CHAPTER 380 Revascularization Techniques for Complex Aneurysms and Skull Base Tumors
After its introduction in 1967 by Donaghy and Yasargil,1 the technique of extracranial-to-intracranial (EC/IC) arterial bypass was envisioned as a surgical strategy to prevent ischemic stroke in patients with carotid and intracranial arterial occlusion. Although the results of the EC-IC Bypass Study Group failed to establish that bypass reduced the risk for stroke,2 the use of bypass coupled with parent artery occlusion provides a means to treat complex unclippable and uncoilable aneurysms and cranial base tumors involving major cerebral arteries that could not be adequately treated otherwise without risking ischemic injury. These techniques for surgical revascularization in the treatment of aneurysms and tumors have been pioneered and later refined by Sundt and colleagues,3,4 Ausman and coworkers,5 Ito,6 Peerless and Hampf,7 Lawton and colleagues,8 Sen and Sekhar,9,10 Martin and colleagues,11–14 and others.
Revascularization For The Treatment Of Aneurysms
The optimal treatment of intracranial aneurysms is surgical clipping or endovascular coiling. However, clipping or coiling of complex, giant, and fusiform aneurysms, which incorporate the parent artery or adjacent arterial branches into the aneurysm base or fundus, may be impossible.3–5,7,8,15–19 Calcification or atherosclerotic thickening of the aneurysm neck or the parent artery can make clipping dangerous or impossible. Additionally, recurrent aneurysms after endovascular coil embolization may be unclippable because of the stenting or obstructing effect of the coils on the aneurysm neck.20
Hypothermic circulatory arrest was first used as a surgical adjunct for complex, giant intracranial aneurysms in the 1960s and was refined dramatically in the 1980s.21–24 This technique has been particularly useful in treating giant posterior circulation aneurysms by allowing the aneurysm to collapse, thereby permitting the surgeon to then reconstruct the aneurysm neck with clips. However, hypothermic circulatory arrest is a complex procedure and carries its own risks.22,23 Even with this method it may be impossible to reconstruct the aneurysm neck in a way that preserves the parent artery or branching vessels, or both. Therefore, parent artery occlusion or aneurysm trapping and distal bypass may often be a superior alternative.
Revascularization For The Treatment Of Skull Base Tumors
The petrous and cavernous internal carotid artery (ICA) can frequently be involved by anterior and middle skull base tumors. The most common tumor types that present this surgical challenge are meningiomas, schwannomas, pituitary adenomas, angiofibromas, and chordomas. Because of the benign nature of these tumor types, the carotid is most frequently partially or completely encased by tumor rather than invaded.25 However, some tumors such as meningiomas, particularly recurrent ones, may be very densely adherent to the ICA. In such cases, complete resection is not possible unless the carotid artery is resected along with the tumor, with or without a revascularization procedure. Malignant skull base and head and neck tumors are more likely to invade the vessel itself. In these cases, radical oncologic resection, if that is an appropriate goal, may require carotid occlusion and resection.26,27
The decision to remove the carotid during resection of skull base tumors is controversial. Some authors advocate ICA resection for cavernous sinus and skull base meningiomas.10,28 However, with benign tumors, many experts prefer to aggressively remove the tumor up to the artery but leave the artery intact, even if this means leaving a small residual of tumor adherent to the vessel.25,29 In the case of benign tumors, representative contemporary series with and without ICA resection show only a small difference in rates of gross total tumor resection and recurrence.10,28,29 Given the efficacy of contemporary stereotactic radiosurgery and fractionated stereotactic radiotherapy, we rarely advocate carotid artery occlusion or excision for the treatment of skull base tumors.
Opposition to ICA resection is fueled by the morbidity associated with such procedures and the treatment alternatives now available for benign lesions. All series of ICA resection and bypass have reported complications associated with these bypasses, particularly with the use of saphenous vein grafts.8,10 The rate of ischemic complications and graft occlusion typically exceeds 10%. Additionally, stereotactic radiosurgery is an effective strategy to treat tumors of the skull base with less likelihood of injury to neurovascular structures. Benign tumors, such as meningiomas and schwannomas, are often very sensitive to radiation treatment.30–33 This argues against aggressive surgical resection with carotid sacrifice for these lesions and supports more conservative surgical debulking and leaving tumor adherent to sensitive structures. There is therefore a growing consensus that the carotid artery should not be resected for most nonmalignant tumors.25,28 Lawton and Spetzler, in a review of ICA sacrifice for the resection of skull base tumors, indicated that they sacrificed the carotid artery and performed revascularization in only 10 of more than 300 patients with anterior skull base tumors.25
The poor prognosis associated with malignant head and neck cancers that can involve the anterior skull base has led some surgeons to consider radical tumor removal with ICA sacrifice.10,26,27 If the carotid—or another major intracranial artery—is the only structure that stands in the way of a complete and potentially curative resection, ICA sacrifice with bypass should be considered. The high rate of morbidity related to carotid artery sacrifice alone, without bypass, and the modest rate of morbidity associated with balloon test occlusion suggest that selective revascularization should be considered when arterial resection is deemed essential to achieve an oncologically meaningful resection.26,27 Preoperative evaluation by angiography and balloon test occlusion (with or without hypotensive provocative testing or cerebral blood flow measurements) may aid in the identification of patients who can tolerate carotid sacrifice. Finally, if the carotid has already ruptured or if rupture appears imminent because of tumor invasion, radionecrosis, or previous surgical arterial injury, ICA resection with revascularization should be strongly considered.
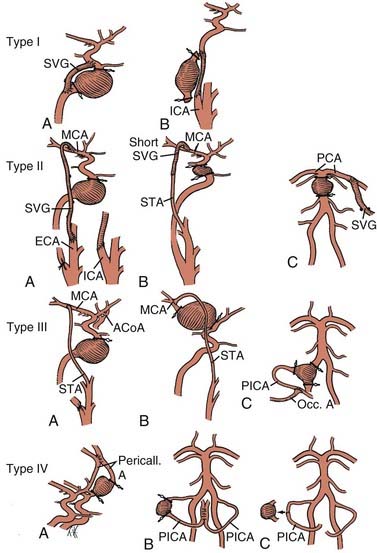
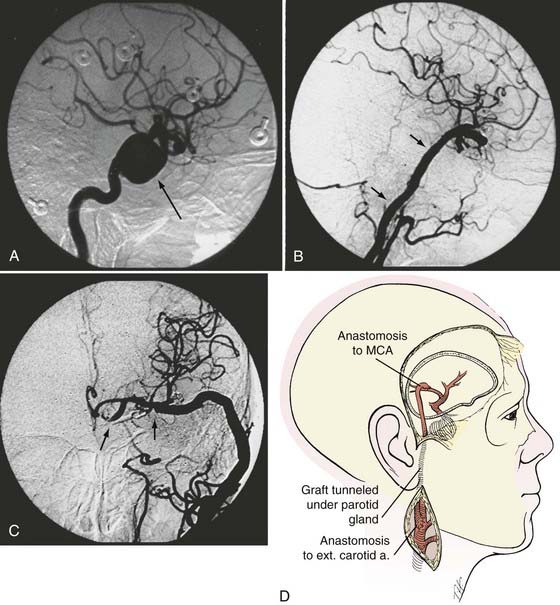
If bypass is deemed necessary in the management of a skull base tumor, the revascularization procedure can be done as a separate, preliminary staged procedure or at the same time as the tumor resection. In general, three types of revascularization can be performed in these situations: the carotid artery can be replaced with an interposition saphenous vein graft (type I), a saphenous vein graft can be placed from the ICA or external carotid artery (ECA) to the middle cerebral artery (MCA) or other intracranial vessel (type II), or the superficial temporal artery (STA) can be anastomosed to the MCA (type III) (Fig. 380-1).
When Is The Collateral Circulation Inadequate And Bypass Necessary?
Anterior Circulation
Elective occlusion or resection of the ICA has been associated with complications in 30% to 45% of cases, but experience with carotid test occlusion suggests that 80% to 90% of patients tolerate ICA occlusion (at least acutely).34,35 Many clinicians advocate a selective approach to surgical revascularization when therapeutic ICA occlusion is planned.36 This approach involves angiographic evaluation of the competence of the circle of Willis and balloon test occlusion coupled with measurement of cerebral blood flow or a hypotensive challenge to identify patients with inadequate or marginal collateral circulation who require bypass in conjunction with parent artery occlusion. Others argue for a universal approach and advocate bypass for all patients who undergo ICA occlusion.8 This strategy is intended to avoid the risk associated with the balloon test occlusion itself, minimize the risk for delayed or chronic cerebral ischemia, and avoid inducing new aneurysms on collateral vessels. Complications related to the balloon test occlusion procedure alone can occur in approximately 3% of patients.37 However, given the relative safety of balloon test occlusion with contemporary endovascular technique, most centers reserve the use of bypass for patients with demonstrably insufficient or marginal collateral circulation in the ICA territory.35,38,39 It should be noted, however, that even if a patient passes a balloon test occlusion, there remains up to a 20% chance of stroke with complete occlusion without a bypass.40
Posterior Circulation
Unclippable and uncoilable posterior circulation aneurysms may require vertebral artery occlusion. In most patients, unilateral vertebral artery occlusion is well tolerated when the contralateral vertebral artery is not hypoplastic and does not terminate in the posterior inferior cerebellar artery (PICA). Bilateral vertebral artery or basilar artery occlusion is associated with a much higher risk for ischemia and should be considered only if blood flow through both posterior communicating arteries is sufficient (>1 mm).18 However, the posterior cerebral artery (PCA) appears to have relatively good collateral potential from leptomeningeal interconnections with the distal temporal and occipital MCA branches. Proximal occlusion of this artery for giant or fusiform arteries is usually well tolerated, with an ischemic deficit (hemianopia) developing in only a few patients.16
Surgical Technique
Intraoperative Monitoring and Management
The patient is allowed to become mildly hypothermic (34°C to 36°C).41 Metabolic suppressive therapy provides cerebral protection during the period of cerebral artery occlusion while the bypass anastomosis is performed.41 We typically use thiopental rather than propofol or etomidate. The efficacy of barbiturates for cerebral protection during transient focal ischemia is supported most strongly by laboratory and clinical evidence.8,41,42 The barbiturates are administered to induce electroencephalographic burst suppression until the anastomosis is complete and the recipient vessels are deoccluded.
Types of Revascularization Procedures
Strategies for cerebral revascularization after parent artery occlusion (see Fig. 380-1) include the use of various vessels as the bypass conduit (e.g., STA, occipital artery, or long or short saphenous vein or radial artery) and the selection of various intracranial arterial sites for the distal anastomosis. These variations can be classified into four types of bypass.12–14
Type I Bypasses—Interposition Vein Grafts
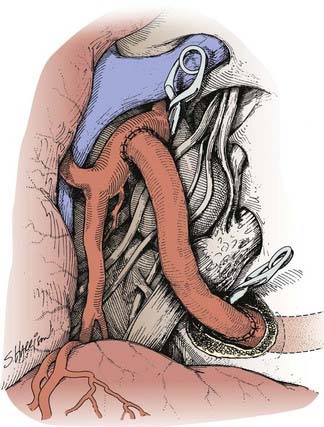
A type I bypass involves an interposition graft from the parent artery proximal to the site of the occlusion to the point immediately distal to the parent artery (Figs. 380-2 and 380-3). The primary example of this type of revascularization is the purely intracranial petrous carotid–to–supraclinoid carotid saphenous vein interposition graft. It is used primarily to reconstruct the carotid artery when it must be resected to remove skull base tumors and to treat giant intracavernous carotid aneurysms.10,43–45 This graft has the disadvantages of being technically complex, requiring a lengthy procedure, and most importantly, necessitating a prolonged period of ICA occlusion. It is associated with a significant complication rate related to graft occlusion and perioperative ischemic brain injury.8,10 A comprehensive description of this procedure is not included because we prefer to use a type II procedure for these indications. Readers are referred to technical descriptions elsewhere.44,45 Of note, carotid artery replacement with a saphenous vein or other interposition graft of this type can be used effectively to reconstruct the cervical carotid artery, below the skull base, if required for the treatment of extracranial carotid aneurysms or neck tumors.27,46–48
Type II Bypasses—Extracranial-to-Intracranial Bypass with a Saphenous Vein Graft or Radial Artery Graft
A type II bypass consists of a saphenous vein interposition graft between the extracranial carotid artery and a major intracranial branch vessel (Figs. 380-4 and 380-5).49 This procedure is used when a major arterial trunk must be occluded to treat a tumor or giant aneurysm and the distal collateral circulation is grossly inadequate (as evidenced by the absence of communicating arteries seen angiographically or by the rapid onset of a deficit during balloon test occlusion).4,8 In such cases, the bypass must replace all the circulation to a major arterial territory, and therefore a large conduit is needed. The normal blood flow of the MCA is about 250 mL/min to the cerebral hemisphere. The blood flow of the PCA is only moderately less. The average STA graft provides blood flow of just 15 to 30 mL/min, although it may increase with time.50–52 Blood flow through a saphenous vein graft typically ranges from 70 to 140 mL/min and can exceed 250 mL/min.51,53 Blood flow through a saphenous vein graft, which averages about 4 to 5 mm in diameter, is high enough to support the circulation in an entire major arterial territory at a level well above the ischemic threshold (particularly when added to the variable contribution through leptomeningeal collateral vessels). Despite these advantages, a vein graft generally has lower long-term patency rates and a higher risk of kinking, and there can be problems with caliber mismatch between the larger vein and smaller intracranial vessels. Alternatively, a radial artery graft can be used, which has a smaller diameter (about 3.5 mm) and a flow rate between 40 and 70 mL/min.51,54 A type II bypass can be a substitute for an STA-MCA bypass when the scalp artery is hypoplastic, diseased, or occluded. In a patient with an aneurysm that can be occluded only proximally, as in the case of some dolichoectatic and fusiform aneurysms, our experience has been that a type II bypass may supply too much flow and can be dangerous. With excessive retrograde filling from a robust saphenous vein graft, the aneurysm can remain patent, continue to enlarge, or even rupture in some cases. In these circumstances, if the distal aspect of the aneurysm must be left unoccluded, we prefer the use of an STA type III bypass when possible.
Extracranial Carotid Artery–to–Middle Cerebral Artery Saphenous Vein Interposition Graft
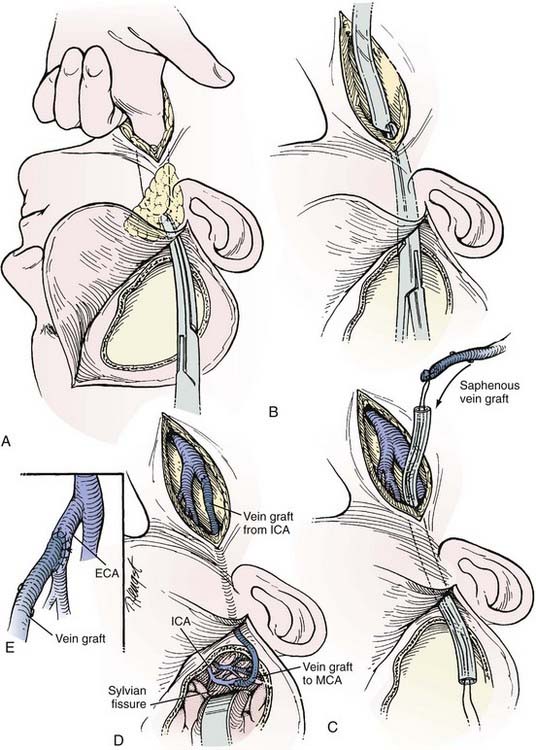
The saphenous vein graft is typically connected end to end to the proximal stump of the ICA or end to side to the ECA (see Figs. 380-4 and 380-5). For the distal anastomosis, we prefer an end-to-side anastomosis to a larger, more proximal MCA branch (M2 or M3 segment) in the sylvian fissure. These vessels better match the size of the saphenous vein and provide a more direct conduit to the entire MCA territory.
After the carotid bifurcation is exposed and a pterional craniotomy performed, the sylvian fissure is opened widely. The ideal M2 or M3 arterial recipient site, free of perforating vessels, is exposed. Next, the saphenous vein is exposed and isolated but left in situ in continuity until just before it is used for the bypass. At our institution, we have a highly experienced cardiac surgery fellow or cardiac surgery physician’s assistant harvest the vein graft by endoscopic technique. Meticulous care is exerted while exposing the vein to avoid trauma that might cause the bypass to thrombose.55–57 The alignment of the vein should be marked with a 6-0 Prolene suture (Ethicon, Johnson & Johnson Professionals, Inc., Somerville, NJ) through the adventitia to define the proper orientation of the vein. This maneuver avoids twisting the vein as it is positioned for the bypass. The vein is ligated proximally and distally, excised, and flushed without overdistention with cool, heparinized saline.
As suggested by Sundt and associates,4 the intracranial anastomosis is performed first. This sequence allows the surgeon to take advantage of slack in the graft, which can be manipulated freely while the back and front walls of the anastomosis are sutured. The terminal 5- to 6-mm portion of the vein graft is trimmed of loose adventitia, and the end is beveled to create an orifice 5 to 6 mm in diameter. After barbiturates are administered and blood pressure is stabilized at 10% to 20% above the patient’s baseline, a 10- to 15-mm length of MCA is occluded between temporary clips. A linear MCA arteriotomy is matched to the diameter of the vein graft orifice. The vein graft is fixed to the MCA branch with 8-0 monofilament nylon sutures, which are used to complete a running closure. After the anastomosis is completed, blood flow is restored, and the barbiturates can be stopped.
As an alternative procedure, a short vein graft can be placed between the STA trunk (exposed at the zygomatic arch) and a proximal M2 or M3 branch of the MCA.58 This procedure is useful when only a short segment of saphenous vein is available or the cervical carotid artery cannot be used as the site of the proximal anastomosis.
External Carotid Artery–to–Posterior Cerebral Artery Saphenous Vein Interposition Graft
An ECA-to-PCA saphenous vein interposition graft is used when the basilar artery or bilateral vertebral arteries are occluded to treat an unclippable basilar artery aneurysm.3,4 Bypass is necessary when the collateral circulation through the posterior communicating arteries is inadequate.18 Because of its difficulty and the substantial associated risks, this procedure is considered only for unclippable basilar aneurysms associated with subarachnoid hemorrhage or intractable progressive symptoms related to a mass effect.
After the intracranial anastomosis is completed, an end-to-side anastomosis is fashioned to the ECA. Sundt and coworkers observed that subdural hygromas develop in a large percentage of patients after this procedure.4 They suggested routinely placing a subtemporal subdural-peritoneal (or atrial) shunt to avoid this complication.
Type III Bypasses—Scalp Artery (Superficial Temporal or Occipital) Extracranial-to-Intracranial Bypass
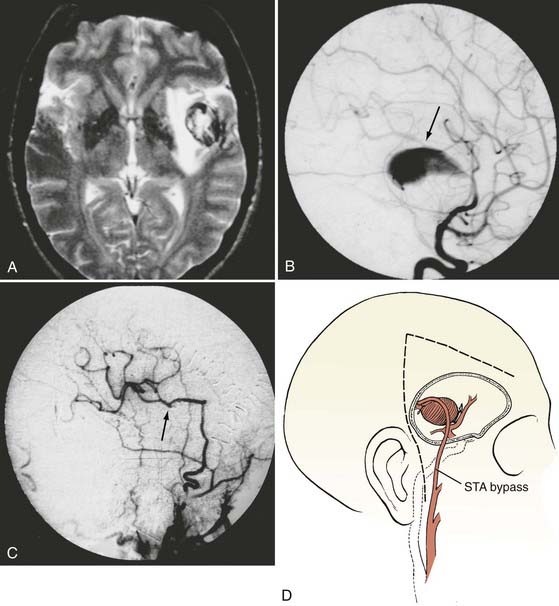
A type III bypass uses a pedicled scalp artery as the donor vessel.5,7,59,60 The STA or occipital artery can be used for these types of bypasses. This procedure is performed when a giant aneurysm requires occlusion of a single, crucial arterial branch or when carotid occlusion is required (for an aneurysm or tumor) and the circle of Willis is only marginally inadequate.61–63 Because the arterial territory at risk is smaller, a lower flow bypass is required than in the case of a type II procedure. These grafts are generally readily available, require only a single anastomosis, and have good patency rates when compared with free vein or arterial grafts. Their chief drawback, however, is their lower flow rate. The STA supplies a flow rate of about 15 to 30 mL/min, although the rate may increase with time after bypass.50–52 The occipital artery has a similar caliber and flow rate but is marginally more difficult to harvest and anastomose. In general, the STA can be used to revascularize the MCA territory, as well as the distal posterior circulation via the superior cerebellar artery or PCA. We have found STA-to-PCA or STA-to–superior cerebellar artery bypass to be adequate when proximal basilar artery occlusion is performed, as long as there is some collateral circulation or the posterior communicating arteries are not atretic. The occipital artery is most commonly used for bypass to the PICA, but it can also be used to revascularize the AICA as well.
Superficial Temporal Artery–to–Middle Cerebral Artery Bypass
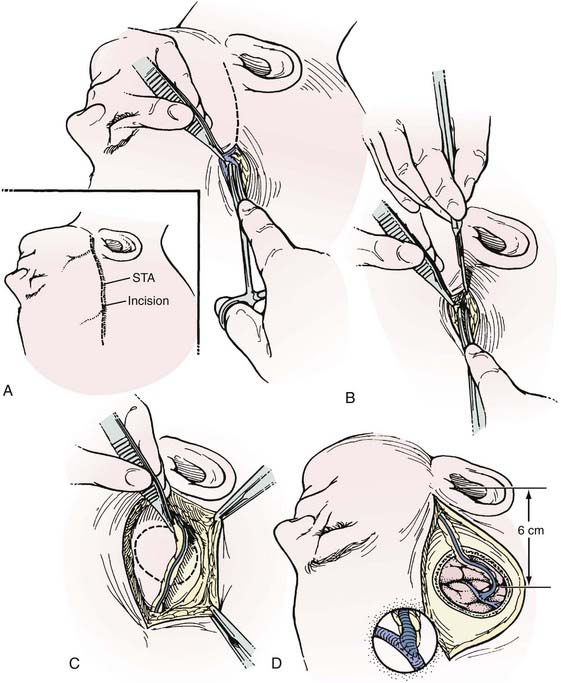
The preoperative angiogram should include an ECA injection to adequately define the patency, course, and caliber of the STA branches on the side that the bypass will be performed. Alternatively, computed tomographic angiography with three-dimensional reconstruction can be used to demonstrate the STA (or the occipital artery). At surgery, the STA branches are identified with a Doppler probe and marked on the scalp (Figs. 380-6 and 380-7). The largest STA branch, as identified on the preoperative angiogram, is selected as the donor vessel. After a linear incision is made over the artery distally, spreading with a small curved hemostat permits the STA, which is located just superficial to the galea, to be identified. The artery is exposed to the zygomatic arch, separated from the adjacent subcutaneous tissue with an adventitial cuff, and left in continuity until detached for anastomosis.
In patients who have two separate MCA branches that arise from the dome of an aneurysm, a single STA bypass may not be sufficient to revascularize the entire MCA territory. In these cases, a “double-barrel” STA bypass in which both the frontal and parietal branches are used can be performed to bypass to two separate MCA branches (Fig. 380-8). In this circumstance, a cutdown incision is made over the posterior STA branch, and the scalp incision is extended anteriorly to the hairline. The scalp flap is elevated in the subgaleal plane, and the anterior STA branch is dissected from the undersurface of the flap.
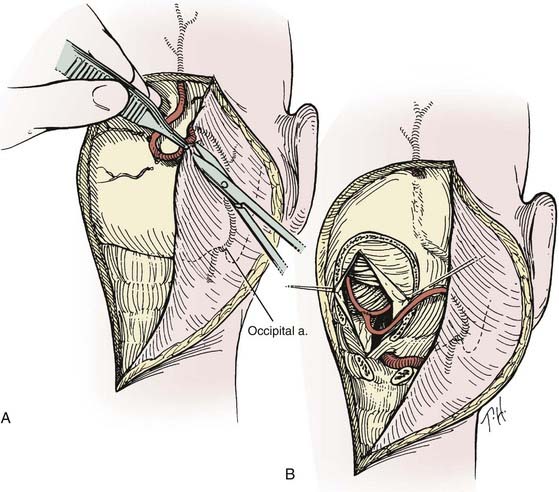
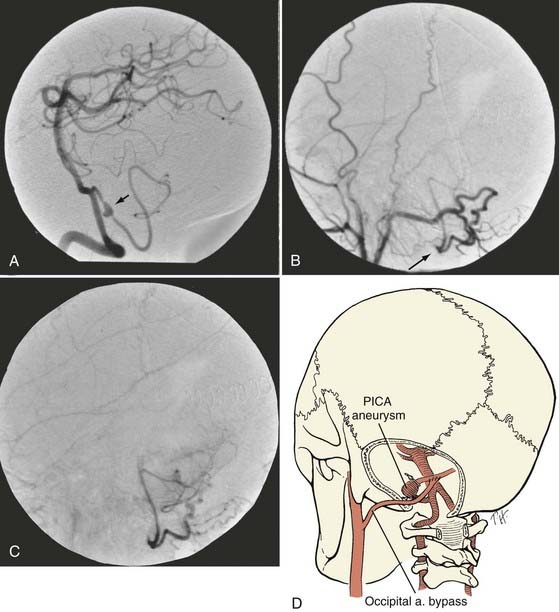
Occipital Artery–to–Posterior Inferior Cerebellar Artery Bypass
The patient is placed in the lateral position with the operative side up (Figs. 380-9 and 380-10). The head is fixed in moderate flexion. The course of the occipital artery is identified with the Doppler probe. A hockey stick incision is made, with the transverse limb located 1 cm above the superior nuchal line. The occipital artery is dissected from the subcutaneous tissue and suboccipital musculature. After initial exposure of the occipital artery at the nuchal line, it is dissected from the undersurface of the myocutaneous suboccipital flap, which is retracted laterally. The vessel is left in continuity until just before it is required for the anastomosis. The occiput, the arch of C1, and the laminae of C2 are exposed. To provide access to the caudal loop of the PICA, the craniotomy is extended from just beyond the midline almost to the region of the occipital condyle. If the vertebral artery is to be exposed intradurally, such as for trapping of a fusiform aneurysm of the vertebral artery, the craniotomy is extended into a far lateral transcondylar exposure. It is often helpful to remove the posterior arch of C1 unilaterally. After the dura is opened, the cerebellar tonsil is elevated, and the PICA loop, which rarely has any branches, can usually be mobilized by carefully dividing the numerous small arachnoid fibers that fix it to the dorsal surface of the medulla. After the PICA loop is exposed and the distal end of the occipital artery is prepared, the patient is given barbiturates. The PICA is occluded and the anastomosis is completed in a fashion similar to that for an STA-MCA bypass. A multilayer meticulous muscle closure is essential because a watertight dural closure cannot be achieved. During the muscle closure, the occipital artery must not become kinked or severely compressed under the suboccipital muscles.
Superficial Temporal Artery–to–Superior Cerebellar Artery Bypass and Superficial Temporal Artery–to–Posterior Cerebral Artery Bypass
An STA-to–superior cerebellar artery bypass or STA-to-PCA bypass may be substituted for the saphenous vein graft–to-PCA bypass when some collateral blood flow is available through small posterior communicating arteries.64 Once the STA is isolated, the incision is extended posteriorly above and behind the ear for the temporal craniotomy. After lumbar drainage of cerebrospinal fluid and elevation of the temporal lobe, the superior cerebellar artery or PCA is exposed lateral to the brainstem. An unbranched length of the recipient vessel is isolated and occluded after barbiturates are administered. A 3- to 4-mm arteriotomy is made along its lateral surface. A sufficiently long length of the STA is isolated and brought down to the recipient artery. The length of the STA must have sufficient slack to allow easy rotation for access to the front and back walls of the anastomosis. The anastomosis is completed in the same fashion as the STA-MCA anastomosis.
Type IV Bypasses—Direct Intracranial Revascularization
A type IV bypass involves an anastomosis between two adjacent cerebral arteries. This type of procedure can involve end-to-end primary reanastomosis after excision of an aneurysm, side-to-side anastomosis of two adjacent intracranial arteries, or an end-to-side anastomosis between two cerebral arteries.5,6,8,65–70 Examples of side-to-side type IV bypasses include PICA-PICA, pericallosal-pericallosal, and MCA-MCA between adjacent MCA branches in the sylvian fissure. We have also performed side-to-side PICA-AICA anastomoses.
Pericallosal-to-Pericallosal Bypass
Pericallosal-to-pericallosal bypass can be used to treat fusiform aneurysms of the proximal pericallosal artery or for giant anterior communicating artery aneurysms that require trapping.6 Interhemispheric exposure to the pericallosal arteries on the dorsal surface of the corpus callosum requires medial expansion of the pterional craniotomy or a separate parasagittal approach. After barbiturates are administered, temporary clips are placed on each pericallosal artery just beyond the genu of the corpus callosum. A 5-mm linear arteriotomy is made along the medial surface of each pericallosal artery. The back walls of the two pericallosal arteries are joined first with running 10-0 nylon suture, followed by closure of the front wall with a second suture. On the back wall, careful planning is required to place the suture so that the knot ends up outside the lumen. This side-to-side anastomosis serves as a new communicating artery.
Other Variations of Intracranial Arterial Reconstruction
Sundt and Piepgras,19 Ausman and colleagues,5 Bederson and Spetzler,66 and others67,69 have described variations of intracranial arterial reconstruction or transposition for the treatment of giant aneurysms. Most such cases have involved large MCA aneurysms, which tend to incorporate distal branches into the base of the aneurysm.
Outcomes
Complications
Another devastating postoperative complication is aneurysmal rupture associated with the hemodynamic changes that accompany arterial reconstruction with a bypass. In our experience, two patients with a giant fusiform basilar trunk aneurysm died of aneurysmal rupture after vein grafts to the PCA were placed.13 In one case, rupture occurred 2 days after surgery, before proximal or distal parent artery occlusion had been performed. In the other, rupture occurred after proximal but not distal parent artery occlusion. In both cases, the hemodynamic stress associated with the presence of the distal high-flow bypass apparently caused the aneurysm to rupture. Other surgeons have reported rupture of giant intracranial aneurysms after distal bypass procedures.71 An additional two patients in our experience demonstrated continued aneurysm growth after vein bypass and proximal occlusion. Both required reduction in flow with encircling fenestrated clips or a Silverstone clamp. These complications emphasize the need to isolate the aneurysm completely from the circulation by trapping whenever possible. If it cannot be trapped completely, we have had better experience with a lower flow distal bypass such as an STA graft, which may reduce the magnitude of hemodynamic stress.
Long-Term Graft Patency
The long-term patency of bypass grafts depends on several factors, the type of graft used chief among them. STA grafts tend to have the greatest patency rates. The International Cooperative EC-IC Bypass Study of 1985 found that the postoperative patency rate of 663 STA-MCA bypasses was 96% at an average follow-up of 55.8 months.2,72 Schick and associates reported a 91% patency rate with a mean follow-up of 2.8 years.72 There is less literature concerning the long-term patency of occipital artery grafts. In smaller clinical series, patency rates have varied from 73% to 94%, but generally with shorter long-term follow-up.63,73
Clinical and experimental data from the cardiac and vascular surgery literature indicate that occlusion of vein grafts increases over time. Ten years after surgery, thrombosis occurs in about 40% of aortocoronary grafts and in more than 50% of femoropopliteal bypasses. When Regli and coworkers used long saphenous vein grafts for cerebral revascularization, they found that the early patency rate (0 to 30 days after surgery) was 88% and that the 1-year cumulative patency rate was 86%.55 At 5 years, the patency rate was 82%; at 13 years, it was 73%. These rates correspond to a mean annual graft failure rate of 1% to 1.5%. Others have shown even poorer long-term patency for vein grafts.72 There are fewer data for radial artery grafts, but it appears that their overall patency is better than that for saphenous vein grafts but marginally inferior to the rate for pedicled arterial grafts. One small study of radial artery bypass grafts for carotid aneurysms in 43 patients had an overall patency rate of 95% at 3 weeks.74 Long-term follow-up was available for only 20 patients, but there were no delayed occlusions. From the cardiac surgery literature, the patency rate has been reported to be 92% at 5 years.75
Results of Bypass for Complex Intracranial Aneurysms
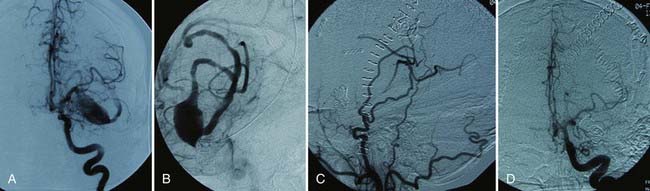
In our series, 21 of 22 saphenous vein bypass grafts were acutely patent, and 69 of 73 STA or occipital grafts were patent (1 of the occluded grafts was successfully revised). All 8 type IV bypasses were patent. Eighty-five patients (86%) had excellent or good outcomes (Glasgow Outcome Scale score of 4 or 5). Nine patients died as a result of perioperative complications (two of whom were Hunt-Hess grade V preoperatively and three of whom died of cardiac causes).13
Using saphenous vein graft bypasses for giant aneurysms, Sundt and coworkers reported an acute graft patency rate of 94% in their more recent experience.4 Excellent or good outcomes were achieved in 80% of anterior circulation aneurysms and in 44% of posterior circulation aneurysms. Lawton and colleagues reported that 93% of 61 patients had good outcomes after revascularization for intracranial aneurysms.8 Sen and Sekhar reported the results in 30 patients who underwent vein grafting with carotid occlusion or resection (primarily for tumors).10 Their rate of graft patency was 86% at 18 months; 4 patients sustained ischemic injury related to the bypass procedure.
Ausman JI, Diaz FG, Sadasivan B, et al. Giant intracranial aneurysm surgery: the role of microvascular reconstruction. Surg Neurol. 1990;34:8-15.
Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656-665.
Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141-162.
Lawton MT, Hamilton MG, Morcos JJ, et al. Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery. 1996;38:83-92.
Martin NA, Kureshi I, Coiteiro D. Bypass techniques for the treatment of intracranial aneurysms. Oper Tech Neurosurg. 2000;3:255-270.
Sekhar LN, Bucur SD, Bank WO, et al. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207-1223.
Sundt TMJr, Piepgras DG. Surgical approach to giant intracranial aneurysms. Operative experience with 80 cases. J Neurosurg. 1979;51:731-742.
Sundt TMJr, Piepgras DG, Houser OW, et al. Interposition saphenous vein grafts for advanced occlusive disease and large aneurysms in the posterior circulation. J Neurosurg. 1982;56:205-215.
Sundt TMJr, Piepgras DG, Marsh WR, et al. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65:439-450.
Sundt TMJr. Maximizing patency and saphenous vein bypass grafts: principles of preparation learned from coronary and peripheral vascular surgery. In: Meyer FB, editor. Sundt’s Occlusive Cerebrovascular Disease. Philadelphia: Saunders; 1994:479-488.
1 Donaghy R, Yasargil MG. Microvascular Surgery. St. Louis: CV Mosby; 1967.
2 Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191-1200.
3 Sundt TMJr, Piepgras DG, Houser OW, et al. Interposition saphenous vein grafts for advanced occlusive disease and large aneurysms in the posterior circulation. J Neurosurg. 1982;56:205-215.
4 Sundt TMJr, Piepgras DG, Marsh WR, et al. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65:439-450.
5 Ausman JI, Diaz FG, Sadasivan B, et al. Giant intracranial aneurysm surgery: the role of microvascular reconstruction. Surg Neurol. 1990;34:8-15.
6 Ito Z. A new technique of intracranial interarterial anastomosis between distal anterior cerebral arteries (ACA) for ACA occlusion and its indication [author’s transl]. Neurol Med Chir (Tokyo. 1981;21:931-939.
7 Peerless SJ, Hampf CR. Extracranial to intracranial bypass in the treatment of aneurysms. Clin Neurosurg. 1985;32:114-154.
8 Lawton MT, Hamilton MG, Morcos JJ, et al. Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery. 1996;38:83-92.
9 Sekhar LN, Sen CN, Lanzino G, et al. Carotid and cranial nerve reconstruction after removal of cavernous sinus lesions. Keio J Med. 1991;40:187-193.
10 Sen C, Sekhar LN. Direct vein graft reconstruction of the cavernous, petrous, and upper cervical internal carotid artery: lessons learned from 30 cases. Neurosurgery. 1992;30:732-742.
11 Coiteiro DN, Oertel M, Martin NA. Revascularization of posterior cerebral circulation. Tech Neurosurg. 2000;6:113-126.
12 Martin NA. The use of extracranial-intracranial bypass for the treatment of giant and fusiform aneurysms. J Stroke Cerebrovasc Dis. 1997;6:242-245.
13 Martin NA. Arterial bypass for the treatment of giant and fusiform intracranial aneurysms. Tech Neurosurg. 1998;4:153-178.
14 Martin NA, Kureshi I, Coiteiro D. Bypass techniques for the treatment of intracranial aneurysms. Oper Tech Neurosurg. 2000;3:255-270.
15 Anson JA, Lawton MT, Spetzler RF. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg. 1996;84:185-193.
16 Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141-162.
17 Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656-665.
18 Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161-173.
19 Sundt TMJr, Piepgras DG. Surgical approach to giant intracranial aneurysms. Operative experience with 80 cases. J Neurosurg. 1979;51:731-742.
20 Gurian JH, Martin NA, King WA, et al. Neurosurgical management of cerebral aneurysms following unsuccessful or incomplete endovascular embolization. J Neurosurg. 1995;83:843-853.
21 Drake CG, Barr HW, Coles JC, et al. The use of extracorporeal circulation and profound hypothermia in the treatment of ruptured intracranial aneurysm. J Neurosurg. 1964;21:575-581.
22 Silverberg GD, Reitz BA, Ream AK. Hypothermia and cardiac arrest in the treatment of giant aneurysms of the cerebral circulation and hemangioblastoma of the medulla. J Neurosurg. 1981;55:337-346.
23 Solomon RA, Smith CR, Raps EC, et al. Deep hypothermic circulatory arrest for the management of complex anterior and posterior circulation aneurysms. Neurosurgery. 1991;29:732-737.
24 Spetzler RF, Hadley MN, Rigamonti D, et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68:868-879.
25 Lawton MT, Spetzler RF. Internal carotid artery sacrifice for radical resection of skull base tumors. Skull Base Surg. 1996;6:119-123.
26 Brisman MH, Sen C, Catalano P. Results of surgery for head and neck tumors that involve the carotid artery at the skull base. J Neurosurg. 1997;86:787-792.
27 Feiz-Erfan I, Han PP, Spetzler RF, et al. Salvage of advanced squamous cell carcinomas of the head and neck: internal carotid artery sacrifice and extracranial-intracranial revascularization. Neurosurg Focus. 2003;14(3):e6.
28 DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
29 Couldwell WT, Kan P, Liu JK, et al. Decompression of cavernous sinus meningioma for preservation and improvement of cranial nerve function. Technical note. J Neurosurg. 2006;105:148-152.
30 Kano H, Niranjan A, Kondziolka D, et al. Stereotactic radiosurgery for trigeminal schwannoma: tumor control and functional preservation. Clinical article. J Neurosurg. 2009;110:553-558.
31 Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-58.
32 Milker-Zabel S, Zabel A, Schulz-Ertner D, et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:809-816.
33 Nishioka K, Abo D, Aoyama H, et al. Stereotactic radiotherapy for intracranial nonacoustic schwannomas including facial nerve schwannoma. Int J Radiat Oncol Biol Phys. 2009;75:1415-1419.
34 Brennan JA, Jafek BW. Elective carotid artery resection for advanced squamous cell carcinoma of the neck. Laryngoscope. 1994;104:259-263.
35 Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863.
36 Hacein-Bey L, Connolly ESJr, Duong H, et al. Treatment of inoperable carotid aneurysms with endovascular carotid occlusion after extracranial-intracranial bypass surgery. Neurosurgery. 1997;41:1225-1231.
37 Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749-754.
38 Heros RC. Revascularization and aneurysm surgery: current techniques, indications, and outcome. (comment to: Lawton MT, Hamilton MV, Morcos JJ, et al). Neurosurgery. 1996;38;:83-94.
39 Piepgras DG. Revascularization and aneurysm surgery: current techniques, indications, and outcome. (comment to: Lawton MT, Hamilton MV, Morcos JJ, et al). Neurosurgery. 1996;38;:83-94.
40 Ryu YH, Chung TS, Lee JD, et al. HMPAO SPECT to assess neurologic deficits during balloon test occlusion. J Nucl Med. 1996;37:551-554.
41 Solomon RA. Principles of aneurysm surgery: cerebral ischemic protection, hypothermia, and circulatory arrest. Clin Neurosurg. 1994;41:351-363.
42 Batjer HH. Cerebral protective effects of etomidate: experimental and clinical aspects. Cerebrovasc Brain Metab Rev. 1993;5:17-32.
43 Linskey ME, Sekhar LN, Sen C. Cerebral revascularization in cranial base surgery. In: Sekhar LN, Janeck IP, editors. Surgery of Cranial Base Tumors. New York: Raven; 1993:45-68.
44 Sekhar LN, Sen CN, Jho HD. Saphenous vein graft bypass of the cavernous internal carotid artery. J Neurosurg. 1990;72:35-41.
45 Spetzler RF, Fukushima T, Martin N, et al. Petrous carotid-to-intradural carotid saphenous vein graft for intracavernous giant aneurysm, tumor, and occlusive cerebrovascular disease. J Neurosurg. 1990;73:496-501.
46 Attigah N, Kulkens S, Zausig N, et al. Surgical therapy of extracranial carotid artery aneurysms: long-term results over a 24-year period. Eur J Vasc Endovasc Surg. 2009;37:127-133.
47 Sessa CN, Morasch MD, Berguer R, et al. Carotid resection and replacement with autogenous arterial graft during operation for neck malignancy. Ann Vasc Surg. 1998;12:229-235.
48 Chazono H, Okamoto Y, Matsuzaki Z, et al. Extracranial-intracranial bypass for reconstruction of internal carotid artery in the management of head and neck cancer. Ann Vasc Surg. 2003;17:260-265.
49 Lougheed WM, Marshall BM, Hunter M, et al. Common carotid to intracranial internal carotid bypass venous graft. Technical note. J Neurosurg. 1971;34:114-118.
50 Bendok BR, Murad A, Getch CC, et al. Failure of a saphenous vein extracranial-intracranial bypass graft to protect against bilateral middle cerebral artery ischemia after carotid artery occlusion: case report. Neurosurgery. 1999;45:367-370.
51 Liu JK, Kan P, Karwande SV, et al. Conduits for cerebrovascular bypass and lessons learned from the cardiovascular experience. Neurosurg Focus. 2003;14(6):e3.
52 Mohit AA, Sekhar LN, Natarajan SK, et al. High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery. 2007;60:ONS105-122.
53 Jafar JJ, Russell SM, Woo HH. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: indications, operative technique, and results in 29 patients. Neurosurgery. 2002;51:138-144.
54 Sekhar LN, Bucur SD, Bank WO, et al. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207-1223.
55 Regli L, Piepgras DG, Hansen KK. Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg. 1995;83:806-811.
56 Sundt TM3rd, Sundt TMJr. Principles of preparation of vein bypass grafts to maximize patency. J Neurosurg. 1987;66:172-180.
57 Sundt TMJr. Maximizing patency and saphenous vein bypass grafts: principles of preparation learned from coronary and peripheral vascular surgery. In: Meyer FB, editor. Sundt’s Occlusive Cerebrovascular Disease. Philadelphia: Saunders; 1994:479-488.
58 Little JR, Furlan AJ, Bryerton B. Short vein grafts for cerebral revascularization. J Neurosurg. 1983;59:384-388.
59 Yasargil MG, Krayenbuhl HA, Jacobson JH2nd. Microneurosurgical arterial reconstruction. Surgery. 1970;67:221-233.
60 Yasargil MG, Yonekawa Y. Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery. 1977;1:22-24.
61 Khodadad G, Singh RS, Olinger CP. Possible prevention of brain stem stroke by microvascular anastomosis in the vertebrobasilar system. Stroke. 1977;8:316-321.
62 Roski RA, Spetzler RF, Hopkins LN. Occipital artery to posterior inferior cerebellar artery bypass for vertebrobasilar ischemia. Neurosurgery. 1982;10:44-49.
63 Sundt TMJr, Piepgras DG. Occipital to posterior inferior cerebellar artery bypass surgery. J Neurosurg. 1978;48:916-928.
64 Ausman JI, Diaz FG, de los Reyes RA, et al. Posterior circulation revascularization. Superficial temporal artery to superior cerebellar artery anastomosis. J Neurosurg. 1982;56:766-776.
65 Quinones-Hinojosa A, Lawton MT. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2008;62:1442-1449.
66 Bederson JB, Spetzler RF. Anastomosis of the anterior temporal artery to a secondary trunk of the middle cerebral artery for treatment of a giant m1 segment aneurysm. Case report. J Neurosurg. 1992;76:863-866.
67 Dolenc V. End-to-end suture of the posterior inferior cerebellar artery after the excision of a large aneurysm: case report. Neurosurgery. 1982;11:690-693.
68 Ito Z. The microsurgical anterior interhemispheric approach suitably applied to ruptured aneurysms of the anterior communicating artery in the acute stage. Acta Neurochir (Wien). 1982;63:85-99.
69 Madsen JR, Heros RC. Giant peripheral aneurysm of the posterior inferior cerebellar artery treated with excision and end-to-end anastomosis. Surg Neurol. 1988;30:140-143.
70 Smith RR, Parent AD. End-to-end anastomosis of the anterior cerebral artery after excision of a giant aneurysm. Case report. J Neurosurg. 1982;56:577-580.
71 Heros RC, Ameri AM. Rupture of a giant basilar aneurysm after saphenous vein interposition graft to the posterior cerebral artery. Case report. J Neurosurg. 1984;61:387-390.
72 Schick U, Zimmermann M, Stolke D. Long-term evaluation of EC-IC bypass patency. Acta Neurochir (Wien). 1996;138:938-942.
73 Ausman JI, Diaz FG, Vacca DF, et al. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J Neurosurg. 1990;72:554-558.
74 Houkin K, Kamiyama H, Kuroda S, et al. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999;90:786-790.
75 Possati G, Gaudino M, Alessandrini F, et al. Midterm clinical and angiographic results of radial artery grafts used for myocardial revascularization. J Thorac Cardiovasc Surg. 1998;116:1015-1021.