Chapter 2. Problems at birth and resuscitation
Paediatric assistance is requested at around a quarter of all deliveries and some form of support is required in 40% of these cases. Intensive resuscitation, respiratory support at least, is required at 3% of all deliveries. This chapter will cover the commoner problems that may lead to difficulties at delivery and in the early postnatal period and will emphasise the standard approach to resuscitation that is taught on the Resuscitation Council Neonatal Life Support Course. 1 With the current quality of antenatal imaging many problems can be detected in pregnancy (60–80% of major and 35% of minor abnormalities by routine screening at 18–20 weeks) and an appropriate early management plan can be initiated. However, unexpected problems will always occur and it is thus essential that all staff have the skills necessary to deal with these situations.
| Neonatal Life Support Algorithm |
| Dry and wrap |
| ↓ |
| Assess – breathing, heart rate, colour, tone |
| ↓ |
| Open airway |
| ↓ |
| Re-assess |
| ↓ |
| Inflation breaths – 5 breaths; 2–3 seconds; 30cmH 2O |
| ↓ |
| Re-assess |
| ↓ |
| Ventilation breaths – for 30 seconds: 30/minute; 25cmH 2O |
| ↓ |
| Re-assess |
| ↓ |
| Cardiac compressions – (assuming good chest movements and no improvement in heart rate) 3 compressions to one breath |
| ↓ |
| Re-assess after 30 seconds |
| ↓ |
| Drugs |
| If the chest fails to move |
| Check head position |
| ↓ |
| Single person jaw thrust |
| ↓ |
| Two person jaw thrust |
| ↓ |
| Clear airway under direct vision |
| ↓ |
| Guedel airway |
QUESTION 1
You are called to a delivery of a term baby where profound decelerations have been seen on the CTG. The baby is born in poor condition and there is thick meconium in the liquor and on the baby’s skin.
i) Which of the following manoeuvres are appropriate? Give one answer.
a. Perineal suction
b. Clamping the chest
c. Tracheal lavage with normal saline
d. Immediate intubation and tracheal suction
e. Dry and wrap.
The baby is not breathing and is white and hypotonic with a heart rate is 30 bpm. Initial T-piece ventilation fails to obtain chest movement.
ii) What two forms of immediate treatment are appropriate?
a. Start cardiac compressions immediately
b. Repeat inflation breaths
c. Continue with ventilation breaths
d. Prepare for drug administration through the umbilical vein
e. Immediate intubation
f. Check head position
g. Visualise the oro-pharynx.
Following tracheal suction, chest movement is obtained. After 30 seconds of ventilation, the heart rate remains at 30 bpm.
iii) What two immediate actions are indicated?
The baby is transferred to the neonatal unit and requires ventilation. Initial ventilatory settings are:
Pressures 30/4, FiO 2 1.0, rate 40, Ti 0.4 s, flow 4 L/min
His initial arterial gas is as follows:
| pH | 7.03 |
| PCO 2 | 10.6 kPa |
| PO 2 | 4.5 kPa |
| BE | –10.6 mmol/L |
| Bicarbonate | 17.5 mEq/L |
v) What ventilation manoeuvres could you do to improve the gas?
a. Increase PIP
b. Increase Ti
c. Increase PEEP
d. Increase oxygen
e. Decrease Te
f. Increase flow
g. Decrease flow.
vi) What additional treatments could be considered?
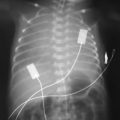
At 2 hours of age, the baby develops a tension pneumothorax. Following drain insertion, the following chest X-ray is obtained.
 |
| Figure 2.1. |
vii) List five abnormalities on the chest x-ray.
At 6 hours the following gas is obtained:
| pH | 7.18 |
| pO 2 | 2.8 kPa |
| pCO 2 | 8.3 kPa |
| BE | –9.6 mmol/L |
| Bicarbonate | 22.1 mEq/L |
Ventilatory settings are:
Pressures 35/5 cmH 20, MAP 17, FiO2 1.0, Ti 0.4 s, rate 60
viii) What is the oxygenation index?
ix) What is the most likely diagnosis?
x) List four possible treatments.
QUESTION 2
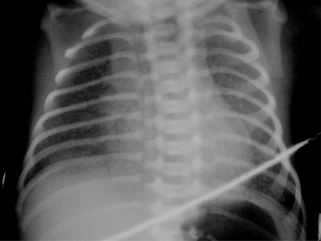
You are asked to review a baby on the postnatal wards 12 hours of age after a difficult delivery. The baby is said to be fractious and is not feeding. As part of a septic screen, a chest x-ray is carried out.
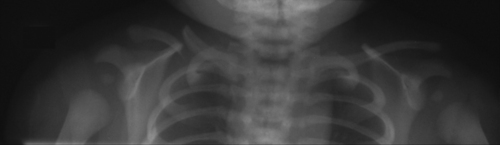 |
| Figure 2.2. |
What abnormality does this x-ray show?
QUESTION 3
On a first day check, a baby is noted to have an absent red reflex.
i) What is the most likely diagnosis?
a. Retinoblastoma
b. Cataract
c. Glaucoma
d. African race.
ii) What is the most important action and why?
QUESTION 4
You are called urgently to the postnatal wards to see a baby. He was born at term by normal vaginal delivery with good Apgars and was feeding well for the first 2 days. Since this morning his mother has noticed that he is floppy, and not interested in feeds. The midwife has just been called to see him as his mother has tried to wake him for a feed and cannot rouse him. He is pale with cold peripheries, capillary refill time is 7 seconds, respiration is shallow, pulses are weak and heart rate is 180 bpm.
i) What are the two most likely diagnoses?
a. Intracranial haemorrhage
b. Sepsis
c. Inborn error of metabolism
d. Hypoglycaemia
e. Hypernatraemia
f. Hypocalcaemia
g. Duct-dependent congenital heart disease
h. Hypoxic–ischaemic encephalopathy.
ii) What two immediate actions would you undertake?
iii) What three investigations would be most useful in directing early management?
iv) What three investigations would be most helpful in establishing the differential diagnosis?
vi) The following blood count is obtained:
| Haemoglobin | 18.6 g/dL |
| WBC | 25×10 9/L |
| Neutrophils | 15×10 9/L |
| Lymphocytes | 8.5×10 9/L |
| Monocytes | 1.1×10 9/L |
| Eosinophils | 0.4×10 9/L |
| Platelets | 155×10 9/L |
With this information what is the most likely diagnosis?
vii) While waiting for an echocardiogram you obtain the chest x-ray shown below.
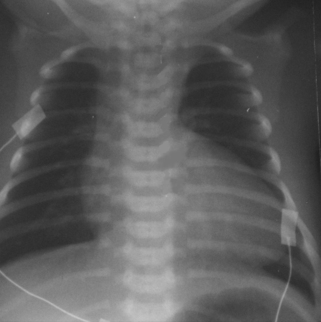 |
| Figure 2.3. |
Describe the abnormalities.
viii) With this information what is your diagnosis and action?
ix) Parents are anxiously waiting for further information. What are you going to tell them?
QUESTION 5
i) Give three indications for postnatal administration of BCG.
ii) Give three contraindications for postnatal administration of BCG.
QUESTION 6
A baby is born following a ventouse delivery. Mother has noticed a swelling on the side of the head. Choose four features that would allow you to discriminate between a caput, cephalhaematoma and a subgaleal haematoma (subaponeurotic haematoma).
a. Location
b. Demarcation
c. Jaundice
d. Clinical time course
e. Petechiae
f. Bruising
g. Erythema
h. Time of presentation
i. History of instrumental delivery
j. Coagulation
k. Blood count.
QUESTION 7
You are asked to see a baby who has the obvious features of a brachial plexus injury.
i) How do you differentiate between an Erb and a Klumpke palsy?
ii) The parents want to know why it happened, how common it is, what treatments are available and what the long-term outcome is likely to be.
QUESTION 8
A baby has been born by difficult instrumental delivery. You are asked to review the baby and skull imaging is performed. The following picture is obtained.
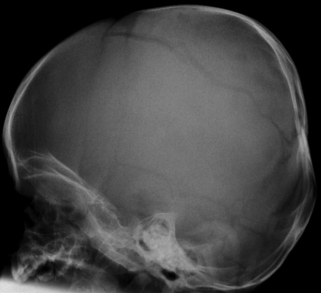 |
| Figure 2.4. |
i) What does the X-ray show?
ii) What management is indicated and why?
QUESTION 9
A baby is born by normal vaginal delivery. A right sided facial palsy is noted and the right eye cannot be fully closed.
i) What is the most likely cause, and what immediate action would you consider?
ii) Which of the following syndromes are associated with a facial palsy? Choose three.
a. Cardiofacial syndrome
b. Edwards syndrome
c. CHARGE syndrome
d. Möbius syndrome
e. Poland sequence
f. Goldenhaar syndrome.
iii) The palsy persists and appears unchanged at an outpatient appointment two weeks later. What course of action would you recommend?
On more detailed examination in clinic, the infant is noted to have an inability to abduct the left eye. In addition there is mild talipes equinovarus and what appears to be a lack of chest wall bulk on the right side. There is also mild micrognathia and microtia.
iv) What syndrome is most likely?
v) What medication used in pregnancy is associated with this syndrome?
vi) What is the most likely long-term outcome if this diagnosis is correct?
QUESTION 10
A term baby presents with an intracranial bleed and gastrointestinal bleeding. He has not had vitamin K prophylaxis. Which of the following coagulation results will confirm the diagnosis of vitamin K deficiency bleeding? Choose three.
i) Normal PT, Normal APPT, Normal Fibrinogen, Normal Platelets
ii) Prolonged PT, Normal APPT, Normal Fibrinogen, Normal Platelets
iii) Prolonged PT, Prolonged APPT, Low Fibrinogen, Low Platelets
iv) Normal PT, Normal APPT, Normal Fibrinogen, Low Platelets
v) Very prolonged PT, Moderately prolonged APPT, Normal Fibrinogen, Increased Platelets
vi) Very prolonged PT, Moderately prolonged APPT, Increased Fibrinogen, Normal Platelets
vii) Normal PT, Prolonged APPT, Decreased Fibrinogen, Normal Platelets
QUESTION 11
A 37 week gestation infant is admitted following an elective caesarean section. She has become tachypnoeic and a radial artery line has been inserted. She is noted to have a bruise on her left leg and a clotting screen is sent. The following result is obtained:
| PT | 17 seconds |
| APPT | 84 seconds |
| Fibrinogen | 2.5 g/L |
| Platelets | 449×10 9/L |
i) What does this coagulation result show?
ii) What abnormal conditions may explain it?
iii) What other explanation is possible?
iv) How would you distinguish between the possible causes?
QUESTION 12
A woman is admitted on to delivery suite at term. The CTG is abnormal with marked decelerations to 40 and a baseline bradycardia. She is taken immediately to theatre for an emergency caesarean section, and the baby is born 15 minutes later. At delivery the baby is white, floppy, and the heart rate is very slow.
i) What are your first actions? List four.
There is no respiratory effort, the heart rate is 20 bpm, and the baby is white and floppy.
ii) What are your next four steps?
The chest is seen to move well; however the heart rate remains at <20 bpm, and the baby is still white and floppy.
iii) What are your next actions? Suggest three.
The baseline heart rate remains at around 20 bpm. Good chest movement continues. You insert an umbilical venous catheter.
iv) What is the first thing you will do after successful placement?
v) Heart rate remains slow. You decide to give resuscitation drugs.
a. What will you use?
b. How much will you give?
c. What order will you give them in and what is your rationale for doing so?
With the drugs you have administered, the heart rate rises to approximately 60 bpm but no further. Ventilation remains effective. The blood tests you send from umbilical venous blood are reported back as:
The heart rate rises a little further to 75 bpm. The baby remains very white and floppy. Further blood results come back with a haemoglobin from the umbilical venous sample of 15.2 g/dL. However, the obstetrician performing the C/S reports that there was ‘an awful lot of blood about’.
vii)
a. What else might you consider?
b. What action would you take?
Following this action, the heart rate rises to >100 bpm but there is still no respiratory effort. The baby remains floppy and does not respond to stimulation. You admit the baby to the neonatal intensive care unit, and initiate positive pressure ventilation at pressures of 20/4, I:E ratio 0.3:0.8 seconds, FiO 2 0.21.
viii) What baseline monitoring would you consider? List six.
ix) What baseline investigations would you perform? List five.
Half an hour after admission, the following results are obtained from an arterial line:
pH 7.3, pCO 2 3.1 kPa, BE –15.5 mmol/L, lactate 9 mmol/L, Hb 10.2 g/dL
x) What three actions would you consider?
The nursing staff report oxygen desaturations on the monitor. They are uncertain as to whether there are associated abnormal movements. The baby remains extremely floppy.
xi) What further baseline investigations would you perform? Name six.
CFAM is commenced and the following trace is obtained.
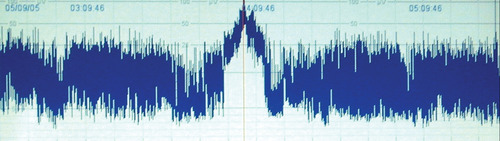 |
| Figure 2.5. |
xii)
a. Describe the trace. Is it normal?
b. What key features have led to your conclusion?
xiii) What treatment modalities would you consider?
xiv)
a. What is your first line medication for this condition?
b. What dose would you prescribe (the baby weighs approximately 3.5 kg)?
Despite this treatment, there is no improvement in the baby’s condition. The CFAM at this point is shown below.
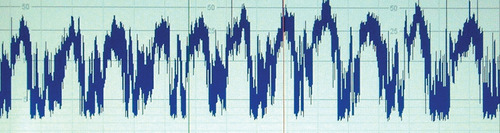 |
| Figure 2.6. |
xv) Describe the trace.
xvi) What do you do now?
xvii) Following this treatment, the baby’s clinical condition seems to stabilise, but the CFAM at this point is below.
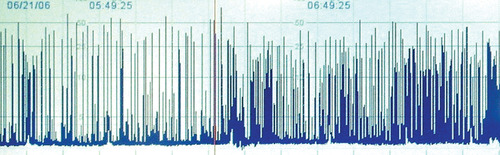 |
| Figure 2.7. |
Describe the trace.
xviii) What other complications may this baby sustain and how would you assess them?
As part as a more detailed assessment, cranial ultrasonography is performed with Doppler studies of the anterior cerebral artery. The cerebral blood flow velocity is more than 3 SD from the mean and the PRI (Pourcelot Resistance Index) is 0.5.
xix) Explain the significance of these observations.
At 24 hours of age, the baby is anuric, urea 9.2 mmol/L, creatinine 165 μmol/L. Blood pressure is persistently low with a mean BP of 28–30 mmHg despite inotropic support. Echocardiography shows very poor myocardial contractility. Assessment of liver function shows markedly elevated transaminases and gamma-GT. The baby is profoundly hypotonic and unresponsive to painful stimuli. A formal EEG is performed and the following trace obtained.
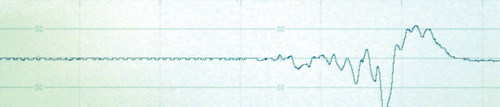 |
| Figure 2.8. |
xx) What does this show?
xxi) What would be the main areas of discussion that you would have with the parents?
QUESTION 13
You are informed of the imminent delivery of a baby at 25 weeks gestation.
i) In the few minutes you have before delivery what specific areas of preparation will you concentrate on? List five.
ii) The infant is delivered and cries at birth. What immediate measure can be taken to minimise heat loss?
iii) What actions should you take to optimise ventilatory support immediately after birth?
QUESTION 14
You are called to see a baby boy on the postnatal wards who has had difficulty feeding. On examination the baby is noted to be floppy and has normal tendon reflexes. There are spontaneous anti-gravity movements. There is no fasciculation.
i) Is this central or peripheral hypotonia?
ii) What could be the likely cause? Choose two.
a. Spinal muscular atrophy
b. Prader–Willi syndrome
c. Hypothyroid
d. Congenital myotonic dystrophy
e. Congenital myasthenia.
On further examination the baby is noted to have undescended testes and almond shape eyes.
iii) What is the most likely diagnosis?
iv) What test would confirm your diagnosis?
v) Which of the following endocrine problems is recognised as a characteristic finding in Prader–Willi Syndrome?
a. Hypothyroidism
b. Hypoinsulism
c. Hypoparathyroidism
d. Hypopituitarism
e. Hypoaldosteronism.
ANSWER 1
i)
e. Dry and wrap.
ii)
f. Check head position.
g. Visualise the oro-pharynx.
Many different treatment recommendations have been made in the past for meconium aspiration. Randomised controlled trials have failed to support any. Appropriate management of meconium aspiration should, as with all other scenarios, follow the normal resuscitation algorithm. There is evidence that suction on the perineum is ineffectual and this procedure cannot be recommended. Intubation and tracheal suction of all infants may be harmful to some and there is no evidence to support tracheal lavage. Clamping of the chest has no effect on a baby that is not breathing and may suppress respiration in an infant who is breathing effectively. The current recommendations therefore are to dry and wrap initially and then to assess respiration. If there is effective respiration, then no action is required, but close monitoring must be continued. If an infant shows no respiratory effort, then the airway must be opened, and cleared under direct vision, with inflation breaths thereafter if spontaneous respiration does not occur. An alternative strategy would be to attempt inflation breaths and only directly visualise if chest movement cannot be obtained, despite the use of appropriate airways opening manoeuvres.
iii) Continue effective ventilation and commence cardiac compressions. There is some debate as to whether cardiac compressions should be started immediately after inflation breaths or after 30 seconds of effective ventilation. Undoubtedly if there is no heart rate or profound bradycardia, then cardiac compressions should be started immediately after chest inflation has been obtained. However, if the infant is moderately bradycardic (60 bpm), it is reasonable to delay the commencement of compressions until a 30 second cycle of ventilation has been completed.
iv) Answer f is correct.
The pH is low (normal values 7.31–7.34 at 1–6 hours) showing an acidotic picture. The PCO 2 is raised (normal values 4.7–6.0 kPa at 1–6 hours) which could account for the low pH but the bicarbonate is also low (normal 20–26 mmol/L) which is also contributing to the acidosis. Thus it is a mixed acidosis.
v) Answers a, c, e and f are correct.
There are several ventilatory manoeuvres that could be tried to improve the gas and the baby’s condition. Increasing the peak inspiratory pressure (PIP) would help oxygenation by increasing the mean airway pressure. Decreasing Te would increase the rate and thus decrease the carbon dioxide level. Increasing PEEP would again increase the mean airway pressure and might improve oxygenation. There is a possibility of gas trapping in meconium aspiration which may be worsened with a higher PEEP. Increasing the flow would increase the mean airway pressure and hopefully oxygenation. A standard flow would be 7 L/minute. Increasing the inspiratory time is not helpful with meconium aspiration as it can lead to air trapping and air leaks. We are unable to increase the oxygen any further as the baby is already in 100% oxygen. Decreasing the flow would lead to a decrease in mean airway pressure and thus worsen oxygenation.
vi) Additional treatments that could be tried are:
High frequency oscillation ventilation. This leads to improved gas exchange and can prevent up to 80% ECMO candidates requiring ECMO.
Surfactant. Meconium inactivates surfactant function and this may be important in the pathophysiology of meconium aspiration. Several studies including a Cochrane Review have shown a reduction in air leaks and a decreased need for ECMO. 2
ECMO – see answer x) for details. 3
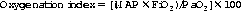
vii) Five abnormalities on the chest x-ray:
a. Partially drained pneumothorax
b. Chest drain in situ
c. Endotracheal tube in situ
d. Transcutaneous monitor
e. Patchy bilateral opacification.
viii) 80.
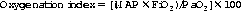
The oxygenation index is an excellent measure of oxygenation in severe pulmonary disease. As the PaO 2 falls below 5–6 kPa (kPa×7.6 = mmHg), the denominator for the OI equation generates an exponential increase in OI. Studies during the introductory phase of ECMO suggested that an OI of greater than 40 on three out of five post-ductal arterial blood gases drawn within a 30-minute period defined a mortality greater than 80%.
ix) Persistent pulmonary hypertension. The gas in this case shows marked hypoxia, but with less severe hypercarbia. The high solubility of CO 2 means that the limited amount of blood entering the lungs is able to exchange CO 2 more effectively than oxygen.
x)
a. Nitric oxide
b. ECMO
c. Tolazoline
d. Prostacycline.
Nitric oxide is of proven benefit in term infants with established PPHN. It is a selective pulmonary vasodilator and there is evidence that a combination of HFOV and NO is better than either modality alone. In those infants who do not respond ECMO may be effective. If NO and HFOV are to be used it is advisable to discuss the individual patient with an ECMO centre early. There is evidence that delaying ECMO can lead to longer ECMO run times and poorer outcome. Prior to nitric oxide, vasodilators such as tolazoline and prostacycline were the available treatments. Vasodilatation was less selective and hypotension was a frequent side effect. If availability of nitric oxide is likely to be delayed, it is still appropriate to use these drugs. There are anecdotal reports of direct intratracheal administration of tolazoline and nebulised prostacycline. There is no evidence to support their use in this fashion. 3
ANSWER 2
The x-ray shows a fractured right clavicle. Clavicle fractures occur in 0.2–5% of newborns. Large birth weight, technically difficult deliveries due to shoulder dystocia and the use of vacuum or forceps are associated with increased risk of clavicle fractures. However, some studies suggest that the majority occur with no reported difficulties during labour. Although these normally heal without complication, there are cases where an osteomyelitis has developed or there has been an associated brachial plexus injury. Re-fracture has been described in the literature, although this is rare. Clinical signs may be minimal movement of the affected limb with irritability, swelling with crepitus, absent Moro reflex or a palpable lump. Newborn fractures require no intervention apart from re-examination and possible repeat x-ray within 2 weeks to ensure healing is occurring. Early specialist intervention is recommended if there has been a brachial plexus injury.
ANSWER 3
i) Cataract is the most likely diagnosis to be picked up on day 1, although retinoblastoma and glaucoma do cause an absent red reflex. Cataracts occur in 2–3 per 10,000 live births. They are associated with congenital infection such as rubella, herpes simplex, toxoplasmosis and cytomegalovirus; syndromes, e.g. Lowes, Conradi’s and Hallermann–Streiff; familial (the majority are autosomal dominant although autosomal recessive and X-linked have been described); and metabolic, such as hypocalcaemia, galactosaemia. Retinoblastoma does result in an absent red reflex but it is rare (the incidence is 1 in 20,000 live births) and average age for diagnosis is 18 months. Congenital glaucoma is infrequent but is a preventable cause of blindness. It presents with a cloudy cornea and progressive globe enlargement. It is sometimes difficult to see the red reflex in a baby of African race, but it is not absent. The corneas of darkly pigmented eyes reflect more light and appear cloudier than light eyes.
ii) Absent red reflex requires an urgent referral to ophthalmology. The best results are obtained when surgery and specialised ophthalmological support are initiated early, before amblyopia has developed. This may occur within six weeks from birth.
ANSWER 4
i)
b. Sepsis.
g. Duct dependent congenital heart disease.
Sudden collapse at 2–3 days in a previously well baby, who has been feeding well and caused no concerns before, should raise the suspicion of a duct dependent heart lesion. Sepsis acquired at the time of birth usually presents earlier than this but cannot be excluded and appropriate treatment should be initiated while the diagnosis is clarified. Metabolic abnormalities may lead to sudden collapse but in these conditions previous good health and good feeding is unlikely. Hypernatraemia is unlikely in a baby who is feeding well. Intracranial haemorrhage initiated at the time of delivery usually leads to a more gradual deterioration. Rarely a catastrophic bleed may lead to sudden collapse, normally shortly after birth. Hypoxic–ischaemic encephalopathy is unlikely to be a cause in a baby who has shown no abnormal signs in the first two days after birth.
ii)
a. Secure airway and support breathing
b. Obtain venous access.
Any seriously ill infant, irrespective of the cause, should be approached using the standard resuscitation algorithm. This infant has shallow respirations and obviously has impaired circulation, but A and B must come before C. This infant may well continue to deteriorate very rapidly and there is no doubt that vascular access will be needed. Peripheral venous access may well not be possible. The umbilical vein may be accessible but may not and if so intra-osseous access may be the only available option. It is important that access is obtained swiftly and repeated futile attempts for peripheral access should be discouraged.
iii)
a. Blood glucose
b. Blood gas
c. Electrolytes
Any baby who has collapsed, irrespective of the cause, is likely to have an abnormal glucose (both low and high are possible). If the glucose is low, a bolus of 10% dextrose 2.5–5 mL/kg should be given intravenously and a continuous infusion started. A blood gas will allow assessment of ventilatory requirements and extent of acidosis. Electrolyte assessment is necessary as abnormalities may lead to collapse but may also be secondary to other causes. Irrespective of whether cause or effect, these must be treated.
iv)
a. Septic screen
b. Metabolic screen
c. Echocardiogram
Ideally a full screen should be carried out on all babies who collapse, including a supra-pubic urine specimen, lumbar puncture, chest X-ray and these should be performed pre-antibiotic administration, provided this does not cause unnecessary delay. As antibiotics will be commenced before any results are available to support a diagnosis of sepsis, a septic screen will not influence early management but is essential to refine the diagnosis. The metabolic screen should include lactate, ammonia, acyl carnitines, amino and organic acids. A more detailed metabolic screen can be tailored to initial results after consultation with a biochemist. Although an alternative diagnosis may have become apparent before any results are available, it is important that these samples are sent early so that results become available relatively soon in the diagnostic process. An echocardiogram is most likely to give the definitive diagnosis of congenital heart disease and will allow assessment of the degree of cardiac compromise. Although chest x-rays may be helpful, even severe cardiac anomalies may have a relatively normal x-ray. An ECG is unlikely to be helpful in this case.
v)
a. Ventilation.
b. Antibiotics.
c. Intravenous fluids.
d. Prostaglandin E2.
This baby has collapsed and respiration is failing. Respiratory support is required and will be guided by blood gas analysis. Antibiotics should be commenced as soon as a septic screen has been performed and it is essential that there are no delays in starting therapy. The most likely organisms to cause postnatal collapse are Group B Streptococcus and E. coli and initial therapy should cover these organisms specifically.
This baby will need maintenance intravenous fluids. The need for a fluid bolus is contentious – the baby is showing signs of shock and a single fluid bolus would not be inappropriate. However, if a cardiac abnormality is responsible for the collapse, further fluid boluses may worsen the situation.
If the baby has collapsed following duct closure in a duct dependent heart lesion, the only effective therapeutic intervention is to re-open the duct using prostaglandin E2 or E1 (E2 is as effective as E1 and significantly cheaper). If an echocardiogram is immediately available, it may be appropriate to withhold treatment until the diagnosis is established. In practice this is often not the case and it is not acceptable to delay starting prostaglandin. In this situation, administration in itself may be diagnostic. The major side effects of Prostin are apnoeas, but as this infant is already ventilated for failing respiration it does not present a risk.
vi) Congenital heart disease. All these parameters are within the normal range for a newborn baby within the first week of life. A relatively high white cell count is normal (10–26×10 9/L) and a high normal neutrophil count (2.7–14.4×10 9/L) is more reassuring than worrying. Babies with severe sepsis leading to collapse are more likely to be neutropenic than have a neutrophilia. Similarly, severe infection is likely to be associated with marked thrombocytopenia rather than a low normal platelet count (150–450×10 9/L). If overwhelming sepsis is excluded, heart disease becomes the most likely diagnosis in a previously well baby.
vii)
a. Heart shape resembles egg on side.
b. Narrow vascular pedicle.
viii)
a. Transposition of the great arteries. Although these are the classical x-ray signs of a transposition, this diagnosis is possible with a relatively normal x-ray.
b. Inform paediatric cardiologists. There is no doubt that this child will need to be managed within a specialised cardiac unit. In many centres early correction is performed and early transfer is indicated.
ix) It will be necessary to discuss the abnormal anatomy and the fact that surgery is required. Parents are likely to want to know why the diagnosis has been missed despite an apparently normal baby check and antenatal scans. It will be necessary to explain the contribution of the normal ductus and the fact that, prior to closure, there would have been no abnormal signs. Although antenatal scanning may detect some babies with this condition, it may not be detected in a significant proportion even with a very skilled ultrasonographer. Providing the baby did not suffer prolonged acidosis during the collapse, it should be possible to maintain stability with the Prostin infusion and successfully transfer to a specialist centre. The management thereafter will be determined by the particular practice of the specialist centre and is probably beyond the scope of your discussion with the parents.
An arterial switch is now the treatment of choice in most cases and operative survival has been reported in some centres to be 95–97% in infants with uncomplicated presentation. Long-term survival following the procedure is excellent, although complications happen in a significant number.
ANSWER 5
i) Current Health Protection Agency recommendations are that BCG should be given to:
a. All infants in areas within the United Kingdom where the incidence of tuberculosis is greater than 40 per 100,000 population per year.
b. Infants, wherever they live, with one or more parent or grandparent born in a country with a tuberculosis incidence of greater than 40 per 100,000.
c. Previously unvaccinated new immigrants from high incidence countries.
Up-to-date prevalence of TB can be found at: http://www.globalhealthfacts.org.
ii)
a. Maternal HIV.
b. Mother has received continuous high dose steroids prior to delivery. In this case BCG should not be administered until adrenal function has been assessed in the infant.
c. BCG should not be given to babies receiving corticosteroids or for at least 3 months after steroid therapy stopped.
ANSWER 6
a. Location.
b. Demarcation.
d. Clinical time course.
h. Time of presentation.
Caput succedaneum is the most common abnormality and is simply positional oedema due to pressure on the scalp at birth. A cephalhaematoma is bleeding between the skull and periosteum, most commonly seen over one or both parietal bones and rarely over the occipital or frontal bones. A subgaleal haematoma or subaponeurotic haematoma is bleeding between the epicranial aponeurosis that connects the frontal and occipital components of the occipitofrontalis muscle. Unconfined expansion allows wide spreading and it is vital to identify as early as possible, before circulatory collapse. The most important factors therefore in discrimination are as above.
A caput occurs on the scalp at point of contact with the cervix and can therefore extend across suture lines. It is vaguely demarcated, with pitting oedema that shifts with time under the influence of gravity. It is at its largest and firmest at the time of birth, softening progressively and usually disappearing within 48–72 hours.
Cephalhaematomas are most likely over the parietal bones and are discrete swellings with distinct margins, which do not cross suture lines. They are due to rupture of the diploic veins which are separate for each cranial bone. They usually increase in size after birth for the first 12–24 hours and then remain stable. They are usually smooth and firm on palpation. They normally resolve over the first 2–3 weeks but can remain for months and may calcify.
A subgaleal haematoma is not confined and therefore may be located across the scalp. It may present as a firm to fluctuant mass which may extend onto the forehead or the back of the neck and behind the ears. Crepitus may be felt particularly at the margins and a fluid wave may be palpable. It will progressively increase in size from birth and may be massive, particularly if associated with a consumptive coagulopathy.
ANSWER 7
i) Brachial plexus injuries occur in 1 in 2300 live births (UK 1998 figure) although figures as high as 4/1000 have been reported in the literature, with 90% being Erb’s palsy (the most frequently affected regions C5, 6, less commonly also involving C7) and 10% Klumpke’s (involvement of C8, T1). Clinical presentation of Erb’s is typically the inability to abduct and externally rotate the shoulder, to flex the elbow and to supinate the forearm. When C7 is also involved neither the wrist nor fingers can be extended giving the classic Waiter’s Tip sign. Involvement of the lower roots in Klumpke’s palsy leads to involvement of the hand with a complete loss of grip, but preservation of movement elsewhere.
ii) In some cases it is thought that it is due to postural factors in utero although evidence for this is unclear. In most cases, it appears to be due to distraction at birth, i.e. pulling appears stretching leading to rupture, but this may be due to the birth process itself and does not have to be attributed to assistance. Risk factors are known to be shoulder dystocia (64% of cases), large birth weight, multiparity, assisted delivery, previous child with obstetric brachial palsy, prolonged labour and breech presentation. It is of interest to note that damage is most likely and prognosis worst in a small baby born by breech delivery, where C5/C6 avulsion occurs in approximately 80% of cases, and surgery is almost always required. Associated injuries are fractured clavicle (10%), fractured humerus (10%), subluxation of cervical spine (5%), spinal cord injury (5%), facial palsy (10–20%) and diaphragmatic palsy (5%).
Affected infants need to be followed up closely to assess the extent of recovery. Physiotherapy should be instigated early to maintain joint mobility and if recovery appears delayed or incomplete, surgery should be considered with referral to a specialist centre. In the absence of significant recovery, earlier referral is indicated. In a large UK study, about half of the infants had recovered fully at about 6 months of age but the remainder showed incomplete recovery, including 2% with no recovery. With appropriately timed surgery, substantial improvement may be reported but there is a significant long-term disability risk which should not be underestimated. 4
ANSWER 8
i) The x-ray shows a linear skull fracture. Linear skull fractures are relatively common compared to depressed fractures and are usually in the parietal region. They commonly follow a forceps delivery or a prolonged, difficult labour. They have also been described following ventouse deliveries. Depressed fractures or ping-pong type fracture leads to an inward buckling of the bone where bone is depressed but not fractured. This injury is most commonly associated with forceps delivery and usually parietal bones are involved. 5
ii) Uncomplicated linear fractures usually do not require treatment and heal within several months without sequelae. They can be associated with extra- and intracranial complications including haemorrhage, although these are rare. Another complication is tearing of the dura and the development of a leptomeningeal cyst. The fracture line may widen rapidly within weeks and infants therefore need a repeat x-ray at a few months of age to detect early widening of the fracture line.
For depressed fractures, some deformities resolve on their own, other reports include the use of digital pressure and use of suction from breast pump and obstetric vacuum extractors. Neuro-surgery is considered if other modalities are unsuccessful.
ANSWER 9
i) The most likely cause is birth trauma which accounts for approximately 80% of all cases. Forceps delivery is associated with approximately 75% of cases but it is of interest to note that in children with long-standing evidence of facial palsy there was no statistical association with the use of forceps and prenatal factors may be implicated. 6 Acutely, the only important aspect is to protect the exposed cornea and therefore artificial tears and patching may be indicated.
ii)
a. Cardiofacial syndrome – true. This is also known as deletion 22q11.2 syndrome, velo-cardio-facial syndrome or Di George syndrome. There are abnormalities in the face and heart (as expected from the name) but there are also problems with mild learning difficulties, slender and hypotonic limbs with hyperextensible joints. Facial palsy is an occasional finding in this syndrome.
b. Edwards syndrome – false. There are characteristic facial features associated with trisomy 18, but facial palsy is not one of them.
d. Möbius syndrome – true. The basic features of Möbius syndrome are mask-like facies with sixth and seventh cranial nerve palsies. Other cranial nerves can also be affected. See answer iv.
e. Poland sequence – false. Poland sequence is a unilateral defect of pectoralis major and syndactyly of the hand. Occasional hemi-vertebra occurs, as do renal anomalies and dextrocardia. Poland sequence has been grouped with Möbius syndrome because of a similar pathogenesis classified as the subclavian artery disruption sequence.
f. Goldenhaar syndrome – false. Also known as oculo-auriculo-vertebral spectrum, this abnormality occurs due to developmental problems with the first and second branchial arch. It is sometimes accompanied by renal, vertebral and ocular abnormalities hence the use of the word ‘spectrum’ in the name. Facial anomalies are marked but the majority of affected individuals are of normal intelligence. Facial palsy is not associated with Goldenhaar syndrome.
iii) Usually some recovery will be evident shortly after birth. In 90% of cases there is spontaneous recovery within four weeks of birth. 7 In infants with no evidence of resolution, referral to a surgeon with a specialised interest in this area is recommended. Surgical decompression is rarely needed but should be strongly considered if there is no recovery either on physical examination or electro-physiologically by five weeks of age.
iv) The combination of facial palsy with an abnormality of eye movement involving at least one cranial nerve is the minimum requirement for a diagnosis of Möbius syndrome. The sixth cranial nerve (abducent) is affected in approximately 75% cases. In approximately one-third of cases there may be defects affecting the lower limbs, facial structures and chest wall anomalies which are typical of Poland syndrome. It is for this reason that some reports suggest an association with facial nerve palsy and Poland syndrome. Experts in the field believe that these cases are Möbius syndrome variants.
v) An association between the use of misoprostol as an abortifacient has been reported to be associated with a 30-fold increase in the incidence of Möbius syndrome.
vi) During infancy, failure to thrive due to feeding problems and aspiration is not uncommon. Approximately 30% patients have mental retardation, which is commonly severe. Approximately one-quarter develop severe autistic symptoms. Speech impairment is very common.
ANSWER 10
Answers ii, v and vi are correct.
In all cases of vitamin K deficiency bleeding (previously haemorrhagic disease of the newborn), clotting studies show prolonged PT, normal platelets and normal fibrinogen. In a severe deficiency the APPT may also be prolonged and platelets and fibrinogen increased.
Other answers:
i) Normal.
iv) Thrombocytopenia – allo- or iso-immune with no other derangement of clotting.
vii) Need repeat sample – results do not match with clinical possibilities.
viii) Inherited deficiency of factor VIII, IX, XI, XII. Also consider heparin contamination.
ANSWER 11
i) The APPT is prolonged. PT is at the upper end of normal for a term infant immediately after birth. Fibrinogen is normal and platelets at the upper end of the normal range.
ii) This result could be explained by an inherited deficiency of factor VIII, IX, XI or XII. These conditions classically result in prolongation of APPT without affecting other parameters.
iii) If this coagulation screen had been performed on a sample taken through the arterial line the possibility of heparin contamination must be considered. This also may lead to prolongation of the APPT without affecting other clotting parameters.
iv) This could be differentiated from an inherited factor deficiency by use of the reptilase test. The reptilase time will be normal when the abnormality is due to heparin contamination but abnormal with inherited factor deficiencies. Assuming that heparin contamination could be discounted as a cause, further tests should be directed at differentiation between the different factor deficiencies. This will normally involve measurement of different factor levels but interpretation may be difficult in the newborn period and expert advice should be sought at the outset.
ANSWER 12
i)
a. Dry and wrap. Despite the severity of the baby’s condition, it is still vitally important to dry and wrap the baby.
b. Call for help.
c. Open the airway. The baby is extremely floppy and therefore airway opening manoeuvres are crucial in the early stage of resuscitation.
d. Assess – colour, tone, heart rate and breathing.
ii)
a. Inflation breaths. 5 at 30 cmH 2O pressure, for 2–3 seconds each to see the chest rise.
b. Ventilation breaths. 30 seconds comprising 15 ventilation breaths.
c. External cardiac massage for 30 seconds.
d. Re-assess – colour, tone, heart rate and breathing.
iv) Send blood for pH and blood gases, haemoglobin and blood glucose.
v)
a. Adrenaline and sodium bicarbonate.
b. 0.1 mL/kg 1 in 10,000 adrenaline.
2–4 mL/kg of 4.2% sodium bicarbonate.
c. There is as yet no consensus on the order of which drugs should be given. Adrenaline is frequently given first and followed by sodium bicarbonate when no or little response is observed. In an acidotic infant it is probably more sensible to administer bicarbonate before adrenaline as binding of adrenaline to its receptor is said to be less effective in an acidotic milieu.
vi)
a. Further bicarbonate.
b. Further adrenaline.
vii)
a. Severe acute blood loss around the time of delivery. This baby has only shown partial response to appropriate resuscitation. It is therefore quite likely that there are further factors contributing. A haemoglobin of 15.2 g/dL is not unduly low but if blood loss is recent, this may well not represent the final haemoglobin once compensatory fluid shifts have occurred. The haemoglobin may well drop to very significant lower levels.
b. Emergency transfusion with O negative CMV negative blood. This should be stored in a fridge on labour ward in Pedipacks.
viii)
a. Heart rate.
b. Respiratory rate.
c. Oxygen saturation.
d. Blood pressure.
e. Urine output.
f. Temperature.
There is already evidence to suggest that this baby has suffered a severe antepartum/peripartum injury. There may be considerable physiological instability, and thus all parameters normally measured in a sick infant should be recorded.
ix)
a. Repeat blood gas (including lactate).
b. Repeat haemoglobin.
c. Baseline coagulation.
d. Baseline U + E.
e. Repeat blood glucose.
If there has been severe blood loss and a major ischaemic event, there may be rapid changes in haemoglobin and other measures. However, gas exchange may be minimally affected.
x)
a. Blood transfusion.
b. Reduction of ventilation to treat respiratory alkalosis.
c. Treatment of metabolic acidosis.
On first impression a pH of 7.3 appears acceptable. However this is obtained through a combination of a metabolic acidosis and a respiratory alkalosis, neither of which are acceptable. In some cases, a moderately affected baby may spontaneously correct the metabolic acidosis, but it cannot be assumed that this will happen. Close monitoring and appropriate intervention is therefore essential.
xi)
a. Glucose
b. Repeat gas
c. Calcium
d. Magnesium
e. Cerebral function monitoring (CFM)
f. Cranial USS
In this clinical scenario, seizure activity may be masked by the severe hypotonia. It is prudent in all cases of suspected seizures to check baseline electrolytes, especially glucose, calcium and magnesium. A repeat blood gas would help determine if there is a respiratory cause for the desaturations. A cranial USS would be useful to act as a baseline against which subsequent scans can be compared. Early scans may well show no significant abnormalities. CFM (cerebral function monitoring – integrated amplitude EEG) is a useful bedside test that is becoming widely available and easy to use with modern systems.
xii)
a. The trace is abnormal.
b. The trace shows a normal upper limit (should be 10–40μV) but the lower limit is depressed (should be >5μV). There is absence of sleep–wake cycling which should normally be seen and there is a marked increase in activity with a narrowing of the width in the middle of the trace. This is clear evidence of seizure activity.
To illustrate these differences compare with the normal trace below.
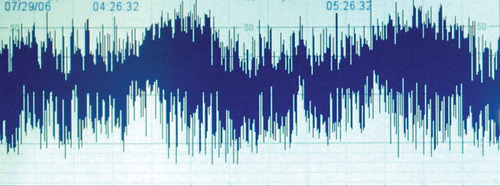 |
| Figure 2.9. |
xiii)
a. Anticonvulsants.
b. Fluid restriction.
c. Controlled hypothermia.
The CFM has shown clear evidence of fits. As there is evidence to suggest that uncontrolled seizure activity is associated with worse long-term outcome, this should be therefore be controlled. Anticonvulsant therapy remains the mainstay of treatment.
Cerebral oedema is likely to accompany a severe asphyxial episode and fluid restriction is normally commenced, although the evidence of a link between fluid intake and cerebral oedema has not been established. Renal compromise is likely in this case and fluid restriction would be a sensible part of management of this complication.
There is evidence to suggest that moderate hypothermia – either whole body or selective head cooling – may have a significant impact on outcome, particularly in moderate asphyxial injury. It is currently recommended that this should only be provided in specialist centres (within 6 hours in clinical trials), and in those without this facility care should be directed towards avoidance of active rewarming. The main UK trial (Total Body Hypothermia trial) finished recruiting at the end of 2006 with publication of provisional results of 18 month follow-up in abstract only at the end of 2008. As meta-analysis of the results of trials has shown a reduction in death or disability (RR 0.76; 95% CI 0.65–0.89) many centres are continuing to provide hypothermia for affected infants. 8
xiv)
a. Phenobarbitone remains the first-line agent for treatment of seizures in many centres. Although this is the case, there is no evidence base to support this.
b. Commonly phenobarbitone is commenced at 20 mg/kg – thus in this case a loading dose of 75 mg should be prescribed.
The current practice is to give further loads of 10 mg/kg to a maximum total of 40 mg/kg.
xv) The CFAM trace shows a lower baseline than normal and there is almost continuous seizure activity.
xvi)
a. Further loading dose of phenobarbitone can be given up to total of 40 mg/kg.
b. Loading dose of phenytoin 20 mg/kg slowly over 30 minutes.
c. Consider other anticonvulsants – lignocaine, midazolam or lorazepam. There is much national variation in the second-line treatment of seizures if phenobarbitone does not control the seizures.
xvii) The CFAM shows severe depression of the baseline with burst suppression. This is suggested by very flat periods on the trace with intermittent short spikes. A continuous EEG would need to be examined to confirm this.
xviii)
a. Renal failure:
Urine output
Dipstick urine for blood and protein
Urea and electrolytes
Urine electrolytes and osmolality.
b. Hepatic failure:
Liver function tests
Coagulation profile.
c. Bone marrow suppression:
FBC and differential.
d. Myocardial dysfunction:
Echocardiography
Ejection fraction calculation
Blood pressure.
Multi-system failure may follow a severe asphyxial insult and it is important that function of different systems is carefully assessed and treatments modified accordingly. Of particular importance is the use of aminoglycoside antibiotics in an infant whose renal function may be very poor.
xix) The use of cerebral ultrasound and Doppler assessment of cerebral haemodynamics has been shown to be of use in predicting the long-term outcome of these infants. A cerebral blood flow velocity above 3 SD from the mean is strongly associated with adverse outcome and is probably a more specific indicator than the Pourcelot Resistance Index (PRI = peak systolic-end diastolic/peak systolic pressure). A low PRI of <0.55 is strongly associated with poor outcome.
xx) Flat or low voltage trace with burst suppression.
xxi) This baby is showing signs of advanced multi-organ failure and an extremely high chance of death or of very severe brain damage if survival occurs. The combination of a low voltage EEG with burst suppression, low PRI on cerebral blood flow Doppler and severe multi-system failure firmly place this baby in this category. Discussion with parents should therefore be centred around the appropriateness of continuing intensive care. Should care be continued, all affected systems must be continuously monitored and a decision to continue care be constantly evaluated and re-assessed. All discussions and decisions should be clearly documented in the baby’s notes, and records should be kept of all personnel involved in discussions.
ANSWER 13
i)
a. Temperature control.
b. Appropriate help.
c. Surfactant.
d. Ventilation.
e. Discussion with parents.
Resuscitation should be well prepared for all infants. This is particularly the case for extremely immature infants. Temperature control is of vital importance and all equipment should be at working temperature well before delivery. Early surfactant is indicated and should be available and warm. Ventilation equipment, both on the resuscitaire and intensive care space the baby will occupy, should be prepared. If there is any chance, discussions with parents should be held, however briefly, before the baby is born.
ii)
a. Plastic occlusive dressings, either in the form of a bag or a sheet, are now widely used to prevent evaporative heat loss. The limited amount of data available strongly supports this intervention.
b. Use of a resuscitaire with a temperature probe. Although efforts must concentrate on prevention of even moderate hypothermia, care should be taken to avoid hyperthermia as well. Use of a resuscitaire on auto mode with measurement of the baby’s temperature is a highly desirable means of optimising body temperature.
c. Resuscitation in a warm ambient environment. Delivery suites are often relatively cool with air conditioning systems producing cold down drafts that may seriously impair the ability to maintain a stable thermal environment. If this situation exists, the resuscitaire should be positioned in a location where external factors can be minimised.
d. Maintenance of supportive environment during transfer to the intensive care unit. Although heaters on modern resuscitaires are extremely effective in maintaining satisfactory body temperature, this no longer works when the resuscitaire is disconnected from the main electrical supply. Transport over any distance should therefore be in a pre-warmed transport incubator. Transport over short distances can be performed on a disconnected resuscitaire, providing the infant is surrounded by an abundance of warmed towels. Heat loss from the head should be minimised by a hat.
iii)
a. Prompt and appropriate resuscitation. It is generally felt that intervention to support respiration should commence immediately in very immature infants. There is no evidence to support an expectant policy and there is little doubt that allowing hypoxia and hypercarbia before resuscitation is commenced is likely to make subsequent resuscitation more difficult.
b. Early surfactant. Randomised controlled trails have clearly shown that very early treatment with surfactant (within first 15 minutes) is associated with significantly better outcome than delaying surfactant until signs of respiratory distress develop. Some practitioners advocate a policy of surfactant administration before inflation breaths. Others recommend securing adequate lung inflation before surfactant administration. Currently available evidence suggests that there is no disadvantage from achieving adequate lung inflation prior to surfactant. Whichever policy is chosen, there is no question that delaying surfactant administration beyond the first few minutes cannot be recommended.
c. Appropriate pressure for inflation and subsequent ventilation breaths. Resuscitation guidelines advocate the use of five prolonged relatively high pressure inflation breaths to recruit an appropriate degree of lung filling. However, these guidelines are specifically derived for resuscitation of term infants. Although adequate lung inflation must still be obtained in premature babies, there are concerns and limited evidence to suggest that early hyperinflation may be damaging to the lungs. To minimise this potential damage, pressure controlled T-piece delivery systems should be used instead of resuscitation bags with poorly controlled inflation pressure and normal breaths should be given. After adequate initial breaths, pressure must be decreased to continue with ventilation breaths. In some preterm infants, with hyaline membrane disease, chest movement may be difficult to ascertain, but a response in heart rate will be obtained.
d. Avoidance of hyperoxia. Evidence for oxygen concentrations to be used in resuscitation of preterm babies is lacking. There is increasing evidence to suggest that there is no detriment from resuscitating term infants in air as opposed to 100% oxygen. Although there is currently no consensus as to the best oxygen concentration in which to initiate resuscitation, there is no reason to suppose that hyperoxia is desirable at this point when we know it is not at any other point. The commonest limiting factor determining management is the availability of an oxygen blender on the resuscitaire. Currently different practitioners are initiating resuscitation in air, or in an arbitrarily selected oxygen concentration and adjusting according to heart rate response. What is certain is that the routine use of 100% oxygen in the resuscitation of preterm infants cannot be recommended. 9
e. Choice of appropriate ventilation strategy. Although there is general agreement that ventilatory support should be provided immediately in extremely premature infants, there are different schools of thought as to how this is best provided. Some practitioners advocate the use of CPAP from birth (some stipulating that the baby be intubated, surfactant administered and then extubated; some are strongly opposed to the destabilisation that an intubation/extubation policy may cause), others prefer to intubate and ventilate first and extubate to CPAP at a later date. The evidence to support any particular philosophy is inconclusive, and is to some extent dependent on the philosophy of an individual unit and the enthusiasm of staff in adhering to that philosophy.
ANSWER 14
i) Central. It is important to distinguish between central and peripheral hypotonia. Central hypotonia is caused by a problem above the lower motor neurone. The floppiness is much more severe than the weakness, and sometimes, despite being very floppy, infants can show strong movements on stimulation. Tendon reflexes are usually preserved and there is no fasciculation which is seen in peripheral hypotonia.
ii)
a. No – SMA causes a peripheral neuromuscular hypotonia and presents with severe weakness as well as hypotonia. SMA also causes muscle fasciculation.
b. Yes – Prader–Willi syndrome could be the cause.
c. Yes – hypothyroidism is unusual in that it can cause both central and peripheral hypotonia.
d. No – congenital myotonic dystrophy causes peripheral hypotonia with marked weakness of respiratory muscles, feeding difficulties and facial diplegia.
e. No – congenital myasthenia occurs in 10–12% of infants born to mothers with myasthenia. The neurological features present are very dramatic and can evolve rapidly with feeding and respiratory problems. Generalised muscle weakness is present in 70% of cases.
iii) Prader–Willi syndrome. Babies with PWS have bitemporal narrowing and ‘almond shaped’ palpebral fissures (although this may become more evident postnatally as opposed to at birth). The hair and the skin can be lightly pigmented compared to the rest of the family. The genitalia are usually hypoplastic. Boys have cryptorchidism, and girls have small labia minora and clitoris.
iv) Chromosomal analysis should be requested looking specifically at the proximal long arm of chromosome 15 (15q11-q13). The majority of cases (about 75%) are caused by a deletion of the paternal copy of the appropriate region of chromosome 15 which can be picked up by fluorescence in situ hybridisation. Approximately 20% of cases are due to uniparental disomy, which occurs when both copies of the chromosomal region are inherited from a single parent, in this case the mother. These cases have a normal FISH study but an abnormal DNA methylation test. Uniparental disomy occurs sporadically, making the recurrence risk extremely low. A small minority of people have a translocation or imprinting irregularity involving chromosome 15. The recurrence risk is less than 1%.
v) Infants with PWS have hypothalamic hypopituitarism which leads to hypogonadism and growth hormone deficiency. The growth hormone deficiency leads to short stature and low muscle bulk. Other associated problems in later life are scoliosis, strabismus and osteoporosis.
vi) Infants with PWS have delayed milestones. The average age for walking alone is about 2 years although there is wide variation in this time. Hearing is normal but strabismus is common. Problems with speech, particularly articulation problems, are widely reported. Parents with babies with PWS describe them as being very quiet and placid babies. They sleep well and the main problems in the early postnatal period tend to be with feeding and weight gain, which persist up to 6 months or longer. Most children with PWS have borderline or moderate learning difficulties. The average IQ is about 70, although affected individuals may find it hard to perform at their IQ level, as emotional and social skills may be less well developed than in their peers. Writing and reading are usually considerably better than number skills and abstract thinking.
vii) In the early neonatal period, feeding and growth must be addressed. Many babies will need tube feeding with high calorie milks. The babies seem to tolerate nasogastric tubes and it is best to avoid gastrostomies as they can lead to significant areas of lipo-atrophy (predominantly abdominal) in later life. The most publicised complication of PWS is obesity. Between 2 and 6 years of age, children develop a hyperphagic phase which appears to be related to a hypothalamic problem affecting the satiety pathway. There has been much research into the specific defect, looking at neuropeptide Y, pro-opiomelanocortin, leptin and ghrelin. The hyperphagia is thought to be due to the decreased ability to feel satiated when the stomach is full. There have been many trials of treatment including appetite suppressant medication, surgery to decrease the gastric volume and behavioural therapy to decrease the feeding issues. Childhood obesity carries significant risk of morbidity including diabetes, heart failure and sleep apnoea. Dietary management is crucial during early childhood with dietetic input and mealtime routines. Growth hormone deficiency affects all individuals with PWS. Growth hormone is being used to improve linear growth and also improves amount of lean muscle mass compared to fat. Evidence shows that early treatment with growth hormone in the first year leads to improved growth and improved motor development. It has also been shown that facial and body appearance can normalise. Selective serotonin reuptake inhibitors can be helpful if the child shows obsessive–compulsive traits that are commonly seen along with other behavioural disorders.
Overall, the management of infants with PWS is best in a multidisciplinary team setting with paediatricians, endocrinologist, dieticians and psychologists. There are good support groups for parents (www.pwsa.co.uk).
REFERENCES
1. Resuscitation at birth. Newborn Life Support Provider Course Manual. Resuscitation Council (UK), London, 2006
2. Soll, RF; Dargaville, P, Surfactant for meconium aspiration syndrome in full term infants, Cochrane review ( 2) ( 2000); CD002054.
3. Hintz, SR; Suttner, DM; Sheehan, AM; et al., Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization, Pediatrics 106 (6) ( 2000) 1339–1343.
4. Evans-Jones, G; Kay, SPJ; Weindling, AM; et al., Congenital brachial palsy: incidence, causes, and outcome in the United Kingdom and Republic of Ireland, Arch Dis Child Fetal Neonatal Ed 88 (2003) F185––F189.
5. King, SJ; Boothroyd, AE; et al., Cranial trauma following birth in term infants., Br J Radiol 71 (1998) 233–238.
6. Laing, JHE; Harrison, DH; Jones, BM; et al., Is permanent congenital facial palsy caused by birth trauma?Arch Dis Child 74 (1) ( 1996) 56–58.
7. Smith, JD; Crumley, RL; Harker, LA, Facial paralysis in the newborn, Otolaryngol Head Neck Surg 89 (6) ( 1981) 1021–1024.
8. Edwards, AD; Azzopardi, DV, Therapeutic hypothermia following perinatal asphyxia, Arch Dis Child Fetal Neonatal Ed 91 (2006) F127–F131.
9. Richmond, S, ILCOR and neonatal resuscitation, Arch Dis Child Fetal Neonatal Ed 92 (2007) F163–F165.