CHAPTER 40 Safety and Outcome in Pediatric Anesthesia
Anesthesiology has served as a model for patient safety and was the first medical specialty to recognize patient safety as an independent problem (Gaba, 2000). The safety of infants and children undergoing general anesthesia has improved considerably since the 1970s, as evidenced by significant decreases in anesthesia mortality despite the fact that more complicated surgical procedures have been performed on sicker children and more premature infants.
Since the 1980s, anesthesiologists’ awareness of and interest in the subject of patient safety has reached a new peak, and a number of new steps have been taken to ensure perioperative patient safety (Keats and Siker, 1985; Smith and Norman, 1987; Runciman, 1988a; Runciman, 1988b). In addition to advanced technology for patient monitoring, standards for basic patient monitoring have been implemented (American Society of Anesthesiologists [ASA], 1986; Eichhorn et al., 1986). Documentation of the quality assurance (QA) process has been emphasized as an integral and essential component for hospital accreditation by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) in the United States. To further improve the quality of patient care, a number of national and international organizations have been created, including the International Committee on Prevention of Anesthesia Mortality and Morbidity, the Anesthesia Patient Safety Foundation, the Australian Patient Safety Foundation, and the ASA Committee on Patient Safety and Risk Management (Cooper and Pierce, 1986; Cooper, 1988; Pierce, 1984, 1988; Runciman, 1988b). The Institute of Medicine of the National Academies is an independent, nonprofit organization that seeks to ask and answer the nation’s questions about health and health care. Their reports, such as the Preventing Medication Errors series, have analyzed complex problems in the modern health care industry and provided solutions that have been widely adopted. Sessions on patient safety topics have also been incorporated into the programs of the American Society of Anesthesiologists and Society for Pediatric Anesthesia annual meetings.
Nevertheless, anesthesia-related morbidity and mortality still do occur during the administration of anesthesia and can happen with any anesthesiologist under various situations. An analysis of anesthesia mishaps from closed anesthesia malpractice claims in the United States, before the new patient monitoring standards with pulse oximetry and capnography were instituted, indicated that at least 80% of the claims consisted of preventable hypoxic damage caused by human errors rather than mechanical failures (Davis, 1984).
Allnutt (1987), a member of the British Army Personnel Research Establishment, examined human factors in anesthesia- related mishaps in comparison with those in military aviation accidents. He stresses that “both pilots and doctors make many errors” (i.e., performance that deviates from the ideal). “Usually there is sufficient slack in the system for the error to be … noticed and corrected, but some apparently innocuous errors are not noticed and some systems are not so forgiving as others” (such as a high performance aircraft in flight). “Thus recovery from a control error when flying at high speed, low level may not be possible, whereas the same error in the cruise [at high altitude] might barely occasion comment.” A basic tenet of Allnutt’s theory is that “all human beings, without any exception whatsoever, make errors and that such errors are a completely normal and necessary part of human cognitive function.” He goes on to state that “to claim exemption on the grounds of being a senior professor [or a] test pilot … or of having 30 years’ experience or 3000 accident-free hours, is the first step on the road to disaster.” The first step toward the prevention of catastrophe is for the pilot or the anesthetist to accept that he or she is as likely as anyone else to make an error (Pierce, 1988).
Anesthesia-related mortality
Overall Anesthesia-Related Mortality
The number of deaths associated with general anesthesia has declined steadily over the past several decades as the standard of anesthesia practice has improved and as advances have been made in instrumentation, anesthetic and adjuvant drugs, and safety monitors and standards. An extensive survey of 10 university hospitals by Beecher and Todd (1954) involving nearly 600,000 anesthetic cases between 1948 and 1952 suggested that mortality primarily attributable to anesthesia occurred in 1:2680 anesthesia cases (3.7:10,000), whereas the overall anesthesia-associated mortality was 1:1580 (6.3:10,000) anesthesia procedures (Graff et al., 1964). A survey by Dornette and Orth (1956) showed similar mortality rates. Data from the Baltimore Anesthesia Study Committee (1953 to 1963) showed an anesthesia-related death rate of 2.7:10,000 cases. During the 1970s and 1980s, statistics on anesthesia-related mortality in the United States were scarce, apparently because of medicolegal concerns. Anesthesia-related mortality from Canadian, British, and European sources during this time ranged from 0.7 to 2.2:10,000 anesthetic procedures (Bodlander, 1975; Harrison, 1978; Hovi-Viander, 1980; Turnbull et al., 1980; Lunn and Mushin, 1982; Hatton et al., 1983; Vickers and Lunn, 1983).
In the 1980s, European and Australian studies showed much lower rates of operative mortality directly attributed to anesthesia. A report from the British Confidential Enquiry into Perioperative Deaths (CEPOD), a survey that was jointly organized by the Associations of Anaesthetists and Surgeons of Great Britain and Ireland during 1985 and 1986 and included more than 480,000 general anesthetic procedures, indicated that mortality attributable to anesthesia alone was 1:185,000 (0.054:10,000) procedures (Buck et al., 1987). However, anesthesia, along with other causes, was thought to be a contributory factor in the death of between 1.4 (surgeons’ estimate) and 9.8 (anesthetists’ estimate) per 10,000 cases. A prospective survey of anesthesia outcome by the French Health Ministry during the time from 1978 to 1982, in which nearly 200,000 general anesthetic cases were documented, revealed an intraoperative and early postoperative death rate solely attributable to anesthesia to be 0.76:10,000 procedures, and an intraoperative death rate of 0.44:10,000 cases (Table 40-1) (Tiret et al., 1986, 1988).
TABLE 40-1 Historical Changes in Anesthesia-Related Mortality (All Ages)*
| Authors (Country) | Years Includedin Study | Incidence per 10,000 |
| Beecher and Todd (United States) | 1948 to 1952 | 3.7 |
| Graff et al. (United States) | 1953 to 1963 | 2.7 |
| Hovi-Viander (Finland) | 1975 | 2 |
| Lunn and Mushin (United Kingdom) | 1978 to 1979 | 1 |
| Tikkanen (Finland) | 1986 | 0.6 |
| Tiret et al. (France) | 1978 to 1982 | 0.76† |
| Buck et al., CEPOD (United Kingdom) | 1985 to 1986 | 0.05† |
| Eichhorn (United States) | 1976 to 1988 (ASA PS 1 or 2) | 0.05† |
| Kawashima (Japan) | 1994 to 1998 | 0.13† |
| Lagasse (United States) | 1995 to 1999 | 0.75 |
| Fasting and Gisvold (Canada) | 1996 to 2000 | 0.12 |
| Irita (Japan) | 1999 to 2002 | 0.1† |
| Lienhart (France) | 2006 | 0.12† |
ASA PS, American Society of Anesthesiologists physical status; CEPOD, Confidential Enquiry into Perioperative Death.
* For complete citations of studies, consult chapter reference list online at www.expertconsult.com.
† Anesthesia primarily responsible only; other factors may be involved.
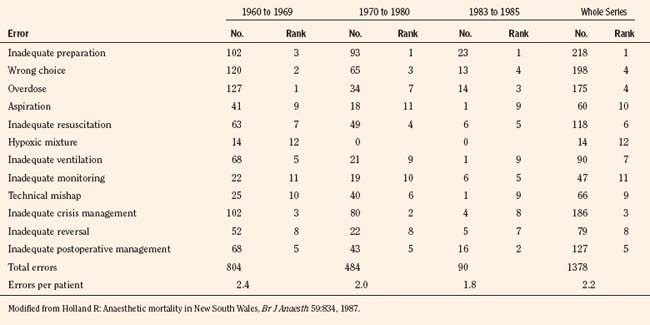
A longitudinal comprehensive anesthesia-related mortality study from New South Wales, Australia, which was continued by the same author using the same criteria since 1960 (interrupted between 1980 and 1983 because of the temporary loss of legal confidentiality), has indeed shown a steady decline in anesthetic mortality from 1.8:10,000 cases in 1960 to 0.38:10,000 cases by 1984 (Holland, 1984, 1987). Similarly, a longitudinal study from South Africa has also shown a decreasing trend in anesthesia-related mortality from 3.3:10,000 between 1956 and 1965 to 0.7:10,000 between 1983 and 1987 (Table 40-2) (Harrison, 1978, 1990).
TABLE 40-2 Anesthesia-Related Mortality: Longitudinal Studies at the Same Institution
| Authors (Country) | Years Included in Study | Incidence per 10,000 |
| Holland (Australia) | 1960 to 1969 1970 to 1980 1983 to 1985 |
1.8 0.97 0.38 |
| Harrison (South Africa) | 1956 to 1965 1967 to 1976 1983 to 1985 |
3.3 2.2 0.7 |
From Holland R: Anaesthetic mortality in New South Wales, Br J Anaesth 59:834, 1987; Harrison GG: Death due to anesthesia at Groote Schuur Hospital, Cape Town: 1956-1987. Part II. Causes and changes in aetiological pattern of anesthetic-contributory death, S Afr Med J 77:416, 1990.
In the United States, Eichhorn (1989) analyzed data from nine Harvard University–affiliated hospitals between 1976 and 1988. He reported 11 major anesthesia-related intraoperative accidents, including five deaths based on more than 1 million anesthetic procedures in relatively healthy patients (ASA PS 1 and 2); the anesthetic mortality was 0.05:10,000 cases; postoperative mortality, including two deaths from halothane hepatitis, was excluded from these statistics. After implementation of patient monitoring standards in 1985, there was only one serious accident (no mortality) in 319,000 general anesthesia procedures (Eichhorn et al., 1986). Of the 11 major accidents, eight cases were considered preventable with proper monitoring, especially with capnography. Unrecognized hypoventilation (seven cases) was the most common cause of major mishaps. Inadequate supervision of residents and nurse anesthetists was also contributory. Although Eichhorn’s statistics were based on a malpractice insurance database and are likely different and considerably lower than the data based on a peer-review process, anesthesia-related safety appears to have improved significantly.
Irita and others (2004) analyzed morbidity and mortality statistics in Japan from 1999 to 2002 from the Japanese Society of Anesthesiologists annual survey. They reviewed 3,855,384 cases completed during that time. The incidence of cardiac arrest and mortality totally attributable to anesthesia management was 0.47 and 0.1 per 10,000 anesthesia procedures. Half of the anesthesia-related deaths were caused by airway or ventilatory problems; the other causes were medication-related and infusion/transfusion accidents.
Practitioners of anesthesiology have institutionalized patient safety in their scientific and governing bodies (such as the ASA Anesthesia Patient Safety Foundation and similar organizations in other countries) (Cooper and Gaba, 2002). In 1999, the Committee on Quality of Health Care in America for the Institute of Medicine published a report entitled To Err Is Human: Building a Safer Health Care System (Kohn et al., 1999). The report stated, “Anesthesia is an area in which very impressive improvements in safety have been made.” This statement was based on the statistics that anesthesia-related mortality rates had decreased from two deaths per 10,000 anesthetic procedures in the 1980s to about 1 death per 200,000 to 300,000 anesthetic procedures administered at the time the report was published (probably quoting the report by Eichhorn [1989]). Such dramatic decreases in anesthetic mortality can be attributed to a variety of mechanisms, including wide acceptance of new monitoring guidelines, improvement in monitoring techniques, safer anesthetic drugs, and adoption of QA mechanisms and other systematic approaches for reducing human and systemic errors (Gaba, 2000; Stoelting, 2000; Lagasse, 2002).
Mortality in Infants and Children
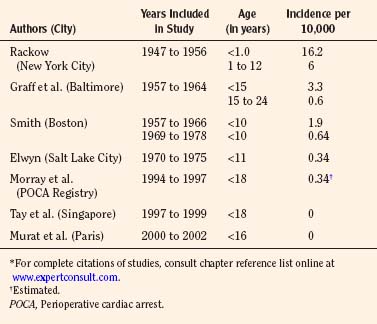
Among the pediatric age group, the anesthesia-related mortality has been reported to be disproportionately high in the literature. In the 1950s, Beecher and Todd (1954) and Stevenson and others (1953) found that accidental deaths resulting from anesthesia were disproportionately high during the first decade of life. Between 1947 and 1956 at the Babies Hospital/Columbia-Presbyterian Medical Center, Rackow et al. (1961) found that the rate of cardiac arrest associated with anesthesia in infants younger than 1 year of age (1 in 617 cases, or 16.2:10,000) was higher than in children aged 1 to 12 years (1 in 1678, or 6.0:10,000) and in adults (1 in 2580, or 3.9:10,000) (Beecher and Todd, 1954). Hypoventilation and hypoxia from ether overdose were among the common causes of death. Smith (1956) emphasized the importance of certain factors contributing to the high anesthetic mortality in pediatric anesthesia. These factors included: lack of proper equipment, improper preoperative rehydration and stabilization, inadequate intraoperative monitoring, error in fluid replacement, and aspiration of vomitus. Today, half a century later, some of these factors are still applicable.
In a report by the Baltimore Anesthesia Study Committee, anesthesia-related mortality for children younger than 15 years of age was found to be 3.3:10,000 cases (vs. 0.6:10,000 for those aged 15 to 24 years) (Phillips and Frazier, 1957; Graff et al., 1964). These authors also found that the ratio of anesthesia deaths to total surgical deaths was higher in the neonatal period than in any other age group. Furthermore, 57% of the deaths related to anesthesia occurred in healthy children (ASA PS 1 and 2). Respiratory problems were implicated in 83% of the anesthesia-related deaths (Graff et al., 1964) (Table 40-3).
In contrast, in a review of 73 anesthesia-related cardiac arrests in children between 1960 and 1972 (33% resulted in death), Salem and others (1975) found that both respiratory (airway obstruction) and cardiovascular causes (blood loss, preoperative anemia, inappropriate injection of succinylcholine and potassium) were equally responsible. In retrospect, most of these accidents were preventable.
In an attempt to improve patient safety during anesthesia in infants and children, a number of important innovations and improvements in perioperative management and monitoring were made by the pioneering pediatric anesthesiologists in the 1950s and 1960s. These innovations include homemade pediatric blood pressure cuffs and precordial stethoscopes (by Robert Smith in Boston) and endotracheal intubation (by Margo Demming in Philadelphia). Fellowship training in pediatric anesthesia also began in several cities in North America and in the United Kingdom in the 1950s and spread across the continent by the early 1970s (see Chapter 41, History of Pediatric Anesthesia).
By the mid-1970s, anesthesia-related morbidity and mortality decreased considerably. Management of known hazards, such as the full stomach, preoperative fever, and hypovolemia, was greatly improved by increased experience and knowledge (Smith, 1975). Smith (1980) reported the anesthesia-related mortality rate of 2:10,000 general anesthesia cases in children (0 to 10 years old) during the decade ending in 1966 at the Children’s Hospital in Boston; the mortality rate decreased to 0.6:10,000 anesthesia cases in the decade ending in 1978. Furthermore, there was a series of 35,710 consecutive tonsillectomies and adenoidectomies, mostly in children, without a single death at the Eye and Ear Hospital of Pittsburgh (Petruscak et al., 1974). There were 7500 consecutive anesthesia procedures for cleft lip and cleft palate repairs without a death at the Children’s Hospital in Boston (Smith, 1975). Elwyn, in his 5-year study between 1970 and 1975 at the Primary Children’s Hospital in Salt Lake City, reported one anesthetic death in 29,101 anesthetic procedures (0.34:10,000) in children under 11 years of age (Smith, 1980). Downes and Raphaely (1979) reported an anesthetic mortality of 0.2:10,000 cases (from a total of 50,000 patients) at Children’s Hospital of Philadelphia. Most fatalities occur during the first year of life, beyond which the risk of mortality is no higher than that in teenagers and young adults (Table 40-3) (Smith, 1975).
Despite advances in pediatric anesthesia, statistics from the 1980s still showed anesthesia-related mortality rates in children that were three to four times higher than in the general patient population, although the mortality rates in children had decreased considerably and appeared to have reached a plateau (Keenan and Boyan, 1985; Gibbs, 1986; Olsson and Hallen, 1988).
As part of a study of closed malpractice claims by the Committee on Professional Liability of the ASA, Morray and others (1993) compared pediatric and adult closed claims and found a different distribution of serious outcomes in children compared with those in adults. Of 2400 closed malpractice claims, 238 (10%) were in the pediatric age group (15 years old or younger). A majority of cases involved children younger than 3 years of age, and 28% of all pediatric cases involved infants younger than 1 year of age. Respiratory events (mostly inadequate ventilation) were more common than among adult claims (43% vs. 30%), and mortality was higher (50% vs. 35%), mostly attributable to inadequate ventilation. Anesthesia care was judged inadequate more often. The authors concluded that 89% of the pediatric claims that were related to inadequate ventilation could have been prevented with proper monitoring through the use of pulse oximetry and capnography (Morray et al., 1993). Jimenez and others (2007) reviewed 532 pediatric claims from 1973 to 2000. They concluded that claims for death (41%) and brain damage (21%) remained the dominant injuries in pediatric anesthesia claims in the 1990s. Half of the claims in 1990 to 2000 involved patients 3 years old or younger, and one fifth of the patients had ASA PS scores of 3 to 5. Cardiovascular (26%) and respiratory (23%) events were the most common damaging events. The proportion of claims reported as preventable by better monitoring decreased to 16% in the 1990s.
Analysis of anesthesia-related incidents reported to the Australian Incident Monitoring Study (AIMS) showed almost identical characteristics among pediatric age groups (van der Walt et al., 1993). Of the first 2000 cases reported, 10% involved infants and children. Incidents involving respiratory and breathing circuit systems accounted for nearly half of the adverse incidents. As with the ASA Closed Claims Project, the Australian reviewers estimated that 89% of all applicable problems in AIMS could have been detected and potentially prevented by the combination of pulse oximetry and capnography (van der Walt et al., 1993).
A study from Singapore, based on a QA database, reports no fatalities among 10,000 consecutive general pediatric anesthetic procedures from 1997 to 1999 (Tay et al., 2001). A 2004 QA database study from Hôpital d’Enfants Armand Trousseau in Paris also reports zero mortality among 24,165 general anesthesia procedures in children between 2000 and 2002 (Murat et al., 2004). On the other hand, from the Pediatric Perioperative Cardiac Arrest (POCA) Registry in the United States between 1994 and 1997, the anesthesia-related mortality rate was estimated to be 0.36:10,000 (Morray et al., 2000) (see related section). Obviously, a large-scale prospective and longitudinal study is needed to determine the overall pediatric anesthesia-related mortality in the early 21st century.
Anesthesia-related morbidity in infants and children
Perioperative Cardiac Arrests
Incidences of POCA have been reported from North America, Europe, and Australia. Estimated incidence of cardiac arrests ranged 17 to 24:10,000, and as with the mortality rates, the rates are three to 10 times higher in infants than in older children (Olsson and Hallen, 1988; Tiret et al., 1988; Cohen et al., 1990). Studies by Keenan and Boyan (1985) and by Morgan and others (1993) also showed higher incidences of cardiac arrest in younger children (younger than 10 to 12 years) than in older children. Most common causes leading to cardiac arrest involved respiratory and cardiovascular systems and included relative drug overdose, vagal stimulation, hypoventilation, and succinylcholine-induced asystole.
Keenan and others (1991) reported the effect of specialty training in pediatric anesthesia on the safety of children, especially in infants. In a single university hospital setting, the incidence of POCA in infants younger than 1 year of age was 19:10,000 with mortality when residents were supervised by nonpediatric attending anesthesiologists, whereas no cardiac arrest occurred when pediatric anesthesiologists were in charge.
Braz and others (2006) looked at the causes of POCA in children at a teaching hospital in Brazil. They reviewed 15,253 anesthesia procedures that took place between 1996 and 2004. There were 35 cardiac arrests (22.9:10,000) and 15 deaths (9.8:10,000). They identified seven anesthesia-related cardiac arrests but no deaths. The main causes of anesthesia-related cardiac arrest were respiratory events (71.5%) and medication-related events (28.5%). Major risk factors for cardiac arrest were neonates and children under 1 year of age with an ASA PS of 3 or poorer, emergency surgery, and general anesthesia.
Flick and others (2007) reviewed pediatric cardiac arrest data from 1998 to 2005 at the Mayo Clinic. A total of 92,881 anesthetics were administered to children under the age of 18 for noncardiac and cardiac procedures during the study period. The incidence of POCA and mortality during noncardiac procedures was 2.9:10,000 and 1.6:10,000, and the incidence of cardiac arrest during cardiac procedures was 127:10,000. The incidence of POCA attributable to anesthesia was 0.65:10,000, representing 7.5% of the 80 POCA. Both cardiac arrests and mortality were highest among neonates undergoing cardiac procedures. Regardless of procedure type, most patients who experienced POCA (88%) had congenital heart disease. Factors found to be associated with mortality included higher ASA PS, age, the need for mechanical ventilation before surgery, and the cause of the POCA.
Pediatric Perioperative Cardiac Arrest Registry
In order to accurately estimate the incidence of cardiac arrests and adverse outcomes, the Pediatric POCA Registry was formed in 1994 under the combined auspices of the ASA Committee on Professional Liability, the Quality Assurance Committee of the Section on Anesthesiology of the American Academy of Pediatrics (AAP) (Morray, 2004). The registry included 63 institutions, of which 75% were university hospitals and 40% were children’s hospitals. All cardiac arrests requiring cardiopulmonary resuscitation during the immediate perioperative period are eligible for inclusion. During the first 4 years of the registry (1994 to 1997), participating institutions administered an estimated total of 1,089,200 anesthetics to children younger than 18 years old (Morray et al., 2000). A total of 289 cardiac arrests were registered, of which 150 cases were considered as anesthesia-related. The mean overall incidence of anesthesia-related cardiac arrest was 1.4:10,000 with a mortality rate of 26% (0.36:10,000). Of the total anesthesia-related cardiac arrests, 55% occurred in infants younger than 11 months of age (Morray et al., 2000).
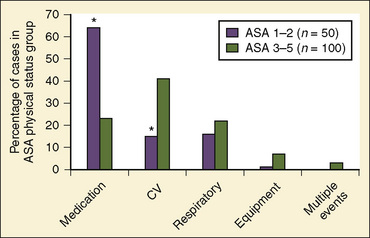
Of the major causes of anesthesia-related cardiac arrests, medication-related (37%) and cardiovascular causes (32%) were most common, together accounting for 69% (Table 40-4). In contrast, the respiratory causes represented only 20%, a marked reduction from the incidence of 43% reported by the ASA Closed Claim Project (Morray et al., 2000). Equipment-related causes comprised 7% of the total (Morray et al., 2000). With regard to the patients’ physical status, 33% were those with ASA PS 1 and 2, a significant decrease from earlier studies of pediatric mortality (57% of deaths), a significant improvement and a move in the right direction, although the percentage is still too high (Graff et al., 1964). Of medication-related cardiac arrests, cardiovascular depression with halothane alone or in combination with other drugs (mostly opioids) accounted for 66% of all medication-related arrests. In healthy children with ASA PS 1 and 2, 64% of cardiac arrests were medication-related in comparison with 23% in those with ASA PS 3 to 5 (Fig. 40-1) (Morray et al., 2000; Mason, 2004). Among the patients who sustained anesthesia-related cardiac arrest in the POCA Registry, death was associated most strongly with an ASA PS 3 to 4 and emergency surgery (Morray et al., 2000). Similar correlations between cardiac arrest or death and ASA PS 3 to 4 were found in the earlier study by Keenan and Boyan (1985).
| Mechanism | Number of Cardiac Arrests |
| Medication-related inhalation agents | 55 (37%) |
| Halothane alone | 26 (46%) |
| Halothane plus an intravenous medication | 11 (20%) |
| Sevoflurane alone | 2 (4%) |
| Intravenous medications | |
| Single | 5 (9%) |
| Combination | 5 (9%) |
| Intravenous injection of local anesthetic | 5 (9%) |
| Succinylcholine-induced hyperkalemia | 1 (2%) |
| Cardiovascular | 48 (32%) |
| Presumed cardiovascular, unclear etiology | 18 (38%) |
| Hemorrhage, transfusion related | 8 (17%) |
| Inadequate/inappropriate fluid therapy | 6 (13%) |
| Arrhythmia | 5 (10%) |
| Hyperkalemia | 4 (8%) |
| Air embolism | 2 (4%) |
| Pacemaker related | 2 (4%) |
| Vagal response | 1 (2%) |
| Pulmonary hypertension | 1 (2%) |
| Tetralogy hypercyanotic spell | 1 (2%) |
| Respiratory | 30 (20%) |
| Laryngospasm | 9 (30%) |
| Airway obstruction | 8 (27%) |
| Difficult intubation | 4 (13%) |
| Inadequate oxygenation | 3 (10%) |
| Inadvertent extubation | 2 (7%) |
| Presumed respiratory, unclear etiology | 2 (7%) |
| Inadequate ventilation | 1 (3%) |
| Bronchospasm | 1 (3%) |
| Equipment related | 10 (7%) |
| Central line | 4 (40%) |
| Breathing circuit | 2 (20%) |
| Peripheral intravenous catheter | 1 (10%) |
| Other | 3 (30%) |
| Multiple events | 5 (3%) |
| Hypothermia | 1 (<1%) |
| Unclear etiology | 1 (<1%) |
Modified from Morray JP et al.: Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry, Anesthesiology 93:614, 2000.
Since the last publication of the POCA Registry that was based on data between 1994 and 1997, more than 200 POCA cases have been added to the registry (from 2000 to 2003), and about one half of these cardiac arrests were found to be anesthesia-related (Morray, 2004). In a preliminary report on this new series of cardiac arrests, the cause profile has changed considerably from that of 1994 through 1997 (Fig. 40-2) (Morray, 2004). Medication-related cardiac arrests decreased markedly, from 37% to 12% of the total causes, primarily because of the near disappearance of cardiovascular depression by inhaled anesthetics causing cardiac arrest (Morray, 2004). These welcome changes appear to coincide with the replacement of halothane with sevoflurane (with its lower incidence of causing myocardial depression and bradycardia) as an anesthetic of choice for induction,. As the consequence of reductions in cardiac arrests with medication (primarily from halothane), cardiovascular causes of cardiac arrest increased relatively, from 32% to 52%. Hypovolemia from hemorrhage and a metabolic consequence of massive transfusion and resultant hyperkalemia were the common causes of cardiac arrest under this category (Morray, 2004). Also, with a reduction in medication-related cardiac arrest in healthy infants, the incidence of cardiac arrest in patients with ASA PS 1 to 2 declined considerably from 33% to 19%, one of the most remarkable differences between the first and second databases (Table 40-5).
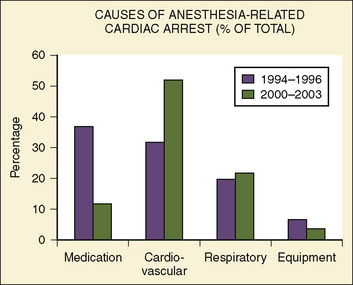
FIGURE 40-2 Causes of anesthesia-related cardiac arrest in children between 1994 and 1996 vs. between 2000 and 2003.
(From Morray JP: Unexpected cardiac arrest in the anesthetized child. Presented at the Society of Pediatric Anesthesia Spring Meeting, March 4-7, 2004.)
TABLE 40-5 Demographic Data from Pediatric POCA Registry Cases, 1994 to 1997 vs. 2000 to 2003
| 1994 to 1997 | 2000 to 2003 | |
| ASA PS | ||
| 1 | 15% | 4% |
| 2 | 18% | 15% |
| 3 | 37% | 46% |
| 4 | 27% | 22% |
| 5 | 2% | 13% |
| Age | ||
| <1 mo | 15% | 13% |
| 1 to 5 mo | 28% | 25% |
| 6 to 11 mo | 13% | 10% |
| 12 mo to 5 yr | 31% | 25% |
| 6 to 18 yr | 13% | 27% |
| Emergency surgery | 21% | 30% |
| Mortality | 26% | 27% |
POCA, Perioperative cardiac arrest; ASA PS, American Society of Anesthesiologists physical status.
Modified from Morray JP: Unexpected cardiac arrest in the anesthetized child. Presented at the Society of Pediatric Anesthesia Spring Meeting, March 4-7, 2004.
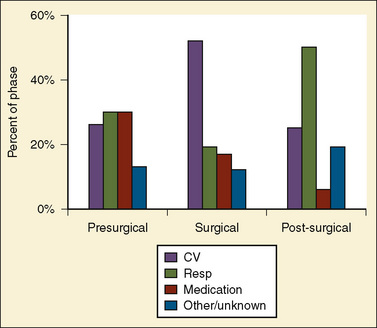
Bhananker and others (2007) subsequently published a study related to cases submitted to the POCA registry between 1998 and 2004. During that time, the registry received 397 reports of POCA in children. Of these cardiac arrests, 193 were judged as anesthesia related. Three quarters of the anesthesia-related arrests occurred in patients of ASA PS 3 to 5. Between 1998 and 2004, cardiovascular causes of cardiac arrest accounted for the highest proportion of anesthesia-related cardiac arrests (41%), in part because of a dramatic decrease in medication-related cardiac arrest. Among these, the most common identifiable cause was hypovolemia related to blood loss. The majority of these cardiac arrests resulted from blood loss that occurred during either spinal fusion or craniotomy and craniectomy. The most common anesthesia-related factors were underestimation of blood loss (48%) and inadequate venous access (22%). As reported in the preliminary communication by Morray (2004) on cases from the POCA Registry between 2000 and 2003, medication-related cardiac arrests decreased dramatically to 18% of all cardiac arrests (12% for 2000 to 2004, see above) from the 37% reported from 1994 to 1997, apparently because of the decrease in the use of halothane for induction of anesthesia. However, it is important to remember that sevoflurane is also a cardiac depressant, and cardiac arrests related to its effects have been identified in the registry. Most cardiac arrests occurred during anesthesia maintenance (58%). Nearly one quarter (24%) occurred in the induction or preinduction phase, and 19% occurred during emergence, transport, or recovery (Fig. 40-3). Eight of 10 cardiac arrests caused by electrolyte imbalance were caused by hyperkalemia from the transfusion of stored blood. The use of fresh blood cells and saline washing of irradiated blood may help in reducing the incidence of transfusion-associated hyperkalemia. Equipment-related cardiac arrests accounted for 5% of the anesthesia-related incidents, and half of these were secondary to central venous catheter complications. Six percent of the causes of cardiac arrest were unknown. Mortality after anesthesia-related cardiac arrest was 28%. The only factors predictive of mortality after cardiac arrest were ASA PS and emergency surgery.
Information about POCA in children (younger than 18 years) between 1988 and 2005 was reported from the Mayo Clinic (Flick et al., 2007). Out of a total of 92,881 anesthesia procedures, of which about 5% were for the repair of congenital heart disease, the incidence of POCA for noncardiac procedures were 2.9:10,000 and the incidence during the cardiac surgery was 127:10,000. The incidence of cardiac arrest attributable to anesthesia, however, was 0.65:10,000 procedures, representing 7.5% of all POCA, much lower than in some of the published reports. Both the incidence of arrest as well as mortality rates were highest among neonates (younger than 30 days’ postnatal life) undergoing cardiac procedures (POCA: 435:10,000; mortality: 389:10,000) (Flick et al., 2007).
Other Perioperative Adverse Events
Computerized data acquisition on nonfatal adverse outcome has become commonplace in most hospitals for QA or quality improvement (QI); such information in pediatric anesthesia has started to appear in the literature. Excellent reviews on this subject have been published (Holzman, 1994; Duncan, 1995).
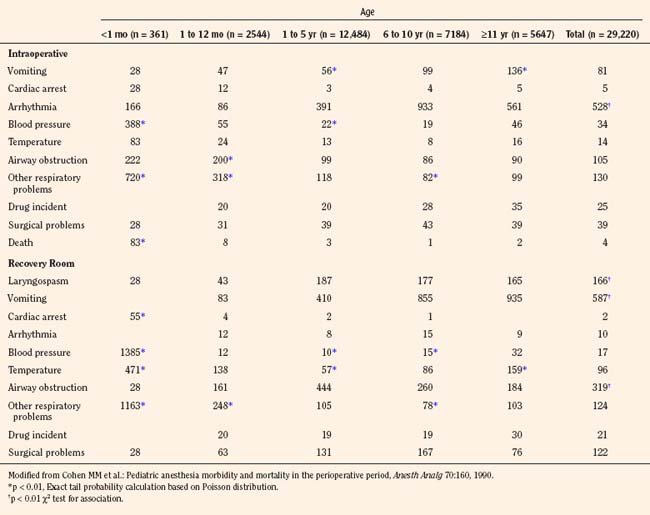
Cohen and others (1990) reviewed perioperative adverse events in over 29,000 children between 1982 and 1987 at Winnipeg Children’s Hospital. Unlike the adult surgical population, a majority of children (70%) were healthy and had no preoperative medical problems. Infants younger than 1 year of age, particularly those younger than 1 month of age (61% of whom underwent intraabdominal, intrathoracic, or major cardiovascular surgery), had a significantly higher intraoperative incidence of airway obstruction and other adverse respiratory events (9.4%) and hypotension (3.9%) than did older children. Among children 1 to 10 years of age, the most common problem was arrhythmias (3.9% to 9.3%). In the recovery room, infants younger than 1 month of age had hypotension (13.9%), respiratory events (11.6%), and abnormal temperature (4.7%). In older children, the most common adverse event in the recovery period was vomiting (5.9%), followed by airway obstruction (3.2%). This study was performed during the pre-sevoflurane era, when essentially all inhalation inductions were performed with halothane, with potent myocardial depression and bradycardia (Table 40-6).
A report from Hôpital d’Enfants Armand Trousseau in Paris was based on a QA database involving over 24,000 pediatric anesthesia cases for a 30 months between 2000 and 2002, when halothane had been completely eliminated from clinical use (Murat et al., 2004). Although this database did not include open-heart and neurosurgical cases, the nature of adverse events and their incidence have changed considerably. As a whole, respiratory events were most common, representing 53% of all intraoperative events (Table 40-7). As with other reports, respiratory events were more common among infants (3.6:10,000 vs. fewer than 1.5:10,000 in older children); in ear, nose, and throat (ENT) surgery than in other surgery; in children who were intubated vs. those who were not intubated, and in those with ASA PS 3 to 5 vs. PS 1 or 2 (Murat et al., 2004). Cardiac events represented 12.5% of all intraoperative events and were mostly observed in sick children (ASA PS 3 to 5).
TABLE 40-7 Respiratory and Cardiac Adverse Events Observed During Anesthesia and in PACU by Age Group
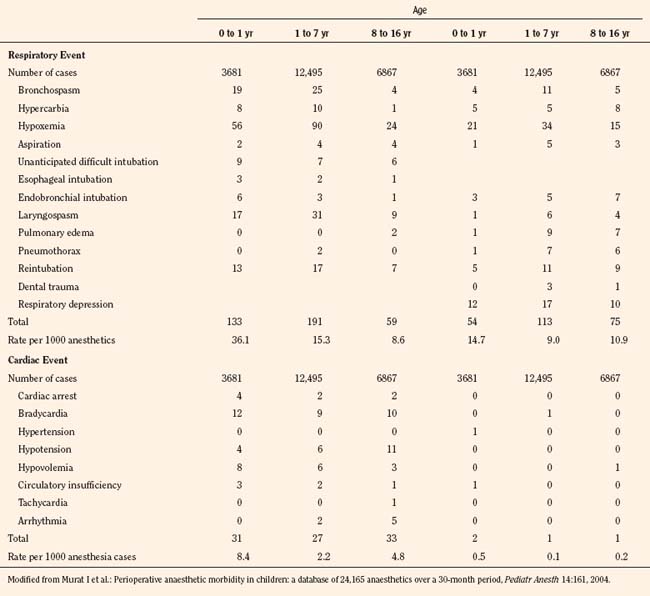
In contrast to earlier reports, the incidence of bradycardia was greatly decreased (13:10,000) and arrhythmias essentially disappeared. There were eight cardiac arrests (3.3:10,000), of which five children were in the ASA PS 3 to 5 category and four were infants 6 months old or younger (Table 40-7). There were no anesthesia-related deaths (Murat et al., 2004). Vomiting was the most common adverse event postoperatively, with an overall incidence of 6%. As with previous studies, vomiting was more common in older children than in infants and occurred more often after ENT surgery compared with other surgery and in children who were intubated vs. those who were not (Murat et al., 2004). Similarly, based on QA data of 10,000 surgical cases, Tay and others (2001) in Singapore found critical perioperative incidents four times higher in infants younger than 1 year of age than in older children (8.6% vs. 2.1%). Respiratory events were most common (77.4%) with laryngospasm accounting for 35.7%. There were no anesthesia-related deaths (Tay et al., 2001).
Down syndrome is the most common autosomal chromosomal disorder in humans. Children with Down syndrome have a number of characteristics that place them at high risk for anesthesia complications, including craniofacial and cardiac anomalies. A study of 488 patients undergoing 930 procedures revealed several anesthesia-related complications. The most common complications were severe bradycardia (3.7%), natural airway obstruction (1.8%), difficult intubation (0.5%), postintubation croup (1.8%), and bronchospasm (0.4%) (Borland et al., 2004). The rates of bradycardia, obstruction, and postintubation croup were statistically significant and more than twice the rate of patients without Down syndrome, suggesting the need for increased vigilance in these patients.
Bradycardia
An outcome study from the Medical College of Virginia examined the incidence of bradycardia in nearly 8000 children younger than 4 years of age (Keenan et al., 1994). Bradycardia (fewer than 100 beats per minute) was more common in infants (1.27%) and decreased with age. The incidence in the group of children who were 4 years old was only 0.16%. Causes of bradycardia included disease or surgery (35%), inhalation anesthesia (35%), and hypoxemia (22%). Of these children, hypotension occurred in 30%, asystole or ventricular fibrillation in 10%, and death in 8%. Significant associated factors predisposing children to bradycardic events, based on multiple logistic regression analysis, were ASA PS, emergency surgery, duration of surgery (longer than 4 hours), and the absence of a trained pediatric anesthesiologist supervising the anesthetic management (Keenan et al., 1994).
Laryngospasm and Bronchospasm
The incidences of laryngospasm and bronchospasm have been studied in a series of large population studies in Stockholm by Olsson and Hallen (1984, 1988). The incidence of laryngospasm in children younger than 9 years of age was 1.7%. The presence of respiratory infection raises the incidence to 9.6%. The incidence of laryngospasm was also increased in patients with obstructive lung disease (6.4%) and in those with a history of previous anesthetic complications (5.5%). The incidence of bronchospasm in the same age group increased from 0.4% to 4.1% in those with respiratory infection. The incidence of bronchospasm was also elevated (2.4%) in patients at high risk (ASA PS 3 or higher) (Olsson, 1987).
Up to 40% of children preparing for anesthesia have an upper respiratory tract infection (URI). Possible effects of recent or current URIs and the incidence of respiratory events have been studied by a number of investigators using parental interviews or written questionnaires. Of more than 1500 children, Schreiner and others (1996) found that patients who developed laryngospasm were more than twice as likely to have an active URI than were patients in the control group without URIs. A survey of more than 2000 children by Parnis and others (2001) did not find statistically significant differences in the long-term outcome of children with a recent history of URI. They did, however, find that orotracheal intubation was associated with an increased probability of respiratory complications compared with the use of a face mask or laryngeal mask airway (LMA). Similarly, in more than 1000 children, Tait and others (2001) found no difference between children with active or recent URIs vs. asymptomatic children, with respect to the incidence of laryngospasm, bronchospasm, or long-term respiratory sequelae. However, children with current or recent URIs had significantly more overall adverse respiratory events, including breath-holding and major desaturation (arterial oxygen saturation [SpO2] of less than 90%). Independent risk factors for adverse respiratory outcome in children with active URIs included tracheal intubation (younger than 5 years of age), history of prematurity, reactive airways disease, parental smoking, surgery involving the airway, and the presence of copious secretions and nasal congestion (Tait et al., 2001). A logistic regression analysis was created to look at the relationship between preoperative URI symptoms and adverse events during emergence from anesthesia. No association was found between particular URI symptoms and the rate of adverse events, but adverse events were increased if peak URI symptoms had occurred within the preceding 4 weeks (Homer et al., 2007).
The LMA has been advocated as an alternative to tracheal intubation for airway management in children with URIs. Von Ungern-Sternberg and others (2007) studied over 800 children having elective surgery with an LMA. A medical history of recent URI within the 2 weeks before anesthesia approximately doubled the risk of laryngospasm, oxygen desaturation, and coughing both intraoperatively and in the recovery room. This risk was further increased in younger children and in children undergoing ENT surgery (Tait et al., 2001). Flick and others (2008) reviewed 130 cases of laryngospasm in children at the Mayo Clinic and found that the use of an LMA was associated with laryngospasm even when adjusted for the presence of upper respiratory tract infections and airway anomaly.
Passive smoke exposure was studied in 405 children undergoing mask anesthesia procedures. The incidence of airway complications during anesthesia or postanesthesia recovery was significantly higher in children with passive smoke exposure. Intraoperative laryngospasm and airway obstruction were 4.9 and 2.8 times, respectively, more likely in children with passive smoke exposure (Jones and Bhattacharyya, 2006). Perioperative assessment of children undergoing surgery should include screening for passive smoke exposure to alert anesthesia providers to potential complications.
Aspiration
Studies before the 1970s reported high morbidity and mortality from pulmonary aspiration of gastric contents (Mendelson, 1946). The Baltimore Anesthesia Study Committee reported a mortality rate of 39% in children associated with pulmonary aspiration (Graff et al., 1964) (see Chapter 13, Induction, Maintenance, and Recovery). Studies reported since the 1980s, however, indicate marked improvements in outcome.
From a computer database covering the years between 1967 and 1985, Olsson and others (1986) reviewed more than 185,000 anesthesia cases in all ages and identified 83 cases of pulmonary aspiration of gastric contents (4.7:10,000 cases). The rate of gastric aspiration in children younger than 9 years of age (8.6:10,000) was nearly three times higher than that in young adults (20 to 49 years old). In 47% of patients with aspiration, pneumonia or atelectasis developed, as confirmed by chest radiograph. The mortality rate in children was relatively low (0.2:10,000) (Olsson et al., 1986). Risk factors associated with aspiration included the skill and experience of anesthetists, a number of coexisting diseases, ASA PS 3 to 5, emergency surgery, nighttime operation, history indicating an increased risk of regurgitation (e.g., esophageal disease, pregnancy), and difficult intubation. Other high-risk categories included children with intestinal obstruction, increased intracranial pressure, increased abdominal pressure, and obesity. Incidence of gastric aspiration was even lower in studies from the French-speaking countries (1.0:10,000) and from Norway (2.9:10,000) in the 1980s (Tiret et al., 1988; Mellin-Olsen et al., 1996). No fatalities were reported.
Borland and others (1998) studied the incidence and outcome of perioperative aspiration during the 5 years between 1988 and 1993 involving over 50,000 general anesthetic cases at Children’s Hospital of Pittsburgh. They identified 52 cases of aspiration (10.2:10,000 cases), of which 25 patients aspirated gastric contents (4.9:10,000) (the rest were blood or pharyngeal secretions). Approximately 80% of aspirations occurred during induction. Most patients were treated aggressively with fiberoptic bronchoscopy through the endotracheal tube (ETT), removal of solid particles, and continuous positive pressure ventilation. Most patients had radiographic evidence of aspiration (e.g. infiltration, pneumonia, atelectasis, or pulmonary edema), but fulminant chemical pneumonitis secondary to aspiration, as reported in early publications, was absent (Mendelson, 1946). No death was attributable to aspiration. Among the different pediatric age groups, the incidence of aspiration was highest among children 6 to 11 years of age (0.22%). Several risk factors for intraoperative aspiration were identified: ASA PS 3 or higher, a history of previous esophageal surgery, and patients with previous chemotherapy undergoing central venous catheter (Broviac catheter) placement. Twenty-nine percent of these children were kept intubated in the postanesthesia care unit (PACU) for several hours or longer, but only 23% of these patients stayed overnight. None of these children developed clinically significant pneumonia, and there were no deaths (Borland et al., 1998).
Similarly, a study from the Mayo Clinic reported a low incidence of aspiration (3.8:10,000). In this report, however, the incidence of aspiration was similar to that of adults (3.1:10,000). There was no serious respiratory morbidity and no associated deaths (Warner et al., 1999). These epidemiologic studies suggest that the incidence of gastric aspiration and associated morbidity and mortality, especially in children, has declined considerably. The risk of aspiration in general, with the exception of the Mayo Clinic report, remained higher in infants and children than in adults (Olsson et al., 1986; Tiret et al., 1988; Warner et al., 1999; Flick et al., 2002).
Postoperative Complications
Postoperative Hypoxemia
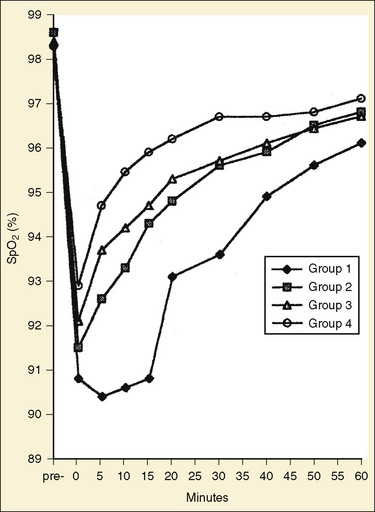
During general anesthesia, static tension of the thoracic inspiratory muscle is abolished and the balance between the outward recoil of the thorax and the inward recoil of the lungs is altered. This change in balance results in the reduction of resting lung volume (functional residual capacity [FRC]), airway closure, collapse of alveoli (microatelectasis), and increased venous admixture, particularly in infants and young children (see Chapter 3, Respiratory Physiology in Infants and Children). By means of pulse oximetry, Motoyama and Glazener (1986) were the first to demonstrate that a large proportion of healthy infants and children undergoing simple elective surgical procedures (42%)become hypoxemic in the PACU. Patients sleeping in the PACU tend to be more hypoxemic and for a longer duration than those who are awake and sitting up, but the presence of hypoxemia is not clinically obvious and does not correlate with the recovery score (Motoyama and Glazener, 1986; Soliman et al., 1988). In a study involving a 1152 healthy infants, children, and adults (ASA PS 1) undergoing plastic surgical procedures, postoperative SpO2 was monitored regularly for 2 hours. The incidences of both moderate (SpO2, 86% to 90%) and severe (SpO2, less than 86%) hypoxemia were the highest among infants (36.6% and 16.7%, respectively), followed by toddlers (20% and 10% in children 1 to 3 years old), children (14% and 3.3%), and adults (8% and 0.6%). The duration of hypoxemia was also significantly longer in infants than in older age groups (Fig. 40-4) (Xue et al., 1996).
Patients also become hypoxemic as often during the short transport from the operating room to the PACU, because the benefit of oxygen breathing to maintain oxygenation lasts only a few minutes (Pullerits et al., 1987). Children with upper respiratory infection and infants younger than 6 months of age are at increasing risk of developing hypoxemia (DeSoto et al., 1988; Kataria et al., 1988; Xue et al., 1996). Most pediatric anesthesiologists, therefore, recommend routine oxygen administration during the transport of children to and in the PACU (Duncan, 1995).
Postoperative Apnea
Postoperative apnea in prematurely born infants has become a major clinical concern since the early 1980s, when the number of premature infants surviving neonatal intensive care started to increase. Apnea is usually defined as the cessation of breathing lasting longer than 15 to 20 seconds or lasting for a shorter duration when associated with bradycardia, cyanosis, or pallor (Thach, 1985). Apneic spells in these infants after simple surgical procedures are mostly central in origin (cessation of respiratory effort), although some infants have mixed (central and obstructive) apneas (Kurth and LeBard, 1991). Postoperative apnea occurs more commonly in infants with a previous history of apnea and those younger than 42 to 44 weeks’ postconception (Liu et al., 1983). Apnea is uncommon after 44 weeks’ postconception, although apnea in older expremature infants (up to 55 weeks’ postconception) has been reported after more extensive surgical procedures (Kurth et al., 1987; Malviya et al., 1993). Malviya and others (1993) recommend that all former premature infants younger than 44 weeks’ postconception be monitored for at least 12 hours postoperatively. Another important risk factor for postoperative apnea appears to be the presence of anemia (Welborn et al., 1991).
In 1995, Coté and others published the results of a meta-analysis of data from eight published reports of postoperative apnea between 1987 and 1993 involving 384 expremature infants after inguinal hernia repair. They concluded that apnea was strongly and inversely correlated both with gestational age and postconceptual age; an associated risk factor was continuing episodes of apnea at home; small-for-gestational-age infants seemed to be somewhat protected from apnea compared with those with normal or large-for-gestational-age infants; anemia (hematocrit less than 30) was a significant risk factor even beyond 43 weeks’ postconceptual age; and relationships of postoperative apnea with history of necrotizing enterocolitis, neonatal apnea, respiratory distress syndrome, bronchopulmonary dysplasia, or operative use of opioids and muscle relaxants could not be determined (Coté et al., 1995). The probability of apnea in infants without anemia who are free of apnea in the recovery room decreases with postconceptual and postnatal ages but is not less than 5% (with 95% confidence limits) until the postconceptual age of 48 weeks (35 weeks’ gestational age) and no less than 1% until postconceptual age of 56 weeks (with a gestational age of 32 weeks) or postconceptual age of 54 weeks (with a gestational age of 35 weeks) (Fig. 40-5) (Coté et al., 1995). In 2008, Murphy and others looked at the incidence of apneas in premature infants after inguinal hernia repair in 126 infants over a 5 years. Their data confirmed both the incidence and the risk factors for postoperative apnea of Coté’s meta-analysis (Murphy et al., 2008).
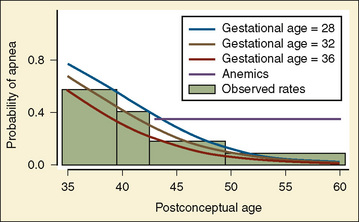
FIGURE 40-5 Probability of postoperative apnea vs. postconceptual and gestational ages of former preterm infants.
(From Coté CJ et al.: Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis, Anesthesiology 82:809, 1995.)
Based on these findings, it is generally recommended in the United States that expremature infants younger than 44 to 46 weeks’ postconception be admitted overnight for monitoring after general anesthesia. Whether the infant with postconceptual age between 46 and 48 weeks or even 52 weeks is admitted overnight depends on the decision made case by case between the anesthesiologist and the surgeon, based on a number of factors. These factors include the general health of the infant and his or her home environment (e.g., parents, passive smoking, and distance from the hospital). However, the decision often depends on the general policies of the hospital administration and insurance providers, and these policies do not necessarily represent the best interest of the patient or the health care providers. In countries like France and Japan, where economic pressure on health care resources is less stringent than in the United States, most infants younger than 60 weeks’ postconception are admitted overnight for monitoring (Murat, 2002).
The incidence of postoperative apnea was reported to be lower after spinal anesthesia alone than after general (halothane or sevoflurane) anesthesia, but spinal anesthesia combined with ketamine increases the incidence of apnea more than that with general anesthesia (Welborn et al., 1990). Caffeine or methylxanthine may be helpful in preventing postoperative apnea and bradycardia in preterm infants (Welborn et al., 1988). Caffeine is usually preferred, because it has been proposed to have fewer hemodynamic consequences, a greater therapeutic index, and a longer half-life. A dose of 10 mg/kg of caffeine appears to be a safe and effective dose for prophylaxis of postoperative apnea. The infants treated with caffeine had a relative risk of 0.09 for postoperative apnea and bradycardia (McNamara et al., 2004). A long-term study on the effects of caffeine for apnea of prematurity (called the CAP Study) has been conducted (Schmidt, 2005).
Upper airway obstruction is an increasingly common indication for adenotonsillectomy in children. Adenotonsillectomy has established effectiveness for the treatment of obstructive sleep apnea (OSA). However, more than 20% of children with OSA have respiratory compromise requiring medical intervention in the postoperative period (Rosen et al., 1994). Nixon and others (2005) studied the postoperative course of children with OSA undergoing adenotonsillectomy surgery. They found that obstructive events occurred postoperatively in all children, but were more common and profound in those with severe OSA preoperatively. They recommend continuous pulse oximetry and overnight admission for children with severe OSA in a unit skilled in pediatric airway management. Schwengel and others (2009) reviewed the current literature on OSA in children and determined several clinical features that predict respiratory compromise after adenotonsillectomy (Box 40-1).
Box 40-1 Predictors of Respiratory Compromise and Persistent Obstructive Sleep Apnea after Adenotonsillectomy
Modified from Blum RH, McGowan FX: Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathology, and anesthetic implications, Paediatr Anaesth 14(1):75–83, 2004; Guilleminault C, Huang YS, Glamman C, et al: Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey, Otolaryngol Head Neck Surg 136(2):169–175, 2007; Gerber ME, O’Connor DM, Adler E, Myer CM 3rd: Selected risk factors in pediatric adenotonsillectomy, Arch Otolaryngol Head Neck Surg 122(8):811–814, 1996; McGowan FX, Kenna MA, Flemming JA, O’Connor T: Adenotonsillectomy for upper airway obstruction carries increased risk in children with a history of prematurity, Pediatr Pulmonol 13(4):222–226, 1992; Fricke BL, Donnelly LF, Shott SR, et al: Comparison of lingual tonsil size as depicted on MR imaging between children with obstructive sleep apnea despite previous tonsillectomy and adenoidectomy and normal controls, Pediatr Radiol 36(6):518–523, 2006.
Postintubation Croup
The major cause of postintubation croup is subglottic injury and edema associated with traumatic intubation, especially with an oversized ETT. Koka and others (1977) made an important observation that the incidence of postintubation croup increases markedly when there is no air leak around the ETT with the airway pressure exceeding 40 cm H2O. Consequently, it has become a standard practice in pediatric anesthesia to choose an ETT that produces air leak around the tube with a pressure lower than 30 cm H2O. With this preventive measure in clinical practice, the incidence of postintubation croup has decreased dramatically from 1% to less than 0.1%, along with reductions in the severity of croup (Litman and Keon, 1991). With the presence of a URI, however, the incidence of airway complications and the tendency for oxygen desaturation increase (Cohen and Cameron, 1991; Rolf and Coté, 1992).
Until the late 1980s and early 1990s, the use of cuffed ETTs was not generally recommended in most pediatric anesthesia textbooks for children younger than 5 to 8 years of age, depending on the authors (Fisher, 1989; Uejima, 1989). The primary reason for it had been that the inner diameter (ID) of a cuffed ETT had to be 1 to 2 sizes (0.5 to 1.0 mm) smaller than the uncuffed tube to accommodate the passage through the larynx of a bulky cuff, and consequently flow resistance would be drastically increased in those children most commonly breathing spontaneously under general anesthesia (with ether) before mechanical ventilation became a common practice (Gronert and Motoyama, 1996). More recently, cuffed ETTs have been used increasingly in young children and even in infants, and such practice has shown to be associated with decreased, rather than increased, incidence of postintubation croup (Motoyama, 2009). This decrease is in part a consequence of choosing a cuffed ETT that is 1 to 2 sizes smaller than a “properly” fitting uncuffed tube, thereby markedly reducing the need for reintubation attempts. (Keine, 1997; James, 2001; Fine and Borland, 2004; Cohen and Motoyama, 2006). Indeed, Murat (2001) reported that after a complete elimination of uncuffed ETTs at the Children’s Hospital in Paris, there was not a single incidence of postintubation croup over 3 years. A new microcuff pediatric ETT has been developed that seals with a high-volume, low-pressure cuff (Dullenkopf et al., 2005). This tube uses an ultrathin, walled polyurethane cuff that is shorter and more distally placed. It can stay away from the cricoid mucosa while the airway is sealed at the upper to midtrachea, and away from the carina even in the neonate, where the posterior membranous wall can stretch and accommodate a complete seal with a low cuff pressure of less than 15 cm H2O without increases in airway complications (Dullenkopf et al., 2005). In a study of 500 children, the microcuffed tube exchange rate was 1.6%, with only 0.4% having postintubation croup requiring therapy (Dullenkopf et al., 2005; Weiss and Gerber, 2006).
Postoperative Nausea and Vomiting
Although rarely life-threatening, postoperative nausea and vomiting (PONV) remains the single most common complication resulting in unscheduled overnight admissions in same-day surgery settings (Cohen et al., 1990; Patel and Rice, 1991). PONV occurs twice as often in children as in adults, increasing until puberty and then decreasing to adult incidence rates. The average incidence of PONV in children over 3 years of age is reported to be over 40%. The incidence of PONV has decreased considerably with newer anesthetics and techniques as well as with more effective medications (Murat et al., 2004). The incidence of PONV is higher after certain types of surgery, including adenotonsillectomy, eye-muscle surgery for strabismus, and orchiopexy. Other factors affecting the incidence of PONV include the gender and age of the child (uncommon in infants), PONV after previous surgery or history of motion sickness, anesthesia techniques (inhaled anesthetics and nitrous oxide vs. intravenous anesthesia with propofol), tracheal intubation, intraoperative opioids, inadequate pain control, gastric distention, and the skill of the anesthesiologist (Patel and Rice, 1991; Martin et al., 1993; Weir et al., 1993; Weinstein et al., 1994; Duncan, 1995; Villeret et al., 2002). A mandatory requirement for oral fluid intake and early ambulation before discharge from the short stay unit has also been associated with increased incidence of vomiting (Schreiner et al., 1992; Weinstein et al., 1994). Serotonin (5-HT3) receptor antagonists (such as ondansetron and granisetron) have shown to be highly effective in preventing or treating PONV and are the preferred first-line agents for children, especially because ondansetron has become available as a less expensive generic drug (Fujii et al., 1996; Patel et al., 1996; Gan et al., 2007; Glass and White, 2007). Prophylactic use of dexamethasone and transdermal scopolomine were also found to be effective (Aouad et al., 2001) (see Chapter 11, Intraoperative and Postoperative Management). Gan and others (2003) developed consensus guidelines for the management of PONV. Strategies to reduce baseline PONV risk factors include the use of regional anesthesia, use of propofol for induction and maintenance of anesthesia, use of intraoperative supplemental oxygen, use of hydration, avoidance of nitrous oxide, avoidance of volatile anesthetics, minimization of intraoperative and postoperative opioids, and minimization of neostigmine (Gan et al., 2007). Droperidol has been effectively used for many years for PONV prophylaxis. However, because of the increased risk of extrapyramidal symptoms, high levels of sedation, and the United States Food and Drug Administration’s (FDA’s) “black box” warning about QT prolongation, droperidol was recommended to be reserved for patients in whom all other therapies have failed and who are being admitted to the hospital (Gan et al., 2003). Children at moderate to high risk for PONV should be given antiemetic prophylaxis with combination therapy with 2 or 3 prophylactic antiemetics from different antiemetic drug classes.
In comparison with PONV, the studies on postdischarge nausea and vomiting (PDNV) have been lacking. Oral opioid analgesics for postoperative pain management are a major factor contributing to PDNV in the ambulatory surgery facilities or after discharge from a hospital (Glass and White, 2007). Long-acting antiemetics (e.g., transdermal scopolamine or palonosetron) may offer an advantage over the commonly used antiemetics (Glass and White, 2007).
Complications of Regional Anesthesia
The potential benefits of peripheral nerve blocks for surgical procedures, when compared with general anesthesia, include improved postoperative analgesia, with an associated decrease in postoperative pain medication use, decreased nausea and vomiting, and quicker recovery and discharge times from the hospital (Hadzic et al., 2004). Regional anesthesia techniques in children have become more popular in the past decade. A survey by the ASA Closed Claims Project on the complication of regional anesthesia revealed that of 2400 closed anesthesia malpractice claims cases, 29 adult patients and 1 pediatric patient who developed cardiac arrest during regional anesthesia, resulting in death or severe brain damage, were identified (Morray et al., 1993). From the analysis of these data, mostly from the time before the ASA monitoring guidelines, including the mandatory use of pulse oximetry and capnography, had been adopted, it can be concluded that cardiac arrest resulting in death or other major outcomes can occur during apparently well-managed spinal or epidural anesthesia in young, healthy patients undergoing relatively minor procedures because respiratory insufficiency from sedation was not recognized; and that pulse oximetry would have given an early warning of respiratory insufficiency (Caplan et al., 1988; Cheney, 1988; Keats, 1988). An examination of the closed claims database from 1980 to 2000 by Lee and others showed that the most common complication associated with peripheral nerve block claims was nerve damage (31%) followed by pneumothorax (25%) and eye damage (18%). Block-needle trauma was the cause of 38% of these nerve injury claims. (Lee et al., 2008). The increasing use of ultrasound for the placement of peripheral nerve blocks has the promise of decreasing the incidence of nerve damage caused by block needle trauma (Marhoffer et al., 2005). Unintentional intravascular injection or signs of local anesthetic toxicity were associated with one third of the 19 claims with death or brain damage. Outcomes from this potentially lethal complication may be improved with the recent introduction of 20% intralipid as a rescue agent for local anesthetic toxicity (Lee et al., 2008).
The Japanese Society of Anesthesiology has conducted annual surveys concerning critical incidents in the operating room since 1994. Irita and others (2005) investigated critical incidents associated with regional anesthesia between 1999 and 2002. In patients receiving regional anesthesia, 628 critical incidents, including 108 cardiac arrests and 45 subsequent deaths were reported. The incidences of cardiac arrest and mortality resulting from anesthetic management were 0.54 and 0.02:10,000 with spinal anesthesia, 0.55 and 0:10,000 with combined spinal-epidural anesthesia, and 0.72 and 0.14:10,000 with epidural anesthesia, respectively (Irita et al., 2005).
Valley and Bailey (1991), in a retrospective survey involving 138 pediatric patients who received caudal morphine, reported 11 patients (8%) with postoperative respiratory depression. All but one incident occurred in infants younger than 12 months of age and within 12 hours of caudal morphine administration (70 mcg/kg). Krane (1988) reported a life-threatening delayed respiratory depression in a 2.5-year-old boy that occurred 3.5 hours after the administration of caudal morphine (100 mcg/kg, a much higher dose than today’s standards of 30 to 50 mcg/kg) for postoperative analgesia. Intravenous naloxone was continued until 16.5 hours after caudal morphine to maintain adequate breathing. Jones and others (1984) used intrathecal morphine in 56 children undergoing open-heart surgery. Respiratory depression occurred (most commonly, 3.5 to 4.5 hours after morphine administration) in six of 27 patients (22%) who received 30 mcg/kg of morphine and in three of 29 children (10%) who received 20 mcg/kg. Patients receiving epidural or intrathecal morphine should therefore be admitted overnight and their respiration continuously monitored.
Giaufré et al. (1996) reported on a 1-year prospective study (1993 to 1994) of morbidity and mortality associated with regional anesthesia by the French Language Society of Pediatric Anesthesia. The study involved over 24,000 regional-block procedures out of about 85,000 pediatric anesthesia cases of which central blocks (approximately 15,000 cases [62%]) were the most common, followed by peripheral nerve blocks (17%) and others (22%). Of the central blocks, caudal block was most common (12,000, or 80% of central block or 50% of the total), followed by lumbar epidural (1700) and spinal anesthesia (500). A total of only 23 incidents was reported, with a morbidity rate of zero for peripheral blocks, 11 (0.7:1000) for caudal blocks, nine (3.7:1000) for lumbar epidural blocks, two (6.8:1000) for sacral epidural blocks, and one (2:1000) for spinal anesthesia (Giaufré et al., 1996). Complications included eight dural punctures (resulting in total spinal block in four cases), six intravascular injections (resulting in seizures or arrhythmias), two overdoses with arrhythmias, and one case of opioid-related apnea. No fatalities were reported. Thus, this study appears to establish the safety of regional anesthesia in children, although there had been some concerns and controversies about the safety of performing regional blocks in children under general anesthesia, because this is widely accepted as an unsafe practice in adults (Giaufré et al., 1996).
More recently, an analysis of a prospective audit of children receiving epidural infusion analgesia (EIA) in the United Kingdom and Ireland has been published; the audit included 10,633 EIA cases over 5 years (2001 to 2005) (Llewellyn and Moriarty, 2007). Of 96 incidents reported, 56 (1:198) were associated with the insertion and maintenance of EIA (mostly of low severity); five incidents were graded as 1 (serious) (1:2000); nine incidents were graded as 2 (moderate) (1:1100). Only one child had residual effects after 12 months after surgery (1:10,000). There were no reported deaths with EIA. There were four cases of compartment syndrome (1:1400), but none of them appeared to be masked by the presence of working epidural analgesia (Llewellyn and Moriarty, 2007).
Complications Arising from Sedation
According to the data compiled by the United States Department of Health and Human Services, more than 80 deaths attributable to midazolam occurred within 3 years after its introduction for clinical use in 1986. Midazolam was used, often in combination with fentanyl, for the sedation of patients undergoing various procedures without the supervision of anesthesiologists (Bailey et al., 1990). Of these deaths associated with midazolam, 78% were respiratory events, with opioids being used in 57% of these cases (Bailey et al., 1990).
A collaborative study by the American Society of Gastrointestinal Endoscopy and the United States Food and Drug Administration, involving over 21,000 endoscopic procedures with sedation, revealed the incidence of serious cardiorespiratory outcome to be 54:10,000 cases with a mortality rate of 3:10,000; that is 10 to 50 times higher than the reported deaths associated with general anesthesia (Tiret et al., 1986, 1988; Buck et al., 1987; Holland, 1987; Eichhorn, 1989; Arrowsmith et al., 1991). These extremely high morbidity and mortality rates of conscious and deep sedations (apparently including inadvertent general anesthesia and deaths) performed by clinicians who were not anesthesiologists and without adequate skills, proper monitoring, or supervision have led the way to the establishment and modifications of the new sedation guidelines by the AAP Section of Anesthesiology (Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics, 1985, 1992). Although the terms adopted (conscious sedation in particular) were misnomers (if not oxymorons), in hindsight the guidelines included the approach similar to that commonly practiced by anesthesiologists; that is, proper fasting, preprocedural history and physical examinations with a special attention to the airways, informed consent, monitoring including pulse oximetry, documentation of drugs used and vital signs during and after the procedure, and discharge criteria (Coté, 2004).
The JCAHO (1992) in the United States modified its regulations and published rules to develop new guidelines in each health care institution it accredits (available at www.jcaho.or.). To further improve patient safety for sedation in accordance with the new JCAHO regulations, a task force by the ASA developed new guidelines for sedation by nonanesthesiologists (Gross et al., 2002). The sedation guidelines were again updated in 2002 (American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists, 2002) with the new terminology (available at www.asahq.org/standard.) and were later incorporated by JCAHO and by the AAP (Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics, 2002). The term conscious sedation has been eliminated, and instead, three stages of procedural sedation are described: minimal, moderate, and deep stages (plus general anesthesia) (Coté, 2004).
Using a QA database created specifically for procedural sedation, Malviya and others (1997) identified 239 adverse outcomes (20% of 1140 children), mostly after receiving recommended doses of chloral hydrate. Oxygen desaturation (5.5%) was the most common adverse outcome, and laryngospasm and apnea occurred in five children. Inadequate sedation occurred in 150 (13.2%) children. These findings appear to indicate that the establishment and enforcement of safety guidelines for procedural sedation have considerably reduced the incidence of adverse outcomes.
Analysis of adverse sedation events reported to the United States Food and Drug Administration (FDA) between 1969 and 1996 by Coté and others (2000) revealed that of 95 incidents reported, 60 ended in death (51) or permanent neurologic injury (9). Although the incidence of respiratory events (about 80% of all events; mostly hypoxia, laryngospasm, and apnea) was similar between hospital and nonhospital settings, inadequate resuscitation (57.1% vs. 2.3%) and death or permanent neurologic injury (92.8% vs. 37.2%) occurred more often in nonhospital vs. in hospital environments. Death or severe adverse outcome occurred disproportionately more often (32 of 95 cases) involving sedation for dental procedures (mostly at nonhospital settings). Ten children sustained death or permanent neurologic injury in the car or at home after being discharged from medical supervision despite deep levels of residual sedation. Unsupervised sedative medication by a parent at home (or by a technician at a facility) caused an additional two cardiac arrests in the car on the way to the hospital or clinic (Coté et al., 2000). The results of this report imply the inadequacy of existing (or the nonexistence of) discharge criteria and their practice. Two reports have further addressed these issues.
Motas and others (2004) studied the efficacy and safety of procedural (light or deep grade) sedation in 86 children under 12 years of age undergoing sedation by nonanesthesia services (for computed tomography scans, cardiac catheterizations, gastrointestinal endoscopy, and dental procedures). A variety of medications were used by different services, including intravenous pentobarbital, intravenous midazolam with fentanyl or meperidine, oral chloral hydrate with meperidine and hydroxyzine, and intramuscular or intravenous ketamine (Motas et al., 2004). An independent observer applied the Bispectral Index (BIS) monitor (40 to 60, general anesthesia; 61 to 70, deep sedation; 71 to 90, minimum, and over 90, awake) and the University of Michigan Sedation Scale (UMSS) (0 to 4 observational scale: 0, awake; 1, minimal sedation [tired, sleepy]; 2, moderately sedated [easily arousable]; 3, deeply sedated [deep sleep, arousable only with strong stimulus]; and 4, unarousable or general anesthesia) at 10-minute intervals for 1 hour. The goal of either light or deep sedation was attained in 53% (BIS) and 72% (UMSS) of cases. Depth consistent with general anesthesia was observed in 35% (BIS) and 0% (UMSS) of patients, and depth consistent with awake state (failure) was observed in 12% (BIS) and 28% (UMSS) of cases. About 8% of patients experienced desaturation and airway events. The patients were often sedated either too deeply or not enough, and the goal of either light or deep procedural sedation was not achieved in large numbers of children.
Malviya and others (2004) assessed the readiness for discharge in 29 children after procedural sedation for echocardiographic examinations with either chloral hydrate (93%) or midazolam with diphenhydramine (7%). A trained observer used a BIS monitor, UMSS scores every 10 to 15 minutes, a Modified Maintenance of Wakefulness Test (MMWT), and the visual observation of the time until the child was able to stay awake for 20 minutes, until revised discharge criteria were met (BIS greater than 90, UMSS of 0 or 1, MMWT greater than 20 minutes). There were moderate correlations among BIS, UMSS, and MMWT (p < 0.01). Revised criteria correctly identified wakefulness (BIS value greater than 90) in 88% of patients. However, when discharged by the nurse, only 55% of patients returned to the baseline BIS value (greater than 90); it took longer to meet the revised criteria (more appropriate and safer) compared with standard criteria (nursing judgment) (75 minutes vs. 13 minutes, p = 0.001).
Thus, Malviya and others (2004) clearly demonstrated that sedation with chloral hydrate can result in prolonged sedation even after the children reached currently used (but unsatisfactory) discharge criteria by nurses, with a potential for airway obstruction and adverse outcome (Coté et al., 2000). The results of this study have several important implications. First, the currently practiced guidelines are inadequate and need changes that make use of more reliable criteria, such as UMSS, MMWT, BIS monitor, or their combinations, to further improve patient safety associated with procedural sedations. Second, the duration of postsedation monitoring should be increased beyond what is currently practiced, and the hospital must respond to increase staffing needs for nurses in the recovery area and to provide additional space for adequate patient observation (quiet space for MMWT) and recovery, changes that have associated cost increases. Third, the anesthesia service should provide additional guidelines to nonanesthesiologists for the proper selection of sedative and hypnotic drugs, with shorter elimination half-life to shorten the recovery time (Coté, 2004). In addition, office-based procedures that are performed with the patient under sedation should either be performed under the care of anesthesiologists within the office setting or moved to hospital-based facilities to further decrease untoward events (see Chapter 35, Anesthesia for Office-Based Pediatric Anesthesia).
Cravero and others (2006) looked at data collected by the Pediatric Sedation Research Consortium (PSRC), a collaborative group of 35 institutions. A total of 26 institutions submitted data on 30,037 sedation and anesthesia procedures outside of the operating room from 2004 to 2005. Serious adverse events were rare in the institutions involved in the study; there were no deaths. Cardiopulmonary resuscitation (CPR) was required once. Less serious events were more common with oxygen desaturations below 90% for more than 30 seconds, occurring 157 times per 10,000 sedations. Unexpected apnea, excessive secretions, and vomiting had rates of 24, 41.6, and 47 per 10,000 encounters, respectively (Cravero et al., 2006). They concluded that pediatric sedation and analgesia for procedures outside of the operating room are unlikely to yield serious adverse outcomes in a collection of institutions with highly motivated and organized sedation services. However, the safety of this practice depends on the system’s ability to manage less serious events (Cravero et al., 2006).
In 2008, Cravero and others looked at the safety of pediatric sedation and anesthesia with propofol for procedures outside of the operating room. Thirty-seven members of the PSRC submitted data on 49,836 propofol sedation and anesthesia cases from 2004 to 2007. There were no deaths, CPR was required twice, and aspiration during sedation or anesthesia occurred four times. Less serious events were more common with O2 desaturation below 90% for more than 30 seconds, occurring 154 times per 10,000 sedation and anesthesia administrations. Central apnea or airway obstruction occurred 575 times per 10,000 sedations. Stridor, laryngospasm, excessive secretions, and vomiting had rates of 50, 96, 341, and 49 per 10,000 encounters, respectively. They concluded that, in the hospital setting of those institutions participating in the PSRC, the reported incidence of serious events in pediatric propofol sedation and anesthesia is low. However, the reported incidence of events that have the potential to harm and that require timely rescue interventions is significant, occurring once per 89 propofol administrations (Cravero et al., 2009).
Bhananker and others (2006) reviewed closed malpractice claims from the ASA database since 1990 to assess the patterns of injury and liability associated with monitored anesthesia care (MAC) compared with general and regional anesthesia. More than 40% of claims associated with MAC involved death or permanent brain damage, similar to general anesthesia claims. Respiratory depression (25%) was the most common damaging mechanism in MAC claims. Nearly half of these claims were judged as preventable by better monitoring.
Common causes of anesthesia-related mishaps
Judgment Errors
If one accepts the theory that “to err is human” and recognizes that “all human beings, without any exception whatsoever, make errors and [errors] are a completely normal and necessary part of human cognitive function,” then it is not surprising to realize that most anesthetic mishaps involve human errors and are preventable (Salem et al., 1975; Cooper et al., 1978; Allnutt, 1987; Holland, 1987; Kohn et al., 1999). Cooper and others (1978), with the use of a carefully structured interview technique with a group of anesthesiologists, collected accounts of incidents in which human error or equipment failure occurred. These investigators found that 82% of incidents were caused by preventable human error; the remaining incidents were mostly caused by equipment failure. Analysis of 238 closed anesthesia malpractice claims involving pediatric patients revealed that 43% of these claims were related to inadequate ventilation and resulted in death or severe permanent neurologic damage (Morray et al., 1993). Morray and others concluded that 89% of these cases could have been prevented if they were monitored with pulse oximetry or capnography.
Holland (1987) compared incidences of various categories of mishaps during his three decades of investigation involving anesthesia-related mortality in patients of all ages (Table 40-8). In the 1960s, anesthetic overdose, wrong choice of anesthetics, inadequate preoperative preparation, and inadequate crisis management dominated as common causes of adverse outcomes, indicating inadequacy (according to today’s standard) of equipment and the physicians’ experience with administering anesthesia. The total incidence of management errors involving fatality was 2.7:10,000 anesthesia procedures. There has been a dramatic decrease in the incidence of management errors with time, as well as changes in the dominant categories of error in management over the decades. In the 1980s, inadequate preparation and inadequate postoperative management ranked high among the categories of errors causing anesthesia-related fatalities. Incidence of management errors decreased to 0.55:10,000 anesthetics, about one fifth that in the 1960s. Factors commonly associated with preventable adverse outcomes (both fatal and nonfatal) include a failure to perform proper review of patients and anesthesia apparatus, distraction (e.g., inattention, haste, fatigue, and boredom), lack of experience, and lack of a skilled assistant (Craig and Wilson, 1981; Derrington and Smith, 1987).
Emergency or Urgent Cases
Inadequate preanesthetic preparation occurs most often with emergency or urgent cases and most commonly represents failure to appreciate the patient’s degree of dehydration and electrolyte imbalance (Holland, 1987). In the CEPOD study cited previously, more than 20% of anesthesia-related deaths occurred after surgery performed as emergencies, and an additional 40% occurred in operations classified as urgent (Buck et al., 1987). In the New South Wales study, the risk of anesthesia-attributable death in patients undergoing emergency anesthesia is 10 times that of the overall incidence (Holland, 1987). In the British CEPOD study, anesthetists were dissatisfied with preoperative preparations in 14% of cases that ended with anesthesia-related deaths, but they presumably were pressured or persuaded by surgeons into administering anesthesia. On the basis of these findings, Lunn and Devlin (1987) emphasize the importance of proper preanesthetic preparation as an important deterrent of tragic outcome. In children, the incidence of bradycardic episodes, both fatal and nonfatal, during anesthesia was reported to be significantly higher among emergency surgical procedures (2.7%) than in elective procedures (1.1%) (Keenan et al., 1994).
Timing of Occurrence
Keenan and Boyan (1985) in a 15-year study of cardiac arrest resulting from anesthesia in a university hospital setting, found 18 of 27 cases that occurred during the induction of anesthesia, whereas the remaining nine occurred intraoperatively. However, in five of six patients in the pediatric age group who sustained cardiac arrest (aged 1 day to 6 years), the cardiac arrest occurred during the maintenance of anesthesia. In most other reports, critical incidents or mishaps, including the cardiac arrests reported from the POCA Registry as discussed earlier in this chapter, have occurred most often during the maintenance period of anesthesia (Cooper et al., 1978; Craig and Wilson, 1981; Gibbs, 1986; Bhananker et al., 2007). The maintenance period is not the quiet interlude between often stormy induction and emergence that many anesthesiologists had for a long time supposed (Epstein, 1978). In a survey involving 112,000 general anesthetic cases in a teaching hospital between 1975 and 1983, the incidence of intraoperative adverse events among adults was lower than events in the PACU (Cohen et al., 1988). In contrast, the incidence of adverse events in children tended to be higher during surgery than in the PACU (Tiret et al., 1988). Machine-patient disconnection was a common occurrence in the 1970s before the disconnect alarm became a standard feature of anesthesia machines, as mandated by the American National Standard Institute Z79 Committee on Anesthesia Equipment (Cooper et al., 1978).
Anesthetic Overdose
Drug overdose as the cause of anesthetic death occurs, although the incidence has decreased since the 1980s and it accounted for only 5.4% of anesthetic deaths reported to the Medical Defense Union of the United Kingdom between 1970 and 1979 (Utting et al., 1979; Holland, 1984, 1987). In a 15-year study between 1969 and 1983 by Keenan and Boyan (1985) an “absolute overdose” (a dose well in excess of the usual clinical range) caused one third of all cardiac arrests resulting from anesthesia. Of six incidents of cardiac arrest in children in this series, absolute overdose with halothane was responsible in five cases; the other resulted from airway obstruction during nitrous oxide-curare anesthesia.
As described earlier, the POCA Registry database revealed that from 1994 to 1997, cardiovascular depression with halothane accounted for 66% of all medication-related cardiac arrests. In healthy children with ASA PS 1 or 2, 64% of all cardiac arrests were medication-related, in comparison with 23% in those with ASA PS 3 to 5 (Morray et al., 2000). The database from 2000 to 2003, when halothane had been replaced by sevoflurane as the primary inhalation induction agent, showed a dramatic decrease in medication-related cardiac arrest, from 37% to 12% of the total arrests, indicating the importance of safer anesthetic drugs for reducing medication-related complications (Fig. 40-2) (Morray, 2004).
Risk Factors in Pediatric Anesthesia
A number of perioperative risk factors specific to infants and children have been identified.
Age
Infants younger than 1 year of age are at higher risk of developing complications (Tiret et al., 1988; Cohen et al., 1990). A prospective survey in France between 1978 and 1982 involving 440 institutions and a total of 40,240 anesthetic procedures found 27 major complications, of which nine occurred in infants (0.43:10,000 cases), an incidence significantly higher than that in children (0.05:10,000) (Tiret et al., 1988). Infants younger than 1 year of age also showed a significantly higher incidence of airway obstruction and other respiratory problems than did older children (Cohen et al., 1990). Cohen and others (1990) reported that the intraoperative incidence of complications in children was about the same as that in adults, but postoperative complications (35%) were twice as common as in adults (17%). The POCA Registry database from 1994 to 1997 revealed that 83 of 150 cases, or 55%, of anesthesia-related cardiac arrests occurred in infants younger than 12 months of age (Table 40-5) (Morray et al., 2000).
Physical Status
Correlation between POCA and ASA PS has been reported in adults (Keenan and Boyan, 1985). In a large-scale prospective survey in France involving infants and children, Tiret and others (1988) showed a highly significant correlation between the ASA PS classification and the incidence of major perianesthetic complications in children (Table 40-9). They also found a highly significant correlation between the perioperative adverse outcomes and the number of coexisting diseases. The POCA Registry database between 1994 and 1997 showed 100 of 150 (66.7%) of all anesthesia-related cardiac arrests occurred among children with ASA PS 3 to 5 (Morray et al., 2000) (Table 40-5).
Emergency Surgery
The French study by Tiret et al. (1988) showed a threefold increase in perioperative complications in pediatric emergency cases compared with the scheduled cases (Table 40-9). Similarly, Keenan and others (1994) found a highly significant increase of bradycardic episodes in children during emergency surgery compared with those with elective procedures (2.7% vs. 1.1%).
Training
Proper training or experience is particularly important for minimizing the incidence of adverse outcome in the field of pediatric anesthesia. In a retrospective study in a large university hospital over a span of 7 years, Keenan and others (1991) reviewed the incidence of cardiac arrests occurring in infants younger than 1 year of age. No anesthesia-related cardiac arrests occurred when trained pediatric anesthesiologists were in charge of pediatric cases. In contrast, when nonpediatric anesthesiologists were supervising, cardiac arrests (including deaths) occurred at a rate of 19.7:10,000 pediatric anesthesia procedures. In a subsequent prospective study from the same institution for the period of 9 years ending in 1992, the incidence of bradycardic episodes was significantly higher when pediatric anesthesiologists were not supervising the trainees administering anesthetics to infants (2.1%) compared with when pediatric anesthesiologists supervised the trainees (0.8%) (Keenan et al., 1994).
Prevention of anesthesia-related mishaps
Preanesthetic Preparation
Preoperatively, the patient’s past medical history, especially the anesthetic history if there is any, should be reviewed thoroughly, and the patient should be examined carefully. The anesthesia machine, accessories, and drugs should also be checked carefully, because failure to perform these steps properly has been identified as a major cause of anesthesia-related mishaps, including death (Holland, 1984, 1987; Derrington and Smith, 1987). In an emergency or urgent situation (perhaps with the exception of exsanguination, cardiac tamponade, or acute upper airway obstruction), clinicians should try to take at least minimal necessary steps (e.g., fluid resuscitation, transfusion, correction of electrolytes, acid-base imbalance, and fever) to stabilize the patient for anesthesia and surgery in a coordinated effort with the surgeon. The anesthesiologist should not yield to unreasonable pressures to start the case before the patient is best prepared under these circumstances. Indeed, hypovolemia and anemia are reported to be the major causes of anesthesia-related cardiac arrest in infants and children (Salem et al., 1975).
If the anesthesiologist is unfamiliar or uncomfortable with uncommon pathophysiology (e.g., an infant with a cyanotic heart disease coming for noncardiac surgery or a child with craniopharyngioma and diabetes insipidus), the anesthesiologist should not hesitate to ask for consultation with a senior anesthesiologist or other specialists. In addition, especially in an emergency situation (such as posttonsillectomy bleeding or acute epiglottitis), an experienced assistant, preferably an attending pediatric anesthesiologist, should be standing by for the induction. Keenan and others (1994) found a significantly lower incidence of bradycardic events with the presence of a pediatric anesthesiologist during induction. In teaching institutions, resident trainees should always be supervised by a pediatric attending anesthesiologist for a pediatric patient. Lack of an experienced assistant has been associated with anesthesia-related mortality and a high rate of morbidity (Craig and Wilson, 1981; Derrington and Smith, 1987; Eichhorn, 1989; Keenan et al., 1994). A reluctance to ask for help, whether out of insecurity, pride, or ill-conceived heroism (or machismo), has no place in a pediatric anesthesia emergency and is the first step toward disaster.
Vigilance
A timely recovery from impending failure is the key to patient safety. It is therefore extremely important to recognize without delay that something is going wrong. Sustained attention or vigilance is essential for patient safety during the maintenance of anesthesia, because critical events most commonly occur at the time of presumably reduced mental and physical workload (Cooper et al., 1978; Craig and Wilson, 1981; Gibbs, 1986; Gaba et al., 1987). One should develop the habit of scanning all of the monitors regularly in an orderly fashion, as a pilot in flight scans the instrument panel at regular intervals, to keep up with minute deviations in the patient’s cardiorespiratory stability. A survey by Cooper and others (1984), however, suggested that at least 33% of critical incidents occurring during the maintenance of anesthesia resulted from errors in judgment rather than from inattentiveness. Vigilance is an important deterrent but by itself is not sufficient for prevention of critical incidents. However, the notion that all human beings, including all anesthesiologists, make mistakes should not be used as an excuse to discount the importance of vigilance, especially in pediatric anesthesia (Allnutt, 1987). The anesthesiologist should aim for near perfection by minimizing factors that adversely affect vigilance, such as fatigue, distraction, and boredom. An audible tone for the pulse and oxygen saturation, as well as an audible tone for capnographic alarm limits and loss of pressure is advocated by the Anesthesia Patient Safety Foundation (APSF) as a method to enhance patient safety and improve vigilance. A better understanding and application of ergonomic principles may improve vigilance and help reduce anesthesia-related mishaps in the future (Gravenstein and Weinger, 1986).
Monitors and Monitoring Standards
The fact that most anesthesia-related fatalities involve human error clearly supports the concept that monitoring devices are essential for patient safety even for the experienced, conscientious, and vigilant anesthesiologist. The ASA Closed Claims Project indicated that in 28% of these cases, proper monitoring already available commercially could have averted the mishaps (Cheney, 1988). Of particular interest to anesthesiologists is that the median cost of legal settlement in cases in which improved monitoring would probably have prevented the complication or death was more than 10 times higher than in cases in which better monitoring would have had no effect on occurrence or outcome (Cheney, 1988). In a similar review involving 238 (10% of 2400 total claims) closed anesthesia malpractice claims concerning children, a vast majority (approximately 89%) of pediatric claims that were related to inadequate ventilation (43% of total) could have been prevented with pulse oximetry, capnography, or both (Morray et al., 1993). The proportion of claims assessed as preventable by better monitoring decreased from an average of 63% in the 1970s to 16% in the 1990s (Jimenez et al., 2007).
In 1985, minimum standards for patient monitoring during anesthesia at Harvard University teaching hospitals were adopted and later published (Eichhorn et al., 1986). Similar but slightly more specific standards for basic intraoperative monitoring, proposed by the ASA, were approved by the House of Delegates in 1986. Both sets of standards specifically mandate that oxygenation, ventilation, circulation, and body temperature be evaluated continually (at regular intervals) or continuously (without interruption). For each of the components, the clear objective to ensure adequacy, followed by specific methods, is stated (see Chapter 10, Equipment; Chapter 11, Monitoring; and Chapter 13, Induction, Maintenance, and Recovery). There is a strong emphasis on combining clinical evaluation and technological methods. Although no specific methodology or instrumentation is mandated for monitoring cardiopulmonary and other indices, the ASA standards strongly encourage quantitative methods, such as pulse oximetry and capnography, over qualitative clinical assessment, with inspection and auscultation for cardiopulmonary monitoring. In addition, the New York State Hospital Code (1988) dictates that pulse oximetry and capnography be used to monitor all patients undergoing general anesthesia to reduce anesthesia-related morbidity and mortality. Eventually, most other states followed with similar laws mandating intraoperative monitoring with pulse oximetry and capnography. Accordingly, most malpractice liability insurers in the United States have required that anesthesiologists follow these standards whenever possible (Orkin, 1989). The APSF endorses the use of audible alarms on physiologic monitors.
Similar standards for basic patient monitoring have also been proposed in the United Kingdom, Australia, and other industrialized countries (Sykes, 1987; Cass et al., 1988; Runciman, 1988b). They are similar to the ASA standards in terms of machine monitoring (i.e., oxygen analyzer, low-flow alarm, and ventilator disconnect alarm). The standards for minimum patient monitoring, however, differ considerably, depending in part on the patient’s physical status and the degree of surgical involvement (i.e., minor, standard, or major operation) (Sykes, 1987).
With the advent of pulse oximetry in the late 1980s, the anesthesiologist’s ability to detect hypoxemia has improved markedly (see Chapters 10, Equipment, and 11, Monitoring). Coté and others (1988, 1991) have demonstrated that major hypoxemic events (arterial saturation of oxygen [Sao2] equal to 80% lasting more than 30 seconds) can occur without visible cyanosis or obvious changes in the cardiorespiratory patterns in children and that pulse oximetry detects such events more quickly than other means of monitoring. Cooper and others (1987), as part of perianesthetic QA activity, found a significant reduction in undesirable anesthesia-related incidents that require intervention after the introduction of pulse oximetry in the operating room.
Technological Improvements
Another technological patient safety strategy is the use of engineered safety devices that physically prevent errors from occurring (Gaba, 2000). An example of this human factor design is the pin index safety system in pneumatic connections, which uses geometric features on the yoke to prevent a gas hose or cylinder from being connected to the wrong gas yoke. This system is not infallible, however, and misconnections can still occur without proper vigilance (Ellett et al., 2009). New technologies have also been developed to improve management of the patient’s airway, including the video laryngoscope, improved fiberoptic laryngoscopes, and LMAs. These devices improve the management of the pediatric (upper) airway in both routine and emergency situations.
According to the APSF, the use of automated information management systems could improve the future ability to link intraoperative events to both short-term and long-term outcomes (Gravenstein, 2001). Collection of real-time data obtained from the millions of anesthetics administered annually worldwide could lead to a better understanding of best anesthesia practices and improved patient safety. Such data collection studies are increasingly common. In pediatric critical care, a collaborative project of over 70 pediatric intensive care units and Virtual Pediatric Systems has accumulated data on over 250,000 critically ill children and is providing benchmarking and quality data to guide critical care practice (Wetzel, 2009). The Society for Pediatric Anesthesia is sponsoring a similar prospective data collection methodology to collect detailed incident data across the field of pediatric anesthesia to provide benchmarking data.
Selection of Safer Anesthetics and Adjuvant Drugs
The POCA Registry data analyses have clearly demonstrated the safety of sevoflurane over halothane as the agent of choice for inhalation induction of anesthesia (Morray, 2004). Between 1994 and 1997, cardiac arrest associated with medication accounted for 37% of all cardiac arrests, from which halothane with or without other drugs accounted for 66% (Morray et al., 2000). From 2000 to 2003, when sevoflurane had replaced halothane as the inhalation induction agent of choice, medication-related causes of cardiac arrests decreased drastically, from 37% to 12%. Concomitantly, the incidence of cardiac arrests among healthy children (ASA PS 1 or 2) decreased from 33% to 19% of all cardiac arrests (Morray, 2004).
The developing nervous system is highly susceptible to neurotoxic insults during rapid synaptogenesis, during the brain growth spurts, and before neurons have migrated to their final destination and fully differentiated (Rabinowicz et al., 1996). In 2003, data were released that clearly showed that modern anesthetics cause widespread neurodegeneration in the developing rat brain (Jevtovic-Todorovic et al., 2003). Since that time, several more studies have confirmed that data by showing anesthesia-related increases in brain cell death and reductions in functional performance in rats. Although the exact mechanism of anesthesia-induced neurodegeneration in animals remains unclear, the evidence points toward the involvement of γ-aminobutyric acid (GABA) and N-methyl-d-aspartic acid (NMDA) receptors. All commonly used anesthetics are thought to exploit their effects on GABA or NMDA receptors to produce unconsciousness and immobility during painful stimulation. Moreover, all commonly used anesthetics that were investigated for their neurodegenerative properties, such as benzodiazepines, ketamine, propofol, nitrous oxide and isoflurane, have been shown to exacerbate neuronal cell death.(Loepke et al., 2008). Although the documented neurotoxic effects of anesthetics in the developing animal model are noteworthy and alarming, the implications of these findings for clinical practice remains uncertain. The complexity of the mammalian nervous-system development complicates the extrapolation of data derived from animal models to humans. The FDA convened an Advisory Committee Meeting in April of 2007 to review all of the available evidence regarding the potential neurodegenerative effects of anesthetics in infant and juvenile animals. The panel determined that, based on the current knowledge and lack of appropriate alternatives, there was no scientific basis to recommend changes in clinical practice. Future research in this area with animals and nonhuman primates is needed to further understand the exact mechanisms behind anesthesia-related neurodegeneration and the long-term effects of modern anesthetic agents on the development of the human brain. Increased research on the mechanisms of anesthetic action are needed to discover drugs with greater specificity or mechanisms of action that do not involve the GABA and NMDA receptors, as well as adjuvant drugs and techniques that may limit their deleterious effects. Until the risk of neurocognitive injury is understood, pediatric surgical specialists, in conjunction with anesthesiologists and pediatricians, should identify surgical procedures that can be delayed until older ages without incurring additional risk (McGowan and Davis, 2008).
Improved Education and Training
The steady decline in anesthesia-related mortality and morbidity over the past several decades has been attributed to increases in better-trained and better-qualified physicians administering anesthesia (Holland, 1987). In a survey of potentially harmful anesthesia-related events, Cooper and others (1978) found that 25% of these events were associated with inadequate training or unfamiliarity with equipment or devices; further training of these anesthetists (presumably trainees) would have prevented some of these events. In addition, an anesthesia-related mortality study from Harvard University has shown that in 8 of 11 cases of fatalities attributed primarily to anesthesia, inadequate supervision of residents, medical students, and nurse anesthetists was considered contributory (Eichhorn, 1989).
As mentioned previously, Keenan and others (1991) reported the importance of specialty training in pediatric anesthesia for the reduction of cardiac arrests in infants. No anesthesia-related cardiac arrests occurred when trained pediatric anesthesiologists were in charge of pediatric cases, whereas cardiac arrests occurred at a rate of 19.7:10,000 when non–pediatric anesthesiologists were supervising trainees. The same investigators also reported in a subsequent prospective study that the incidence of bradycardic episodes was significantly higher when pediatric anesthesiologists were not supervising the trainees anesthetizing infants (2.1%) than when trained pediatric anesthesiologists were in charge (0.8%) (Keenan et al., 1994). These results emphasize the importance of training and experience for a better anesthetic outcome.
There has been an explosion of interest in patient safety over the last four decades, and sessions on patient safety are now common in the scientific programs of the ASA and SPA annual meetings. There is also an increasing amount of patient-safety research being done each year, with the APSF awarding grants for research funds since its inception in 1985. The results of this research are reported in anesthesiology journals, scientific and educational meetings, and textbooks. The availability of these data have raised physician awareness of patient safety. Realistic patient and infant simulators were introduced in the 1980s. Since that time there have been great advances in simulator technology and science, and these simulators are available at many anesthesia training programs and as a component of many scientific meetings. Anesthesia has become the leader in the application and adoption of simulators that provide strong patient safety implications through education, training, and research (Stoelting and Khuri, 2006).
Quality Assurance
Documentation of the QA or QI process, especially that of fatal and morbid events, has been mandated in an effort to reduce the risk to and improve the outcome of patients undergoing anesthesia. In a pilot prospective study, Currie and others (1988) found the QA survey helpful in identifying nonfatal, nonmorbid, often transient events for peer review soon after such events took place. The survey demonstrated disproportionately high incidences of nonfatal events occurring with pediatric and emergency or out-of-hours cases. Induction and maintenance periods seem to be equally hazardous, up to four times more so than the emergence and recovery periods. As noted in other studies, airway events were most common (53%) (Cooper et al., 1978, 1984; Craig and Wilson, 1981). The authors concluded that the advantage of their method lies for the most part in its inherent lack of inertia: rapid evaluation, response, and feedback in a confidential and nonjudgmental atmosphere, resulting in the improvement of patient care in a unique manner (Cooper et al., 1978; Currie et al., 1988). QA or QI programs should be continued to prevent adverse events caused by human factors or errors (active failure) by learning from events and “near misses” as a group. Equally important, the QA program is the process of continually evaluating the environment in which anesthesia is practiced, and identifying systemic problems (environmental factors or latent failures) responsible for or potentially causing adverse events, which have not been obvious, so as to implement strategies or new guidelines to improve the structure or environment and prevent future occurrence of adverse events.
Environmental factors (latent failures) may play a major role and may be responsible for adverse outcomes impeding the safety and quality of patient care. These factors may include inadequate safety mechanisms (e.g., poor organization, unclear drug labeling), inadequate or nonfunctional monitoring systems, and poor communication among the surgeons, anesthesiologists, and operating room personnel. Indeed, in an analysis of 110 adverse events from more than 13,000 anesthetic procedures, Lagasse and others (1995) identified only 8% caused by human errors, whereas 92% of adverse events were attributable to system errors.
The impact of anesthetic management characteristics on severe morbidity and mortality in patients (between 62 and 65 years of age) was analyzed by the Dutch Association of Anesthesiology (Arbous et al., 2005). In a case-controlled study in the cohort of nearly 870,000 cases between 1995 and 1997, 807 patients who remained comatose or died within 24 hours of undergoing general anesthesia were identified and compared with 883 control cases without severe outcome. The incidence of postoperative death was 8.8:10,000 in this age group. Anesthetic management factors that were statistically significantly associated with a decreased risk included: equipment check with a protocol and checklist (odds ratio: 0.64), documentation of the equipment check (odds ratio: 0.61), a directly available anesthesiologist (odds ratio: 0.46), no change of anesthesia provider during the case (odds ratio: 0.44), two rather than one person present at emergence (odds ratio: 0.69), reversal of muscle relaxant or of the combination of relaxant and opioid (odds ratio: 0.1 and 0.29, respectively), and postoperative pain medication vs. no pain medication (Arbous et al., 2005).
Critical events related to anesthesia occur commonly during the practice of anesthesia. A well-trained, motivated, vigilant anesthesiologist normally detects these events in time and takes proper measures to prevent disaster. Anesthesia-related accidents causing catastrophic injury or death, therefore, are rare, especially in relation to the rate of such potentially harmful events. Because humans are imperfect and make mistakes as part of normal human cognitive behavior, catastrophic accidents can occur almost at random to any anesthesiologist (Allnutt, 1987). The availability of both machine and patient monitors and adherence to monitoring standards are indispensable.
Summary
Anesthesia-related mortality has declined steadily over the past several decades as the percentage of general anesthetics administered by trained anesthesiologists has increased and as newer and safer anesthetics and adjuvant drugs, anesthetic equipment, and monitoring and safety standards in the industrialized nations have improved. Yet, anesthesia-related adverse events remain relatively higher in infants younger than 1 year of age than in older children and adults. As in adults, anesthesia-related catastrophe is still caused predominantly by judgment errors and environmental factors that are potentially preventable (Lagasse, 2002). Critical incidents occur more often during the induction and maintenance of anesthesia than during emergence and recovery. Emergency and urgent surgeries are associated with a disproportionately high incidence of anesthesia-related adverse events, in part because of inadequate preanesthesia patient preparation.
Analyses of closed malpractice insurance claims as well as the POCA Registry sponsored by the ASA have been informative in developing a future strategy for preventing anesthesia-related mishaps. Emphasis on strict monitoring standards and QA surveillance, developed and sustained since the late 1980s, seem valuable both in preventing near misses and in heightening anesthesiologists’ awareness of such events. For patient safety in pediatric anesthesia, better clinical training, adequate preanesthetic preparations, vigilance, and adherence to monitoring standards are essential. Anesthesiologists must remain motivated and continue to pursue harmless anesthesia, yet at the same time be proud leaders of patient safety (Cooper and Gaba, 2002).
Arbous S., Meursing A.E., van Kleef J.W., et al. Impact of anesthesia management characteristics on severe morbidity and mortality. Anesthesiology. 2005;102:257-268.
Allnutt M.F. Human factors in accidents. Br J Anaesth. 1987;59:856-864.
American Society of Anesthesiologists. Standards for basic intraoperative monitoring. 1986. Oct http://www.asahq.org/publicationsAndServices/standards/02.pdf#2
American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Pediatric guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017.
Arrowsmith J.B., Gerstman B.B., Fleischer D.E., et al. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:421.
Aouad M.T., Siddik S.S., Rizk L.B., et al. The effect of dexamethasone on postoperative vomiting after tonsillectomy. Anesth Analg. 2001;92:636-640.
Bailey P.L., Pace N.L., Ashburn M.A., et al. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826-830.
Beecher H.K., Todd D.P. A study of the deaths associated with anesthesia and surgery based on a study of 599,548 anesthesias in ten institutions 1948–1952, inclusive. Ann Surg. 1954;140:2.
Bhananker S.M., Posner K.L., Cheney F.W., et al. Injury and liability associated with monitored anesthesia care. Anesthesiology. 2006;104:228-234.
Bhananker S.M., Ramamoorthy C., Geiduschek J.M., et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. 2007;105:344-350.
Bodlander F.M.S. Deaths associated with anesthesia. Br J Anaesth. 1975;47:36.
Borland L.M., Sereika S.M., Woelfel S.K., et al. Pulmonary aspiration in pediatric patients during general anesthesia: Incidence and outcome. J Clin Anesth. 1998;10:95-102.
Borland L.M., Colligan J., Brandom B.W. Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Pediatr Anesth. 2004;14:733-738.
Braz L.G., Braz J.R., Modolo N.S., et al. Perioperative cardiac arrest and its mortality in children: a 9-year survey in a Brazilian tertiary teaching hospital. Pediatr Anesth. 2006;16:860-866.
Buck N., Devlin H.B., Lunn J.N. The report of the confidential inquiry into perioperative deaths. London: Nuffield Provincial Hospitals Trust/King’s Fund, 1987.
Caplan R.A., Ward R.J., Posner K., et al. Unexpected cardiac arrest during spinal anesthesia: a closed claims analysis of predisposing factors. Anesthesiology. 1988;68:5-11.
Cass N.M., Crosby W.M., Holland R.B. Minimal monitoring standards. Anaesth Intensive Care. 1988;16:110.
Cheney F.W. Anesthesia: potential risks and causes of incidents. In: Gravenstein JS., Holzer JF., editors. Safety and cost containment in anesthesia. Boston: Butterworths, 1988.
Cohen I.T., Motoyama E.K. Pediatric intraoperative and postoperative management. In: Motoyama EK., Davis PJ., editors. Smith’s anesthesia for infants and children. ed 7. Philadelphia: Elsevier; 2006:38-42.
Cohen M.M., Cameron C.B. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72:282-288.
Cohen M.M., Duncan P.G., Tate R.B. Does anesthesia contribute to operative mortality? JAMA. 1988;260:2859-2863.
Cohen M.M., Cameron C.B., Duncan P.G. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70:160-167.
Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics. Guidelines for the elective use of conscious sedation, deep sedation and general anesthesia in pediatric patients. Pediatrics. 1985;76:317-321.
Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89:1110-1115.
Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: Addendum. Pediatrics. 2002;110:836-838.
Cooper J.B. Conference report: 1986 Meeting of the International Committee for Prevention of Anesthesia Mortality and Morbidity. Can J Anaesth. 1988;35:287.
Cooper J.B., Gaba D. No myth: Anesthesia is a model for addressing patient safety (editorial). Anesthesiology. 2002;97:1335-1337.
Cooper J.B., Pierce E.C.Jr. Safety foundation organized: statement of purpose. Anesthesia Patient Safety Foundation Newsletter. 1986;1:1.
Cooper J.B., Newbower R.S., Kitz R.J. An analysis of major errors and equipment failures in anesthesia management: considerations for prevention and detection. Anesthesiology. 1984;60:34-92.
Cooper J.B., Cullen D.J., Nemeskal R., et al. Effects of information feedback and pulse oximetry on the incidence of anesthesia complications. Anesthesiology. 1987;67:686-694.
Cooper J.B., Newbower R.S., Long C.D., et al. Preventable anesthesia mishaps: a study of human factors. Anesthesiology. 1978;49:399-406.
Coté C.J. Discharge criteria for children sedated by nonanesthesiologists. Anesthesiology. 2004;100:207-209.
Coté C.J., Goldstein E.A., Coté M.A., et al. A single-blind study of pulse oximetry in children. Anesthesiology. 1988;68:184-188.
Coté C.J., Rolf N., Liu L.M.P., et al. A single-blind study of pulse oximetry and capnography in children. Anesthesiology. 1991;74:980-987.
Coté C.J., Zaslevsky A., Downes J.J., et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis. Anesthesiology. 1995;82:809-822.
Coté C.J., Nottermann D.A., Karl H.W., et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105:805-814.
Craig J., Wilson M.E. A survey of anaesthetic misadventures. Anaesthesia. 1981;36:933-936.
Cravero J.P., Blike G.T., Beach M.L., et al. Incidence and Nature of Adverse Events During Pediatric Sedation/Anesthesia for Procedures Outside the Operating Room: Report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;118:1087-1096.
Cravero J.P., Beach M.L., Blike G.T., et al. The Incidence and Nature of Adverse Events During Pediatric Sedation/Anesthesia With Propofol for Procedures Outside the Operating Room: A Report From the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795-804.
Currie M., Pybus D.A., Torda T.A. A prospective survey of anaesthetic critical events: a report on a pilot study of 88 cases. Anaesth Intensive Care. 1988;16:103-107.
Davis D.A. An analysis of anesthetic mishaps from medical liability claims. Int Anesthesiol Clin. 1984;22:31-42.
Derrington M.C., Smith G. A review of studies of anaesthetic risk, morbidity and mortality. Br J Anaesth. 1987;59:815-833.
DeSoto H., Patel R.I., Soliman I.E., et al. Changes in oxygen saturation following general anesthesia in children with upper respiratory infection signs and symptoms undergoing otolaryngological procedures. Anesthesiology. 1988;68:276-279.
Dornette W.HL., Orth O.S. Death in the operating room. Anesth Analg. 1956;35:545.
Downes J.J., Raphaely R.C. Anesthesia and intensive care. In Ravitch MM., Welch KJ., Benson CD., et al, editors: Pediatric surgery, ed 3, Chicago: Year Book Medical Publishers, 1979.
Dullenkopf A., Gerber A.C., Weiss M. Fit and seal characteristics of a new paediatric tracheal tube with high volume-low pressure polyurethane cuff. Acta Anesthesiol Scand. 2005;49:232-237.
Duncan P.G.. Clinical pearls-pediatric anesthesia. IARS Review Course Lectures. Anesth Analg. 1995(Suppl):9. March
Eichhorn J.H. Prevention of intraoperative anesthesia accidents and related severe injury through safety monitoring. Anesthesiology. 1989;70:572-577.
Eichhorn J.H., Cooper J.B., Cullen D.J., et al. Standards for patient monitoring at Harvard Medical School. JAMA. 1986;256:1017-1020.
Ellett A.E., Shield J.C., et al. A near miss: a nitrous oxide-carbon dioxide mix-up despite current safety standards. Anesthesiology. 2009;110(6):1429-1431.
Epstein R.M. Morbidity and mortality from anesthesia: a continuing problem. Anesthesiology. 1978;49:388-389.
Fasting S., Gisvold S.E. Data recording of problems during anaesthesia: presentation of a well-functioning and simple system. Acta Anaesthesiol Scand. 1996;40:1173-1183.
Fine G.F., Borland L.M. The future of the cuffed endotracheal tube. Pediatr Anesth. 2004;14:38-42.
Fisher D.M. Anesthesia equipment for pediatrics. In: Gregory GA., editor. Pediatric anesthesia. New York: Churchill Livingstone; 1989:464-465.
Gronert B.J., Motoyama E.K. Induction of anesthesia and endotracheal intubation. In: Motoyama EK., Davis PJ., editors. Smith’s anesthesia for infants and chidlren. ed 6. St Louis: Mosby; 1996:281-312.
Flick R.P., Sprung J., Harrison T., et al. Perioperative cardiac arrest in children between 1988 and 2005 at a tertiary referral center. Anesthesiology. 2007;106:226-237.
Flick R.P., Schears G.J., Warner M.A. Aspiration in pediatric anesthesia: is there a higher incidence compared with adults? Curr Opin Anesthesiol. 2002;15:323-327.
Flick R.P., Wilder R.T., Pieper S.F., et al. Risk factors for laryngospasm in children during general anesthesia. Pediatric Anesthesia. 2008;18(4):289-296.
Fujii Y., Toyooka H., Tanaka H. Antiemetic efficacy of granisetron and metoclopramide in children undergoing ophthalmic or ENT surgery. Can J Anaesth. 1996;43:1095-1099.
Gaba D.M. Anesthesiology as a model for safety in health care. Br Med J. 2000;320:785-788.
Gaba D.M., Maxwell M., DeAnda A. Anesthetic mishaps: breaking the chain of accident evolution. Anesthesiology. 1987;66:670-676.
Gan T.J., Meyer T., Apfel C.C., et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62-71.
Gan T.J., Meyer T.A., Apfel C.C., et al. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105(6):1615-1628.
Giaufré E., Dalens B., Gombert A. Epidermiology and morbidity of regional anesthesia in children: a one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg. 1996;83:904-912.
Gibbs J.M. The Anaesthetic Mortality Assessment Committee 1979-1984. N Z Med J. 1986;99:55-59.
Glass P.A., White P.F. Practice guidelines for the management of postoperative nausea and vomiting: past, present and future. Anesth Analg. 2007;105:1528-1529.
Graff T.D., Phillips O.C., Benson D.W., et al. Baltimore Anesthesia Study Committee, factors in pediatric anesthesia mortality. Anesth Analg. 1964;43:407.
Gravenstein J.S. Information systems: new role of information in anesthesia patient safety highlighted in a series of articles by technology experts. APSF Newsletter. 2001:16. Summer
Gravenstein J.S., Weinger M.B. Why investigate vigilance? J Clin Monit. 1986;2:145-147.
Gross J.B., Bailey P.L., Caplan R.A., et al. Practice guidelines for sedation and analgesia by non-anesthesiologists: a report by the American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology. 2002;96:1004-1017.
Hadzic A., Arliss J., Kerimoglu B., et al. A comparison of infraclavicular nerve block vs. general anesthesia for hand and wrist day-case surgeries. Anesthesiology. 2004;101:127-132.
Harrison G.G. Death attributable to anaesthesia. Br J Anaesth. 1978;50:1041.
Harrison G.G. Death due to anesthesia at Groote Schuur Hospital, Cape Town-1956–1987. Part II. Causes and changes in aetiological pattern of anesthetic-contributory death. S Afr Med J. 1990;77:416-421.
Hatton F., Tiret L., Vourc’h G., et al. Morbidity and mortality associated with anaesthesia. French survey: preliminary results. In: Vickers MD., Lunn JN., editors. Mortality in anaesthesia. Berlin: Springer-Verlag; 1983:25.
Holland R. Anaesthesia-related mortality in Australia. Int Anesthesiol Clin. 1984;22:61.
Holland R. Anaesthetic mortality in New South Wales. Br J Anaesth. 1987;59:834-841.
Holzman R.S. Morbidity and mortality in pediatric anesthesia. Pediatr Clin North Am. 1994;41:239-256.
Homer J.R., Elwood T., Peterson D.O., et al. Risk factors for adverse events in children with colds emerging from anesthesia: a logistic regression. Pediatric Anesthesia. 2007;17:154-161.
Hovi-Viander M. Death associated with anesthesia in Finland. Br J Anaesth. 1980;52:483-489.
Irita K., Kawashima Y., Iwao Y., et al. Annual mortality and morbidity in operating rooms during 2002 and summary of morbidity and mortality between 1999 and 2002 in Japan. Masui. 2004;53(3):320-335.
Irita K., Kawashima Y., Morita K., et al. Critical incidents during regional anesthesia in Japanese Society of Anesthesiologists-certified training hospitals. Masui. 2005;54(4):440-449.
James I. Cuffed tubes in children. Pediatr Anesth. 2001;11:259-263.
Jevtovic-Todorovic V., Olney J.W. PRO: Anesthesia-induced developmental neuroapoptosis: status of the evidence. Anesth Analg. 2008;106:1659-1663.
Jevtovic-Todorovic V., Hartman R.E., Izumi Y., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
Jimenez N., Posner K.L., Cheney F.W., et al. An update on pediatric anesthesia liability: a closed claims analysis. Anesth Analg. 2007;104:147-153.
Joint Commission on Accreditation of Healthcare Organizations. Joint Commission Accreditation Manual on Hospitals. vol 3. Oak Brook Terrace, IL: JCAHO; 1992.
Jones D.T., Bhattacharyya N. Passive smoke exposure as a risk factor for airway complications during outpatient pediatric procedures. Otolaryng-Head Neck Surg. 2006;135:12-16.
Jones S.EF., Beasley J.M., MacFarlane W.R., et al. Intrathecal morphine for postoperative pain relief in children. Br J Anaesth. 1984;56:137-140.
Kataria B.K., Harmik E.V., Michard R., et al. Postoperative arterial oxygen saturation in the pediatric population during transportation. Anesth Analg. 1988;67:280-282.
Kawashima Y., Takahashi S., Suzuki M., et al. Anesthesia-related mortality and morbidity over a 5-year period in 2,363,038 patients in Japan. Acta Anaesthesiol Scand. 2003;47:809-817.
Keats A.S. Anesthesia mortality: a new mechanism. Anesthesiology. 1988;68:2-4.
Keats A.S., Siker E.S. International Symposium on Preventable Anesthetic Morbidity and Mortality, Boston, Oct. 8-10, 1984. Anesthesiology. 1985;63:349.
Keenan R.L., Boyan C.P. Cardiac arrest due to anesthesia: a study of incidence and causes. JAMA. 1985;253:2373-2377.
Keenan R.L., Shapiro J.H., Dawson K. Frequency of anesthetic cardiac arrest in infants: effect of pediatric anesthesiologists. J Clin Anesth. 1991;3:433-437.
Keenan R.L., Shapiro J.H., Kane F.R., et al. Bradycardia during anesthesia in infants: an epidemiologic study. Anesthesiology. 1994;80:976-982.
Khine HH., Corddry DH., Kettrick RG., et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997;86:627-631.
Kohn L., Corrigan J., Donaldson M., editors. Committee on Quality of Health Care in America: To err is human: building a safer health care system in America. Institute of Medicine. National Academy Press; 1999:241.
Koka B.V., Jeon I.S., Andre J.M., et al. Postintubation croup in children. Anesth Analg. 1977;56:501-505.
Krane E.J. Delayed respiratory depression in a child after caudal epidural morphine. Anesth Analg. 1988;67:79-82.
Kurth C.D., LeBard S.E. Association of postoperative apnea, airway obstruction, and hypoxemia in former premature infants. Anesthesiology. 1991;75:22-26.
Kurth C.D., Spitzer A.R., Broennle A.M., et al. Post-operative apnea in preterm infants. Anesthesiology. 1987;66:483-488.
Lagasse R.S. Anesthesia safety: model or myth? Anesthesiology. 2002;97:1609-1617.
Lagasse R.S., Steinberg E.S., Katz R.I., et al. Defining quality of perioperative care by statistical process control of adverse outcomes. Anesthesiology. 1995;82:1181-1188.
Lee L.A., Posner K.L., Cheney F.W., et al. Complications associated with eye blocks and peripheral newve blocks: an American Society of Anesthesiologists closed claims analysis. Reg Anesth Pain Med. 2008;33(5):416-422.
Lienhart A., Auroy Y., Pequignot F. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105:1087-1097.
Litman R.S., Keon T.P. Postintubation croup in children. Anesthesiology. 1991;75:1122-1123.
Liu L.M., Coté C.J., Goudsouzian N.G., et al. Life-threatening apnea in infants recovering from anesthesia. Anesthesiology. 1983;59:506-510.
Llewellyn N., Moriarty A. The national pediatric epidural audit. Pediatr Anesth. 2007;17:520-533.
Loepke A.W., Soriano S.G. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681-1707.
Loepke A.W., McGowan F.X., Soriano S.G. CON: The toxic effects of anesthetics in the developing brain: the clinical perspective. Anesth Analg. 2008;106:1664-1669.
Lunn J.N., Devlin H.B. Lessons from the confidential enquiry into perioperative deaths in three NHS regions. Lancet. 1987;2:1384-1386.
Lunn J.N., Mushin W.W. Mortality associated with anaesthesia. London: Nuffield Provincial Hospitals Trust, 1982.
Malviya S., Swartz J., Lerman J. Are all preterm infants younger than 60 weeks postconceptual age at risk for postanesthetic apnea? Anesthesiology. 1993;78:1076-1081.
Malviya S., Voepel-Lewis T., Tait A.R. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85:1207-1213.
Malviya S., Voepel-Lewis T., Ludomirsky A., et al. Can we improve the assessment of discharge readiness? Anesthesiology. 2004;100:218-224.
Marhoffer P., Greher M., Kapral S. Ultrasound guidance in regional anesthesia. Br J Anesth. 2005;94:7-17.
Martin T.M., Nicolson S.C., Bargas M.S. Propofol anesthesia reduces emesis and airway obstruction in pediatric outpatients. Anesth Analg. 1993;76:144-148.
Mason L.J. An update on the etiology and prevention of anesthesia-related cardiac arrest in children. Pediatr Anesth. 2004;14:412-416.
McGowan F.X., Davis P.J. Anesthetic related neurotoxicity in the developing infant: of mice, rats, monkeys and, possibly, humans. Anesth Analg. 2008;106:1599-1602.
McNamara D.G., Nixon G.M., Anderson B.J. Methylxanthines for the treatment of apnea associated with bronchiolitis and anesthesia. Paediatr Anaesth. 2004;14:541-550.
Mellin-Olsen J., Fasting S., Gisvold S.E. Routine preoperative gastric emptying is seldom indicated: a study of 85,594 anaesthetics with special focus on aspiration pneumonia. Acta Anaesthesiol Scand. 1996;40:1184-1188.
Mendelson C.L. Aspiration of stomach contents into lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;51:889-892.
Morgan C.A., Webb R.K., Cockings J., et al. The Australian Incident Monitoring Study. Cardiac arrest: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:626-637.
Morray J.P.. Unexpected cardiac arrest in the anesthetized child. Presented at Society of Pediatric Anesthesia spring meeting March 4–7. 2004.
Morray J.P., Geiduschek J.M., Caplan R.A., et al. A comparison of pediatric and adult anesthesia closed malpractice claims. Anesthesiology. 1993;78:461-467.
Morray J.P., Geiduscheck J.M., Ramamoorthy C., et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesthesiology. 2000;93:6-14.
Motas D., McDermott N.B., Vansickle T., et al. Depth of consciousness and deep sedation attained in children as administered by nonanesthesiologists in a children’s hospital. Paediatr Anaesth. 2004;14:256-260.
Motoyama E.K. The shape of the pediatric larynx: cylindrical or funnel shaped? Anesth Analg. 2009;108:1379-1381.
Motoyama E.K., Glazener C.H. Hypoxemia after general anesthesia in children. Anesth Analg. 1986;65:267-272.
Murat I. Cuffed tubes in children: a 3-year experience in a single institution. Paediatr Anaesth. 2001;11:748-749.
Murat I. Mortality, morbidity and outcome. In: Bissonette B., Dalens B., editors. Pediatric anesthesia: principles and practice. New York: McGraw-Hill; 2002:1465-1478. Chapter 79
Murat I., Constant I., Maud’Huy H. Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30 month period. Paediatr Anaesth. 2004;14:158-166.
Murphy J.J., Swanson T., Ansermino M., et al. The frequency of apneas in premature infants after inguinal hernia repair: do they need overnight monitoring in the intensive care unit? J Pediatr Surg. 2008;43:865-868.
New York State Hospital Code. 1988. Section 405.13, endorsed by the New York State Hospital Review and Planning Council, June 9 approved by the Commissioner of the New York State Department of Health for Jan. 1, 1989, implementation
Nixon G.M., Kermack A.S., McGregor C.D., et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2005;39:332-338.
Olsson G.L. Bronchospasm during anesthesia: a computer-aided incidence study of 136,929 patients. Acta Anesthesiol Scand. 1987;31:244-252.
Olsson G.L., Hallen B. Laryngospasm during anesthesia: a computer-aided incidence study in 136,929 patients. Acta Anaesthesiol Scand. 1984;28:567-575.
Olsson G.L., Hallen B. Cardiac arrest during anaesthesia: a computer-aided study in 250,543 anaesthetics. Acta Anaesthesiol Scand. 1988;32:653-654.
Olsson G.L., Hallen B., Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30:84-92.
Orkin F.K. Practice standards: the Midas touch or the emperor’s new clothes? Anesthesiology. 1989;70:567-571.
Parnis S.J., Barker D.S., van der Walt J.H. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
Patel R.I., Rice L.J. Special considerations in the recovery room of children from anesthesia. Int Anesthesiol Clin. 1991;29:55-68.
Patel R.I., Verghese S.T., Hannallah R.S., et al. Fast-tracking children after ambulatory surgery. Anesth Analg. 2001;92:918-922.
Petruscak J., Smith R.N., Breslin P. Mortality related to ophthalmological surgery. Surv Anesthesiol. 1974;18:87.
Phillips O.C., Frazier T.M. Baltimore Anesthesia Study Committee: organization and preliminary report. Anesthesiology. 1957;18:33.
Pierce E.C.Jr. Analysis of anesthetic mishaps: historical perspectives. Int Anesthesiol Clin. 1984;22:116.
Pierce E.C.Jr. Safety in anesthesia. Curr Opin Anaesth. 1988;1:532.
Practice guidelines for sedation and analgesia by non-anesthesiologists. a report by the American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology. 1996;84:459.
Pullerits J., Burrows F.A., Roy W.L. Arterial desaturation in healthy children during transfer to the recovery room. Can J Anaesth. 1987;34:470-473.
Rabinowicz T., DeCourten-Myers G.M., et al. Human cortex development: estimates of neuronal numbers indicate major loss during gestation. J Neuropathol Exp Neurol. 1996;55:320-328.
Rackow H., Salanitre E., Green L.T. Frequency of cardiac arrest associated with anesthesia in infants and children. Pediatrics. 1961;28:697.
Rolf N., Coté C.J. Frequency and severity of desaturation events during general anesthesia in children with and without upper respiratory infection. J Clin Anesth. 1992;4:200-203.
Rosen G.M., Muckle R.P., Mahowald M.W., et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93(5):784-788.
Runciman W.B. Monitoring and patient safety: an overview. Anaesth Intensive Care. 1988;16:11-13.
Runciman W.B. The Australian Patient Safety Foundation. Anaesth Intensive Care. 1988;16:114.
Runciman W.B., Ilsley A.H., Holland R.B., et al. Symposium issue: monitoring and patient safety. Anaesth Intensive Care. 1988;16:1-116.
Salem M.R., Bennett E.J., Schweiss J.F., et al. Cardiac arrest related to anesthesia: contributing factors in infants and children. JAMA. 1975;233:238-241.
Schmidt B. Methylxanthine therapy for apnea of prematurity: evaluation of treatment benefits and risks at age 5 years in the International Caffeine for Apnea of Prematurity (CAP) Trial. Biol Neonate. 2005;88:208-213.
Schreiner M.S., Nicholson S.C., Martin T., et al. Should children drink before discharge from day surgery? Anesthesiology. 1992;76:528-533.
Schreiner M.S., O’Hara I., Markakis D.A., et al. Do children who experience laryngospasm have an increased risk of upper respiratory tract infection? Anesthesiology. 1996;85:475-480.
Schwengel D.A., Sterni L.M., Tunkel D.E., et al. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109:60-75.
Smith G., Norman J., editors. Symposium on complications and medico-legal aspects of anesthesia. Br J Anaesth. 1987;59:813.
Smith R.M. Some reasons for the high mortality in pediatric anesthesia. N Y State J Med. 1956;56:2212.
Smith R.M. The pediatric anesthetist, 1950-1975. Anesthesiology. 1975;43:144.
Smith RM., editor. Anesthesia for infants and children, ed 4, St. Louis: CV Mosby, 1980.
Soliman I.E., Patel R.I., Ehrenpreis M.B., et al. Recovery scores do not correlate with postoperative hypoxemia in children. Anesth Analg. 1988;67:53-56.
Stevenson H.E., Reid L.C., Hinton J.W. Some common denominators in 1200 cases of cardiac arrest. Ann Surg. 1953;137:731.
Stoelting R. APSF response to IOM medical error report. Anesthesia Patient Safety Foundation Newsletter. 2000;15:1.
Stoelting R.K., Khuri S.F. Past accomplishments and future directions: risk prevention in anesthesia and surgery. Anesthesiol Clin. 2006;24(2):235-253.
Sykes M.K. Essential monitoring. Br J Anaesth. 1987;59:901-912.
Tait A.R., Malviya S. Anesthesia for the child with an upper respiratory tract infection: still a dilemma? Anesth Analg. 2005;100:59-65.
Tait A.R., Malviya S., Voepel-Lewis T., et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
Tay C.LM., Tan G.M., Ng S.BA. Critical incidents in paediatric anaesthesia: an audit of 10,000 anaesthetics in Singapore. Paediatr Anaesth. 2001;11:711-718.
Thach B.T. Sleep apnea in infancy and childhood. Med Clin North Am. 1985;69:1289-1315.
Tiret L., Desmonts J.M., Hatton F., et al. Complications associated with anaesthesia: a prospective survey in France. Can Anaesth South J. 1986;33:336.
Tiret L., Nivoche Y., Hatton F., et al. Complications related to anaesthesia in infants and children: a prospective survey of 40,240 anaesthetics. Br J Anaesth. 1988;61:263-269.
Turnbull K.W., Fancourt-Smith P.F., Banting G.C. Death within 48 hours of anaesthesia at the Vancouver General Hospital. Can Anaesth Soc J. 1980;27:159-163.
Uejima T. Cuffed endotracheal tubes in pediatric patients. Anes. Analg. 1989;68:423-.
Utting J.E., Gray T.C., Shelley F.C. Human misadventure in anaesthesia. Can Anaesth Soc J. 1979;26:472-478.
Valley R.D., Bailey A.G. Caudal morphine for postoperative analgesia in infants and children: a report of 138 cases. Anesth Analg. 1991;72:120-124.
van der Walt J.H., Sweeney D.B., Runciman W.B., et al. Pediatric incidents in anaesthesia: an analysis of 2000 incidents reports. Anaesth Intensive Care. 1993;21:655-658.
Vickers M.D., Lunn J.N. Mortality in anaesthesia. Berlin: Springer-Verlag, 1983.
Villeret I., Laffon M., Duchalais A., et al. Incidence of postoperative nausea and vomiting in paediatric ambulatory surgery. Paediatr Anesth. 2002;12(8):712-717.
Von Ungern-Sternberg B.S., Boda K., Schwab C., et al. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology. 2007;107:714-719.
Warner M.A., Warner M.E., Warner D.O., et al. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90:66-71.
Weinstein M.S., Nicholson S.C., Schreiner M.SS. A single dose of morphine sulfate increases the incidence of vomiting after outpatient inguinal surgery in children. Anesthesiology. 1994;81:572-577.
Weiss M., Gerber A.C. Cuffed tracheal tubes in children: things have changed. Paediatr Anaesth. 2006;16:1005-1007.
Weir P.M., Munro H.M., Reynolds P.I. Propofol infusion and the incidence of emesis in pediatric outpatient strabismus surgery. Anesth Analg. 1993;76:760-764.
Welborn L.G., deSoto H., Hannallah R.S., et al. The use of caffeine in the control of post-anesthetic apnea in former premature infants. Anesthesiology. 1988;68:796-798.
Welborn L.G., Rice L.J., Hannallah R.S., et al. Postoperative apnea in former preterm infants: prospective comparison of spinal and general anesthesia. Anesthesiology. 1990;72:838-842.
Welborn L.G., Hannallah R.S., Luban N.LC., et al. Anemia and postoperative apnea in former premature infants. Anesthesiology. 1991;74:1003-1006.
Wetzel R.C. Who is doing what to whom: a large prospective study of propofol anesthesia in children. Anesth Analg. 2009;108:695-698.
Xue F.S., Huang Y.G., Tong S.Y., et al. A comparative study of early postoperative hypoxemia in infants, children, and adults undergoing elective plastic surgery. Anesth Analg. 1996;83:709-715.