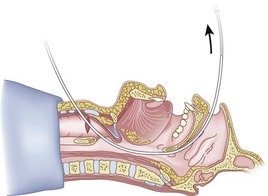
Chapter 20 Retrograde Intubation Techniques
I History
The first reported case of retrograde intubation (RI) was by Butler and Cirillo in 1960.1 The technique involved passing a red rubber catheter cephalad through the patient’s previously existing tracheostomy. When the catheter exited the oral cavity, it was tied to the endotracheal tube (ETT), allowing it to be pulled into the trachea.
The first person to perform RI as presently practiced was Waters, a British anesthesiologist in Nigeria.2 In 1963, he reported treating patients who had cancrum oris, an invasive gangrene that deforms the oral cavity, severely limiting mouth opening. His technique involved passing a standard Tuohy needle through the cricothyroid membrane (CTM) and feeding an epidural catheter cephalad into the nasopharynx. He “fished” the catheter out of the nasopharynx through the nares, using a hook he devised. The epidural catheter was then used as a stylet to guide the ETT through the nares and into the trachea.
Over the ensuing years, RI did not gain clinical acceptance because of its invasiveness and the potential for complications from the CTM puncture. After 1964, when fiberoptic technology became available, RI was irregularly but occasionally discussed in the literature.1–165 In 1993, RI was designated as part of the anesthesiologist’s armamentarium by the American Society of Anesthesiologists (ASA) Difficult Airway (DA) Task Force.3
The term retrograde intubation, used by Butler and Cirillo, is a misnomer.4 The technique is actually a translaryngeal guided intubation, but for historical reasons we continue using the name retrograde intubation.
II Indications
The RI technique has been used both in the hospital setting and in prehospital mobile units (in the field).5,6 It has been employed with both anticipated and unanticipated DAs2,5–20; after failure to intubate by conventional means (direct laryngoscopy,6,9,11 blind nasal intubation,10,11 bougie,13,21 laryngeal mask airway [LMA], and fiberoptic laryngoscopy18,22–24); and in both humans and animals.25,26 In the literature, including my own experience (31 patients), there have been approximately 807 cases (670 patients and 137 cadavers) in which RI was used as a means of securing the airway. Although in most cases RI has been used to place a single-lumen ETT, one case report described placement of a double-lumen ETT through RI.7
A wide variety of airway diseases have necessitated RI (Box 20-1). RI has been most frequently associated with limited range of motion of the neck (153 trauma victims with potential cervical spine injury), and its use has been reported in facial trauma. It has been employed in both adults and pediatric patients with success (Box 20-2).
Box 20-1
Number of Retrograde Intubations in the Literature (539 Patients and 137 Cadavers)
From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.
Box 20-2
Characteristics of Retrograde Intubations (RIs) in the Literature
Number of adult patients (RI): 509
Number of pediatric patients (RI): 30
Age range: 1 day (weight, 2.9 kg) to 84 yr
From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.
Not all reports have described the amount of time required to perform the technique, but in one study involving emergency medical service personnel (paramedics and registered nurses) using training mannequins, the average time was 71 seconds (range, 42 to 129 seconds).27 Barriot and Riou described 13 patients with maxillofacial trauma who could not be intubated in the field using direct laryngoscopy (six attempts; average time, 18 minutes).5 Intubation was subsequently performed in these patients on the first RI attempt, with an average time of less than 5 minutes. An additional 6 patients were intubated in less than 5 minutes when RI was used as the initial method of choice. Slots and colleagues reported a modified technique using a Mini-Trach II set on 20 cadavers with an average time for intubation of 6.7 seconds (range, 3 to 10 seconds); it was subsequently used on an emergency basis on 3 patients with an average time of 10 seconds. The investigators concluded that the RI technique was a rapid, efficacious method for intratracheal intubation of trauma patients, especially patients with maxillofacial trauma.28
Historical indications for RI are the following:
1. Failed attempts at laryngoscopy, LMA, or fiberoptic intubation (FOI)
2. Urgent establishment of an airway where visualization of the vocal cords is prevented by blood, secretions, or anatomic derangement in scenarios in which ventilation is still possible
3. Elective use when deemed necessary in clinical situations such as unstable cervical spine, maxillofacial trauma, or anatomic anomaly
III Contraindications
Contraindications to RI have been cited, often anecdotally (Box 20-3). Most are relative contraindications and can be divided into four categories: unfavorable anatomy, laryngotracheal disease, coagulopathy, and infection.
A Unfavorable Anatomy
Because in most cases RI is performed above or below the cricoid cartilage, absolute lack of access to this region, as in patients with severe flexion deformity of the neck, poses a contraindication if not an impossibility.29,30 For the same reason, the patient with nonpalpable landmarks,31–33 obesity,34 overlying malignancy,32 or large thyroid goiter should be approached cautiously.32 Shantha reported a case of RI in a patient with a large thyroid goiter.35 After failure of conventional intubating methods (including FOI), the surgeons dissected down to the CTM and subsequently passed the catheter cephalad. Thirteen cases of RI have been reported in obese patients without major complications.12,18,34,36
B Laryngotracheal Disease
Theoretically, laryngotracheal stenosis may contraindicate RI because narrowing of the trachea or larynx could be made worse by either the needle puncture or the catheter.2,31 However, RI has been used in patients with laryngeal cancer,37 epiglottitis,38 and laryngeal edema resulting from burn injuries.16,24 It should not be used if laryngeal tracheal stenosis is present directly under the intended puncture site.
C Coagulopathy
Preexisting bleeding diathesis should be considered a relative contraindication.31,32,39–44 Although there is a potential for bleeding, the CTM is considered to be a relatively avascular plane (see later discussion). A small, self-limited hematoma was reported in a patient who underwent a coronary artery bypass grafting with intraoperative heparin and postoperative disseminated intravascular coagulation.9
D Infection
RI in the presence of preexisting infection over the puncture site or in the path of the puncture, as in pretracheal abscess or Ludwig’s angina, could result in transmittal of bacterial flora into the trachea and should be avoided. This, again, should be considered a relative contraindication, because transtracheal aspiration is performed to obtain a sputum sample in patients with pneumonia despite the possibility of pretracheal abscess (see “Complications”).31,32,43,45–47
IV Anatomy
The performance of RI requires basic anatomic knowledge of the cricoid cartilage (Fig. 20-1) and the structures above and below it to minimize complications and failure. Indeed, regardless of the intubation technique planned, the cricoid cartilage and CTM should be identified preoperatively in every patient.48 Cartilage and membrane, vascular structures, and the thyroid gland are relevant anatomic structures.
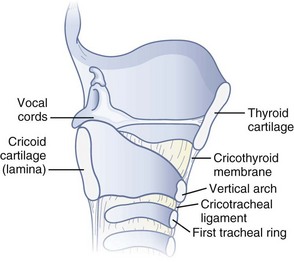
Figure 20-1 Anatomy of the cricoid cartilage. Midsagittal view of the larynx and trachea.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
A Cartilage and Membrane
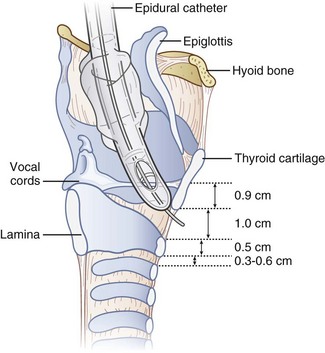
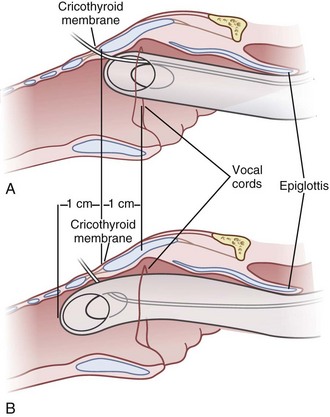
The cricoid cartilage has the shape of signet ring (see Fig. 20-1). It consists of a broad, flat, posterior plate called the lamina and a narrow, convex, anterior structure called the arch.49,50 In most cases the cartilage can be easily palpated by identifying the thyroid notch and running a finger down the midline in a caudad direction until a rigid, rounded structure is encountered. The vertical height of the arch is 0.5 to 0.7 cm (Fig. 20-2).50 The CTM connects the superior border of the arch to the inferior border of the thyroid cartilage and measures approximately 1 cm in height and 2 cm in width.49,51 The lateral borders are the paired cricothyroid muscles.45 The cricotracheal ligament connects the inferior border of the arch to the upper border of the first tracheal ring and measures 0.3 to 0.6 cm in height.52 The distance between the inferior border of the thyroid cartilage and the vocal cords varies with gender but is approximately 0.9 cm.53
B Vascular Structures
There are paired major blood vessels above and below the cricoid cartilage: the cricothyroid artery and the superior thyroid artery (Fig. 20-3).
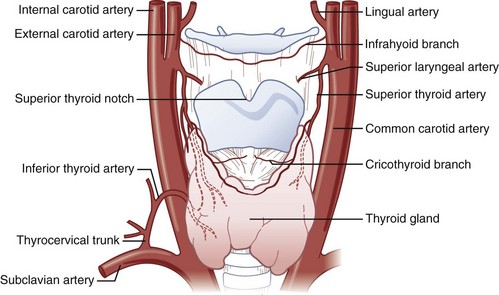
Figure 20-3 Vascular anatomy above and below the cricoid cartilage.
(Modified from Naumann H, editor: Head and neck surgery, Philadelphia, 1984, WB Saunders.)
The cricothyroid artery,50,51,53–55 a branch of the superior thyroid artery, runs along the anterior surface of the CTM, usually close to the inferior border of the thyroid cartilage. In some cases, the cricothyroid arteries anastomose in the midline and give rise to a descending branch that feeds the middle lobe of the thyroid gland when present. On the basis of dissections that I have performed on cadavers, the cricothyroid artery becomes insignificant in size as it approaches the midline. No major venous plexus could be found mentioned in the literature, and none was found in my own dissections.
The anterior branch of the superior thyroid artery runs along the upper border of the thyroid isthmus to anastomose with its counterpart from the opposite side.50,53–55 The inferior thyroid artery also anastomoses with the superior thyroid artery at the level of the isthmus. The arteries are remarkable for their large size and frequent anastomoses. In fewer than 10% of the population, an unpaired thyroid artery ascends ventral to the trachea (from either the aortic arch or the brachiocephalic artery) to anastomose at the level of the isthmus. It is usually small but may be very large. A rich venous plexus is formed in and around the isthmus.
C Thyroid Gland (Isthmus, Pyramidal Lobe)
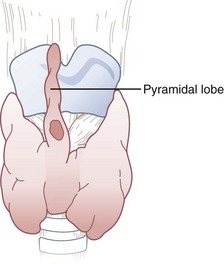
The isthmus of the thyroid gland (see Fig. 20-3) is rarely absent and generally lies anterior to the trachea between the first and fourth tracheal rings (usually between the second and third), although there are many variations. Its size and vertical height vary; the average vertical height and depth are 1.25 cm. Extending from the isthmus, the highly vascular pyramidal lobe (Fig. 20-4) is well developed in one third of the population. It is found more frequently on the left of the midline and may extend up to the hyoid bone (the upper continuation is usually thyromuscular).25,49,50,54,55
V Physiology
Sympathetic stress responses—increased heart rate, blood pressure, intraocular pressure, and intracranial pressure and elevated catecholamine levels—have been reported with laryngoscopy, endotracheal intubation, coughing, translaryngeal local anesthesia, laryngotracheal anesthesia, and FOI. Therefore, concern is appropriate when performing RI in patients with coronary artery disease, elevated intraocular pressure, or elevated intracranial pressure.29,30,56–62 It is reasonable to argue, however, that RI, performed skillfully, is not more stimulating than any other technique for managing the airway.
No apparent significant changes in hemodynamics were reported in multiple case reports of patients with cardiac disease (congenital anomalies, ischemic coronary artery disease, valvular disease, pericarditis, and congestive heart failure) who underwent RI, both awake with topical anesthesia and under general anesthesia.9,11,15,17,38,63–68 Casthely and colleagues reported on 25 patients with DA due to rheumatoid arthritis who underwent open heart surgery (coronary artery bypass graft and valve replacement).9 The patients had invasive monitors (Swan-Ganz catheters and peripheral arterial lines) placed preoperatively. The initial 24 patients underwent a cardiac induction of anesthesia (diazepam 10 mg, fentanyl 25 to 30 µg/kg, and pancuronium 0.1 mg/kg) before rigid laryngoscopy followed by RI. Comparison of hemodynamic responses to rigid laryngoscopy (Macintosh and Miller blades) versus RI demonstrated that the former approach was more stressful (Table 20-1). Patient 25 underwent RI before induction of anesthesia after application of topical anesthesia with no significant hemodynamic response.
| Laryngoscopy | Retrograde Intubation | |
|---|---|---|
| HR | Increase | No change from baseline |
| MAP | Increase | No change from baseline |
| CI | Decrease | No change from baseline |
| PCWP | Increase | No change from baseline |
| ECG | 3-mm ST depression | No change from baseline |
CI, Cardiac index; ECG, electrocardiogram (ST segment changes in lead V5); HR, heart rate; MAP, mean arterial pressure; PCWP, pulmonary capillary wedge pressure.
Modified from Casthely PA, Landesman S, Fynaman PN: Retrograde intubation in patients undergoing open heart surgery. Can Anaesth Soc J 32:661, 1985.
Two case reports documented patients with a previous history of DA and intracranial pathology (pseudotumor cerebri and intracranial tumor with elevated intracranial pressure) who underwent elective, awake RI after topical anesthesia with no evidence of further increase in intracranial pressure.8,14 I myself, unmedicated except for topical lidocaine, underwent awake RI with no significant hemodynamic changes.48
VI Techniques
A Preparation
1 Positioning
The ideal position for RI is the supine sniffing position with the neck hyperextended.69,70 In this position, the cervical vertebrae push the trachea and cricoid cartilage anteriorly and displace the strap muscles of the neck laterally. As a result, the cricoid cartilage and the structures above and below it are easier to palpate. RI can also be performed with the patient in a sitting position,48 which may be the only position in which some patients can breathe comfortably. Potential cervical spine injury or limited range of motion of the cervical spine may necessitate RI with the neck in a neutral position, which is a well-documented practice (see Box 20-1).
2 Skin Preparation
Although most documented RIs have not been elective, every effort should be made to perform RI using aseptic technique. Recommendations have been made for prophylactic antibiotics in diabetic or immunocompromised patients, who may be more susceptible than others to infection.71
3 Anesthesia
1. Translaryngeal anesthesia during intravenous sedation or general anesthesia2,8–10,16,18,72
2. Translaryngeal anesthesia with superior laryngeal nerve block5,6,73
3. Translaryngeal anesthesia with topicalization of the pharynx (aerosolized or sprayed)7,14,74,75
4. Glossopharyngeal nerve block and superior laryngeal nerve block with nebulized local anesthetic17
(Refer to Chapter 11 for a detailed description of neural blockade of the airway.)
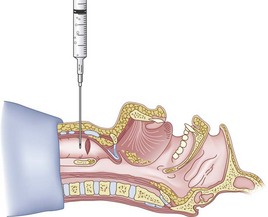
In my own experience,48 an awake RI can be performed using translaryngeal anesthesia (4 mL 2% lidocaine) supplemented with topicalization (nebulized or sprayed local anesthetics) of the pharynx and hypopharynx. Special caution should be exercised when performing the translaryngeal anesthesia, because coughing, grunting, sneezing, or swallowing causes the cricoid cartilage to travel cephalad, with the potential for breaking the needle in the trachea.76,77 To avoid this, one can insert a 20-G angiocatheter and remove the needle before injecting the local anesthetic.
4 Entry Site
The transtracheal puncture for RI can be made either above or below the cricoid cartilage. The CTM is relatively avascular and has less potential for bleeding (see “Anatomy”). The disadvantages of the CTM are that initially only 1 cm of ETT is actually placed below the vocal cords, and the angle of entry of the ETT into the trachea is more acute. An initial puncture performed at the cricotracheal ligament or lower affords the added advantage of allowing the ETT to travel in a straighter path as well as allowing a longer initial length of ETT below the vocal cords. The disadvantage is that this site (below the cricoid cartilage) has more potential for bleeding (although none has been reported). Both entry sites have been used successfully. In cadaver studies, the success rate for RI was higher with less vocal cord trauma when the cricotracheal ligament rather than the CTM was used. Vocal cord trauma has not been reported in living patients.78,79
B Classic Technique
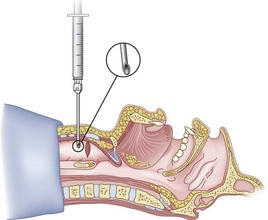
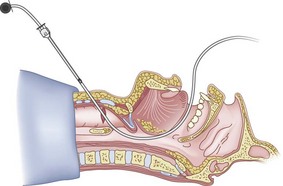
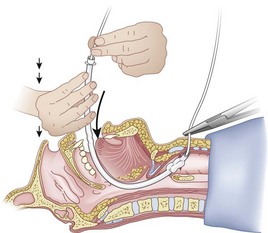
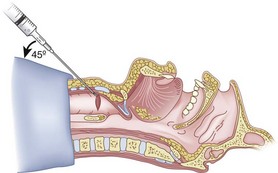
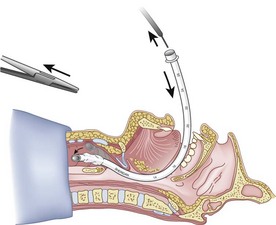
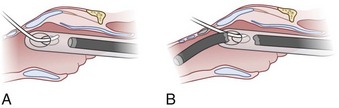
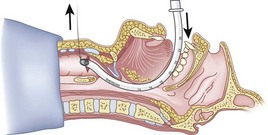
After positioning (Fig. 20-5), skin preparation, and anesthesia, a right hand–dominant person should stand on the right side of the supine patient. The left hand is used to stabilize the trachea by placing the thumb and third digit on either side of the thyroid cartilage. The index finger of the left hand is used to identify the midline of the CTM and the upper border of the cricoid cartilage.
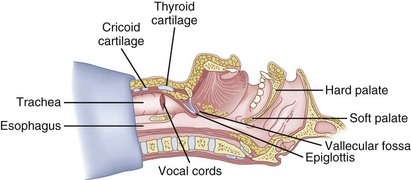
Figure 20-5 Classic technique. Midsagittal view of the head and neck.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
Because the Tuohy needle is blunt, a small incision through the skin and subcutaneous tissue with a no. 11 scalpel blade is recommended. Because of the significant force required to perforate the skin and the CTM, there is a risk of perforating the posterior tracheal wall as well. This has been verified in cadaver studies with the use of a fiberoptic bronchoscope (FOB).34
The right hand then grasps the Tuohy needle and saline syringe like a pencil (using the fifth digit to brace the right hand on the patient’s lower neck) and performs the puncture, aspirating to confirm placement in the lumen of the airway (Figs. 20-6 and 20-7).
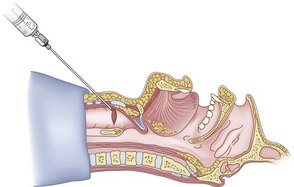
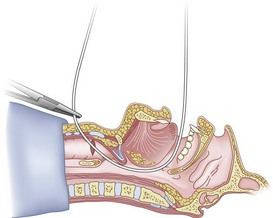
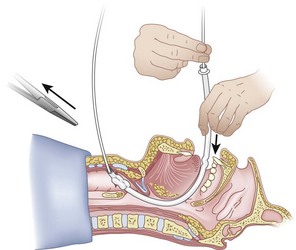
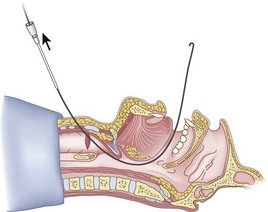
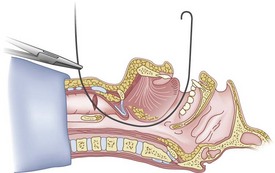
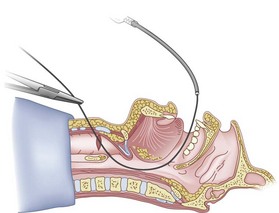
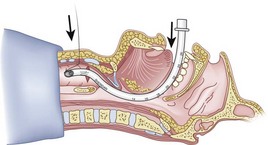
Once the Tuohy needle is in place, the epidural catheter is advanced into the trachea (Fig. 20-8). When advancing the epidural catheter, it is important to have the tongue pulled anteriorly to prevent the catheter from coiling up in the oropharynx. The catheter usually exits on its own from either the oral (Fig. 20-9) or the nasal cavity. A hemostat should then be clamped to the catheter at the neck skin line to prevent further movement of the epidural catheter. If the catheter has to be retrieved from the oropharynx, my preferred instrument is a nerve hook (V. Mueller NL2490, Baxter, Deerfield, IL). Magill forceps have been used, but these were designed to grasp large structures such as an ETT and may not grip the relatively small catheter (the distal tips of the forceps do not completely occlude); in addition, they may traumatize the pharynx. Arya and associates described an innovative atraumatic method of retrieving catheters from the oral pharynx in patients with limited mouth opening. They used a “pharyngeal loop” that they devised from a ureteral guidewire that was threaded through a 3-mm uncuffed polyvinyl chloride ETT and doubled up to form a loop.80
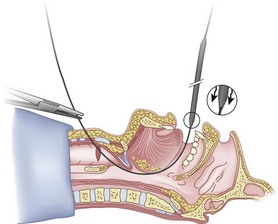
Originally, the catheter was threaded through the main distal lumen (beveled portion) of the ETT. Bourke and Levesque modified the technique by threading the catheter through the Murphy eye (Fig. 20-10), reasoning that this would allow an additional 1 cm of ETT to pass through the cords.81 Lleu and coworkers,78,79 in cadaver studies, showed that using the cricotracheal ligament as the puncture site in combination with threading the epidural catheter through the Murphy eye enhanced success compared with the original technique.
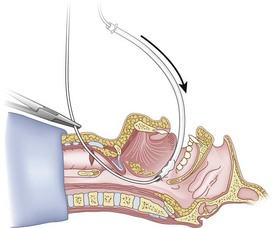
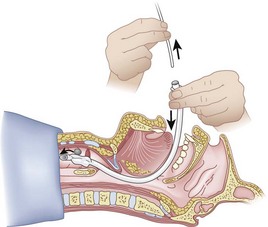
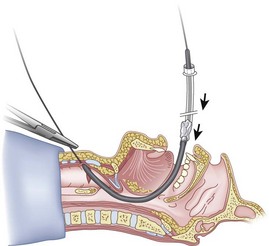
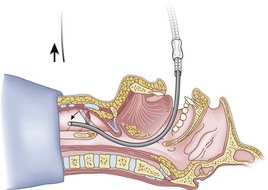
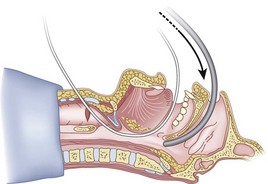
When the ETT is being advanced over the epidural catheter (Figs. 20-11 through 20-13), a moderate amount of tension should be employed.13,40 Excessive tension pulls the ETT anteriorly, making it more likely to be caught up against the epiglottis, vallecula, or anterior commissure of the vocal cords. If there is difficulty in passing the opening of the glottis, the ETT can be rotated 90 degrees counterclockwise or exchanged for a smaller tube.13,23,34
Ideally, one would like to verify that the ETT is below the vocal cords before removing the epidural catheter (Fig. 20-14; see Fig. 20-13). The methods of verification are the following:
1. By direct vision, using the FOB (see “Fiberoptic Technique”)
2. If the patient is breathing spontaneously, by listening to breath sounds through the ETT
3. By capnography, using a fiberoptic elbow adapter connected to a capnograph82
C Guidewire Technique
The modified technique using a guidewire was developed because the flexible epidural catheter is prone to kinking.4,7,13–15,19,23–25,31–33,38,39,59–63,72–74,84–91 Equipment consists of an 18-G angiocatheter, a J-tip guidewire with 0.038 inch outer diameter (OD) and 110 to 120 cm in length, and a guide catheter (Fig. 20-15).
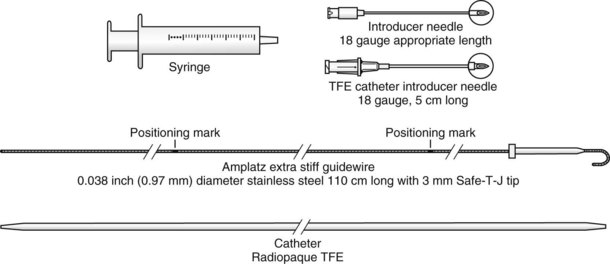
Figure 20-15 Guidewire technique. Retrograde kit TFE (Teflon guide catheter).
(From Cook Incorporated, Bloomington, IN.)
Use of a guidewire offers the following advantages:
1. The J tip tends to be less traumatic to the airway.24,39,74,87
2. Retrieval of the guidewire from the oral or nasal cavity is easier.33,74,87
3. The guidewire is less prone to kinking.92
4. The guidewire can be used with the FOB (see “Fiberoptic Technique”).
5. The guidewire is easy to handle.32,73,87
6. The technique takes less time to perform than the classic technique.27,33
Discrepancy between the external diameter of the guidewire and the internal diameter (ID) of the ETT allows a “railroading” effect to occur, with the tip of the ETT catching peripherally on the arytenoids or vocal cords instead of going straight through the cords. Sliding the guide catheter over the guidewire from above (antegrade) when it has exited the mouth or nose increases the external diameter of the guidewire,82 and use of the guide catheter in combination with a smaller-diameter ETT allows the ETT to enter the glottis in a more centralized position with respect to the glottic opening.
Various types of antegrade guide catheters have been used: FOBs (described in the next section), nasogastric tubes,34,93 suction catheters,34,94 plastic sheaths from Swan-Ganz catheters,88 Eschmann stylets,13 and tube changers.24,89 The Cook Critical Care retrograde guidewire kit (Cook Incorporated, Bloomington, IN) contains a tapered antegrade guide catheter, which is my choice of antegrade guide catheter (see Fig. 20-15) and is used here to describe the basic guidewire and antegrade guide catheter technique.
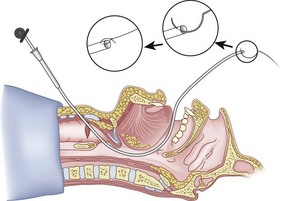
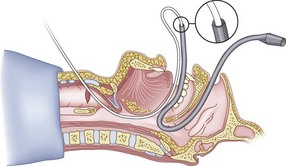
The guidewire and antegrade guide catheter technique is as follows. The trachea is identified (Figs. 20-16 to 20-18) with the syringe and 18-G angiocatheter. The J wire is then fed through the intratracheal catheter (Fig. 20-19) until it passes out the mouth (Fig. 20-20). The guidewire is clamped at the neck skin line, and the tapered antegrade guide catheter is fed over the guidewire (Fig. 20-21) until the antegrade guide catheter reaches the CTM (Fig. 20-22). The ETT is then fed over the antegrade guide catheter (Figs. 20-23 and 20-24) and the antegrade guide catheter is removed (Fig. 20-25). The standard Cook RI set has been modified to include a tapered antegrade guide catheter with distal sideports and Rapi-Fit adapters (Arndt Airway Exchange Catheter) to allow oxygenation and ventilation of the patient and to facilitate placement of an ETT.
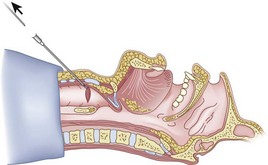
Figure 20-18 Guidewire technique. Advance the sheath of angiocatheter cephalad, and remove the needle.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
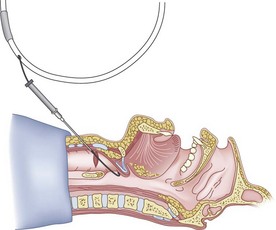
Figure 20-19 Guidewire technique. Advance the J-tip guidewire through the angiocatheter sheath.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
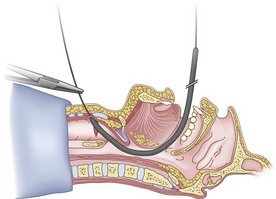
Figure 20-22 Guidewire technique. Advance the guide catheter to the cricothyroid membrane.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
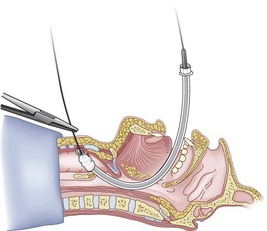
Figure 20-24 Guidewire technique. Advance the endotracheal tube through the vocal cords and up against the cricothyroid membrane.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
Three other modifications have been reported using guide catheters. First, the guidewire is removed when the guide catheter abuts against the CTM (see Fig. 20-21). The guide catheter alone is then advanced farther into the trachea and used as a stylet for the ETT.88,72,89 Second, an RI is performed with the bare guidewire (or epidural catheter) alone. When the ETT abuts the CTM, the guide catheter is advanced through the ETT from above (antegrade), passing distally to the carina.11 The guidewire is then removed, and the guide catheter is again used as a stylet for the ETT. In my opinion, the best results from a blind RI are obtained by using the cricotracheal ligament as a puncture site and the technique in which the guide catheter is passed over the guidewire. Third, an LMA has been used as conduit for the exit of the guidewire; a jet stylet is then placed over the guidewire, and both LMA and guidewire are removed, leaving the jet stylet as a guide catheter for the ETT. The benefits of this technique include a longer time for ventilation and the ability to place a larger ETT than the LMA would have accommodated.95
D Fiberoptic Technique
The FOB is a versatile tool for the anesthesiologist,54 but like RI, has its limitations. In some cases, the combination of two techniques, when previously either technique alone has failed, allows achievement of tracheal intubation.14,15,19,34,38,63,73,82 The combination of RI with direct laryngoscopy or RI using FOB can improve the chance of successful intubation.14,15,19,38,63,73 The advantages of passing an FOB antegrade over a guidewire placed by RI are as follows:
1. The OD of the guidewire and the ID of the suction port of the FOB form a tight fit that prevents railroading between the two cylinders, allowing the FOB to follow a straight path through the vocal cords without being caught on anatomic structures.
2. The FOB acts as a large antegrade guide catheter (see “Guidewire Technique”) and prevents railroading of the ETT.
3. When the FOB has passed over the wire through the vocal cords, it can be advanced freely beyond the puncture site to the carina, which eliminates the problem of distance between vocal cords and puncture site.
4. Use of the FOB allows placement of the ETT under direct vision.
5. The FOB can be used by less experienced operators.
6. Oxygen can be delivered continuously through the FOB with the guidewire still in place (see “Pediatrics”).
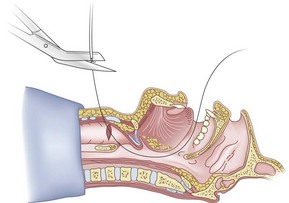
Preparation of the FOB should be completed before RI is initiated. The rubber casing from the proximal portion of the suction port must be removed (to allow the guidewire to exit from the FOB handle), and the FOB should be armed with the appropriate-sized ETT (6.5 to 7.0 mm ID). The RI is performed using the guidewire technique (Fig. 20-26), and the FOB is then passed antegrade over the guidewire like a guide catheter (Figs. 20-27 through 20-30). Once the tip of the FOB abuts the CTM (Fig. 20-31), there are the following options:
1. Option 1: Remove the guidewire distally (Fig. 20-32) or proximally (through the fiberoptic handle) and, after advancing under direct vision to the carina, intubate (Figs. 20-33 and 20-34). Removal of the guidewire distally is less likely to dislodge the FOB from the trachea, but anecdotal reports suggest that removal proximally should decrease the incidence of infection resulting from oral contaminants.71,96
2. Option 2: Instead of removing the guidewire, allow it to relax caudad into the trachea, advance the FOB below the cricoid cartilage, and then remove the guidewire. This allows a greater length of the FOB in the trachea before the guidewire is removed.
3. Option 3: Remove the guidewire proximally only until it is seen through the FOB to have popped out of the CTM and into the trachea; then advance the guidewire through the FOB to the carina. The FOB can then be advanced antegrade over the guidewire to the carina. (Caution: Be sure both ends of the guidewire are floppy.)
4. Option 4: Tobias used a fiberoptic technique that did not make use of the suction port of the FOB.19 A standard RI was performed, feeding the guidewire or epidural catheter first through the main lumen (bevel) of the ETT and immediately exiting out of the Murphy eye. The ETT was then advanced to the level of the CTM (Fig. 20-35). At this time, the FOB was advanced proximally through the ETT to the level of the carina. The retrograde guidewire was then removed, and a standard FOI was performed.
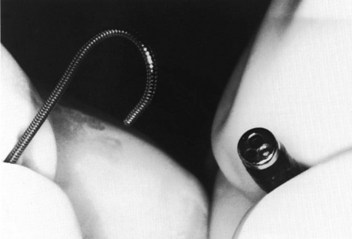
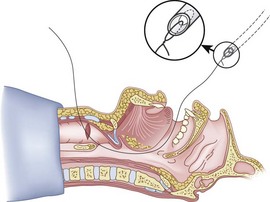
Figure 20-27 Fiberoptic technique. Close-up view of J tip of guidewire and distal tip of fiberoptic bronchoscope.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
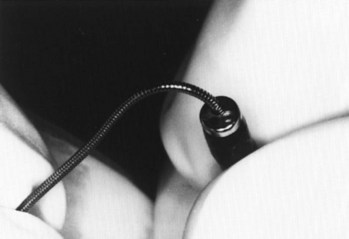
Figure 20-28 Fiberoptic technique. Close-up view of J tip being fed into suction port of fiberoptic bronchoscope.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)

Figure 20-29 Fiberoptic technique. J tip exiting from handle of fiberoptic bronchoscope.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
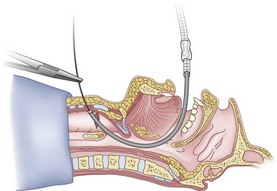
Figure 20-31 Fiberoptic technique. Advance fiberoptic bronchoscope to the cricothyroid membrane.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
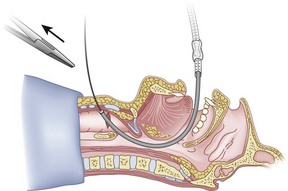
Figure 20-32 Fiberoptic technique. Have an assistant remove the hemostat (arrow).
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
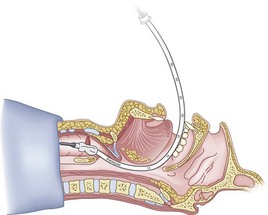
Figure 20-34 Fiberoptic technique. Continue as for a standard fiberoptic intubation.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
E Silk Pull-Through Technique
Various pull-through techniques have been described using epidural catheters,39,97 central venous pressure catheters,67 monofilament sutures,75 and Fogarty catheters98; the silk technique is also a pull-through technique.34,36,48 The basic principle involves advancing the epidural catheter retrograde as in the classic technique, attaching it to a length of silk, attaching the length of silk to the tip of the ETT, and then using the catheter-silk combination to pull the ETT into the trachea. The silk technique offers the following advantages:
1. The necessary equipment is readily available in the operating room.
2. The silk is intimately attached to the ETT, eliminating railroading.
3. Multiple attempts at intubation are allowed without having to repeat the procedure (CTM puncture) if it fails initially.
4. Oxygen can be delivered through the ETT using a standard anesthesia circle system, and in-line capnography can be used to verify placement of the ETT.
5. If necessary, postoperative reintubation can be accomplished using the silk, which is left in place until the time of discharge from the recovery room.
The silk technique employs the principles and equipment of the classic technique, with the addition of a length of silk suture (3-0 nylon monofilament may also be used). Once the epidural catheter (which is used only to place the silk) is out of the oral or nasal cavity, the silk suture is tied to the cephalad end of the catheter (Fig. 20-36), and the silk is pulled antegrade through the CTM (Fig. 20-37). The epidural catheter is cut off and discarded (see Fig. 20-37), and the cephalad end of the silk is tied to the Murphy eye (Fig. 20-38). The silk suture is then used to pull the ETT gently into the trachea (Fig. 20-39). If a floppy epiglottis causes obstruction, one can deliberately intubate the esophagus; as the ETT is being gently withdrawn from the esophagus, tension applied simultaneously to the distal end of the silk pops the tip of the ETT anteriorly, lifts up the epiglottis, and allows the ETT to enter the larynx. When the ETT abuts against the CTM, the tension on the silk suture is released and the ETT is passed further to enter the trachea (Fig. 20-40). If a nasal intubation is required, a urologic catheter can be used to cause the epidural catheter to exit the nasal rather than the oral cavity (Figs. 20-41 through 20-44).
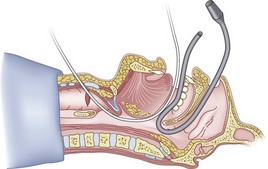
Figure 20-42 Silk pull-through technique. The tip of the urologic catheter is retrieved from the oral cavity.
(From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.)
VII Pediatrics
In pediatric patients, the physician is faced with the formidable problems of small anatomic structures that are difficult to palpate, immature anatomic structures such as anterior larynx and narrow cricoid cartilage, congenital anomalies, and pathologic disorders that intimately affect the airway (e.g., acute epiglottitis). Because the pediatric airway is different, concerns have been raised about whether RI is indicated or contraindicated in infants.69,70,74,99 Some have claimed that RI is dangerous but without citing any clinical supportive evidence.99 The number of articles and case reports in the medical literature on the subject of RI in the pediatric population is limited.2,10,11,16,20,34,38,40,69,70,73,74,81,99
RI has been used with both anticipated and unanticipated pediatric DA, primarily after failure of conventional intubating techniques (blind nasal intubation, direct laryngoscopy, or FOI). The technique used is the same as in the adult, but a higher incidence of difficulties has been reported, including problems in cannulating the ETT and inability to pass the ETT through the glottic opening.11,73,100
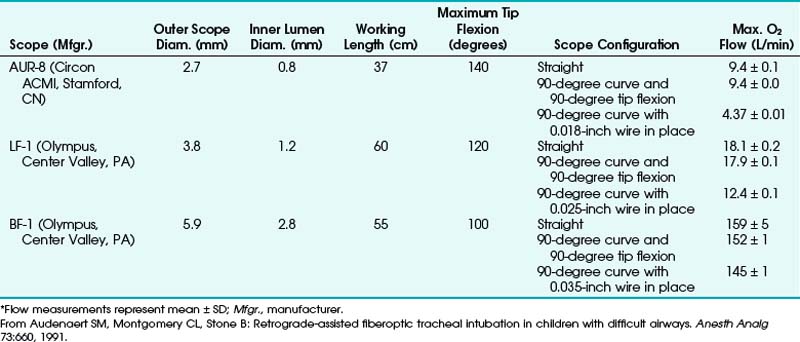
In some cases, combined techniques have offered more success than blind RI. In one case report of a 16-year-old patient with acute epiglottitis, intubation was accomplished only by RI combined with rigid laryngoscopy; the retrograde catheter marked a path through an otherwise completely distorted anatomy.38 The largest pediatric series with the highest success rate was reported by Audenaert and colleagues73; in that series, RI was performed for 20 pediatric patients, aged 1 day to 17 years, with DAs primarily resulting from congenital anomalies (Table 20-2). The authors’ preferred approach, a combination of FOI with retrograde guidewire, offers the following advantages:
3. Ability to insufflate oxygen through the suction port of the FOB with the guidewire in place (Table 20-3)
4. No hanging up of the ETT in the glottis
5. No need to rely on anatomic landmarks to guide the FOB into the trachea
TABLE 20-2 Summary of Clinical Approaches to Pediatric Patients with Airways Difficult to Manage Clinically*
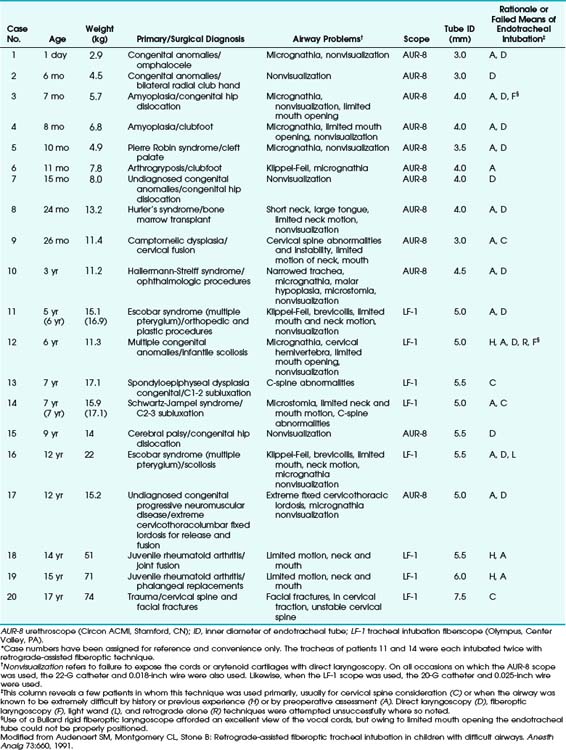
No major complications were reported, and the technique was considered a valuable addition to pediatric airway management.73
Przybylo and Stevenson described a unique method of managing the airway in a pediatric patient for closure of a tracheocutaneous fistula. The patient was a 4-year-old girl with severe micrognathia and limited temporomandibular joint motion and mouth opening who required a tracheostomy in the neonatal period for upper airway obstruction. Attempts at oral-nasal FOI were unsuccessful; the FOB was passed through the existing tracheocutaneous fistula in a cephalad direction, into the nasal cavity, and the FOB was then used as a stylet for nasal intubation.101
VIII Complications and Cautions
Although it has been demonstrated that transtracheal needle puncture is safe and associated with only minor complications (Box 20-4), numerous potential complications of RI have been cited in the literature (Box 20-5). Documented complications of RI are relatively few, and most were self-limited (Box 20-6). The most common complications were bleeding and subcutaneous emphysema.5,6,9,20,31,37,39,40,92
Box 20-5
Potential Complications of Retrograde Intubation
From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.
Box 20-6
Reported Complications of Retrograde Intubation
From Sanchez AF: The retrograde cookbook, Irvine, 1993, University of California, Department of Anesthesia.
* Refers to catheter traveling in a caudad direction toward the lungs instead of cephalad toward the oral cavity.
† Refers to Dr. Waters’ technique of retrieving catheter from nasopharynx using a self-made hook (Waters DJ: Guided blind endotracheal intubation: For patients with deformities of the upper airway. Anaesthesia 18:158, 1963).
A Bleeding
Insignificant bleeding (4 to 5 drops of blood) has been observed with CTM puncture during RI.40,92 Even a patient who had received heparin intraoperatively and had postoperative disseminated intravascular coagulation experienced only a small, self-limited hematoma after the procedure.9 Controversy exists with respect to making the puncture below the cricoid cartilage because of a greater potential for bleeding.2,39,79 Three studies involving 57 patients who underwent RI with punctures at the cricotracheal ligament or between the second and third tracheal rings showed no evidence of major bleeding.6,20,39 There are, however, scattered reports of severe hemoptysis after transtracheal needle puncture with resultant hypoxia, cardiorespiratory arrest, dysrhythmias, and death.41,44,102–104 Two patients had epistaxis after nasal intubation (no vasoconstricting agent was used).25,40 The following measures have been suggested to decrease the potential for bleeding:
B Subcutaneous Emphysema
Subcutaneous emphysema localized to the area of a transtracheal needle puncture site is common but self-limited.6,20,30,42,76,82,102–108 In severe cases, air may track through the fascial planes of the neck, leading to tracheal compression with airway compromise, pneumomediastinum, and pneumothorax.29,30,29,30,42,44,77,106,108,109 Accumulation of air occurs gradually (1 to 6 hours) after a transtracheal puncture.42,106 Severe subcutaneous emphysema has been attributed to use of a large-bore needle, multiple CTM punctures, and exposure of the puncture site to persistent elevated intratracheal pressure (coughing, grunting, or sneezing). In addition, pneumomediastinum has been reported in patients who underwent transtracheal puncture with a needle and was attributed to elevated endotracheal pressure (paroxysmal coughing and sneezing).41–43 When the patient has been intubated by the retrograde technique, elevated peak inspiratory pressure (PIP) and elevated end-expiratory pressure (PEEP) should not increase the likelihood of these complications intraoperatively, because the puncture site is located above the ETT cuff, so that the area of the initial puncture site is not exposed to high pressure. Lee and coauthors reported on a patient with a history of noncardiogenic pulmonary edema and atelectasis who, after RI, received 7.5 cm H2O with PEEP with no complications (PIP values were not reported).65
C Other Complications
Other reported self-limited complications were breath holding and travel of the catheter (a straight, flexible guidewire) caudally.20,40,84
Complications that were not self-limited were as follows:
1. Trigeminal nerve trauma,12 which the author suspected was due to multiple laryngoscopies
2. Guidewire fracture, in which wire had to be surgically removed64
3. Loss of hook (the type that was originally used by Waters and is no longer used)2,40,100
4. Pneumothorax, which necessitated use of a chest tube42
5. Pretracheal abscess in diabetic patients after multiple punctures at the CTM requiring incision and drainage71
IX Conclusions
International awareness (in multiple specialties) of the value of RI in selected clinical settings has increased.110,111 In my opinion, RI is a valuable additional airway management technique and should be included as part of the armamentarium of health care providers involved in the care of seriously injured or ill patients.
X Clinical Pearls
• Have the equipment readily available!
• Know your anatomy and practice daily: During your daily patient airway evaluation, make sure to palpate and note the location of the arch of the cricoid cartilage on every patient (important for RI and for emergency cricothyrotomy).
• Positioning of the patient and, more importantly, positioning of the anesthesiologist should be practiced on the mannequin until it becomes second nature.
• The initial puncture site through the cricothyroid membrane (CTM) should be as close to the upper border of the arch of the cricoid cartilage as possible. This allows for a longer length of the ETT to be below the vocal cords before removal of the guidewire (preventing the ETT from buckling backward into the esophagus).
• Use an ETT with smaller inner diameter (6.5 to 7.0 mm ID) to prevent “railroading.” I believe many of the failures are caused by the use of a large ETT that gets caught on the arytenoids (similar to the problem found during FOIs).
• Although it is more time consuming and more cumbersome to perform, the silk pull-through technique is my method of choice because it addresses most of the challenges encountered (railroading, potential need to perform multiple attempts, and ability to reintubate the patient postoperatively).
All references can be found online at expertconsult.com.
14 Gupta B, McDonald JS, Brooks HJ. Oral fiberoptic intubation over a retrograde guidewire. Anesth Analg. 1989;68:517.
27 Van Stralen DW, Rogers M, Perkin RM, et al. Retrograde intubation training using mannequin. Am J Emerg Med. 1995;13:50.
33 Stern Y, Spitzer T. Retrograde intubation of the trachea. J Laryngol Otol. 1991;105:746.
35 Shantha TR. Retrograde intubation [letter]. Br J Anaesth. 1983;55:855.
37 Guggenberger H, Lenz G, Heumann H. Success rate and complications of a modified guided blind technique for intubation in 36 patients. Anaesthesist. 1987;36:703.
52 Shantha TR. Retrograde intubation using the subcricoid region. Br J Anaesth. 1992;68:109.
73 Audenaert SM, Montgomery CL, Stone B. Retrograde-assisted fiberoptic tracheal intubation in children with difficult airways. Anesth Analg. 1991;73:660.
95 Arndt GA, Topp J, Hannah J, et al. Intubation via the LMA using a Cook retrograde intubation kit. Can J Anaesth. 1998;45:257.
132 Latto IP, Rosen M. Management of difficult intubation. In: Latto IP, Rosen M. Difficulties in tracheal intubation. Philadelphia: Baillière Tindall, 1984.
136 Linscott MS, Horton WC. Management of upper airway obstruction. Otolaryngol Clin North Am. 1979;12:351.
152 Sanchez AF. Retrograde intubation. Anesthesiol Clin North Am. 1995;13:439.
1 Butler FS, Cirillo AA. Retrograde tracheal intubation. Anesth Analg. 1960;39:333.
2 Waters DJ. Guided blind endotracheal intubation: For patients with deformities of the upper airway. Anaesthesia. 1963;18:158.
3 ASA Difficult Airway Task Force. Practice guidelines for management of the difficult airway. Anesthesiology. 1993;78:597.
4 King HK, Wang LF, Wooten DJ. Endotracheal intubation using translaryngeal guided intubation vs percutaneous retrograde guidewire insertion [letter]. Crit Care Med. 1987;15:183.
5 Barriot P, Riou B. Retrograde technique for tracheal intubation in trauma patients. Crit Care Med. 1988;16:712.
6 Mahiou P, Bouvet FR, Korach JM: Intubation retrograde [abstract R26]. Presented at the Proceedings of the Thirty-First Congress de Intubation Tracheal, Paris, July 14, 1983.
7 Alfery DD. Double-lumen endobronchial tube intubation using retrograde wire technique [letter]. Anesth Analg. 1993;76:1374.
8 Amanor-Boadu SD. Translaryngeal guided intubation in a patient with raised intracranial pressure. Afr J Med Med Sci. 1992;21:65.
9 Casthely PA, Landesman S, Fynaman PN. Retrograde intubation in patients undergoing open heart surgery. Can Anaesth Soc J. 1985;32:661.
10 Borland LM, Swan DV. Difficult pediatric endotracheal intubation: A new approach to the retrograde technique. Anesthesiology. 1981;55:577.
11 Cooper CMS, Murray-Wilson A. Retrograde intubation: Management of a 4.8 kg. 5-month infant. Anaesthesia. 1988;42:1197.
12 Faithfull NS. Injury to terminal branches of the trigeminal nerve following tracheal intubation. Br J Anaesth. 1985;57:535.
13 Freund PR, Rooke A, Schwid H. Retrograde intubation with a modified Eschmann stylet [letter]. Anesth Analg. 1988;67:596.
14 Gupta B, McDonald JS, Brooks HJ. Oral fiberoptic intubation over a retrograde guidewire. Anesth Analg. 1989;68:517.
15 Lechman MJ, Donahoo JS, Macvaugh H. Endotracheal intubation using percutaneous retrograde guidewire insertion followed by antegrade fiberoptic bronchoscopy. Crit Care Med. 1986;14:589.
16 Manchester GH, Mani MM, Master FW. A simple method for emergency orotracheal intubation. Plast Reconstr Surg. 1972;49:312.
17 Pintanel T, Font M, Aguilar JL, et al. Intubation orotracheal retrograde [cartas al director]. Rev Esp Anestesiol Reanim. 1988;35:344.
18 Rossini L. The tunneling technique: An approach to difficult intubations. J Am Assoc Nurse Anesth. 1984;52:189.
19 Tobias R. Increased success with retrograde guide for endotracheal intubation [letter]. Anesth Analg. 1983;62:366.
20 VanNiekerk JV, Smalhout B. Retrograde endotracheal intubation using a catheter. Ned Tijdschr Geneeskd. 1987;131:1663.
21 Dennison PH. Four experiences in intubation of one patient with Still’s disease. Br J Anaesth. 1978;50:636.
22 Bissinger U, Guggenberger H, Lenz G. A safer approach to retrograde-guided fiberoptic intubation. Anesth Analg. 1996;82:1108.
23 Heller EM, Schneider K, Saven B. Percutaneous retrograde intubation. Laryngoscope. 1989;99:555.
24 Hines MH, Meredith JW. Modified retrograde intubation technique for rapid airway access. Am J Surg. 1990;159:597.
25 Corleta O, Habazettl H, Kreimeier U. Modified retrograde orotracheal intubation technique for airway access in rabbits. Eur Surg Res. 1992;24:129.
26 Sanchez AF: Retrograde intubation in the llama. Presented at the American Association of Zoological Veterinarians annual meeting, St. Louis, November 3, 1993.
27 Van Stralen DW, Rogers M, Perkin RM, et al. Retrograde intubation training using mannequin. Am J Emerg Med. 1995;13:50.
28 Slots P, Vegger P, Bettger H, et al. Retrograde intubation with a Mini-Trach II kit. Acta Anaesth Scand. 2003;47:274.
29 Ovassapian A. Fiberoptic tracheal intubation. Ovassapian A, ed. Fiberoptic airway endoscopy in anesthesia and critical care, ed 3, New York: Raven Press, 1990.
30 Ovassapian A. Topical anesthesia. In: Ovassapian A, ed. Fiberoptic airway endoscopy in anesthesia and critical care. New York: Raven Press, 1990.
31 Guggenberger H, Lenz G. Training in retrograde intubation [letter]. Anesthesiology. 1980;69:292.
32 McNamara RM. Retrograde intubation of the trachea. Ann Emerg Med. 1987;16:680.
33 Stern Y, Spitzer T. Retrograde intubation of the trachea. J Laryngol Otol. 1991;105:746.
34 Sanchez AF: The retrograde cookbook. Presented at the First International Symposium on the Difficult Airway, Newport Beach, California, June 6, 1993.
35 Shantha TR. Retrograde intubation [letter]. Br J Anaesth. 1983;55:855.
36 Sanchez AF: Preventing the difficult from becoming the impossible airway: Retrograde intubation. Presented at the annual meeting of the American Society of Anesthesiologists, Las Vegas, Nevada, October 16, 1990.
37 Guggenberger H, Lenz G, Heumann H. Success rate and complications of a modified guided blind technique for intubation in 36 patients. Anaesthesist. 1987;36:703.
38 Heslet L, Christensen KS, Sanchez R, et al. Facilitated blind intubation using a transtracheal guide wire. Dan Med Bull. 1985;32:275.
39 Abou-Madi MN, Trop D. Pulling versus guiding: A modification of retrograde guided intubation. Can J Anaesth. 1989;36:336.
40 Akinyemi OO. Complications of guided blind endotracheal intubation. Anaesthesia. 1979;34:590.
41 Kalinske RW, Parker RH, Brandt D. Diagnostic usefulness and safety of transtracheal aspiration. N Engl J Med. 1967;276:604.
42 Poon YK. Case history number 89: A life-threatening complication of cricothyroid membrane puncture. Anesth Analg. 1976;55:298.
43 Ries K, Levison ME, Kaye D, et al. Transtracheal aspiration in pulmonary infection. Arch Intern Med. 1974;133:453.
44 Unger KM, Moser KM. Fatal complication of transtracheal aspiration: A report of two cases. Arch Intern Med. 1973;132:437.
45 Deresinski SC, Stevens DA. Anterior cervical infections: Complications of transtracheal aspirations. Am Rev Respir Dis. 1974;110:354.
46 Green DC, Strait GB. A complication of transtracheal anesthesia: Nocardia cellulitis. Ann Thorac Surg. 1969;8:561.
47 Yoshikawa TT, Chow AW, Montgomerie JZ, et al. Paratracheal abscess: An unusual complication of transtracheal aspiration. Chest. 1974;65:105.
48 Sanchez AF. ASA airway safety video: II. Cricothyroid membrane. Park Ridge, IL: American Society of Anesthesiologists; 1992.
49 Caparosa RJ, Zavatsky AR. Practical aspects of the cricothyroid space. Laryngoscope. 1957;67:577.
50 Clemente C, ed. Gray’s anatomy, ed 13, Philadelphia: Lea & Febiger, 1985.
51 Kress TD, Balasubramaniam S. Cricothyroidotomy. Ann Emerg Med. 1982;11:197.
52 Shantha TR. Retrograde intubation using the subcricoid region. Br J Anaesth. 1992;68:109.
53 Naumann H. Head and neck surgery. Philadelphia: WB Saunders; 1984.
54 Anson BJ. Morris human anatomy: A complete system treatise, ed 12. New York: McGraw-Hill; 1966.
55 Bergman R, Thompson S, Afifi AK, et al. Compendium of human anatomic variation: Text, atlas, and world literature. Baltimore: Urban & Schwarzenberg; 1988.
56 Barash PG, Cullen BF, Stoelting RK. Clinical anesthesia. Philadelphia: JB Lippincott; 1989.
57 Cuchiara RF, Black S, Steinkeler JA. Anesthesia for intracranial procedures. In: Barash PG, Cullen BF, Stoelting RK. Clinical anesthesia. Philadelphia: JB Lippincott; 1989:652.
58 Kaplan JA. Postoperative respiratory care. In: Kaplan JA, ed. Thoracic anesthesia. New York: Churchill Livingstone, 1983.
59 Miller RD. Anesthesia. vol 2. ed 2. New York: Churchill Livingstone; 1990.
60 Shapiro HM, Drummond JC. Neurosurgical anesthesia and intracranial hypertension. ed 3. Miller RD, ed. Anesthesia. New York: Churchill Livingstone; 1990;vol 2:854.
61 Stone DJ, Gal TJ. Airway management. ed 3. Miller RD, ed. Anesthesia. New York: Churchill Livingstone; 1990;vol 2:1105.
62 Wedel DJ, Brown DL. Nerve blocks. ed 3. Miller RD, ed. Anesthesia. New York: Churchill Livingstone; 1990;vol 2:235.
63 Carlson CA, Perkins HM. Solving a difficult intubation. Anesthesiology. 1986;64:537.
64 Contrucci RB, Gottlieb JS. A complication of retrograde endotracheal intubation. Ear Nose Throat J. 1989;69:776.
65 Lee YW, Lee YS, Kim JR. Retrograde tracheal intubation. Yonsei Med J. 1987;28:228.
66 Maestro CM, Andujar MJJ, Sancho CJ, et al. Intubacion retrograda en un paciente con una malformacion epiglotica [cartas al director]. Rev Esp Anestesiol Reanim. 1988;35:344.
67 Raza S, Levinsky L, Lajos TZ. Transtracheal intubation: Useful adjunct in cardiac surgical anesthesia. J Thorac Cardiovasc Surg. 1978;76:721.
68 Schmidt SI, Hasewinkel JV. Retrograde catheter-guided direct laryngoscopy. Anesthesiol Rev. 1989;16:6.
69 France NK, Beste DJ. Anesthesia for pediatric ear, nose and throat surgery. In: Gregory GA, ed. Pediatric anesthesia. ed 2. New York: Churchill Livingstone; 1989:169.
70 Gregory GA. Induction of anesthesia. Gregory GA, ed. Pediatric anesthesia, ed 2, New York: Churchill Livingstone, 1989.
71 Beebe DS, Tran P, Belani KG, Adams GL. Pretracheal abscess following retrograde tracheal intubation. Anaesthesia. 1995;50:470.
72 King HK, Wang LF, Khan AK. Translaryngeal guided intubation for difficult intubation. Crit Care Med. 1987;15:869.
73 Audenaert SM, Montgomery CL, Stone B. Retrograde-assisted fiberoptic tracheal intubation in children with difficult airways. Anesth Analg. 1991;73:660.
74 Dailey RD, Simon B. Retrograde tracheal intubation. In: Purcell T, ed. The airway emergency management. St. Louis: Mosby; 1992:84.
75 Harrison CA, Wise CC. Retrograde intubation [letter]. Anaesthesia. 1988;43:609.
76 Newman J, Schultz S, Langevin RE. Bronchography by cricothyroid catheterization. Laryngoscope. 1965;75:774.
77 Willson JKV. Cricothyroid bronchography with a polyethylene catheter: Description of a new technique. AJR Am J Roentgenol. 1959;81:305.
78 Lleu JC, Forrler M, Forrler C, et al. L’intubation oro-trachéale par void rétrograde. Ann Fr Anesth Reanim. 1989;8:632.
79 Lleu JC, Forrler M, Pottecher T. Retrograde intubation using the subcricoid region [letter]. Br J Anaesth. 1983;55:855.
80 Arya VK, Dutta A, Chari P, et al. Difficult retrograde endotracheal intubation: The utility of a pharyngeal loop. Anesth Analg. 2002;92:470.
81 Bourke D, Levesque PR. Modification of retrograde guide for endotracheal intubation. Anesth Analg. 1974;53:1013.
82 Benumof JL. Management of the difficult adult airway. Anesthesiology. 1991;75:1087.
83 Hung OR, al-Qatari M. Light-guided retrograde intubation. Can J Anaesth. 1997;44:877.
84 Criado A, Planas A. Intubacion orotraqueal retrograda [cartas al director]. Rev Esp Anestesiol Reanim. 1988;35:344.
85 Dhara SS. Retrograde intubation: A facilitated approach. Br J Anaesth. 1992;69:631.
86 Gerenstein RI. J-wire facilitates translaryngeal guided intubation [letter]. Anesthesiology. 1992;76:1059.
87 Gerenstein RI, Arria-Devoe G. J-wire and translaryngeal guided intubation [letter]. Crit Care Med. 1989;17:486.
88 King HK. Translaryngeal guided intubation using a sheath stylet. Anesthesiology. 1985;63:567.
89 King KK, Wang LF, Khan AK, Wooten DJ. Antegrade vs retrograde insertion introducer for guided intubation in needle laryngostomized patient. Can J Anaesth. 1989;36:252.
90 Roberts KW. New use for Swan-Ganz introducer wire [letter]. Anesth Analg. 1981;60:67.
91 Yealy DM, Paris PM. Recent advances in airway management. Emerg Med Clin North Am. 1989;7:83.
92 Powell WF, Ozdil T. A translaryngeal guide for tracheal intubation. Anesth Analg. 1967;46:231.
93 Luhrs R, Fuller E. A case study: The use of trans-tracheal guide for a patient with a large protruding oral myxoma. J Am Assoc Nurse Anesth. 1987;55:81.
94 Harmer M, Vaughan R. Guided blind oral intubation [letter]. Anaesthesia. 1986;35:921.
95 Arndt GA, Topp J, Hannah J, et al. Intubation via the LMA using a Cook retrograde intubation kit. Can J Anaesth. 1998;45:257.
96 Rizzi F, Ambroselli V, Mezzetti MG. Sull’impiego dell’intubazione retrograda in emergenza. Minerva Anestesiol. 1991;57:1705.
97 Kubo K, Takahashi S, Oka M. A modified technique of guided blind intubation in oral surgery. J Maxillofac Surg. 1980;8:135.
98 Carlson RR, Sadove MS. Guided non-visualized nasal endotracheal intubation using a transtracheal Fogarty catheter. Ill Med J. 1973;143:364.
99 Levin RM. Anesthesia for cleft lip and cleft palate. Anesthesiol Rev. 1979;6:25.
100 Akinyemi OO, John A. A complication of guided blind intubation. Anaesthesia. 1974;29:733.
101 Przybylo HJ, Stevenson GW. Retrograde fibreoptic intubation in a child with Nager’s syndrome. Can J Anaesth. 1996;43:697.
102 Hemley SD, Arida EJ, Diggs AM, et al. Percutaneous cricothyroid membrane bronchography. Radiology. 1961;76:763.
103 Schillaci RF, Iacovoni VE, Conte RS, et al. Transtracheal aspiration complicated by fatal endotracheal hemorrhage. N Engl J Med. 1976;295:488.
104 Spencer CD, Beaty HN. Complications of transtracheal aspiration. N Engl J Med. 1972;286:304.
105 Hahn HH, Beaty HN. Transtracheal aspiration in the evaluation of patients with pneumonia. Ann Intern Med. 1970;72:183.
106 Massey JY. Complications of transtracheal aspiration: A case report. J Ark Med Soc. 1971;67:254.
107 Radigan LR, King RD. A technique for the prevention of postoperative atelectasis. Surgery. 1960;47:184.
108 Won KH, Rowland DW, Croteau JR, et al. Massive subcutaneous emphysema complicating transcricothyroid bronchography. Am J Roentgenol Radium Ther Nucl Med. 1967;101:953.
109 van der Laan KT, Ballast B, Wouters B, van Overbeek JJ. Retrograde endotracheale intubatie met behulp van een catheter. Ned Tijdschr Geneeskd. 1987;131:2324.
110 Levitan RM, Kush S, Hollander JE. Devices for difficult airway management in academic emergency departments: Results of a national survey. Ann Emerg Med. 1999;33:694.
111 Morton T, Brady S, Clancy M. Difficult airway equipment in English emergency departments. Anaesthesia. 2000;55:485.
112 Benumof JL. Retrograde intubation [letter]. Anesthesiology. 1992;76:1060.
113 Bissinger U, Guggenberger H, Lenz G. Retrograde-guided fiberoptic intubation in patients with laryngeal carcinoma. Anesth Analg. 1995;81:408.
114 Bowes WA, 3rd., Johnson JO. Pneumomediastinum after planned retrograde fiberoptic intubation. Anesth Analg. 1994;78:795.
115 Cossham PS. Difficult intubation. Br J Anaesth. 1985;57:239.
116 Dhara SS. Guided blind endotracheal intubation [letter]. Anaesthesia. 1980;35:81.
117 Diaz J. The difficult intubation kit. Anesth Rev. 1990;17:49.
118 Dubey PK, Kumar A. A device for cricothyrotomy and retrograde intubation. Anaesthesia. 2000;55:702.
119 Eidelman L, Pizov R. A safer approach to retrograde-guided fiberoptic intubation. Anesth Analg. 1996;82:1108.
120 Faithfull NS. Retrograde intubation. Summary of papers presented at the sixth European Congress. Anaesthesia. 1982;1(Suppl 22):458.
121 Faithfull NS, Erdmann W, Groenland THN. Alternatieve intubatie routes. Ned Tijdschr Anaesth Medewerkers. 1985;25:8.
122 Finucane BT, Santora AH. Principles of airway management. Philadelphia: FA Davis; 1988.
123 Gordon RA. Anesthetic management of patients with airway problems. Int Anesthesiol Clin. 1972;10:37.
124 Graham WP, III., Kilgore ES, III. Endotracheal intubation in complicated cases. Hosp Physicians. 1987;3:60.
125 Greaves JD. Endotracheal intubation in Still’s disease. Br J Anaesth. 1979;51:75.
126 Harvey SC, Fishman RL, Edwards SM. Retrograde intubation through a laryngeal mask airway. Anesthesiology. 1996;85:1503.
127 Jagtap SR, Malde AD, Pantvaidya SH. Anaesthetic considerations in a patient with Fraser syndrome. Anaesthesia. 1995;50:39.
128 King HK. Translaryngeal guided intubation [letter]. Anesth Analg. 1985;64:650.
129 King HK. Translaryngeal guided intubation: A lifesaving technique. A review and case report. Anesth Sin. 1984;22:279.
130 King HK, Khan AK, Wooten DJ. Translaryngeal guided intubation solved a critical airway problem. J Clin Anesth. 1988;1:112.
131 King KH, Wang LF, Khan AK. Soft and firm introducers for translaryngeal guided intubation [letter]. Anesth Analg. 1989;68:826.
132 Latto IP, Rosen M. Management of difficult intubation. In: Latto IP, Rosen M. Difficulties in tracheal intubation. Philadelphia: Baillière Tindall, 1984.
133 Lau HP, Yip KM, Liu CC. Rapid airway access by modified retrograde intubation. J Formos Med Assoc. 1996;95:347.
134 Layman PR. An alternative to blind intubation. Anaesthesia. 1983;38:165.
135 Lin BC, Chen IH. Anesthesia for ankylosing spondylitis patients undergoing transpedicle vertebrectomy. Acta Anaesthesiol Sin. 1999;37:73.
136 Linscott MS, Horton WC. Management of upper airway obstruction. Otolaryngol Clin North Am. 1979;12:351.
137 Lopez G, James NR. Mechanical problems of the airway. J Clin Anesth. 1984;36:118.
138 Mahajan R, Sandhya X, Chari P. An alternative technique for retrograde intubation. Anaesthesia. 2001:56.
139 Matot I, Hevron I, Katzenelson R. Dental mirror for difficult nasotracheal intubation. Anaesthesia. 1997;52:780.
140 Mclean D. Guided blind oral intubation [letter]. Anaesthesia. 1982;37:605.
141 Miller RD. Endotracheal intubation. Miller RD, ed. Anesthesia, ed 3, New York: Churchill Livingstone, 1986.
142 Morais RJ, Kotsev SN, Hana SJ. Modified retrograde intubation in a patient with difficult airway. Saudi Med J. 2000;21:490.
143 Parmet JL, Colonna-Romano P, Horrow JC, et al. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg. 1998;87:661.
144 Payne KA. Difficult tracheal intubation. Anaesth Intensive Care. 1980;8:84.
145 Poradowska-Jeszke M, Falkiewicz H. Intubation rétrograde chez un nourrisson atteint de maladie de Pierre Robin. Cah Anesthesiol. 1989;37:605.
146 Ramsay MAE, Salyer KE. The management of a child with a major airway abnormality. Plast Reconstr Surg. 1981;67:668.
147 Reynaud J, Lacour M, Diop L, et al. Intubation tracheale guidée a bouche fermée (technic de D.J. Waters). Bull Soc Med Afr Noire Lang Fr. 1967;12:774.
148 Riou B. Intubation difficile. In: Société Francaise d’Anesthésie Réanimation: Conference d’actualisation, July 3, 1990. Paris: Masson; 1990.
149 Roberts JR. Clinical procedures in emergency medicine. Philadelphia: WB Saunders; 1985.
150 Roberts KW, Solgonick RM. A modification of retrograde wire-guided, fiberoptic-assisted endotracheal intubation in a patient with ankylosing spondylitis. Anesth Analg. 1996;82:1290.
151 Salem MR, Mathrubhutham M, Bennett EJ, et al. Difficult intubation. N Engl J Med. 1976;295:879.
152 Sanchez AF. Retrograde intubation. Anesthesiol Clin North Am. 1995;13:439.
153 Scurr C. A complication of guided blind intubation [letter]. Anaesthesia. 1975;30:411.
154 Seavello J, Hammer GB. Tracheal intubation in a child with trismus pseudocamptodactyly (Hecht) syndrome. J Clin Anesth. 1999;11:254.
155 Sellers W. Finding a use for the lumen in the Portex Tracheal Tube Guide. Anaesthesia. 2003;58:190.
156 Stehling SC. Management of the airway. In: Barash PG, Cullen BF, Stoelting RK. Clinical anesthesia. Philadelphia: JB Lippincott; 1989:652.
157 Stordahl A, Syrovy G. Blind endotracheal intubation over retrograde. Tidsskr Nor Laegeforen. 1986;106:1590.
158 Talyshkhanov KK. Retrograde intubation of the trachea. Anesteziol Reanimatol. 1992;5–6:58–59.
159 Van Stralen D, Perkin RM. Retrograde intubation difficulty in an 18-year-old muscular dystrophy patient. Am J Emerg Med. 1995;13:100.
160 Ward CF, Salvatierra A. Special intubation techniques for the adult patient. In: Benumof JL, ed. Clinical procedures in anesthesia and intensive care. Philadelphia: JB Lippincott; 1992:156.
161 Wijesinghe HS, Gough JE. Complications of a retrograde intubation in a trauma patient. Acad Emerg Med. 2000;7:1267.
162 Williams JB, Sahni R. Performance of retrograde intubation in a multiple-trauma patient. Prehosp Emerg Care. 2001;5:49.
163 Williamson R. Pediatric intubation: Retrograde or blind [letter]? Anaesthesia. 1988;42:802.
164 Yoneda I, Nakamura M, Satoh T. A simple method of retrograde intubation. Masui (Jpn J Anesthesiol). 1991;40:124.
165 Yonfa AE, Waite PD, Ballard JB, et al. Retrograde approach to nasotracheal in a child with severe Pierre Robin syndrome. Anesthesiol Rev. 1983;10:28.