Rhizobium, Ochrobactrum, and Similar Organisms
1. Describe the general characteristics of the organisms discussed in this chapter, including their normal habitat, Gram stain characteristics, and morphology.
2. List the types of diseases associated with each organism.
3. Compare and contrast the Gram stain appearance of the various species.
4. Create an algorithm that outlines the major tests used to differentiate Achromobacter spp., Alcaligenes xylosoxidans, Ochrobactrum anthropi, and Rhizobium radiobacter.
Epidemiology
As environmental organisms, these bacteria are rarely encountered in human specimens or infections. When they are encountered, they are found on contaminated medical devices or are isolated from immunocompromised or debilitated patients. Of the organisms listed in Table 23-1, Rhizobium radiobacter, and O. anthropi are the species most commonly encountered in the clinical setting. Ochrobactrum intermedium is phenotypically indistinguishable from O. anthropi. The other bacteria have rarely been discovered in clinical material, and several have never been established as the cause of human infection.
TABLE 23-1
| Species | Habitat (Reservoir) | Mode of Transmission |
| “Achromobacter” group | Uncertain, probably environmental; may be part of endogenous flora of the ear and gastrointestinal tract | Unknown Nosocomial infections related to contaminated disinfectants, dialysis fluids, saline solution, and water |
| Rhizobium radiobacter | Environmental, soil and plants; not part of human flora | Contaminated medical devices, such as intravenous and peritoneal catheters |
| CDC group EF-4b | Animal oral and respiratory flora; not part of human flora | Animal contact, particularly bites or scratches from dogs and cats |
| Paracoccus yeei | Environmental; not part of human flora | Identified in human peritonitis |
| Psychrobacter immobilis | Environmental, particularly cold climates such as the Antarctic; not part of human flora | Unknown. Rarely found in humans. Has been found in fish, poultry, and meat products |
| CDC group OFBA-1 | Uncertain, probably environmental; not part of human flora | Unknown. Rarely found in humans |
| Ochrobactrum anthropi | Uncertain, probably environmental; found in water and hospital environments; may also be part of human flora | Uncertain. Most likely involves contaminated medical devices, such as catheters or other foreign bodies, or contaminated pharmaceuticals. Also can be acquired in community by puncture wounds |
| Shewanella putrefaciens Shewanella algae |
Environmental and foods; not part of human flora | Unknown, rarely found in humans Isolated from abscesses and wounds |
Pathogenesis and Spectrum of Disease
Because these organisms rarely cause human infections, little is known about what, if any, virulence factors they may produce to facilitate infectivity (Table 23-2). The fact that R. radiobacter and O. anthropi infections frequently involve contaminated medical materials and immunocompromised patients, and rarely, if ever, occur in healthy hosts, suggests that these bacteria have relatively low virulence. One report suggests that R. radiobacter is capable of capsule production. The ability of O. anthropi to adhere to the silicone material of catheters may contribute to this organism’s propensity to cause catheter-related infections. No known virulence factors have been described for CDC group EF-4b. Infection appears to require traumatic introduction by a puncture wound, bite, or scratch, which indicates that the organism itself does not express any invasive properties.
TABLE 23-2
Pathogenesis and Spectrum of Disease
| Species | Virulence Factors | Spectrum of Disease and Infections |
| “Achromobacter” group | Unknown | Rarely isolated from humans. Isolates have been recovered from wounds, blood, respiratory and gastrointestinal tract. |
| Rhizobium radiobacter | Unknown. One blood isolate described as mucoid, suggestive of exopolysaccharide capsule production. | Exposure of immunocompromised or debilitated patient to contaminated medical devices resulting in bacteremia and, less commonly, peritonitis, endocarditis, or urinary tract infection. |
| CDC group EF-4b | Unknown | Infected bite wounds of fingers, hands, or arm leading to cellulitis or abscess formation. Systemic infections are rare. |
| Paracoccus yeei | Unknown | No infections described in humans. Rarely encountered in clinical specimens. |
| Psychrobacter immobilis | Unknown | Rare cause of infection in humans. Has been described in wound and catheter site infections, meningitis, and eye infections. |
| CDC group OFBA-1 | Unknown | Rarely isolated from clinical specimens; found in blood, respiratory, wound, and catheter specimens. |
| Ochrobactrum anthropi | Unknown. Exhibits ability to adhere to silicone catheter material in a manner similar to staphylococci. | Catheter- and foreign body–associated bacteremia. May also cause pyogenic infections, community-acquired wound infections, and meningitis in tissue graft recipients. Patients are usually immunocompromised or otherwise debilitated. |
| Shewanella putrefaciens | Unknown | Clinical significance uncertain; often found in mixed cultures. Has been implicated in cellulites, otitis media, and septicemia; also may be found in respiratory tract, urine, feces and pleural fluid |
For both R. radiobacter and O. anthropi, bacteremia is the most common type of infection (see Table 23-2); peritonitis, endocarditis, meningitis, urinary tract, and pyogenic infections are much less commonly encountered. R. radiobacter is frequently isolated from blood, peritoneal dialysate, urine, and ascitic fluid. Cellulitis and abscess formation typify the infections resulting from the traumatic introduction of CDC group EF-4b into the skin and subcutaneous tissue.
Although other species listed in Table 23-2 may be encountered in clinical specimens, their association with human infection is rare, and their clinical significance in such encounters should be carefully analyzed.
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Direct Detection Methods
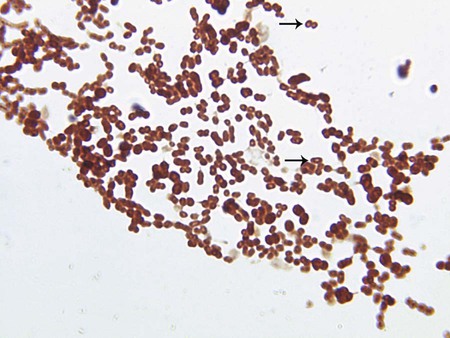
Other than Gram staining, no specific procedures are required for direct detection of these organisms in clinical material. Ochrobactrum spp., CDC group OFBA-1, and CDC group Ic are slender, short to long rods, and CDC group O-3 are thin, medium to slightly long, curved rods with tapered ends, resembling a sickle. R. radiobacter is a short, pleomorphic rod. Psychrobacter immobilis, CDC group EF-4b, and Paracoccus yeei are coccobacilli. P. yeei has a characteristic O appearance on Gram staining (Figure 23-1). Shewanella putrefaciens organisms are long, short, or filamentous rods.
Cultivation
Media of Choice
Colonial Appearance
Table 23-3 presents descriptions of the colonial appearance and other distinguishing characteristics (e.g., hemolysis and odor) of each genus when grown on 5% sheep blood or MacConkey agar.
TABLE 23-3
Colonial Appearance and Characteristics
| Organism | Medium | Appearance |
| “Achromobacter” group | BA | Flat, spreading and rough colonies |
| Mac | NLF; biovar F does not grow | |
| CDC group EF-4b | BA | No distinctive appearance, but cultures smell like popcorn |
| Mac | NLF | |
| CDC group Ic | BA | No distinctive appearance |
| Mac | NLF | |
| CDC group O-3 | BA | Circular, entire, translucent, very punctate |
| Mac | NLF, may grow poorly or not at all | |
| CDC group OFBA-1 | BA | Beta-hemolytic |
| Mac | NLF | |
| Ochrobactrum anthropi | BA | Resembles colonies of Enterobacteriaceae, only smaller |
| Mac | NLF | |
| Paracoccus yeei | BA | Growth frequently mucoid |
| Mac | NLF | |
| Psychrobacter immobilis | BA | No distinctive appearance but usually does not grow well at 35°C; grows best at 20°C; cultures (saccharolytic strains) smell like roses |
| Mac | NLF | |
| Rhizobium radiobacter | BA | No distinctive appearance |
| Mac | NLF (mucoid pink after extended incubation [>48 hr]) | |
| Shewanella putrefaciens | BA | Convex, circular, smooth; occasionally mucoid; lavender greening of blood agar; soluble brown to tan pigment |
| Mac | NLF |
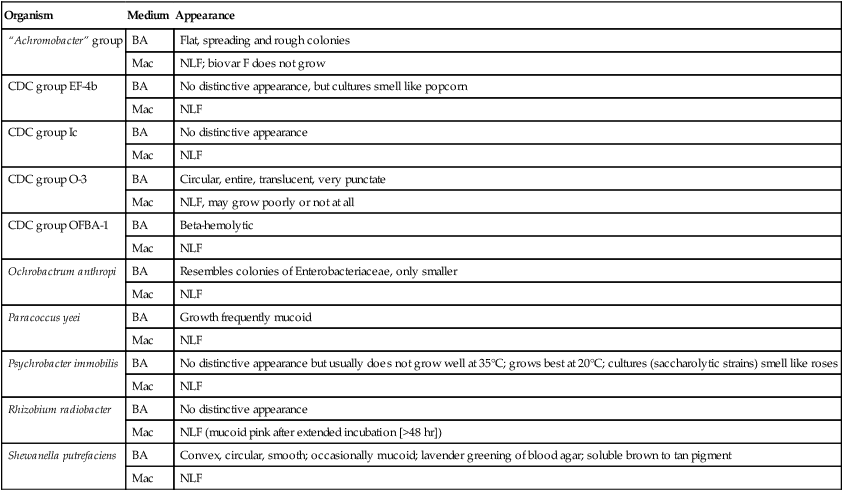
BA, 5% sheep blood agar; Mac, MacConkey agar; NLF, non–lactose fermenter.
Approach to Identification
The key biochemical reactions used to presumptively differentiate among the genera discussed in this chapter are provided in Table 23-4. However, definitive identification of these organisms often requires performing an extensive battery of biochemical tests not commonly available in many clinical microbiology laboratories. Therefore, full identification of clinically relevant isolates may require identification by a reference laboratory.
TABLE 23-4
Key Biochemical and Physiologic Characteristics
| Organism | Oxidizes Glucose | Oxidizes Xylose | Oxidizes Mannitol | Nitrate Reduction | Gas from Nitrate | Arginine Dihydrolase | Esculin Hydrolyzed | Growth on Cetrimide |
| “Achromobacter” groupa,b | + | + | v | + | + | + | + | v |
| CDC group EF–4b | + | − | − | + | − | − | − | ND |
| CDC group Ic | + | − | − | + | − | + | − | + |
| CDC group O–3 | + | + | − | − | − | − | + | ND |
| CDC group OFBA–1c | + | + | (+) | + | + | + | − | + |
| Ochrobactrum anthropib | + | + | v | v | v | v | v | − |
| Paracoccus yeei | + | + | − | + | v | v | − | − |
| Psychrobacter immobilisd | (+) | (+) | − | v | − | v | − | − |
| Rhizobium radiobacter | + | + | + | v | − | − | + | − |
| Shewanella putrefaciense | v | − | − | + | − | − | − | − |
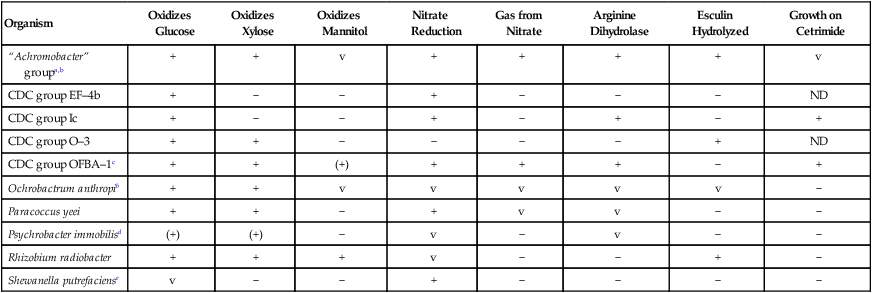
Compiled from data in Weyant RS, Moss CW, Weaver RE et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1996, Williams & Wilkins; and Young JM, Kuykendall LD, Martínez-Romero E et al: A revision of Rhizobium Frank, 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn, 1942 and Allorhizobium undicola de Lajudie et al, 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis, Int J Syst Evol Microbiol 51:89, 2001.
ND, No data available; v, variable; +, >90% of strains are positive; −, >90% of strains are negative; (+), delayed.
aIncludes biovars B, E, and F; F does not grow on MacConkey agar.
bUsually motile by peritrichous flagella.
Comments Regarding Specific Organisms
Although the EF portion of the CDC group EF-4b designation stands for eugonic fermenter (an organism that grows well on common laboratory media), most CDC group EF-4b strains oxidize glucose, so the designation as a eugonic fermenter is a misnomer. P. yeei, formerly CDC group EO-2 (a eugonic oxidizer), has a biochemical profile very similar to that of the saccharolytic, nonhemolytic Acinetobacter spp., except that the latter are oxidase negative (see Chapter 21 for more information about this genus).
Antimicrobial Susceptibility Testing and Therapy
No validated susceptibility testing methods are available for the organisms discussed in this chapter. Although many of these organisms grow on the media and under the conditions recommended for testing of more commonly encountered bacteria (see Chapter 12 for more information on validated testing methods), no standardized reference exists for antimicrobial resistance for these organisms. The lack of validated in vitro susceptibility testing methods does not allow definitive treatment and testing guidelines to be given for the organisms listed in Table 23-5. Although susceptibility data for some of these bacteria can be found in the literature, the lack of understanding of potential underlying resistance mechanisms prohibits the validation of the data. Review Chapter 12 for preferable strategies used to provide susceptibility information and data when validated testing methods do not exist for a clinically relevant bacterial isolate.
TABLE 23-5
Antimicrobial Therapy and Susceptibility Testing
| Species | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods* | Comments |
| “Achromobacter” group | No definitive guidelines. Human infections are rare. | Resistant to narrow-spectrum penicillins, other cephalosporins, and aminoglycosides. | Methods are not standardized. Methods used may include broth macrodilution and microdilution, agar dilution, breakpoint, and Etest. | |
| Rhizobium radiobacter | Optimal therapy uncertain. Treatment involves removal of foreign body. Potentially active agents include ceftriaxone, cefotaxime imipenem, gentamicin, and ciprofloxacin | Yes | Not available | Grows on susceptibility testing media, but standards for interpretation of results do not exist. |
| CDC group EF-4b | No definitive guidelines. Potentially active agents include penicillin, ampicillin, ciprofloxacin, and ofloxacin | Unknown; some cephalosporins may be less active than the penicillins. | Not available | Limited clinical data |
| Paracoccus yeei | No definitive guidelines | Unknown | Not available | No clinical data |
| Psychrobacter immobilis | No definitive guidelines. Usually penicillin susceptible. |
Unknown | Not available | Limited clinical data |
| CDC group OFBA-1 | No definitive guidelines | Unknown | Not available | No clinical data |
| Ochrobactrum anthropi | Optimal therapy uncertain. Treatment involves removal of foreign body. Potentially active agents include trimethoprim-sulfamethoxazole, ciprofloxacin, and imipenem; aminoglycoside activity variable | Commonly resistant to all penicillins and cephalosporins. | Not available | Grows on susceptibility testing media, but standards for interpretation of results do not exist. |
| Shewanella putrefaciens | No definitive guidelines. Generally susceptible to various antimicrobial agents | Often resistant to ampicillin and cephalothin | Not available |
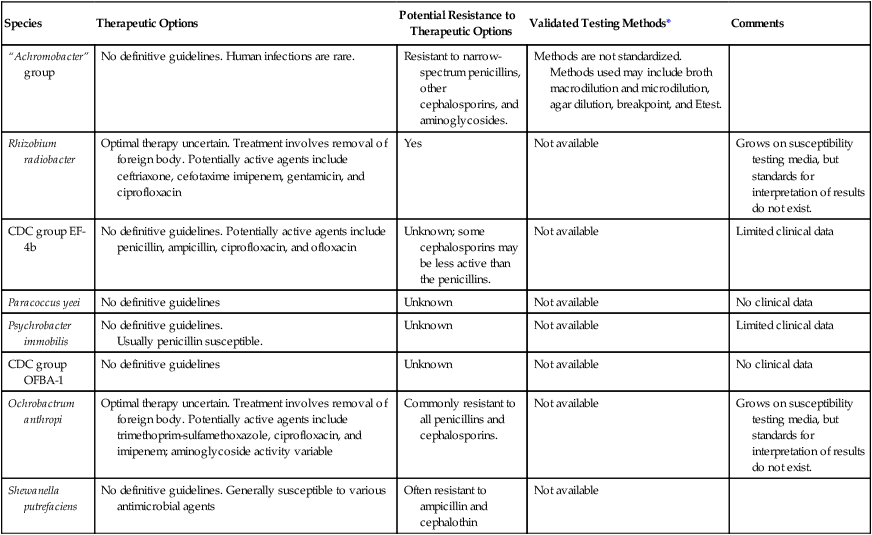
*Validated testing methods include standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and commercial methods approved by the U.S. Food and Drug Administration (FDA).
Because R. radiobacter and O. anthropi infections are frequently associated with implanted medical devices, therapeutic management of the patient often involves removal of the contaminated material. Although definitive antimicrobial therapies for these infections have not been established, in vitro data suggest that certain agents could be more effective than others (see Table 23-5). Most strains of R. radiobacter are susceptible to cephalosporins, carbapenems, tetracyclines, and gentamicin.