Chapter 114 Retinopathy of Prematurity
Introduction
Retinopathy of prematurity (ROP) affects thousands of children each year. Fortunately, ROP causes blindness in only a small percentage. ROP was previously called “retrolental fibroplasia.” The original work in the 1950s of Kinsey et al.1 and Patz and Kinsey2 showed that oxygen contributes to the tissue change described as ROP. It was believed that if the arterial oxygen was kept within prescribed guidelines, ROP might be eliminated. However, with the ability of neonatologists to keep alive newborns of very low birth weight and very young gestational age, there has been a resurgence of ROP despite tight control of the partial pressure of oxygen.
Oxygen’s role in ROP has come under extensive investigation. Many workers have shown in animal models, that immature vessels characteristic of ROP can be produced but not retinal detachment.3–6 Clinical observations by many investigators have shown that ROP can be seen in 85–90% of children of low birth weight when exposed to oxygen.7 Similar clinical pictures have been seen in full-term infants, in familial exudative vitreoretinopathy, and in infants of mothers who are cocaine users.8,9
Recently, the role of vascular endothelial growth factor (VEGF) in ROP has been more appreciated. VEGF under control of oxygen concentration or retinal ischemia can overcome the genetic mandate to form normal retinal vasculature and instead contribute to neovascularization or development of abnormal intraretinal vascular patterns in the retinal periphery.10
Classification system
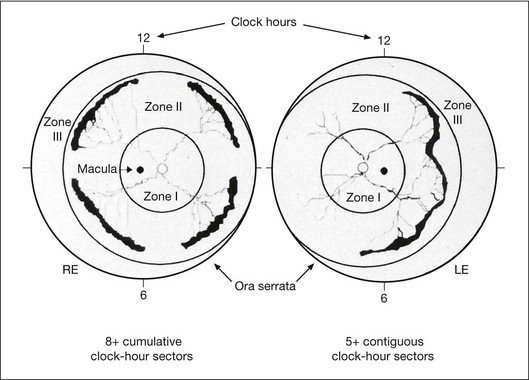
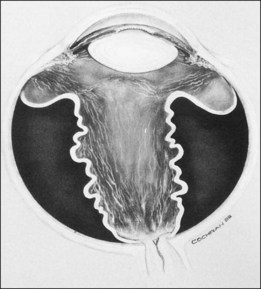
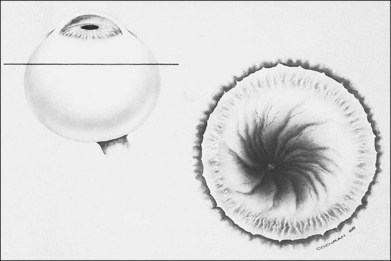
In the past, there were several classifications of ROP, which led to much confusion between ophthalmologists, neonatologists, and pediatricians. However, an International Classification of Retinopathy of Prematurity (ICROP) has now been adopted. It describes three zones of the eye. Zone I uses the optic nerve as the center of a circle, and the radius is defined as twice the distance between the foveola and the optic nerve. Zone II uses as a radius, the distance between the nasal ora serrata in the horizontal meridian and the center of the optic nerve. All of the remaining retina is zone III (Fig. 114.1); that is, zone III is present in all meridians except at the nasal horizontal meridian.
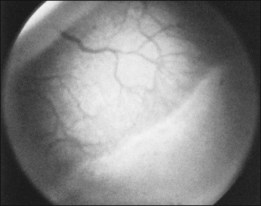
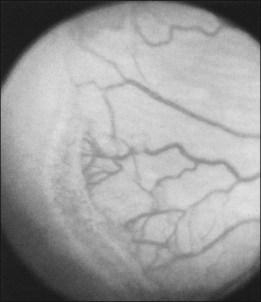
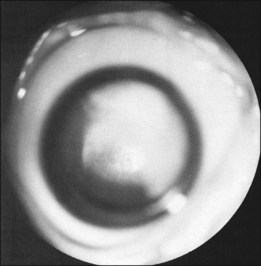
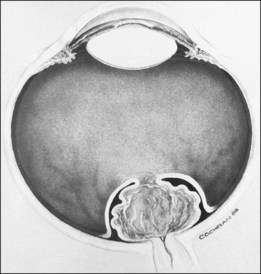
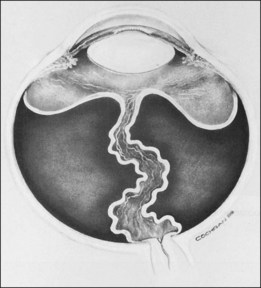
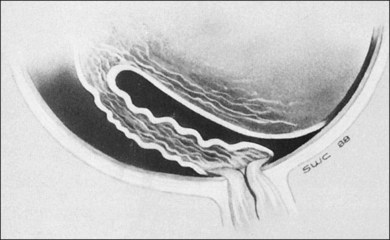
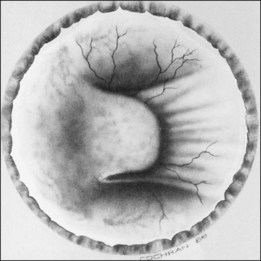
The ICROP defines five stages of ROP. The process of ROP begins at the junction between the vascular and avascular retina. ROP is described as stage 1 if a narrow white line is present at the junction. Stage 2 is a ridge of activity that shows thickening of this line (Fig. 114.2). In addition to this thickening, there is sometimes a ruddy appearance of a shunt within this ridge. Stage 3 involves the growth of vessels from the retina toward the vitreous cavity immediately posterior to and contiguous with the ridge (Fig. 114.3). Stage 4 is a partial retinal detachment and is subclassified as 4A, with the macula attached, and 4B, with the macula detached. Stage 5 implies a total detachment of the vascularized retina (Fig. 114.4) and can be classified further depending on the opening or closure of the anterior and posterior aspects. Even with significant retinal detachment causing a white retrolenticular appearance, the very far peripheral avascular retina often remains attached (Fig. 114.5). The avascular peripheral retina, however, may not be visually functional.
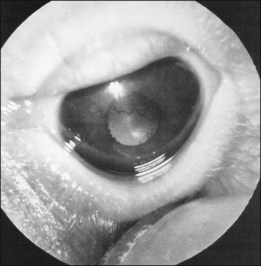
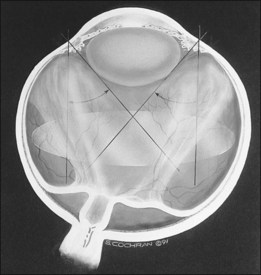
Finally, the ICROP addresses the problem of “plus disease.” It is not a disease different from ROP but is a descriptive term for dilated and tortuous vessels of the posterior pole. In addition, the anterior segment in plus disease often shows dilated iris vessels (Fig. 114.6). These iris vessels may not be true neovascularization of the anterior segment but may represent dilation of an existing tunica vasculosa lentis.11–13 This anterior segment vessel dilation appears to be a manifestation of a generalized intraocular increased VEGF concentration.
• First, ROP presenting completely in zone 1 or posterior zone 2 as mentioned above usually has a more aggressive course and this type of ROP may require more aggressive ablative treatment.
• Second, the stage 3 neovascularization of ROP may appear differently depending on the zone of involvement: (1) mid and anterior zone 2 and zone 3 neovascularization is always posterior to the ridge tissue and growing into the vitreous cavity and (2) zone 1 and posterior zone 2 can be flat, lying along the retinal surface, without the typical ridge tissue features.
• Third, plus disease is a function of an open shunt vessel contained deep within the ridge tissue. As this arteriovenous shunt is established, the vessels become dilated in the periphery. With the advent of wide-angle photography, this image can be easily appreciated. Although “plus disease” is a posterior pole finding and should remain so, the dilated peripheral vessels have been referred to as “pre-plus” and suggests that the eye is at higher risk of developing frank “plus disease.”
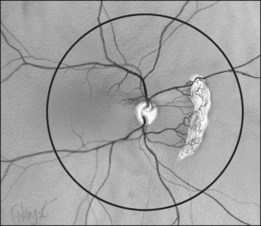
In order to try to identify eyes at high risk of progression, the authors have adopted a modification of the ICROP system. Namely, we designate the number of clock hours of vessels that are entirely in zone 1 by a subscript. For example, if all 12 clock hours are in zone 1 that would be written zone 112. Additionally, if flat neovascularization is present, we use another subscript, “F.” Stage 3F denotes flat neovascularization is present. Since these vessels lie flat on the retina and appear anterior to the shunt vessels, the drawing of the findings show the extent of the clock hours of the flat stage 3 (Fig. 114.7).
Histophysiologic features, clinically relevant cell biology, and pathophysiology
Stages 1 and 2
Much work has been done to promote the understanding of the early changes at the junction of the vascular and avascular retina. Foos8 speaks of a vanguard and rearguard of cells that contribute to the tissue changes.3,11 These cells have been infrequently studied in their active form because most of these neonates survive and the eyes do not become available for study. Other authors13 have described the cells in this region as “spindle shaped.” These spindle-shaped cells are found in many proliferative processes, and this descriptive term does not help define the cell biology. Histologic features of the avascular and vascularized retina reveal a difference in the retina’s thickness, with the posterior vascular retina being somewhat thicker than the more peripheral avascular retina.
Terry14 was perhaps the first to note vitreous abnormalities in children with ROP. The vitreous, which is normally firm and dense in the newborn, is synergetic and organized into fluid lacuna and sheets of collagen in ROP. This vitreous abnormality seems to be present in children with lesser stages of ROP and certainly is present in children with more advanced stages. The significance of these vitreous abnormalities may relate to both development of exudative and tractional detachments and to development of late rhegmatogenous retinal detachments. ROP should be thought of as a vascular vitreoretinopathy. Stage 1 ROP is the visible white line that separates the vascular from the avascular retina. In stage 2 that line widens and may become a salmon color as the shunt vessel opens.
Stage 3
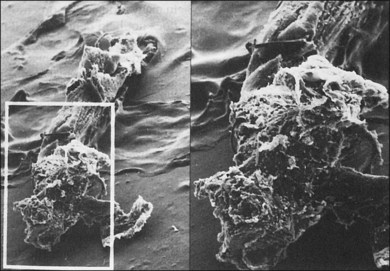
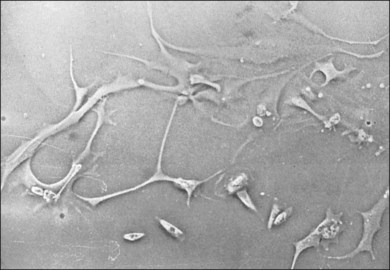
The classical concept of neovascularization in ROP is that a retinal vasoconstriction secondary to oxygen administration or an increase in oxygen concentration as seen with birth alone is followed by vasodilation and associated vascular budding after oxygen withdrawal. However, some infants show significant vasodilation or plus disease even while receiving supplemental oxygen. No matter what the mechanism, the neovascularization that develops in stage 3 ROP has interesting features. One is that the active vessels are usually present at the posterior ridge of tissue. Also, there is often a directional orientation of the vessels toward the posterior apex of the lens in zones 2 and 3. One of the most curious findings of the more posterior ROP is the lack of ridge tissue seen in eyes with extensive shunts and plus disease. The concept of Foos and spindle cells is based on eyes with changes in mid-zone 2 and the absence of ridge tissue is a posterior finding. One suggestion is that the angioblast cells and the astrocyte cells do not meet until the progenitor cells have progressed from the area of the disc into mid-zone 2 in the animal models of ROP.15 This still leaves the difference in appearance of the flat stage 3 neovascularization in zone 1. It may be that the secondary vitreous formed by vascularized retina may dictate at least in part the position of the neovascular fronds. The more posterior smaller-volume vitreous cavity may produce enough secondary vitreous to press the frond along the retinal surface. As is known from diabetic retinopathy, these fronds tend to grow on the posterior hyaloid surface. As the vascularized retina expands, the volume of secondary vitreous needed to fill the hemisphere of the vitreous cavity may not keep pace and allow vessels to grow along the anterior surface of the developing secondary vitreous toward the center of vitreous cavity or toward the lens. This organization of vitreous collagen or hypocellular gel contraction requires few cells to organize large amounts of collagen, as seen in eye tissue samples with stage 5 ROP (Fig. 114.8). In tissue culture, cells have been identified that have the ability to organize large amounts of collagen (Fig. 114.9). It is believed these cells organize anterior vitreous collagen into a plane that allows the vessels to grow on a surface anteriorly toward the posterior aspect of the lens.
Distortion of retinal vascular architecture
Machemer16 suggested that avascular retina is more elastic because it lacks retinal vessels and that this helps explain why the peripheral avascular retina can stretch over large areas of retinal pigment epithelium (RPE). In contrast, we believe that the organization of the cortical vitreous collagen, which is formed by and intimately attached to the vascularized retina, makes this stretching of the vascularized retina difficult. This inelastic cortical vitreous collagen and retinal ridge inhibits stretching of the vascularized retina. It is the absence of the cortical collagen over areas of avascular retina that allows the avascular retina to be stretched over large areas of RPE. Circumferential stretching of the retina is often recognized because of distortion of retinal vessels and is associated with an incomplete retinal ridge.
Stages 4 and 5
ROP has both exudative retinal detachments and tractional retinal detachments. In the evolution of retinal detachments of ROP, at least three factors are involved. The first is the existence of permeable, leaky blood vessels, as seen in stage 3 ROP. These vessels, within the shunt and posterior to the ridge, are capable of supplying large amounts of proteinaceous fluid to both the vitreous cavity and subretinal space. Second, as shown by Ashton and Cook,17 the neovascularization that is commonly thought of as growing only into the vitreous cavity also can be seen growing into the subretinal space (Hirose, pers. comm.). Third, these blood vessels can bleed into both the vitreous cavity and subretinal space. Foos18 showed that eyes with vitreous hemorrhage are susceptible to retinal detachment and seem to have a worse prognosis for total retinal detachment.
Okamoto et al.19 have shown in a murine model that neovascularization into the subretinal space from the retina is produced in areas of VEGF promoter. Cells from the retinal ridge or regressing hyaloid and tunica vasculosa lentis organize vitreous collagen that is tightly attached to the retina. This creates traction on these permeable vessels and results in additional leakage of fluid or blood from these vessels. In children who develop stage 4 ROP, the retinal ridge is the most common location for retinal detachment to begin. Detachments without traction in the area of the ridge often settle spontaneously.20
In eyes with ROP, cells that organize the vitreous appear to migrate into the vitreous cortex from the ridge of retina between avascular and vascular retina and from the area of the optic disc. This means that manipulation of these two areas is important to resolve the tractional component of retinal detachment. It also may be that the cells of the primary vitreous or tunica vasculosa lentis, or both, now called “ocular fetal vasculature,” may contribute cells to this hypocellular gel contraction. The genetically determined apoptosis of the cellular elements of the primary vitreous and tunica vasculosa lentis may be clinically slowed or reversed under the influence of increased VEGF. The higher the concentration of VEGF, the less the apoptosis proceeds, and the more cells are available to organize vitreous collagen and contribute to retinal traction.21
We, and colleagues, have grown cells in tissue culture from the retrolenticular membrane of stage 5 ROP eyes. These cells have been studied by immunofluorescent techniques and appear to be predominately neuroglial in origin. Some cells seemed very immature, perhaps representing multipotential cells that have migrated from the retina into the vitreous cavity. These cells show the ability to organize large amounts of collagen in vitro, as well as the ability to produce collagen. When these cells are tested for collagen organization against cells from both proliferative vitreoretinopathy and tractional diabetic retinal detachment, the cells of ROP are able to organize much more collagen than the proliferative vitreoretinopathy cells.22,23 This organization of vitreous collagen into sheets is supported by the original observations of Terry14 in eyes with ROP.
Development of stage 4A ROP retinal detachments
In stage 4A ROP, the detachment begins at the ridge of the retina. Fluid can leak posteriorly under the retina and cause the posterior retina to detach as well. When a primarily exudative retinal detachment develops, the retinal surface is smooth and shows no evidence of epiretinal proliferation or peaked folds. This is a significant observation in our clinical experience, eyes with smooth anterior retinal surfaces, despite large areas (four disc areas) of retinal detachment, sometimes flatten spontaneously over several months. When the retina flattens, however, the RPE is often disrupted, and many eyes do not have useful vision. As cells migrate into the vitreous cortex along the surface of the vascularized retina, both anteriorly from the ridge and posteriorly from the disc, they can form folds in the retina. These cells can convert a predominately exudative retinal detachment to a predominately tractional detachment. Machemer,16 Charles,24 and others often discussed the peripheral retinal “trough,” or anterior loop traction, associated with ROP. The vitreous is organized in a fashion that allows the retina to form a trough in the far periphery. As retinal detachment in ROP evolves, its configuration depends partly on the symmetry of the retinal ridge. A ridge that contracts evenly and is located an equal distance from the disc in all meridians results in a detachment that closes centrally.
Subretinal fluid can have several clinical manifestations. It can be opalescent or red, being either serum or blood in the subretinal space. This fluid may have a toxic effect on the neurosensory retina and RPE, in addition to the expected degenerative effect on the photoreceptors from retinal detachment itself. This subretinal fluid has been found to have high concentrations of hemoglobin and iron, both bound and unbound.25,26 In addition, cholesterol is often found in the subretinal space, either crystallized or in solution. Opaque subretinal fluid, after even spontaneous reattachment, appears to leave the eye with disrupted RPE and presumably a poor visual result.
Clinical considerations
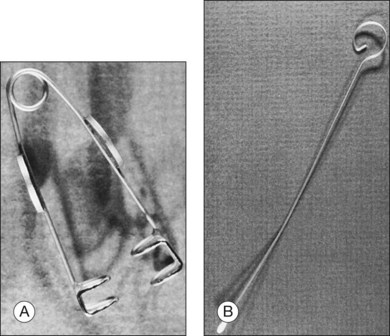
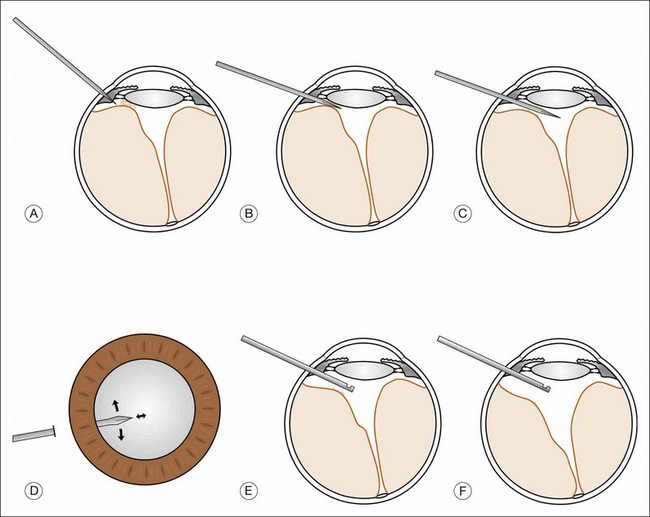
In the results of the Cryotherapy for Retinopathy of Prematurity study,27 and Early Treatment for Retinopathy of Prematurity study,28 a positive treatment effect was reported, and it was suggested that infants be examined at 4–6 weeks after birth and before 31 weeks PMA if they are 1500 g or less birth weight. These infants need to be examined meticulously, including indirect ophthalmoscopy, for vascularization of the anterior segment, pupillary dilation, plus disease, zone of eye involvement, and stage of the disease process. The authors have found that the Alfonso lid speculum (Fig. 114.10A) and Kelge swab (Fig. 114.10B), accompanied by the indirect small pupil ophthalmoscope and 28 diopter lens, are the best instruments to examine the peripheral retina in the neonate. We do not use topical anesthesia because this can cause clouding of the cornea.
Photographic imaging
The Photo-ROP (Photographic Screening for Retinopathy of Prematurity) study and that of Ells et al. evaluated and confirmed the utility of photographic imaging in ROP screening.29,30 This photographic imaging allows a very good representation of the posterior pole and the mid periphery of the eyes of premature infants. Photographic documentation in diabetes, age-related macular degeneration, and other retinal vascular disease is well known.31–33 and photographic documentation of ROP has been available for several years and is equally valuable. Treatment in ROP in large part is driven by zone 1 and particularly zone 2 findings, well seen with photography.29
The interpretation of these pictures requires they be obtained and managed in a timely fashion and read by a qualified reader. Retinopathy of prematurity is a time-dependent disease, allowing development of stage 1 to stage 5 to occur in as little as 2 weeks.34–36 FocusROP (FocusROP, LLC, Troy, MI), an internet-based, HIPAA-compliant, secure website using certified and expert readers, has been developed to handle these images. This website (www.FocusROP.com), receives the uploaded digital information obtained by trained individuals in the neonatal care centers and immediately notifies a previously certified local ophthalmologist to read these images. The follow-up algorithm contained in the software program allows only a very conservative examination schedule.
Cryotherapy
The Cryotherapy for Retinopathy of Prematurity study defined eyes with plus disease showing five contiguous or eight discontiguous clock hours of stage 3 ROP (Fig. 114.1) as reaching a threshold for cryotherapy. Cryotherapy was defined as use of a cryoprobe to treat the avascular retina but not the ridge.37 This technique was found to be effective in reducing by 50% the unfavorable outcome of retinal fold and retinal detachment.
As mentioned earlier, eyes with zone I disease tend to have the worst prognosis, and eyes with zone I involvement and extensive plus disease are said to have Rush disease. Rush disease refers to the tempo of ROP, which can go from stage 1 to stage 5 in only 2 weeks.34–36 Zone I cryotherapy, however, requires extensive treatment and risks occlusion of the central retinal artery for a significant time.
Cryotherapy has the potential to induce future retinal problems in these patients, who have an increased risk for rhegmatogenous retinal detachment as adults, with or without treatment.27 Trese has seen several eyes that developed cataract, hypotony, and iris depigmentation.26 These changes were seemingly caused by anterior segment ischemia following peripheral cryotherapy treatment. Cryotherapy is no longer considered the treatment of choice with the advent of the indirect laser.
Indirect laser photocoagulation
Several reports have shown peripheral ablation by laser (argon blue-green and diode) to be effective in the treatment of ROP. This is perhaps not surprising in that peripheral ablation, no matter how performed, should have a similar effect on the course of the disease. Iverson et al.,38 Landers et al.,39 and McNamara et al.40 have shown a positive treatment effect for the use of peripheral laser ablation. This type of treatment can be performed using topical anesthesia and may be performed in the nursery, eliminating the need for the infant to be transported to the operating room. The diode laser has significant physical advantages because of its portable nature.41
The Early Treatment Retinopathy Of Prematurity study (ETROP)
The ETROP28 used a risk management program RM-ROP2 to evaluate each eye. If the risk generated by the program exceeded 0.15, the eye was randomized to treatment. The eyes eventually were analyzed and using an ICROP-based classification system, they were grouped into type 1 and type 2:
Anti-vascular endothelial growth factor therapy
Multiple reports and series have demonstrated the use of intravitreal bevacizumab, an anti-VEGF agent originally approved by the FDA for the treatment of metastatic colon cancer,43 for cases of ROP concerning stages 3+, 4, and 5. Intravitreal bevacizumab has been used as monotherapy as well as in combination with conventional laser therapy or with vitrectomy.44–58 Contraction of membranes, with resulting acceleration of retinal detachment, can occur when intravitreal bevacizumab is administered too late (at stages 4 and 5).47,51
A case report demonstrated the use of intravitreal ranibizumab, an anti-VEGF agent originally approved by the FDA for the treatment of neovascular age-related macular degeneration, in combination with laser photocoagulation for a patient with bilateral, zone 1, stage 3 with plus ROP.59,60 The patient demonstrated full regression but developed bilateral retinal detachments 1 month later. The safety and efficacy of pharmacological agents, such as bevacizumab, are currently being evaluated in large, prospective, randomized trials.61,62
Therapeutic oxygen
Increasing oxygen has been shown to reduce VEGF activity. Because of this effect, the STOP-ROP study was undertaken to increase the oxygen saturation in infants whose transcutaneous oxygen saturation is less than 94% in hopes that the increased oxygen would decrease VEGF activity and allow the genetically driven development of retinal vessels to regain control. The STOP-ROP study did not show a statistically significant effect.63–67
Stages 4 and 5 preoperative evaluation
Preoperative evaluation of a child with stage 4 or 5 ROP necessitates special considerations. It may be difficult to determine the visual function of the child. The examiner or parent often forms a clinical impression on the basis of the child’s behavior after exposure to a bright light. Many authors68 have tried to define electrophysiological criteria for visual acuity in infants. In our experience, the awake visual evoked potential (VEP) or electrical evoked potential (EEP) seems the most reliable. The VEP is perhaps the most easily accessible and reliable piece of clinical information. The VEP is valuable if a child is without clinical light perception vision but does have a recordable VEP. With this, Trese believes that such a child has an objective demonstration of functional retina, and he would proceed with surgery. In the absence of a VEP response and clinical light response in screening a child for surgical intervention, he believes that these children have a small likelihood of a favorable visual result after surgery. It is possible for children to have a clinical light response and a nonrecordable VEP (false-negative exam).
Surgical therapy
Scleral buckling
Scleral buckling has been suggested for stage 4 ROP. In two series it has been suggested that scleral buckling for stage 4 eyes has a success rate of 66–70% for stage 4A and 67% for stage 4B retinal detachment.26,69 Encirclement is performed with or without drainage of subretinal fluid. If subretinal fluid is not drained, a paracentesis must be performed. Although scleral buckling has not to date been studied in a randomized, prospective clinical trial, data from the Cryotherapy for Retinopathy of Prematurity study suggest that if stage 4 retinal detachment involves eight of 34 ROP sectors of the retina, there is an 88% chance of progression to stage 5 ROP.70 It would seem from these data that scleral buckling would be a reasonable alternative in a predominately effusive stage 4 ROP retinal detachment. With the use of laser, predominately effusive eyes are rare.
Lens-sparing vitrectomy for 4A ROP
In addition to the changes in the appearance of the eyes requiring peripheral ablation over the last 15 years, the appearance of retinal detachment following laser treatment has also changed. With less effusion from laser treatment than cryo treatment, there is less blood in the subretinal space and the eyes are vascularly quieter at an earlier time, allowing vitreous intervention in a vascularly quiet eye where the macula has not detached (stage 4A). This basic principle of retinal detachment repair “that a macula-on retinal detachment is an urgent operation” is also true in ROP. The natural history part of the CRYO-ROP Study showed that a child with an 8 sector 4A ROP detachment at their due date (40 weeks PMA) had a high risk of going on to an unfavorable outcome or total retinal detachment (stage 5).70 Two studies have shown 90% or better anatomic success rates with LSV.71,72
Vitrectomy for 4B ROP
Results for 4B ROP detachments can also be broken down anatomically and visually. A total of 76% of eyes reattached part or all of the retina. Some 15% achieved 20/60–20/300 vision; 30% achieved 20/60–20/800 vision; 48% achieved 20/60–20/1900 (ambulatory) vision, and 72% of eyes achieved 20/60-LP vision. A total of 28% of eyes had no light perception (NLP), despite surgery.70 These results compare favorably with the natural history.
Lensectomy, vitrectomy, and membrane peeling
Detachments in eyes with stage 5 have many features not seen in other retinal detachments. The configuration of the retinal detachment can vary greatly between different quadrants of the same eye. The peripheral retinal trough can be shallow or deep (Fig. 114.14). Depending on differential contraction along the ridge, the funnel can be closed tightly centrally or can be eccentrically displaced toward the periphery in a crescent pattern (Fig. 114.15). It is also common to see the retina posterior to the ridge detached in a spiral configuration caused by differential, circumferential traction (Fig. 114.16). This causes confusion to the novice surgeon, who believes that radial division of epiretinal tissue is safe. Radial division when a spiral configuration is present is dangerous, and it is important to follow the spiral back toward the optic nerve.
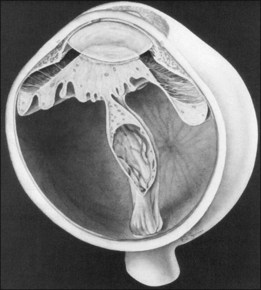
Fig. 114.14 In the same eye the peripheral trough can have a variety of depths.
(Courtesy of Eugene de Juan, MD.)
At the posterior pole, the retina most often shows dragging across the optic nerve head, causing a triple layer of retinal tissue (Fig. 114.17). This often interferes with a good view of the optic nerve. Thorough and careful dissection should be carried out to the posterior pole. This configuration can have an exaggerated form of retinal detachment with a large fold of retina dragged over the disc, often in the horizontal meridian (Fig. 114.18). This fold of retina can be dragged along the top of a radial fold, continuing far anteriorly, which can leave large areas of usually avascular retina attached (Fig. 114.19). If the retina surrounding this fold is attached, it is impossible to flatten the retina; however, if the fold of detached retina is over detached retina, surgical flattening is possible.
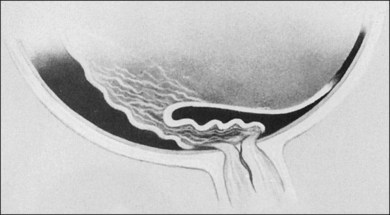
Fig. 114.17 Dragging of the retina across the disc, leaving in some areas a triple thickness of retina.
Enzymatic manipulation of the vitreoretinal junction
A subset of pediatric vitreoretinal surgery requires successful peeling of preretinal membranes or the posterior hyaloid to achieve repair. The vitreoretinal adhesion is mediated in part by laminin and fibronectin.73 Autologous plasmin enzyme (APE) cleaves both laminin and fibronectin and produces vitreous liquefaction and posterior vitreous detachment in adult eyes after intravitreal injection.74 Plasmin enzyme may facilitate the removal of such membranes and reduce the risk of creating an iatrogenic retinal break during membrane peeling.75,76 In young children who cannot tolerate an intravitreal injection in the clinic, APE is injected into the vitreous cavity approximately 30 min before the start of surgery.
Microplasmin (Thrombogenics Inc, Dublin, Ireland) is a recombinant form of plasmin, which retains the enzymatic activity of autologous plasmin.77 Clinical trials in adults have shown that microplasmin can safely relieve vitreoretinal traction, and a clinical trial using microplasmin in pediatric vitreoretinal surgical cases is ongoing.78
Surgical approach
The surgical techniques used in stage 5 are confined to closed lensectomy, pars plicata vitrectomy (with membrane peeling accompanied occasionally by drainage of subretinal fluid), and rarely, scleral buckling. Open-sky vitrectomy with intracapsular lensectomy and membrane peeling has also been used. The bulk of our experience has been in closed vitrectomy, and we reserve the open-sky vitrectomy for those eyes in which the anterior segment is clouded and will not allow the use of the closed vitrectomy technique. Open-sky vitrectomy has the advantage of allowing two-handed dissection through the large anterior incision, and it allows surgery in eyes with clouded corneas.79
Lens-sparing vitrectomy
The development of the infusion light pipe, miniaturized contact lenses, which can be used on the anterior surface of the eye, and the binocular indirect ophthalmoscopy (BIOM) noncontact system, allowing wide-angle viewing in children with a smaller anterior segment, for visualization in the phakic state are perhaps the most significant changes in pediatric vitreoretinal surgery in the recent past (Fig. 114.20). A wide-view infusion light pipe used with the BIOM allows visualization and peripheral dissection without the need for scleral indentation, a practice that can create retinal tears in children with redundant retina. These developments also allow us to deal with the tractional detachment of the posterior pole, leaving the lens in position (Fig. 114.21). Children having this type of procedure can retain clear lenses for many years. Current follow-up suggests that lenses can remain clear at least 4–5 years.80
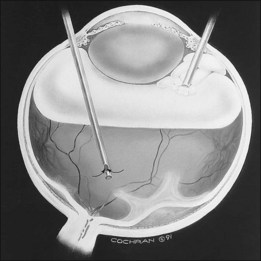
Fig. 114.20 Infusion light pipe at the time of delivering air into the eye during phakic vitrectomy in an infant.
In addition, the visual rehabilitation in these children, without the complications of aphakia, allows us to treat unilateral tractional detachment, as well as bilateral tractional detachments, more effectively. This greatly reduces the refractive rehabilitation and increases the cooperation of the patient and family with conventional forms of refractive and amblyopia therapy. Children with this level of tractional detachment and ROP can achieve levels of vision in the 20/100 to 20/60 level, assuming the central nervous system is able to process visual information.70,81 Children who are operated with a true 4A retinal detachment can achieve visual acuity of 20/20.72
Ab interno incision
A modification of the lens-sparing vitrectomy technique, called an “ab interno incision,” is used in infants in which the surgical entry space between the lens and the retina is too small for current vitrectomy instrumentation.82 In circumstances where retinal folds, organized vitreous, and/or fibrotic tissue are in close approximation to the lens for several clock hours, standard lens-sparing entry into the eye may not be possible. In this setting, an ab interno incision is used. With this technique, once the sclera is entered, the microvitreoretinal (MVR) blade is first directed carefully posterior and then inserted into the space or tissue between the retina and posterior lens capsule (Fig. 114.22A–C). Once the MVR blade is located between the posterior lens capsule and retinal tissue, anterior retinal traction is relieved and a posterior relaxation of the retina is immediately apparent. The incision can be extended for many clock hours by sweeping in the surgical space parallel to the lens capsule using the sclerotomy as a pivot point or by sliding the blade like a saw to release any tractional vectors (Fig. 114.22A,D). Damage to the retina or lens is a possible complication of this technique and care should be taken to avoid violating the lens equator or causing an unintentional retinal break. This provides standard vitrectomy instrumentation a safe entry to complete the posterior vitrectomy (Fig. 114.22A,E,F) and allows for dissection along the retinal surface once more surgical space is created.
Closed lensectomy pars plicata vitrectomy
Because the infant’s eye does not have a pars plana, entry into the eye in the closed vitrectomy is through the pars plicata, iris root, or limbus.24,68,83,84 After entry into the eye, a complete lensectomy is performed, including capsular removal in eyes with open funnel configuration. Trese believes the main reason for failure caused by reproliferation and redetachment often can be traced to incomplete removal of lens epithelium. A two-handed cross action opening of the retrolenticular tissue is performed with two disposable no. 26 needles. This causes little peripheral traction. The resulting central vertical slit in the membrane is then extended using intraocular scissors with continuous Healon infusion (Fig. 114.23). Then two-handed dissection with forceps and scissors is used to divide the retrolenticular tissue.85
Because our surgical goal is to flatten the posterior pole, this is one of the most important areas for dissection. Often, the smaller folds across the optic nerve are left alone. After the central funnel of detached retina is opened, the anterior peripheral trough is approached. Two-handed dissection is used to delaminate this tissue at the ridge where possible. Some ridge tissue cannot be dissected (Fig. 114.24).
After surgery, the child is placed face down for 24 hours. This is done to flatten the posterior retina quickly and displace the subretinal fluid. The subretinal fluid is often very viscous and contains cholesterol crystals. The bulk of subretinal fluid reabsorbs in 2–4 months. As soon as the posterior retina flattens, the child is given spectacles and begins vision training. When both open-sky and closed vitrectomy are used, the anatomic success rate has been 50% for reattachment of zone I of the retina in eyes without peripheral ablation. The reattachment rates vary from 60% to 70% reattachment of zone I with open-funnel retinal detachments to 26% reattachment rates for closed-funnel detachments (Hirose, pers. comm.).68,79,86,87 We reserve reoperation for eyes that have shown visual improvement and later develop redetachment of the posterior pole of the eye.
The long-term anatomic and visual results with a nearly 4-year follow-up showed that appropriate intervention with peripheral ablation and 4B/5 retinal detachment intervention yielded 76% of eyes with attachment of the posterior pole and 15% of eyes with 20/300–20/60 vision; 30% of eyes with 20/800 to 20/60 vision; 48% of eyes with 20/1900–20/60 vision, and 72% of eyes with light perception or better. Some 30% required more than one operation, other than peripheral ablation.70 With laser ablation alone and 4A lens-sparing vitrectomy, 90% anatomic success and function as good as 20/20 can be achieved. The visual results of stage 5 ROP suggest that timing of intervention is important. Intervening as early as possible after retinal detachment in a vascularly inactive eye is more favorable than waiting for spontaneous vascular involution. The belief is that this period of visual deprivation may be irretrievable in these young infants.87 The ideal time is when the eye is vascularly quiet and the macula is still attached (stage 4A).
1 Kinsey VE, Arnold HJ, Kalina RE, et al. PaO2 levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics. 1977;60:655–668.
2 Patz A, Kinsey VE. Retrolental fibroplasia: the pediatrician’s dilemma. Pediatrics. 1971;48:509–510.
3 Ashton N. The pathogenesis of retrolental fibroplasia. Ophthalmology. 1979;86:1695–1699.
4 Garner A, Ashton N. Vaso-obliteration and retrolental fibroplasia. Proc R Soc Med. 1971;64:774–777.
5 Hammer HM, Noble BA, Harcourt RB, et al. Ophthalmic findings in very low birth weight children. Trans Ophthalmol Soc UK. 1985;104:329–331.
6 Phelps DL, Rosenbaum AL. The role of tocopherol in oxygen-induced retinopathy: kitten model. Pediatrics. 1977;59:998–1005.
7 Flynn JT, O’Grady GE, Herrera J, et al. Retrolental fibroplasias. I. Clinical observations. Arch Ophthalmol. 1977;95:217–223.
8 Foos RY. Acute retrolental fibroplasia. Graefes Arch Clin Exp Ophthalmol. 1975;195:87–100.
9 Teske MP, Trese MT. Retinopathy of prematurity-like fundus and persistent hyperplastic primary vitreous associated with maternal cocaine use. Am J Ophthalmol. 1987;103:719–720.
10 Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228.
11 The Committee for the Classification of Retinopathy of Prematurity: an international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134.
12 Committee for the Classification of Retinopathy of Prematurity: an international classification of retinopathy of prematurity. Int Ophthalmol. 1985;8:3–10.
13 Kretzer FL, Hittner HM, Godio LB. Ultrastructural evaluation of the retina in retinopathy of prematurity and correlations with vitamin E therapy. Curr Eye Res. 1984;3:881–882.
14 Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in infants born prematurely. III. Studies in development and regression of hyaloid artery and tunica vasculosa lentis. Am J Ophthalmol. 1942;25:1409.
15 Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–1228.
16 Machemer R. Description and pathogenesis of late stages of retinopathy of prematurity. Ophthalmology. 1985;92:1000–1004.
17 Ashton N, Cook C. Studies on developing retinal vessels. I. Influence of retinal detachment. Br J Ophthalmol. 1955;39:449–456.
18 Foos RY. Chronic retinopathy of prematurity. Ophthalmology. 1985;92:563–574.
19 Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–291.
20 Garfinkel RA, Trese MT. Spontaneous resolution of retinal detachment in retinopathy of prematurity (unpublished).
21 Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028.
22 Mancini MA, Kennedy A, Frank RN, et al. A cell line derived from non-neoplastic human retinal cells. Invest Ophthalmol Vis Sci. 1987;28:S290.
23 Trese MT, Lin LR, Blumenkranz MS, et al. Cell biology of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1987;28:S204.
24 Charles S. Vitreous surgery for retinopathy of prematurity (ROP). In: Syllabus: Retinopathy of Prematurity Conference, Washington, DC, 1981;2:858–63.
25 Loh A, Hadziahmetovic M, Dunaief JL. Iron homeostasis and eye disease. Biochim Biophys Acta. 2009;1790:637–649.
26 Trese MT. Visual results and prognostic factors for vision following surgery for stage V retinopathy of prematurity. Ophthalmology. 1986;93:574–579.
27 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–479.
28 Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity. Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1696.
29 The Photographic Screening for Retinopathy of Prematurity Cooperative Group. The photographic screening for retinopathy of prematurity study: primary outcomes. Retina. 2008;28:S47–S54.
30 Ells AL, Holmes JM, Astle WF, et al. Telemedicine Approach to Screening for Severe Retinopathy of Prematurity – A Pilot Study. Ophthalmology. 2003;110:2113–2117.
31 Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Age-Related Eye Disease Study Research Group. Ophthalmology. 2000;107:2224–2232.
32 Azen SP, Irvine AR, Davis MD, et al. The validity and reliability of photographic documentation of proliferative vitreoretinopathy. Ophthalmology. 1989;96:352–357.
33 Pugh JA, Jacobson JM, Van Heuven WA, et al. Screening for diabetic retinopathy. The wide-angle retinal camera. Diabetes Care. 1993;16:889–895.
34 International Committee for the Classification of Retinopathy of Prematurity, The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999.
35 Morizane H. Initial sign and clinical course of the most severe form of acute proliferative retrolental fibroplasias (type 1) [in Japanese]. Nippon Ganka Gakkai Zasshi. 1976;80:54–61.
36 Quiram PA, Capone A, Jr. Current understanding and management of retinopathy of prematurity. Curr Opin Ophthalmol. 2007;18:228–234.
37 Tasman W, Brown GC, Naidoff M, et al. Cryotherapy for active retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 1987;225:3–4.
38 Iverson DA, Trese MT, Orgel IK, et al. Laser photocoagulation for threshold retinopathy of prematurity (Letter). Arch Ophthalmol. 1991;109:1342.
39 Landers MB, III., Toth CA, Semple HC, et al. Treatment of retinopathy of prematurity with argon laser photocoagulation. Arch Ophthalmol. 1992;110:44–47.
40 McNamara JA, Tasman WS, Brown GC, et al. Laser photocoagulation for stage 3 retinopathy of prematurity. Ophthalmology. 1991;98:576–580.
41 Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity: a randomized study. Ophthalmology. 1993;100:238–244.
42 Gilbert WS, Quinn GE, Dobson V, et al. for the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1996;114:1085–1091.
43 Smith LEH. Through the eyes of a child: understanding retinopathy through ROP: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–5182.
44 Chung EJ, Kim JH, Ahn HS, et al. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007;245:1727–1730.
45 Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38:233–237.
46 Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, et al. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina. 2008;28(Suppl):S19–S25.
47 Honda S, Hirabayashi H, Tsukahara Y, et al. Acute contraction of the proliferative membrane after intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1061–1063.
48 Lalwani GA, Berrocal AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008;28:S13–S18. [Erratum, Retina 2009;29:127.]
49 Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008;92:1450–1455.
50 Mintz-Hittner HA, Kuffel RR, Jr. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28:831–838. [Erratum, Retina 2008;28:1374.]
51 Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, et al. Paradoxical vascular-fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye (Lond). 2010;24:931–933.
52 Law JC, Recchia FM, Morrison DG, et al. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS. 2010;14:6–10.
53 Lee JY, Chae JB, Yang SJ, et al. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010;248:1257–1262.
54 Altinsoy HI, Mutlu FM, Güngör R, et al. Combination of laser photocoagulation and intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2010. Epub:20337366
55 Dorta P, Kychenthal A. Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina. 2010;30(Suppl):S24–S31.
56 Ahmed AE, Channa R, Durrani J, et al. Early experience with intravitreal bevacizumab combined with laser treatment for retinopathy of prematurity. Middle East Afr J Ophthalmol. 2010;17:264–267.
57 Nazari H, Modarres M, Parvaresh MM, et al. Intravitreal bevacizumab in combination with laser therapy for the treatment of severe retinopathy of prematurity (ROP) associated with vitreous or retinal hemorrhage. Graefes Arch Clin Exp Ophthalmol. 2010;248:1713–1718.
58 Wu W-C, Yeh P-T, Chen S-N, et al. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in Taiwan. Ophthalmology. 2011;118:176–183.
59 Resenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;335:1419–1431.
60 Jang SY, Choi KS, Lee SJ. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J AAPOS. 2010;14:457–459.
61 Mintz-Hittner HA, Kennedy KA, Chuang AZ, for the BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615.
62 Trese MT. Vision Research Foundation clinical trial: Pan-VEGF blockade for the treatment of retinopathy of prematurity. Bethesda: National Library of Medicine; 2000. Online. Available at http://clinicaltrials.gov/ct2/show/NCT01232777 (accessed: June 30, 2011)
63 Bremer DL, Rogers GL, Bell H, et al. The efficacy of vitamin E in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 1986;23:132–136.
64 Hittner HM, Rudolph AJ, Kretzer FL. Suppression of severe retinopathy of prematurity with vitamin E supplementation: ultrastructural mechanism of clinical efficacy. Ophthalmology. 1984;91:1512–1523.
65 Phelps DL, Rosenbaum AL, Isenberg SJ, et al. Tocopherol efficacy and safety for preventing retinopathy of prematurity: a randomized, controlled, double-masked trial. Pediatrics. 1987;79:489–500.
66 Schaffer DB, Johnson L, Quinn GE, et al. Vitamin E and retinopathy of prematurity: follow-up at one year. Ophthalmology. 1985;92:1005–1011.
67 The STOP-ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I. Primary outcomes. Pediatrics. 2000;105:295–310.
68 Trese MT. Surgical results of stage V retrolental fibroplasia and timing of surgical repair. Ophthalmology. 1984;91:461–466.
69 Greven C, Tasman W. Scleral buckling in stages 4B and 5 retinopathy of prematurity. Ophthalmology. 1990;97:817–820.
70 Trese MT, Droste PJ. Long-term postoperative results of a consecutive series of stages 4 and 5 retinopathy of prematurity. Ophthalmology. 1998;105:992–997.
71 Capone A, Jr., Trese MT. Lens-sparing vitreous surgery for tractional 4A retinopathy of prematurity retinal detachments. Ophthalmology. 2001;108:2058–2070.
72 Prenner JL, Capone A, Jr., Trese MT. Visual outcomes after lens-sparing vitrectomy for stage 4A retinopathy of prematurity. Ophthalmology. 2004;111:2271–2273.
73 Kohno T, Sorgente N, Ishibashi T, et al. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987;28:506–514.
74 Liotta LA, Goldfarb RH, Brundage R, et al. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41(11 Pt 1):4629–4636.
75 Margherio AR, Margherio RR, Hartzer M, et al. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology. 1998;105:1617–1620.
76 Wu WC, Drenser KA, Trese MT, et al. Pediatric traumatic macular hole: results of autologous plasmin enzyme-assisted vitrectomy. Am J Ophthalmol. 2007;144:668–672.
77 Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004;45:641–647.
78 Williams GA. Results from the MIVI III (Microplasmin for Vitreous Injection) trial. Hong Kong: World Ophthalmology Congress; 2008.
79 Tasman W, Borrone RN, Bolling J. Open-sky vitrectomy for total retinal detachment in retinopathy of prematurity. Ophthalmology. 1987;94:449–452.
80 Maguire AM, Trese MT. Lens-sparing vitreoretinal surgery in infants. Arch Ophthalmol. 1992;110:284–286.
81 Maguire AM, Trese MT. Visual results of lens-sparing vitreoretinal surgery in infants. J Pediatr Ophthalmol Strabismus. 1993;30:28–32.
82 Trese MT, Capone A, Jr. Surgical approaches to infant and childhood retinal diseases: invasive methods. In: Hartnett ME, Trese MT, Capone A, Jr., et al. Pediatric retina. Philadelphia: Lippincott Williams & Williams; 2005:359–364.
83 Lightfoot D, Irvine AR. Vitrectomy in infants and children with retinal detachments caused by cicatricial retrolental fibroplasia. Am J Ophthalmol. 1982;94:305–312.
84 Lin LR, Hartzer M, Blumenkranz M, et al. Hypo-cellular gel contraction: ultrastructural studies on human epiretinal membranes – comparison with experimentally contracted gels. Invest Ophthalmol Vis Sci. 1987;28:S207.
85 Trese MT. Two-hand dissection technique during closed vitrectomy for retinopathy of prematurity. Am J Ophthalmol. 1986;101:251–252.
86 Machemer R. Closed vitrectomy for severe retrolental fibroplasia in the infant. Ophthalmology. 1983;90:436–441.
87 Trese MT. Surgical therapy for stage V retinopathy of prematurity: a two-step approach. Graefes Arch Clin Exp Ophthalmol. 1987;225:266–268.