CHAPTER 65 Revision of Failed Total Elbow Arthroplasty with Osseous Integrity
INTRODUCTION
A number of surgical options are available to treat failed elbow arthroplasty. In this chapter, we emphasize the indications and the surgical techniques for prosthetic reimplantation of the joint not requiring graft augmentation. Revision requiring osseous augmentation is discussed in the subsequent chapter (Chapter 66). The general problems of reimplantation for failed elbow arthroplasty are placed in perspective of alternate treatment possibilities that are more thoroughly reviewed elsewhere (see Chapters 67 and 69).
CLASSIFICATION OF FAILURE
There are several ways of categorizing failed prior arthroplasty, each with different implications for treatment (Table 65-1).
TABLE 65-1 Revision Options as a Function of Initial Arthroplasty Type and Osseous Integrity
| Index Arthroplasty | Osseous Integrity | Revision Options |
|---|---|---|
| Resection | Variably deficient humerus and/or ulna metaphysis | Insert into mature? bone; may require long flange |
| Fusion | Adequate | Treat as primary arthroplasty |
| Interposition | Adequate | Treat as primary arthroplasty |
| Implant failure |
* Strut and impaction grafting may be combined in some procedures.
Failure of a nonimplantation procedure such as interposition or resection arthroplasty is a relatively straightforward matter, and the treatment and characteristics are no different from those of an acute fracture with bone loss (see Chapters 56 and 57). In this chapter, we deal with failed total elbow arthroplasty with adequate osseous integrity for a direct reimplantation and cementation. In Chapter 66, the options for osseous augmentation are discussed.18,20,24
SEPTIC FAILURE
This is mentioned here to emphasize the need to exclude a septic process as the cause of implant failure. A high index of suspicion must be developed. The value of preoperative screening as with sedimentation rates and C-reactive protein (CRP) is well accepted. One recent study demonstrated a sedimentation rate over 23 mm/hr and a CRP in excess of 13.5 mg/L correlates with infection with a specificity and sensitivity of 85% and 90% respectively.11 Radiolucency of the bone-cement interface may be due to either mechanical failure or sepsis, but implant loosening is not usually associated with a septic joint unless the process is a chronic one.21 Infection of a well-fixed device is particularly bothersome, because removing the prosthesis may result in fracture of bone that poses major reconstructive problems for the surgeon, but commonly sepsis is managed with a staged reimplantation. The important consideration is to avoid excessive use of bone graft in this setting. This topic was reviewed recently by Cheung and colleagues, and is discussed in detail in Chapter 62.3
DEVICE FAILURE
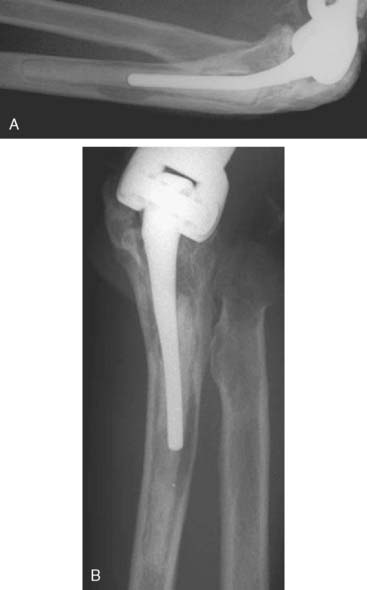
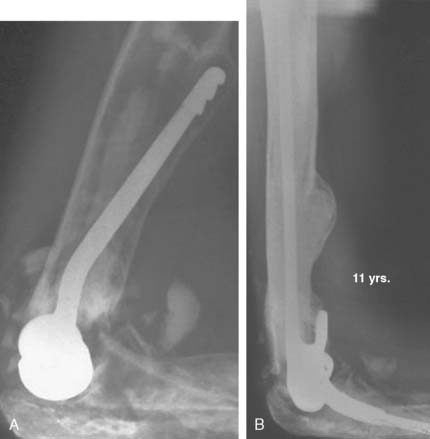
Device failure may involve the stem or the articular coupling elements of the device. High-density polyethylene articular components do have a tendency to wear,15 but this typically does not cause loosening of the implant. Because the semiconstrained implant is being used for broader indications and loosening is much less of a problem, articular wear is increasingly being recognized over long-term follow-up.10,15 Lee et al15 assessed the experience of almost 900 linked Coonrad/Morrey devices. Only 1.5% required reoperation for isolated bushing wear. The authors make the distinction between bushing wear that is managed by simply exchanging the bushing and loosening that typically occurs as a result of loosening and osteolysis that was seen principally in the precoat ulnar component design. This design has not been used for more than 7 years (Fig. 65-1).
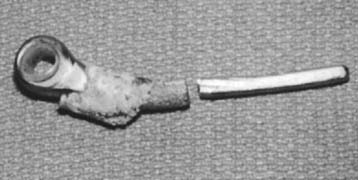
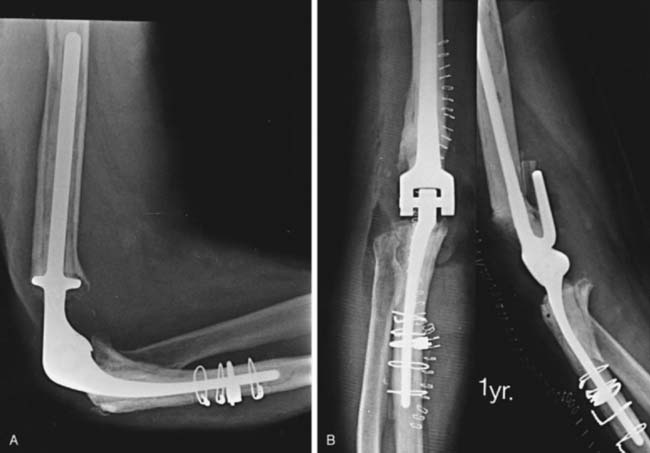
Implant fracture is an uncommon complication that usually follows excessive activity or a single significant trauma. This has occurred with the Coonrad-Morrey device owing to the stress-riser effect of the surface treatment of the titanium implant and in patients with excessive physical demands (Fig. 65-2).25
Athwal et al1 reviewed the Mayo experience with fractured components. The technique of removing well-fixed stems and reinsertion into an expansion of the cement mantle was successful in about 80% of patients.
INSTABILITY
With unlinked prostheses, instability is not uncommon (see Chapter 61). Delayed or chronic subluxation is associated with excessive activity, improper initial balance, or implant wear.7,21
If acute instability is due to ligament insufficiency or improper “balance” and does not respond to a period of immobilization, revision with a linked implant should be considered. If early dislocation is due to malpositioning of the components, immediate revision should be performed. In questionable cases, examination under fluoroscopy may show the cause of the instability and allow the management strategy to be defined (Fig. 65-3).
The selection of unlinked implant has been predicated to some extent on the assumption that a reoperation or revision would prove more reliable after this type of design. Until recently, this hypothesis has not been proven. A review of Mayo’s 30-year experience with linked and unlinked implants appears to confirm this assumption but only if the revision was from an unlinked to a linked device (Fig. 65-4).16 Specifically, reoperation of all unlinked implants resulted in a more predictable outcome than did revision after all failed linked implants. However, the linked revisions were to a great extent in patients with post-traumatic arthritis. There was no difference in success of revision when only the rheumatoid patient was considered. Furthermore, it was also demonstrated that the need to revise the unlinked implant occurred at a significantly greater frequency than did the need to reoperate on a linked device (Fig. 65-5).16
LOOSENING
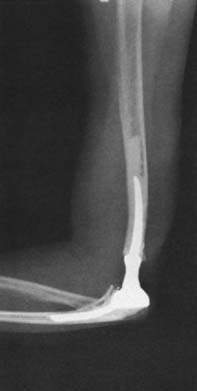
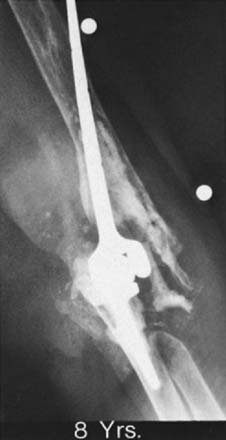
The early experience with total elbow arthroplasty demonstrated loosening of the humeral component about twice as often as loosening of the ulnar component.9,12,21 This difference is not observed with the Coonrad-Morrey device because loosening currently occurs more often at the ulna than at the humerus.4,10,16 Pain is a typical but not a universal feature. Different expressions of failure are recognized. One is a loose implant with pain but few or no x-ray changes and no bone resorption (Fig. 65-6). The other is gross instability and obvious loosening due to bone destruction, with minimal or no pain. Particulate debris results in cortical thinning or “ballooning” of the humerus or the ulna. Resorption of a significant amount of bone can pose major problems with the reconstructive procedure, especially when the bone loss is complicated by fracture (Fig. 65-7). The fracture may be “silent” due to chronic thinning or acute. The latter fracture is not caused by loosening and may heal without a need for implant revision. The topic of periprosthetic fracture is dealt with in detail in Chapter 66 because strut graft is often employed in its treatment.
TREATMENT OPTIONS AND INDICATIONS
As noted earlier, if the failure requires removal of components, treatment possibilities may be categorized according to the integrity of the bone (see Chapter 70), resection (see Chapter 67) and interposition (see Chapter 69). All of these methods may be used to salvage failed prosthetic arthroplasty and are discussed elsewhere. Both reimplantation and nonreimplantation options depend largely on the amount of bone present (Table 65-2). Management of osseous deficiency has been discussed by Sanchez-Sotelo et al,24 and Mansat et al.,20 and is discussed in detail in Chapter 66.
| Bone Stock | Options |
|---|---|
| Adequate | Arthrodesis, resection, interposition, total elbow arthroplasty, semiconstrained prosthesis |
| Inadequate | Resection, allograft, total elbow arthroplasty, semiconstrained (long-flanged) prosthesis composite (total elbow arthroplasty with allograft), custom-made prosthesis |
INTERPOSITION ARTHROPLASTY
Several types of interposition arthroplasty are described, which, if they fail, may be revised depending on the amount and quality of available bone, and the presence of sepsis. Chapter 60 discusses interposition arthroplasty, and Chapter 67 reviews nonimplantation salvage procedures for failed elbow replacement. Blaine et al2 reviewed the Mayo experience of revising failed interposition arthroplasty with the linked total elbow implant. The average Mayo elbow performance score increased from 32 to 80.4 points. The authors reported 9 of 12 (75%) satisfactory outcomes with elbow replacement following failed interposition arthroplasty.
REIMPLANTATION PROCEDURES
General Considerations
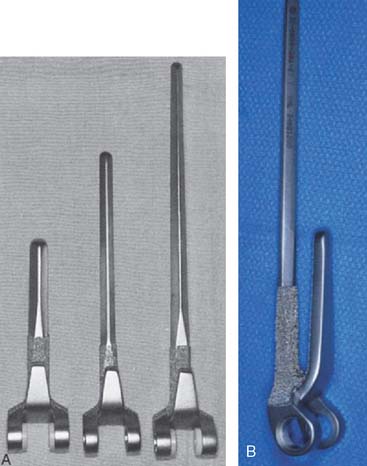
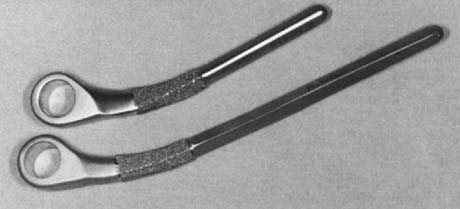
The Coonrad-Morrey implant is made with 6- and 8-inch humeral stems, either of which may be used for the purpose of bypassing a shorter stemmed device, depending on the amount of available bone and the need for long-stemmed fixation (Fig. 65-8). An extended ulnar component is available for problems of proximal ulnar bone deficiency (Fig. 65-9).
Noncustom Semiconstrained Revision Implant Design
The Coonrad-Morrey elbow replacement has in our practice been an ideal noncustom option for the resected joint after failed total elbow arthroplasty or after trauma. The range of sizes and lengths of the humeral (see Fig. 65-8) and ulnar (see Fig. 65-9) components is a most useful feature. Furthermore, the anatomic axis of rotation is restored even with resection at the level of the roof of the olecranon fossa (Fig. 65-10). Shortening of the humerus of up to 2 cm proximal to the olecranon fossa is accepted without resorting to allograft augmentation or the need for a custom implant.8,13,23 Long-flanged implants have been very valuable because they allow various depths of insertion. Overall, up to 8 cm of distal humerus deficiency can be addressed with reimplantation of an off-the-shelf device. If the bone loss is greater than the long flange can accommodate, either a custom implant or a component reconstruction with an interposed distal humeral allograft may be considered (see Chapter 66). The particular value of the design of the Coonrad-Morrey device is that the intramedullary cement fixation is enhanced by an extramedullary bone graft placed behind the flange. This flange design resists forces that tend to displace the implant posteriorly as well as rotatory forces (Fig. 65-11). For most applications of distal deficiency we have resorted to using a longer flange, grafting bone behind the flange, and inserting the implant to less than its full depth (Fig. 65-12).
PREOPERATIVE PLANNING
SURGICAL TECHNIQUE
Exposure
Skin coverage in this group of patients is sometimes compromised, even at the time of the initial procedure. Use of the previous incision is recommended, if possible. Skin flaps should be kept to a minimum (see Chapter 36). A local or a remote pedicle flap may be necessary to ensure adequate wound healing. Preoperative discussion with a microsurgeon or a plastic surgeon is particularly helpful if any question exists.
Ulnar and Radial Nerves
The ulnar nerve must “always” be identified in the revision procedure. In many instances, it will already have been transferred anteriorly; nevertheless, it must be properly identified and protected during the procedure. If ulnar neuropathy is already present and is a significant problem, decompressing the nerve and freeing it of scar tissue has been rewarding in a number of patients. If it is asymptomatic, simply identifying and avoiding injury is appropriate. The radial nerve is identified in all instances and is exposed and protected when cement is removed from the humerus. The radial nerve is vulnerable if the cortex is violated or even from heat polymerization or ultrasonic removal of polymethyl methacrylate. Zook et al29 emphasized the vulnerability of the radial nerve when revising a humeral component. These authors observed that the radial nerve can erode through osteolytic bone, making it extremely vulnerable when removing the intramedullary membrane.
Triceps
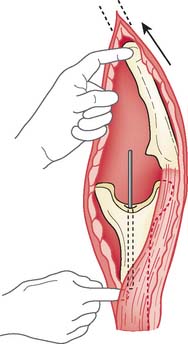
Instead of reflecting the triceps from the olecranon, if at all possible, a true triceps-sparing approach is used and the triceps is not detached from the ulna. This approach is discussed in detail in Chapter 7. The triceps is elevated both medially and laterally from the humerus after the ulnar and radial nerves have been protected. The prosthetic device is identified, and the coupling mechanism is removed (Fig. 65-14). The humerus and the ulna are then dissociated. The humerus is usually delivered through the lateral margin of the triceps, protecting the ulnar nerve. In some instances, the humerus may be easier to expose from the medial margin of the triceps, in which case the ulnar nerve must be carefully released more proximally, protected, and brought either laterally or medially to the humerus. If a triceps-sparing approach has been used, the ulna must be rotated axially with some flexion and extension so that the surgeon can visualize and palpate the humerus and the ulna (Fig. 65-15). We have used this approach successfully for the last 8 years. If, however, there is less experience with elbow exposure in several or the major lesion is at the ulna, or the ulna is angulated, the triceps must be reflected to ensure adequate visualization.
Soft Tissue
It is increasingly being recognized that the soft tissue must be “balanced.” The soft tissue envelope about the joint is no less important at the time of the revision. Hence, we do not feel obliged to reattach the flexor or extensor muscle groups to the distal humeral shaft during distal humeral resection. Loss of attachment of the flexor or extensor muscle mass goes unnoticed by the patient.
REVISING THE HUMERUS
The need for revision of well-fixed implants is uncommon unless osteolyses is present. Nonetheless, well-fixed humeral implants can be reliably removed to date by performing a truncated trapezoid type of osteotomy in the posterior aspect of the cortex (Fig. 65-16). This allows the removal of cement around the implant. The implant is then safely removed without fracturing the humerus. After cement has been removed, bone is reapplied. If the revision device employs a flange, the flange engages the anterior cortex; thus, the removal of the posterior bone is well tolerated. It is important to leave both medial and lateral humeral condyles in any instance in which the humerus is removed because this allows subsequent resection arthroplasty should the elbow become infected.
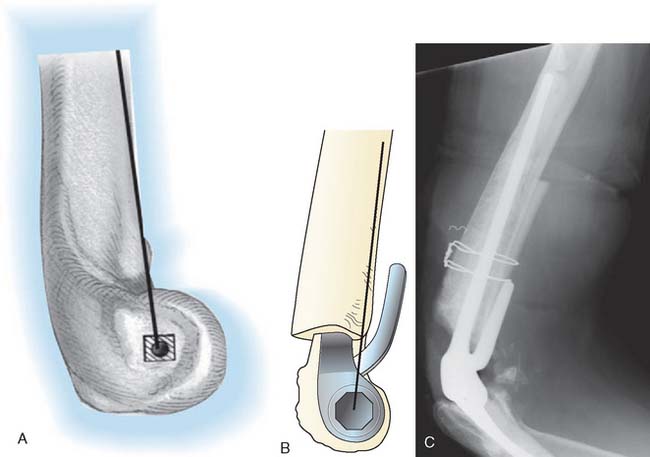
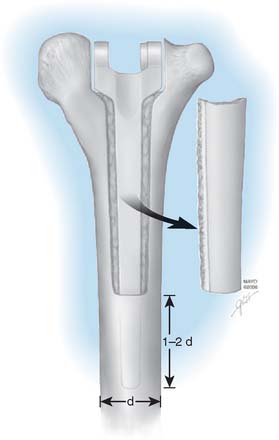
We have found that one helpful technique is to place a 3-mm drill through well-fixed cement and into the medullary canal. A guide pin is then introduced into the hole made in the cement, and the cement is removed by serial flexor flexible reamers. This may then be further expanded with burrs, ultrasonic devices, or osteotomes. However, if the drill is not placed central through the cement and down the canal, eccentric reaming can still violate the cortex and damage the nerve (Fig. 65-17). In this instance, use of an ultrasonic device is recommended. Once again, great care is made to avoid penetration of the humeral cortex, particularly in the region of the radial nerve.
LOOSE PROSTHESES
Well-Fixed Prostheses
If the prosthesis has not become loose, removing it may result in some bone loss or in fracture. To minimize damage to the bone, adequate exposure of the distal humerus should be achieved. Removing a trapezoid shape of cortical bone posteriorly allows access to the cement and removal of the humeral stem (see Fig. 65-16). Removing as much cement as possible allows the device to be removed more easily. A pin placed across the yoke allows easy removal with the hip extraction devices. Once the prosthesis is removed, if the cement is firmly fixed to bone, the cemented mantle is expanded to receive the revision device. However, no attempt is made to remove cement that is well fixed and that does not interfere with reinsertion.
REVISING THE ULNA
With well-fixed implants, the ulna may also be osteotomized. A linear saw cut is made medially approximately three quarters of the distance of the length of the implant. The blade cuts not only the bone but also the cement. An osteotome is used to pry the cement from the implant. If the ulna fractures, it may be reliably fixed to circlage wires. The canal is prepared as described earlier for the humerus. A drill bit is placed through the intact cement, and the canal is enlarged with serial reaming. Attention is paid to the bowing of the ulna, which occurs about 5 cm distal to the coronoid. It is extremely difficult to avoid a small defect in the ulna, but this is usually of no consequence. Yet, it should be avoided if at all possible. Extensive subcutaneous exposure allows palpation of the cortex, which helps to remove cement and perform the needed reconstruction with less risk of penetrating the cortex (Fig. 65-18).
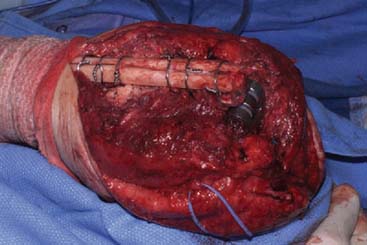
FIGURE 65-18 The subcutaneous nature of the ulna allows safe and easy exposure for a host of revision techniques
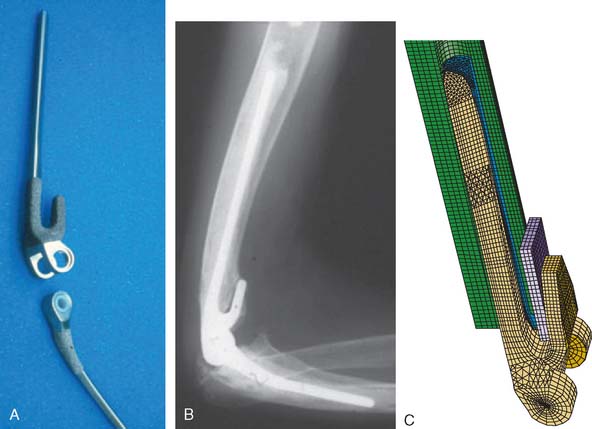
Fractured Component
A specific technique is required in those in which an implant has fractured (see Fig. 65-2). The well-fixed portion of the stem is freed of cement with a high-speed “pencil” burr. If the fractured component cannot be reached with needle-nose type pliers, then a controlled osteotomy as described earlier, is carried out. Once the stem has been removed, if the cement remains well fixed to the bone, the cement is expanded in such a way as to receive the stem. A simple recementing and reinsertion of the implant is carried out. Cerclage wire is employed to stabilize an osteotomy.
Closure
Regardless of the particular revision option selected, the triceps should be left attached to the ulna when possible. If it is reflected, it should be attached to bone with the technique described in Chapter 53. The tourniquet is deflated to allow meticulous hemostasis, and the skin is carefully inspected. If there is any question about the adequacy of the cutaneous circulation, the elbow is splinted in extension for 3 to 4 days and then reinspected. The decision to drain the wound turns on the amount of bleeding and the quality of the skin. If necessary, an extension cast or splint may be used for 3 to 4 weeks.
POSTOPERATIVE MANAGEMENT
If reimplantation has been performed, careful inspection of the skin after 3 days and initiation of motion is recommended. The elbow is rested in a molded splint when not being actively exercised by the patient. If marked swelling is present, motion is deferred. If the bone is cracked or if an allograft has been used, protection in a cast brace for 4 to 8 weeks does not compromise the range of motion eventually achieved.
RESULTS
Several studies have emerged documenting the outcomes of revision procedure. One recent study with the use of a linked device for a revision of both linked and unlinked implants report a 5-year survival rate of 64%. Eleven of the 30 patients in this series ultimately required reoperation.26 The successful revision of the unlinked implant with yet another unlinked implant was reviewed by Van der Lugt et al.28 These authors revealed eight of 24 aseptic failures, with two of 24 becoming infected. Overall, however, the 5-year success rate was considered to be 74%, with the revision implants remaining in situ. A report from the Scandinavian experience of 23 patients revised with a linked implant estimated a 5-year survival of 83% using the Coonrad/Morrey implant. The study concluded that this was a satisfactory device and a fairly reliable procedure.27 Others have documented a short-term follow-up with approximately a 50% satisfactory and 50% unsatisfactory outcomes after 14 revision procedures. These authors also use an unlinked implant to revise the failed unlinked device.6
MAYO EXPERIENCE
Initial Experience
A study of 10 years’ experience with 33 consecutive revision elbow arthroplasties at our institution, all of which used some version of the Coonrad implant, was reported in 1987.22 All patients were evaluated at least 3 years after surgery (average follow-up 61 months, range 3 to 13 years). The range of motion averaged 25 to 130 degrees of flexion, 50 degrees of pronation, and 60 degrees of supination. Complications developed in 20 patients (67%). Three patients incurred an infection, and prosthesis loosening occurred in seven. Eleven (33%) required reoperation. At the time of that review, 55% of the initial revision elbows were still in place and were considered to have satisfactory results. During the course of this study, an additional surgical procedure was performed on 11 elbows. At the time of last review, 73% of all of the elbows were considered satisfactory by current radiographic and clinical standards.22
Subsequent Experience
A subsequent assessment of our 15-year experience using the Coonrad-Morrey device exclusively as a revision implant was reported 10 years ago by King and associates.14 The use of the “noncustom-flanged” device has increased the success rate: 38 of 41 (93%) elbows were functional at an average of 6 years after surgery. Although 14 (35%) had cortical penetration at revision in this series, the advent of ultrasonic cement removal has decreased the frequency of this problem in recent years. Of concern, three patients had injury to the radial nerve, which in two was due to extravasation of cement from a cortical defect and in one occurred during cement removal from the humeral canal with power instruments. Two had revision for bushing wear, each at 6 years, and two others had aseptic loosening due to particulate debris from the plasma-sprayed surface.
CURRENT EXPERIENCE REVISITED
We are currently in the process of reassessing the experience with reinsertion of implants with recementing and reinserting the implant in intact bone (Fig. 65-19).19 The preliminary results of 39 procedures indicate just less than a third required another operation within 5 years. In some instances, the failure of this technique is predicated on the poor quality of the bone, which can be anticipated preoperatively. Based on this study, reimplantation in the face of poor quality is associated with both loosening and fracture and should be avoided.
1 Athwal G.S., Morrey B.F. Revision total elbow arthroplasty for prosthetic fracture. J. Bone Joint Surg. 2006;88:2017.
2 Blaine T.A., Adams R., Morrey B.F. Total elbow arthroplasty after interposition arthroplasty for elbow arthritis. J. Bone Joint Surg. 2005;87A:286.
3 Cheung E.V., Adams R.A., Morrey B.F. Reimplantation of a total elbow prosthesis following resection arthroplasty for infection. J. Bone Joint Surg. 2008;90A:589.
4 Cil, A., Veillette, C. J., Sanchez-Sotelo, J., and Morrey, B. F.: Linked elbow replacement: A salvage procedure for distal humeral nonunion. J. Bone Joint Surg. Am. 2008 (in press).
5 Coonrad R.W. Seven-year follow-up of Coonrad total elbow replacement. In: Inglis A.E., editor. Upper Extremity Joint Replacement (Symposium on Total Joint Replacement of the Upper Extremity, 1979). St. Louis: C.V. Mosby Co., 1982.
6 Ehrendorfer S. Elbow revision arthroplasty in the situation of bone loss using an unlinked long-stem prosthesis. J. Hand Surg. 1999;24A:1337.
7 Ewald F.C., Scheinberg R.D., Poss R., Thomas W.H., Scott R.D., Sledge C.B. Capitellocondylar total elbow arthroplasty: two- to five-year follow-up in rheumatoid arthritis. J. Bone Joint Surg. 1980;62A:1259.
8 Figgie H.E., Inglis A.E., Mow C. Total elbow arthroplasty in the face of significant bone stock or soft tissue losses: preliminary results of custom-fit arthroplasty. J. Arthroplasty. 1986;1:71.
9 Figgie H.E., Inglis A.E., Ranawat C.S., Rosenberg G.M. Results of total elbow arthroplasty as a salvage procedure for failed elbow reconstructive operations. Clin. Orthop. Relat. Res. 1987;219:185.
10 Gill D., Morrey B.F. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis: A 10- to 15-year follow-up study. J. Bone Joint Surg. 1998;80:1327.
11 Greidanus N.V., Masri B.A., Garbuz D.S., Wilson S.D., McAlinden M.G., Xu M., Duncan C.P. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J. Bone Joint Surg. 2007;89:1409.
12 Inglis A.E. Revision surgery following a failed total elbow arthroplasty. Clin. Orthop. Relat. Res. 1982;170:213.
13 Inglis A.E., Pellicci P.M. Total elbow replacement. J. Bone Joint Surg. 1980;62A:1252.
14 King G.J.W., Adams R.A., Morrey B.F. Total elbow arthroplasty: revision with use of a non-custom semiconstrained prosthesis. J. Bone Joint Surg. 1997;79A:394.
15 Lee B.P., Adams R.A., Morrey B.F. Polyethylene wear after total elbow arthroplasty. J. Bone Joint Surg. 2005;87A:1080.
16 Levy, J. C., Loeb, M., Chuinard, C., Adams, R. A., and Morrey, B. F.: Effectiveness of revision following linked versus unlinked total elbow arthroplasty. (in press).
17 Levy, J. C., and Morrey, B. F.: A survivorship of unlinked and linked total elbow arthroplasty for rheumatoid arthritis: A comparative study. J. Bone Joint Surg. Am. (in press).
18 Loebenberg M.I., Adams R.A., O’Driscoll S.W., Morrey B.F. Impaction grafting in revision total elbow arthroplasty. J. Bone Joint Surg. 2005;87A:99.
19 Malone, A., Sanchez-Sotelo, J., Adams, R., and Morrey, B.: Revision of total elbow arthroplasty by exchange cementation. (Submittion for publication, 2008).
20 Mansat P., Adams R.A., Morrey B.F. Allograft-prosthesis composite for revision of catastrophic failure of total elbow arthroplasty. J. Bone Joint Surg. 2004;86A:724.
21 Morrey B.F., Bryan R.S. Complications of total elbow arthroplasty. Clin. Orthop. Relat. Res. 1982;170:204.
22 Morrey B.F., Bryan R.S. Revision total elbow arthroplasty. J. Bone Joint Surg. 1987;69A:523.
23 Rosenfeld S.R., Anzel S.H. Evaluation of the Pritchard total elbow arthroplasty. Orthopedics. 1982;5:713.
24 Sanchez-Sotelo J., O’Driscoll S., Morrey B.F. Periprosthetic humeral fractures after total elbow arthroplasty: Treatment with implant revision and strut allograft augmentation. J. Bone Joint Surg. 2002;84A:1642.
25 Schneeberger A.G., Adams R., Morrey B.F. Semiconstrained total elbow replacement for the treatment of posttraumatic arthritis and dysfunction. J. Bone Joint Surg. 1997;79A:1211.
26 Shi L.L., Zurakowski D., Jones D.G., Koris M.J., Thornhill T.S. Semiconstrained primary and revision total elbow arthroplasty with use of the Coonrad-Morrey prosthesis. J. Bone Joint Surg. 2007;89A:1467.
27 Sneftrup S.B., Jensen S.L., Johannsen H.V., Sojbjerg J.O. Revision of failed total elbow arthroplasty with use of a linked implant. J. Bone Joint Surg. 2006;88B:78.
28 van der Lugt J.C., Rozing P.M. Outcome of revision surgery for failed primary Souter-Strathyclyde total elbow prosthesis. J. Shoulder Elbow Surg. 2006;15:208.
29 Zook J., Ward W.G.Sr. Intraosseous radial nerve entrapment complicating total elbow revision. J. Arthroplasty. 2001;16:919.