk factors associated with this high rate of adverse outcome included pneumothorax, prolonged exposure to mechanical ventilation, and educational and economic disadvantage. The authors suggest that improvements in neonatal care to reduce duration of ventilation and avoidance of pneumothoraces might improve neurodevelopmental outcome for ELBW infants. 11 When counselling parents about a normal head scan, it is important to emphasise that all premature babies will need long-term follow-up to assess growth and neurodevelopment.
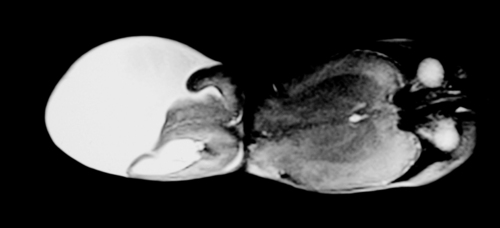
8.
a. This is an MRI scan of an infant’s head and neck. There is a large lesion at the back of the skull, with protrusion of cerebral and meningeal tissue into the sac.
b. This infant has an occipital encephalocele, a congenital defect of the skull which has allowed herniation of brain tissue into the sac. A cerebral meningocele only contains meninges and CSF. The lesion shown above clearly contains brain as well, thus is an encephalocele; 75% of encephaloceles are occipital (as in this case), the other 25% are frontal.
c. Encephaloceles are seen in a variety of chromosomal disorders – trisomy 13 and 18 in particular and also with deletions of 13q and 16q. They can be seen in several syndromes such as Meckel–Gruber (polycystic kidneys, polydactyly, cleft lip and palate), Dandy–Walker (hydrocephalus, absence of cerebellar vermis, cranial nerve palsies), Joubert (cerebellar vermian aplasia, ataxia, coloboma, and renal cysts) and others.
d. Severe intellectual and motor deficits are seen with microcephaly. There is also weakness and spasticity. Intellectual impairment is seen more often in posterior encephaloceles. The long-term outlook depends on presence of brain tissue in the sac, development of hydrocephalus, development of the brain (e.g. presence of microcephaly) and the presence of other brain malformations such as microgyria, optic pathway dysgenesis. 3
9.
a. This is a coronal midline view of a cranial ultrasound. It shows bilateral dilatation of the anterior and posterior horns of the lateral ventricles. The third ventricle is not clearly seen. The parenchyma of the brain looks normal.
b. This is most likely due to resolving haemorrhage in the ventricles. There is a blood clot on the wall of the right ventricle.
c. This baby needs regular follow-up scans to look for progressive dilatation of the ventricles. The scans should be carried out weekly, at a minimum, with a shorter time between scans if the dilatation is significant. The optimum way to assess this is to measure the ventricular index. This is the distance between the midline and the lateral border of the lateral ventricle in a coronal view in the plane of the 3rd ventricle. There is a chart of normal ranges. 12 The baby should be assessed clinically with regular head circumference measurements (plotted on growth charts) and assessment of the fontanelle and sutures. Serial assessment with scans is crucial as the clinical signs of hydrocephalus (rapid head growth, full fontanelle) do not appear for days, or even weeks, after the dilatation has started.
10.
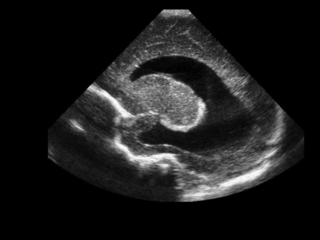
a. The scan is a sagittal view of a cranial ultrasound scan. It shows a uniformly dilated lateral ventricle. This scan would be consistent with post-haemorrhagic hydrocephalus.
b. Continued monitoring is essential and if head circumference continues to increase progressively further from the 95th centile the only option that can be considered is the removal of some cerebrospinal fluid. The exact point at which this should be considered is uncertain and there is considerable variation between practitioners as to when they will start drainage. Repeated taps by insertion of a needle cannot be recommended and appears to be associated with a worse long-term prognosis thus leaving the options of insertion of a reservoir or shunt. Treatment with acetazolamide and furosemide has been associated with worse outcome and no benefit has been demonstrated following a DRIFT procedure (drainage, irrigation and fibrinolytic therapy). 13
11.
a. This is a cranial USS showing a coronal section, in the midline. There is mild asymmetry of the lateral ventricles, which are widely separated and of abnormal shape.
b. This baby has agenesis of the corpus callosum (ACC).
c. ACC can be an isolated abnormality, which usually means there is no obvious neurological abnormality. Its prevalence is therefore unknown in the general population as it may be asymptomatic. It can be associated with other abnormalities, and this may change the outcome, e.g. Chiari malformation and neuronal migration disorders. With other abnormalities, the prognosis is considered to be poor. Isolated ACC has a much more variable prognosis with some infants having normal neurodevelopment to others having severe neurological handicap. Infants with ACC require careful neurodevelopmental follow-up. Infants with this finding on USS warrant an MRI to look for other abnormalities.
12.
a. This is an antenatal scan of a fetus.
b. It shows a cross section of the fetus and the chest. The rib ends are clearly visible, as is the heart. The most striking abnormalities are the growths on the outer chest wall which can be seen bilaterally. From the ultrasound it is difficult to say what the masses are. Doppler would be useful to see if these are haemangiomas.
c. Serial antenatal scans would be needed to assess the rate of growth of the masses. Depending on the size, mode of delivery may be affected. Detailed postnatal examination would be necessary plus imaging, initially ultrasound followed by CT or MRI for more detail and involvement of underlying structures.
13.
a. This a parasagittal view of a cranial ultrasound. The obvious abnormality is a cystic lesion in the floor of the lateral ventricle, known as a subependymal cyst.
b. This occurs as a germinal matrix haemorrhage resolves and is replaced by a cavity. The bright white line above the cyst, which can extend around the whole of the ventricle, is due to haemosiderin-laden macrophages. These post-haemorrhagic cystic lesions are most commonly detected at the caudothalamic notch. They are often tear-shaped and measure 2–11 mm in size. 14
c. Generally, infants with subependymal cysts have no long-term sequelae. However, these have occurred after a germinal haemorrhage and therefore there does appear to be an increased risk of long-term sequelae as discussed in detail in the answers to question 6.
14.
a. There is an equal and symmetrical signal within the posterior limb of the internal capsule (PLIC), and there is asymmetry of signal in the basal ganglia. There is also some mild cortical highlighting.
b. This infant has suffered an episode of perinatal asphyxia. The usual pattern seen on an MRI scan with perinatal asphyxia is loss of the normal signal intensity with the PLIC. Abnormalities within the basal ganglia and thalami, and cortical highlighting are also seen following asphyxial episodes. The prevalence of changes in the basal ganglia and thalami are thought to be due to the high metabolic rate in this region due to myelination.
15.
a. This is a renal ultrasound scan. It shows a large hyperechogenic kidney. As a rule of thumb the kidney measurements should be the number of gestation weeks in millimetres. There are areas of different echogenicity, with loss of the normal intrarenal architecture. The kidney contains multiple tiny cysts measuring 1–2 mm each, giving the ‘bright’ appearance.
b. The likely diagnosis is autosomal recessive polycystic kidney disease (ARPCKD). Multi-cystic dysplastic kidneys have a different appearance with the classical appearance of numerous cysts resembling a bunch of grapes with no normal renal parenchyma seen.
c. Severe early problems for these babies may develop due to pulmonary hypoplasia because of inadequate liquor volume in utero. Antenatal scans can show enlarged hyperechoic kidneys from 14 weeks gestation, along with oligohydramnios and an empty bladder. Infants may require prolonged periods of ventilation which can be difficult. If infants survive the acute neonatal period, then up to 33% will develop end-stage renal failure in childhood, necessitating dialysis and eventual renal replacement.
d. ARPCKD is associated with hepatic duct ectasia, cysts and peripheral fibrosis. A gene locus on 6p21 has now been found.
16.
a. This is a sagittal section of a head ultrasound scan. It shows a small cyst at the front of the lateral ventricle.
b. The most likely diagnosis is a connatal cyst. Synonyms for connatal cysts are coarctation of the lateral ventricles and frontal horn cysts. Connatal cysts are located at or just below the superolateral angles of the frontal horns or body of the lateral ventricles and are mainly anterior to the foramina of Monro. They can be hard to differentiate from subependymal cysts that are located below the external angle and posterior to the foramen of Monro. They represent a normal variant due to approximation of the walls of the frontal horns of the lateral ventricles proximal to their external angles. When the ventricular walls are close enough to touch each other, the most external portion of the ventricle acquires a round shape which on ultrasound appears to be a cyst. 15
c. Connatal cysts have a reported incidence of 0.7% in low birth weight preterm infants and are thought to be benign. These cysts have been reported to resolve in follow-up studies. 16
17.
a. There are large intraventricular haemorrhages in both lateral ventricles. The temporal horns of the ventricles are distended. There is extension of the clot into the frontal horns, especially on the left. There is also an area in the left periventricular region which is suspicious of a venous infarct.
b. The lateral ventricle still contains some blood and there is now dilatation of the frontal horns. There are bilateral parenchymal echodensities lying posteriorly around the temporal horns, consistent with venous infarction. One paper described this pattern of infarction, however only with unilateral lesions and associated with mild to moderate intraventricular haemorrhage. The survivors did not have a uniform type of cerebral palsy but had a lower Bayley Mental Development Index. Some developed temporal lobe epilepsy. 17 The infant in the case above obviously has bilateral large intraventricular haemorrhages associated with bilateral venous infarcts. This is an unusual lesion and data regarding long-term prognosis is sparse.
18.
a. This MRI scan shows agenesis of the corpus callosum. The lateral ventricles are abnormal and crescentic in shape. They are shifted laterally resulting in the formation of a large midline interhemispheric subarachnoid space. The abnormal shape is due to deformation by fibres of the cerebral hemisphere that were meant to cross in the corpus callosum, but due to the agenesis, run longitudinally as the bundles of Probst.
b. Agenesis of the corpus callosum can be an isolated finding but can be associated with other structural brain anomalies. It is seen in Aicardi, Apert’s, Smith–Lemli–Opitz, Goldenhaar, Fryns’, Meckel–Gruber, Zellweger’s and Walker–Warburg syndromes. It is also seen with inborn errors of metabolism and in fetal alcohol syndrome. It is therefore important not only to look for other anomalies on the MRI scan, but to look at the infant for anomalies in other systems.
c. Agenesis of the corpus callosum involves a spectrum of abnormalities including abnormalities in the pericallosal nervous tissue. It is thus difficult to give the parents a definitive idea of long-term outlook as the wide range of abnormalities will also imply a wide spectrum of intelligence and of associated neurodevelopmental problems. Normal intelligence is not unusual, but severe compromise may be present, especially if other abnormalities are present.
19.
a. The scan shows a congenital diaphragmatic hernia. The heart is visible in the chest and there are loops of bowel present at the same level.
b. An experienced neonatal team should attend the delivery of an infant with a know CDH. Affected babies should be delivered in a tertiary centre where paediatric surgery is accessible. Rapid intubation with gentle ventilation is recommended, with permissive hypercapnia. The lungs are frequently hypoplastic and pneumothoraces are common. It is known that infants with CDH are surfactant deficient and some reports have shown that exogenous surfactant could be beneficial. More recent papers have shown that in term infants the use of surfactant leads to higher ECMO use, more chronic lung disease and lower survival rates. 18 The use of nitric oxide as a pulmonary vasodilator has also been used in the treatment of infants with CDH. It can cause a rapid improvement in oxygenation but the effect is usually transient. It has been not shown to improve survival19 but there are varying reports on whether it decreases the need for ECMO. 19 and 20 A Cochrane review in 2001 found no clear data to support use in infants with CDH. 21 There are however cases where a trial of nitric oxide is warranted.
c. It is extremely difficult to accurately predict the chance of survival in these infants. The first paper to describe survival in a surgical series was in 1946 with 100% survival. 22 Clearly it depends on whether the infant is stable enough to be operated on. There are a group of infants who are difficult to ventilate from birth due to severe pulmonary hypoplasia and die within the first few days without surgery. If infants are operated on, their survival increases up to 75% in some series. 23 Left-sided defects are more common and have a better outcome than right-sided defects. Late presenting defects have a better prognosis due to less lung hypoplasia and pulmonary hypertension.
20.
a. The scan shows dilatation of the pelvis (prominent echo-poor component) surrounded by normal renal cortex. This is consistent with hydro-nephrosis. Both kidneys are visible in this scan, one of top of the other. The upper kidney is the abnormal one. The lower kidney is difficult to assess in this view; however the renal pelvis does appear prominent.
b. Approximately 1% of fetuses present some dilatation of their urinary tract in utero. 24 The significance of mild antenatally detected hydro-nephrosis remains controversial. Further investigations are warranted if scans show a progressive enlargement or the anteroposterior diameter is >10 mm, as this is more likely to be associated with pathology. Studies have show that mild dilatation (4–10 mm) progresses to hydronephrosis (>10 mm) in approximately a quarter of cases, 25 and other groups have shown that it can be associated with vesicoureteral reflux. 26 This infant should have a postnatal scan to assess the degree of dilatation, and if the dilatation is progressive, should be commenced on prophylactic antibiotics and a micturating cystogram performed to look for obstruction in the urinary tract. 27
21.
a. This antenatal scan shows an abdominal wall defect. It is an exomphalos because of its covering of omphaloperitoneal membrane, and its relationship to the umbilical cord. The dark area within the fetal abdomen could represent a displaced stomach, or dilated bowel. The mass (with the markers on) is the exomphalos. The mass is within the base of the umbilical cord which can be seen continuing from it on the right of the image.
b. Exomphalos is a spectrum of anomalies ranging from a small defect with gut herniating into the cord to a large defect (exomphalos major), where bowel and liver are enclosed in the sac. It is frequently associated with other anomalies and echocardiography, karyotyping, and in some cases contrast studies should therefore be performed to assess gut rotation. It can be associated with other midline defects including involvement of diaphragm, heart and bladder.
c. 30% of cases of exomphalos are associated with chromosomal abnormalities, particularly trisomy 13 and 18, and less so 21, 28 and there is a higher rate of chromosomal abnormalities in smaller defects that do not include the liver. 29 More than half of infants with exomphalos have other malformations, with cardiac defect being the most common. 30 There is also an association with neural tube defects. 31 The most well recognised association is with Beckwith–Wiedemann syndrome (omphalocele, macroglossia and hyperinsulinism).
22.
a. This is a CT scan.
b. The interhemispheric fissure is deviated to the left. There is an area of high attenuation along both leaves of the tentorium cerebelli which is more pronounced on the right. There appears to be a widening of the extra-axial space on the right with a density compatible with the presence of blood. Although a ventricular space can be seen on the left none is visible on the right. This could be due to a degree of swelling of the right side of the brain causing both midline deviation and ventricular compression. These findings are consistent with a subdural haematoma on the right side.
c. In the most severe forms outlook is poor with neurological abnormalities in approximately half at follow-up. This is presumably due to related cerebral injury. In less severe forms the majority do well.
23.
a. It is a T2 scan. Following application of a magnetic field, the time required for a certain percentage of the tissue’s nuclei to realign is termed ‘Time 1’ or T1, which is typically about 1 second. T2 imaging employs a spin echo technique, in which spins are refocused to compensate for local magnetic field inhomogeneities. In practical terms this has a major effect on the appearance of the scans. In T1 weighting white matter appears white and grey matter grey while CSF appears black. In T2 imaging this is reversed with CSF appearing white.
b. The scan shows severe hydrocephalus with very marked reduction of the cortical mantle. The inter-ventricular septum is absent. Although cranial ultrasound is an ideal way to measure ventricular dilatation, more detailed imaging with MRI is usually carried out prior to shunt insertion. The diagnosis in this case is Dandy–Walker malformation. This is associated with severe hydrocephalus, as seen in this scan.
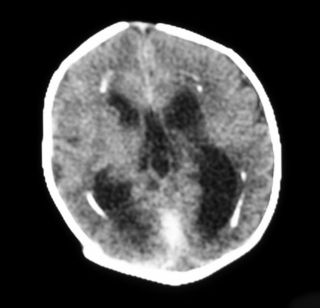
24.
a. This is a CT scan of the brain. The striking abnormalities are ventriculomegaly and the areas of brightness (same as bone) in the periventricular regions due to calcification.
b. This scan is consistent with congenital cytomegalovirus.
c. Urine should be sent for CMV detection by PCR, and blood for serology for CMV IgM. This has a sensitivity of approximately 70%.
For further information on CMV see Chapter 10, questions 1 and 2.
25.
a. The two abnormalities seen on the scan are marked asymmetry of the cortex, with one hemisphere being much smaller than the other and with asymmetry of the lateral ventricles.
b. This is likely to be secondary to a middle cerebral artery infarct in the neonatal period and the scan shows the long-standing deficit.
26.
a. There is a large mass at the back of the skull which seems to be separate from the brain contents. The brain looks relatively normal and the mass seems to be full of vessels and covered with skin.
b. It is difficult to say from the scan what the diagnosis is, but the most likely is an arterio-venous malformation (as was the case here) as no extension of brain tissue into the sac is visible.
27.
This is a coronal view of a cranial scan. There is extensive haemorrhage seen on the right with complete obliteration of the right ventricle. There is deviation of the midline with the left ventricle pushed to the far left of the image. This baby has suffered a large intraventricular bleed with parenchymal extension. There are three clinical syndromes described by Volpe. 4 This baby has had a catastrophic deterioration, presenting with pallor, acidosis and a change in ventilation. Other signs can be a fall in blood pressure or feed intolerance. If these changes occur along with a drop in haematocrit, a full fontanelle or seizures, a GMH-IVH is likely to have occurred as in this case. The saltatory syndrome is more common and more gradual in onset. This generally presents with a change in type of movements and seizures can occur. Most GMH-IVH however are asymptomatic – in fact up to 50% have no obvious clinical signs.
REFERENCES
1. Rennie, JM, Neonatal cerebral ultrasound. ( 1997)Cambridge University Press, Cambridge.
2. Rutherford, M, MRI of the neonatal brain. ( 2001)WB Saunders, Oxford.
3. In: (Editors: Levene, M.I.; Chervenak, F.A.; Whittle, M.) Fetal and neonatal neurology and neurosurgery3rd edn. ( 2001)Churchill Livingstone, Edinburgh.
4. Volpe, JJ, Neurology of the newborn. ( 2001)WB Saunders, Oxford.
5. Sreenan, C; Bhargava, R; Robertson, CM, Cerebral infarction in the term newborn: clinical presentation and long-term outcome, J Pediatr 137 (3) ( 2000) 351–355.
6. Estan, J; Hope, P, Unilateral neonatal cerebral infarction in full term infants, Arch Dis Child Fetal Neonatal Ed 76 (2) ( 1997) F88–F93.
7. Mercuri, E; Rutherford, M; Cowan, F; et al., Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic resonance imaging study, Pediatrics 103 (1) ( 1999) 39–46.
8. Guzzetta, F; Shackelford, GD; Volpe, S; et al., Periventricular intraparenchymal echodensities in the premature newborn: critical determinants of neurologic outcome, Pediatrics 78 (1986) 995–1006.
9. de Vries, LS; Roelants-van Rijn, AM; Rademaker, KJ; et al., Unilateral parenchymal haemorrhagic infarction in the preterm infant, Eur J Paediatr Neurol 5 (2001) 139–149.
10 Patra, K; Wilson-Costello, D; Taylor, HG; et al., Grades I–II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment, J Pediatr 149 (2) ( 2006) 169–173.
11 Laptook, AR; O’Shea, TM; Shankaran, S; Bhaskar, B, NICHD Neonatal network. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents, Pediatrics 115 (3) ( 2005) 673–680.
12 Levene, MI, Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound, Arch Dis Child 56 (12) ( 1981) 900–904.
13 Whitelaw, A; Evans, D; Carter, M; et al., Randomised clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid, Pediatrics 119 (2007) e1071–e1078.
14 Larcos, G; Gruenewald, SM; Lui, K, Neonatal sub-ependymal cysts detected by sonography: prevalence, sonographic findings, and clinical significance, Am J Roentgenol 162 (1994) 953–956.
15 Rosenfeld, DL; Schonfeld, SM; Underberg-Davis, S, Coarctation of the lateral ventricles: an alternative explanation for subependymal pseudocysts, Pediatr Radiol 27 (1997) 895–897.
16 Pal, BR; Preston, PR; Morgan, ME; et al., Frontal horn thin walled cysts in preterm neonates are benign, Arch Dis Child Fetal Neonatal Ed 85 (2001) F187–F193.
17 Govaert, P; Smets, K; Matthys, E; et al., Neonatal focal temporal lobe or atrial wall haemorrhagic infarction, Arch Dis Child Fetal Neonatal Ed 81 (3) ( 1999) F211–F216.
18 Van Meurs, K, Congenital Diaphragmatic Hernia Study Group. Is surfactant therapy beneficial in the treatment of the term newborn infant with congenital diaphragmatic hernia?J Pediatr 145 (3) ( 2004) 312–316.
19 Neonatal Inhaled Nitric Oxide Study Group (NINOS), Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia, Pediatrics 99 (6) ( 1997) 838–845.
20 Neonatal Inhaled Nitric Oxide Study Group (NINOS), Inhaled nitric oxide in term and near-term infants: neurodevelopmental follow-up of the neonatal inhaled nitric oxide study group, J Pediatr 136 (5) ( 2000) 611–617.
21 Finer, NN; Barrington, KJ, Nitric oxide for respiratory failure in infants born at or near term, Cochrane Database Syst Rev ( 2) ( 2001); CD000399.
22 Gross, RE, Congenital hernia of the diaphragm, Am J Dis Child 71 (1946) 580–592.
23 Boloker, J; Bateman, DA; Wung, JT; et al., Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair, J Pediatr Surg 37 (3) ( 2002) 357–366.
24 Cachat, F; Ramseyer, P; Meyrat, BJ; et al., Antenatally detected hydronephrosis: practical approach for the pediatrician, Rev Med Suisse 1 (7) ( 2005) 505–506; 509–12.
25 Persutte, WH; Koyle, M; Lenke, RR; et al., Mild pyelectasis ascertained with prenatal ultrasonography is pediatrically significant, Ultrasound Obstet Gynecol 10 (1) ( 1997) 12–18.
26 Gloor, JM; Ramsey, PS; Ogburn, PL; et al., The association of isolated mild fetal hydronephrosis with postnatal vesicoureteral reflux, J Matern Fetal Neonatal Med 12 (3) ( 2002) 196–200.
27 John, U; Kähler, C; Schulz, S; et al., The impact of fetal renal pelvic diameter on postnatal outcome, Prenat Diagn 24 (8) ( 2004) 591–595.
28 Reddy, VN; Aughton, DJ; DeWitte, DB; et al., Down syndrome and omphalocele: an under recognized association, Pediatrics 93 (3) ( 1994) 514–515.
29 Benacerraf, BR; Saltzman, DH; Estroff, JA; et al., Abnormal karyotype of fetuses with omphalocele: prediction based on omphalocele contents, Obstet Gynecol 75 (1990) 317–319.
30 Hughes, MD; Nyberg, DA; Mack, LA; et al., Fetal omphalocele: prenatal US detection of concurrent anomalies and other predictors of outcome, Radiology 173 (1989) 371–376.
31 Calzolari, E; Bianchi, F; Dolk, H; et al., Omphalocele and gastroschisis in Europe: a survey of 3 million births 1980–1990. EUROCAT Working Group, Am J Med Genet 28 (58) ( 1995) 187–194.