Chapter 133 Retinal Metastases
Introduction
Retinal metastases represent a small percentage of intraocular malignancy secondary to systemic cancers, even though 10% of patients who die of cancer have been found to have intraocular metastases.1–4 While relatively rare, the diagnosis of retinal metastases can be challenging, as its presentation can mimic other etiologies. High clinical suspicion and use of appropriate diagnostic techniques are vital in order to successfully diagnose and manage these challenging cases. This chapter provides a review of the literature of this rare clinical entity and management recommendations.
Metastatic cascade
Metastases are responsible for most cancer deaths.5 The understanding of metastases is limited due to the “hidden” nature of this process, as it occurs inside the body and is difficult to observe.5 Although many tumor cells are shed daily into the bloodstream or lymphatic system in those with cancer, the evidence is unclear on the fate of these tumor cells.6,7 Some models suggest that the majority do not survive in the circulation, while others suggest that most can survive and extravasate.5,6,8–10 For tumor metastasis to develop, a series of biological steps must be completed for a tumor cell to grow at a different site.5,6,10–15
Dissociation, invasion, and intravasation
For tumor cells to invade the circulation, they must dissociate from the primary tumor.5,6,10–14 On a molecular level, the dissociation is initiated by an array of motility factors11,12 and requires modulation of the expression of cadherins and integrins.5,6,13–16 Degradation of the extracellular matrix by proteolytic enzymes (primarily matrix metalloproteases and the plasminogen activator system) facilitates invasion of the surrounding connective tissue components.5,6,13,15,17–23 Metalloproteases also modulate cell adhesion in their local environment and help release growth factors from their stores.18,19,21 Then, tumor cells must penetrate the basement membranes of endothelial cells to enter the blood and lymphatic circulation.5,6,15 This step again requires well-coordinated proteolysis, as well as mechanical deformation and locomotion of the tumor cells.5,6,14,15 If this complex interaction is regulated successfully, certain populations of cells can break through the matrix and endothelium to reach the blood stream.
Hematogenous dissemination
The destination within the eye of circulating tumor cells may depend on several factors. Tumor size, vascular circulatory patterns and organ-specific factors which encourage tumor growth (so-called seed and soil factors) all may play a role in the location of metastases.5,6,15 Reese emphasized that, although tumor emboli are more prominent in the uvea, more than 90% of infectious emboli involve the retina.24 Because large emboli (e.g., tumor emboli) travel along the vessel wall in the slower-moving part of the blood stream, they are more likely to enter vessel branches, such as the short ciliary arteries. Small emboli (e.g., bacterial emboli) travel in the central, faster-moving part of the blood stream towards the terminal vessels, such as the central retinal artery.25 The marked vascularity of the posterior choroid relative to that of the retina (as noted above) could also contribute to more frequent choroidal involvement as metastatic tumors to the retina and optic disc, supplied by the central retinal artery, are rare.3,4
Extravasation and angiogenesis
Although circulation patterns and tumor size may play a role in implantation, metastatic growth may require organ-specific factors which facilitate tumor cell survival.5,15,16,26–28 Animal models of metastases support the role of both vascular flow patterns and organ compatibility factors in the development of metastases.5,29–31 Tumor cell anchorage at a target organ site depends on shear-resistant attachment to local endothelium. Various integrins and selectins have been identified which appear to mediate such specialized tumor cell adhesion under dynamic flow conditions.5,16,32,33
The delivery of the tumor cell to the site of metastasis depends on mechanical flow, but the growth or survival can depend on organ specific molecular interactions. These interactions can encourage tumor cell growth via expression of growth factors, and altering the gene expression of tumor cells.5,6,34–37 Expression of specific chemokine receptors by tumor cells may also target tumor cells to specific organs which express the specific ligands for these receptors.5,15,38–41 This match could lead to chemokine signal activation of genes which would encourage tumor cell growth.5,15 Specific interactions unique to retinal metastasis to this point have not been identified.
After the colony at the secondary site is established, angiogenesis will again play a key role in the continued growth of the tumor. The onset of angiogenesis involves an alteration in the balance between positive and negative regulators. In vivo experiments with human tumor cell lines have shown that both vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF) have direct roles in tumor-associated angiogenesis,42 and FGF-2 also are the two major angiogenic factors in the retina and increased expression of both has been identified in retinal tumors.43–47 VEGF not only regulates tumor-associated angiogenesis in response to local hypoxia and various cytokines, but may regulate breakdown of the blood–ocular barrier in ocular melanoma and other tumors.43,47–50 FGF-2 is a potent mitogen of choriocapillary endothelial cells, and FGF acts synergistically with VEGF to stimulate tumor angiogenesis.44,51–53
Review of case reports
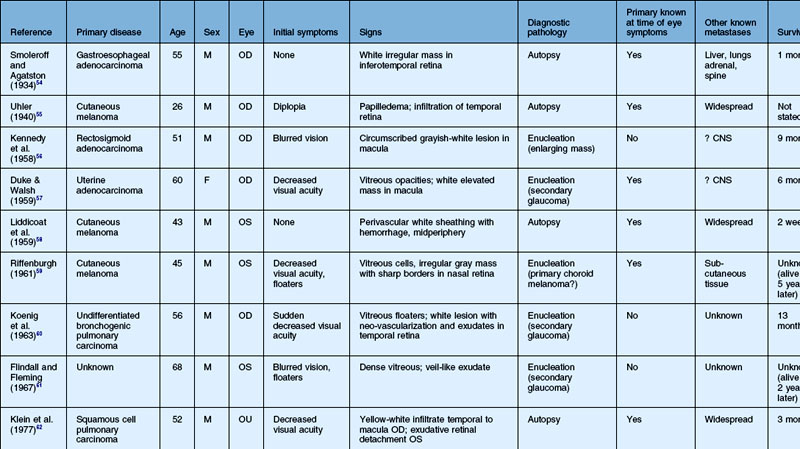
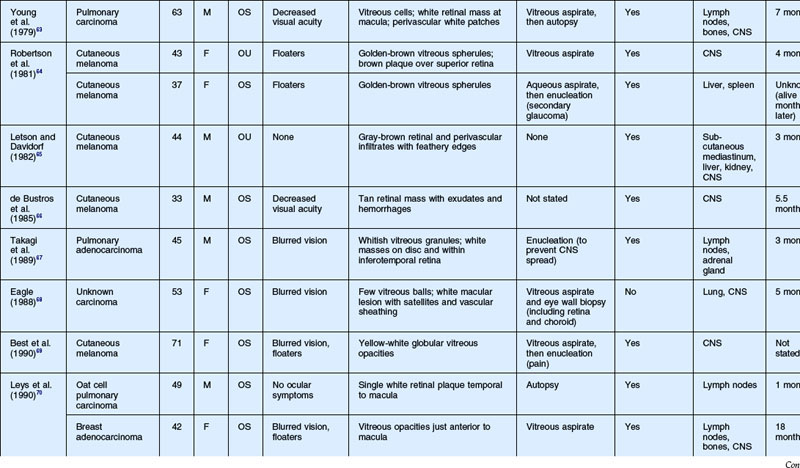
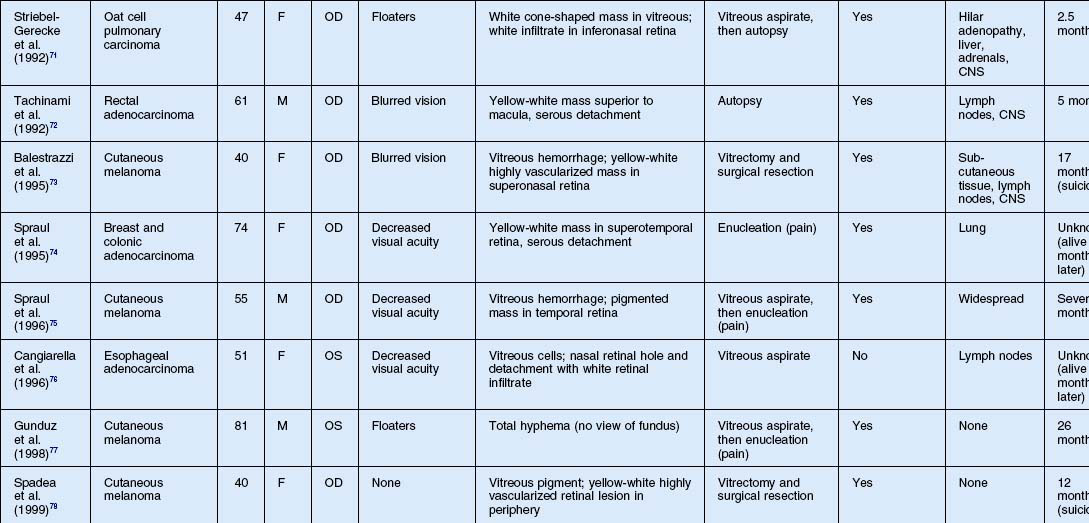
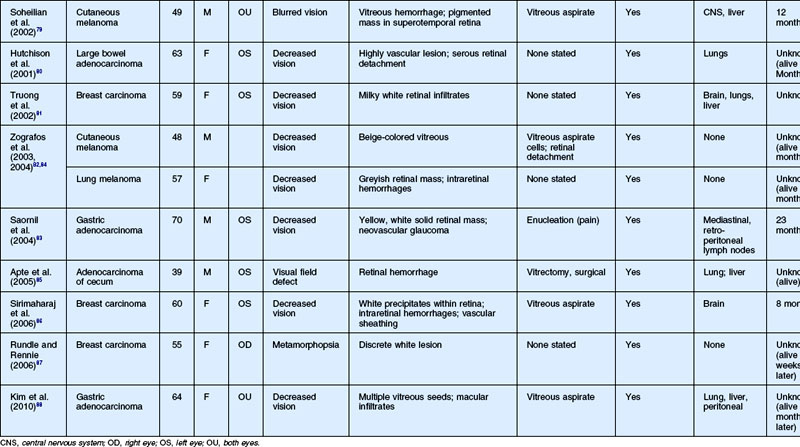
As noted above, retinal metastasis represents a small percentage of intraocular metastasis. Thus, no large single-center review has been published detailing outcomes associated with retinal metastasis. Publications in the literature have been limited to case reports.54–88 The first was published in 1934 and was diagnosed at autopsy.54 Until 1979, the diagnosis of retinal metastasis was made either by enucleation or at autopsy. Since 1979, diagnosis of retinal metastasis has been made via biopsy including vitreous, chorioretinal, retinal biopsies, clinical inspection or surgical removal.63 Although the case reports reviewed provide detail in presentation and treatment of retinal metastases, follow-up is limited for many of the publications. The clinical appearance, symptoms and outcomes from published case reports will be reviewed below and are displayed in Table 133.1.
Clinical findings
Symptoms
The most common visual complaint among those with retinal metastases is decreased or blurred vision (Table 133.1). Floaters are also a common complaint. Other symptoms include pain, diplopia, and red eye. Some cases presented without any specific visual symptoms.54,58,65 The underlying primary source of the metastases did not have a bearing on the type of visual symptom reported by the patient.
Signs
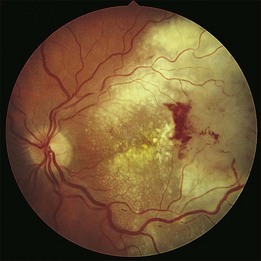
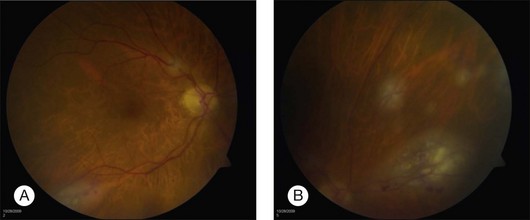
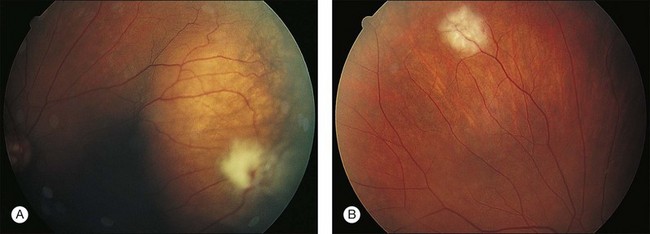
The clinical appearance of retinal metastases can vary based on the primary tumor and level of invasion of the tumor. Metastatic melanoma typically presents as a pigmented lesion within the retina with irregular borders and flat appearance (Fig. 133.1). Carcinomas tend to be non-pigmented, white or yellow in appearance and vary in size (Fig. 133.2). Some metastatic carcinomas can appear with significant mass and an elevated appearance with surrounding subretinal fluid.
Intraretinal hemorrhage and exudates have been described in association with these lesions (Figs. 133.3, 133.4). Subretinal hemorrhage has also been described as a presenting feature of metastatic carcinoma.85 Perivascular infiltrates can also be seen in some cases (Fig. 133.2). Retinal hemorrhages and exudates are thought to occur secondary to damage to the retinal microvasculature. Additionally, subretinal fluid has been described in several cases of retinal metastases. The appearance of subretinal fluid or exudative retinal detachment warrants an evaluation for a choroidal lesion.
Vitreous cells are often seen in association with retinal metastases. These cells are usually pigmented in cases of metastatic melanoma and can be large. Vitreous cells in melanoma cases can be floating throughout the vitreous cavity (described as “brown spherules” or “globular vitreous opacities”).64,69 In contrast, vitreous cells associated with metastatic carcinoma tend to be white and confined to the area overlying retinal involvement.68,70
Other signs include secondary glaucoma from tumor invasion into the ciliary body, iris or anterior chamber angle.57
Differential diagnosis
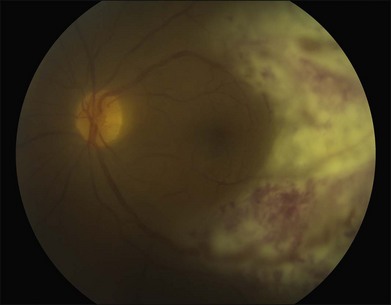
The differential diagnosis of a metastatic retinal lesion is varied, based on the initial appearance of the lesion. Choroidal metastasis with overlying retinal metastasis should be excluded initially (Fig. 133.5). For non-pigmented white retinal lesions seen in metastatic carcinoma, the differential includes inflammatory and infectious diseases such as toxoplasmosis chorioretinitis, cytomegalovirus (CMV) retinitis, herpetic-associated retinitis, syphilitic retinitis, endogenous endophthalmitis, sarcoidosis, multifocal choroiditis, birdshot choroidopathy, acute posterior multifocal placoid pigmentary epitheliopathy (APMPPE), intraocular lymphoma and other collagen vascular diseases. For pigmented lesions such as seen with metastatic melanoma the differential also includes choroidal tumors including melanoma and metastatic choroidal tumors, neovascular macular degeneration. Given the appearance of exudates and hemorrhages in some cases, retinal microvascular diseases such as hypertensive retinopathy, diabetic retinopathy, retinopathy secondary to anemia are also possible diseases. Finally, conditions that may simulate the vitreous floaters of retinal metastases include granulomatous uveitides, amyloidosis, asteroid hyalosis, or intraocular lymphoma.69,70
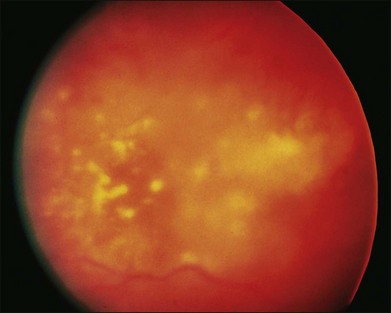
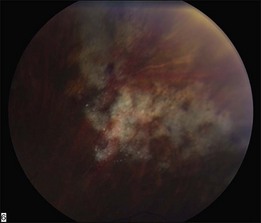
In the setting of systemic malignancy and a potentially compromised immune system, the differential of infectious retinitis warrants further discussion. CMV retinitis typically occurs in patients with CD4 counts <50 cells/µl.89 The appearance of CMV retinitis may begin as a small white retinal infiltrate than can expand to a fluffy white lesion which is perivascular in origin with intraretinal hemorrhages. CMV retinitis can also appear as a granular lesion with atrophic appearance of retina and a leading edge of retinitis with faint whitening and minimal hemorrhage. There is typically minimal vitreous inflammation and the disease tends to spread slowly (approximately 250 µm/week) (Fig. 133.6).89
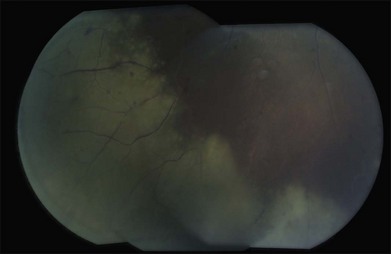
In contrast, herpetic retinitis tends to be rapidly spreading with confluent areas of outer retinal whitening which progress to full-thickness retinitis.89,90 Typically, the peripheral retina is affected first with circumferential progression. The lesions do not usually follow the retinal vessels like CMV retinitis, though an occlusive retinal vasculitis is typically seen.89,90 Significant vitreous inflammation is seen in those with a competent immune system, while those with a compromised immune system can have mild inflammation. Optic disc edema is often seen. The contralateral eye is often also affected (Fig. 133.7).
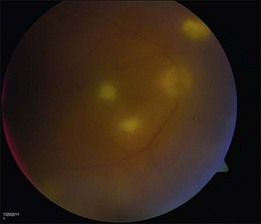
Toxoplasmosis retinochoroiditis can also present as a whitish-yellow retinal lesion. Typically, a chorioretinal scar will be seen in cases of reactivation with surrounding whitening or other satellite lesions.89 Vitreous inflammation is usually seen. In some cases, no previous scar is seen and a solitary white chorioretinal lesion with irregular borders will be noted. A multifocal retinitis can be seen in patients who are immune compromised (Fig. 133.8).89
Diagnostic evaluation
A patient presenting with a retinal lesion with a history of systemic malignancy warrants a metastatic workup. In some case reports, the retinal metastasis was the presenting system in a previously undiagnosed malignancy.76 This presentation is rare and most patients present with a previous history of malignancy. A complete history and physical exam, with review of systems, should be performed. Coordination with the patient’s oncologist is imperative in evaluating for potential metastases. Given the potential for infectious etiologies including endogenous endophthalmitis, a review of recent illnesses, history of fever, chills, or other signs of infections should be reviewed.
Blood work, including serum chemistry, liver function panel, complete blood count, should be ordered.91 Depending on the appearance of the lesion, serum markers for infectious disease such as syphilis (rapid plasmin reagin (RPR) and fluorescent treponemal antibody absorption (FTA-Abs)), toxoplasmosis (anti-toxoplasmosis IgG and IgM antibodies), toxocara should be drawn. Blood cultures may also be drawn. Serum markers for malignancy such as carcinoembryonic antigen (CEA) are not clearly associated with intraocular malignancy, thus their use may be limited in this setting.89
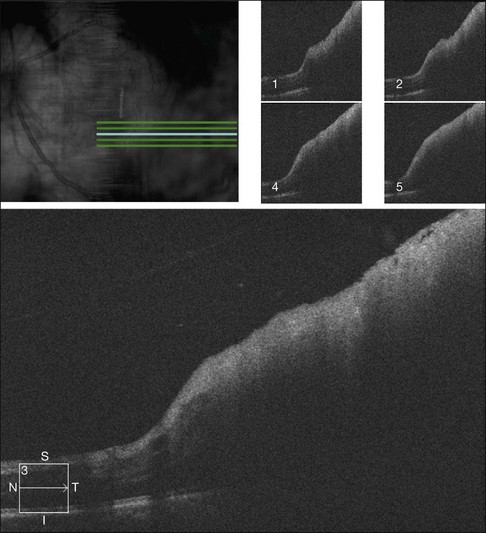
Diagnostic imaging is usually warranted in these patients. B-scan ultrasonography can assist in determining if there is potential choroidal disease with secondary retinal disease. B-scan ultrasonography has shown intermediate to high internal reflectivity on A-scan and dense thickening of the retina on B-scan.69,70,74,75,78,79 However, ultrasonographic analysis of retinal metastases is limited by the thin size of most retinal lesions and the lack of established criteria given the rarity of such cases. Although fluorescein angiography is not diagnostic, it is helpful in differentiating metastatic tumors from non-neoplastic conditions.4 Prominent vascularity is a conspicuous feature of metastatic cancer, and progressive hyperfluorescence in the late phases of the angiogram is characteristic (Fig. 133.9). Optical coherence tomography can also be used to confirm the involvement of the retina (Fig. 133.10). Several case reports describe a thickened retina with hyper-reflectance without any noted changes in the underlying choroid.81
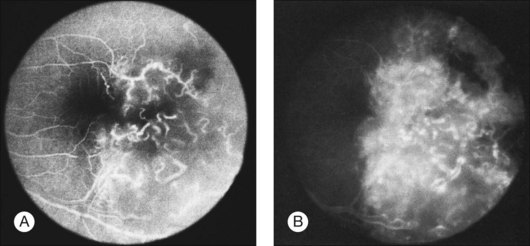
Fig. 133.9 Fluorescein angiogram of the metastatic lesion shown in Figure 133.2. (A) In an early frame, the retinal vasculature is clearly visible, with early hyperfluorescent leakage. (B) In a late frame, staining of the retinal mass is evident.
Ultimately, to make the diagnosis of retinal metastasis, tissue needs to be obtained. This can be done in a number of ways. Prior to obtaining a sample, coordination with an experienced ocular pathologist is recommended in order to identify the correct handling of the specimen and the specific amount of tissue required. A vitreous or aqueous aspirate can be the only sample required to make a diagnosis in those patients with significant vitreous or aqueous cells. Fine-needle aspiration biopsy (FNAB) of intraocular lesions can also be used to establish a diagnosis. Both the reliability and safety of FNABs have been demonstrated with no increase in tumor-related mortality rates.91–93 In some cases however, pars plana vitrectomy with retinal biopsy may be needed. In cases where the diagnosis is unclear and the differential includes infectious etiologies, vitrectomy offers the ability to obtain a substantial sample. In those particular cases, vitreous specimens could be sent for viral and toxoplasmosis polymerase chain reaction (PCR) identification. Tissue biopsy during a vitrectomy offers the ability to directly visualize the area in question and obtain a significant sample. With any sample an experienced pathologist is needed to handle and accurately review the biopsy.91
Treatment
Treatment of metastatic retinal disease varies based on a number of factors. The type of primary tumor, sites of additional metastases, the area of tumor involvement within the eye, vision and the presence of pain all will determine the treatment for the eye. Eyes with poor vision and which are in severe, intractable pain should be considered for enucleation.4,94 External beam irradiation can be effective in controlling intraocular metastatic tumors, thus enucleation is usually only reserved for select cases. In multiple case reports, external beam irradiation has demonstrated effectiveness in controlling tumor size and leading to tumor regression.63,66,71,80,86 In one case, autopsy studies of one eye revealed eradication of the tumor (metastatic pulmonary adenocarcinoma) within the retina, although the retinal architecture had been destroyed by prior tumor invasion.63
When patients are asymptomatic and the intraocular tumor is well-controlled, systemic chemotherapy alone has been considered, but results in patients with retinal metastases have been discouraging. With systemic chemotherapy, three cases of cutaneous melanoma and one case of rectal adenocarcinoma metastatic to the retina failed to respond.64,65,71,72 Additionally, one case of adenocarcinoma of the cecum failed to respond to multiple cycles of chemotherapy, but responded after excision and palliative radiation.85 With systemic and intravitreal chemotherapy, one reported case of breast carcinoma metastatic to the retina displayed partial regression of the vitreous involvement, but retinal detachment with massive vitreous traction occurred.70
Surgical resection has also been described as potential therapy. In one case, as noted above, the lesion was removed via vitrectomy and retinectomy and orbital radiation was performed postoperatively. After 3 months of follow-up, the eye and vision were stable.85 Two reported cases of isolated retinal metastasis secondary to cutaneous melanoma were treated with vitrectomy and surgical resection.73,78 In one case, surgical resection of the vascularized mass in the superotemporal retina, with systemic chemotherapy, resulted in resolution of blurred vision with 20/20 visual acuity. However, brain metastases developed within 1 year, and the patient committed suicide 3 months later.73 In the other case, surgical resection of a peripheral lesion preserved vision for 12 months, until the patient committed suicide.78 For more peripheral isolated lesions, surgical resection and vitrectomy may offer an alternative to external beam irradiation.
Plaque radiotherapy has proven to be effective in the treatment of well-circumscribed, isolated choroidal metastases.66,89,95 However, its use in patients with retinal metastasis has not been described. Photodynamic therapy has been reported as a treatment of a presumed isolated retinal metastasis secondary to breast cancer.87 However, follow-up was limited to only 8 weeks with tumor regression noted and stable visual acuity.
Prognosis
External beam radiation can reduce tumor size and salvage eyes prior to death.63,66,71,86 Several eyes with intractable pain and/or glaucoma required enucleation. Visual acuity results vary and depend on the extent of macular involvement. In those eyes with small lesions in the periphery, there is a chance to maintain good visual acuity as opposed to those eyes with large macular involvement. Despite improved diagnostic techniques and new treatment modalities, the prognosis remains poor.
1 Albert DM, Rubenstein RA, Scheie HG. Tumor metastases to the eye. I. Incidence in 213 patients with generalized malignancy. Am J Ophthalmol. 1967;63:723–726.
2 Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol. 1971;85:673–675.
3 Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch Ophthalmol. 1974;92:276–286.
4 Shields JA, Shields CL. Intraocular tumors: a text and atlas. Philadelphia: WB Saunders; 1992:208–210. 223
5 Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572.
6 Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastases in breast cancer – clinical applications. Nat Rev Clin Oncol. 2010;7:693–701.
7 Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:512–516.
8 Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with125 I-5-iodo-2’-deoxyuridine. J Natl Cancer Inst. 1970;45:773–782.
9 Wong CW, Lee A, Shientag L, et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61:333–338.
10 Podsypanina K, Du YC, Jeklinger M, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844.
11 Hart IR, Goode NT, Wilson RE. Molecular aspects of the metastatic cascade. Biochem Biophys Acta. 1989;989:65–84.
12 Jiang WG, Puntis MCA, Hallett MB. Molecular and cellular basis of cancer invasion and metastatic implications for treatment. Br J Surg. 1994;81:1576–1590.
13 Kurschat P, Mauch C. Mechanisms of metastasis. Clin Exp Dermatol. 2000;25:482–489.
14 Engers R, Gabbert HE. Mechanisms of tumor metastasis: cell biological aspects and clinical implications. J Cancer Res Clin Oncol. 2000;126:682–692.
15 Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284.
16 Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213.
17 Cottam DW, Rennie IG, Woods K, et al. Gelatinolytic metalloproteinase secretion patterns in ocular melanoma. Invest Ophthalmol Vis Sci. 1992;33:1923–1927.
18 Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43:S42–S51.
19 Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis, and metastasis. Trends Cell Biol. 2001;11:S37–S43.
20 Dano K, et al. Plasminogen activation and cancer. Thromb Haemost. 2005;93:676–681.
21 Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174.
22 Harbeck N, Kates RE, Schmitt M, et al. Urokinase-type plasminogen activator and its inhibitor type 1 predict disease outcome and therapy response in primary breast cancer. Clin Breast Cancer. 2004;5:348–352.
23 Riisbro R, Christensen IJ, Piironen T, et al. Prognostic significance of soluble urokinase plasminogen activator receptor in serum and cytosol of tumor tissue from patients with primary breast cancer. Clin Cancer Res. 2002;8:1132–1141.
24 Reese AB. Tumors of the eye, 2nd ed. New York: Harper & Row; 1963.
25 Ferry AP. Metastatic carcinoma of the eye and ocular adnexa. Intl Ophthalm Clin. 1967;7:615–658.
26 Miyasuka M. Cancer metastasis and adhesion molecules. Clin Ortho Rel Res. 1995;312:10–18.
27 Mooy CM, Luyton GP, de Jong PT, et al. Neural cell adhesion molecule distribution in primary and metastatic uveal melanoma. Hum Pathol. 1995;26:1185–1190.
28 Zhu D, Cheng CF, Pauli BU. Mediation of lung metastasis of murine melanomas by a lung-specific endothelial cell adhesion molecule. Proc Natl Acad Sci USA. 1991;88:9568–9572.
29 Hart IR. eed and soil’ revisited: mechanisms of site specific metastasis. Cancer Metastasis Rev. 1982;1:5–16.
30 Zetter BR. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990;322:605–612.
31 Fidler IJ. Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis. Surg Oncol Clin N Am. 2001;10:257–269.
32 Pilch J, Habermann R, Felding-Habermann B. Unique ability of integrin alpha(v)beta 3 to support tumor cell arrest under dynamic flow conditions. J Biol Chem. 2002;277:21930–21938.
33 Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090.
34 Radinsky R. Modulation of tumor cell gene expression and phenotype by the organ-specific metastatic environment. Cancer Metastasis Rev. 1995;14:323–338.
35 Fidler IJ. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592.
36 Radinsky R. Molecular mechanisms for organ-specific colon carcinoma metastasis. Eur J Cancer. 1995;31A:1091–1095.
37 Radinsky R, Ellis LM. Molecular determinants in the biology of liver metastasis. Surg Oncol Clin N Am. 1996;5:215–229.
38 Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568.
39 Campbell JJ, Butcher EC. Chemokines in tissue specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341.
40 Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nature Rev Immunol. 2002;2:175–184.
41 Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56.
42 Hori A, Sasada R, Matsutani E, et al. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991;51:6180–6184.
43 Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993;362:841–844.
44 Zubilewicz A, Hecquet C, Jeanny JC, et al. Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: a comparative study with VEGF. Oncogene. 2001;20:1403–1413.
45 Baird A, Esch F, Gospodarowicz D, et al. Retina- and eye-derived growth factors: partial molecular characterization and identity with acidic and basic fibroblast growth factor. Biochem. 1985;24:7855–7860.
46 Chen CH, Chen SC. Evidence for the presence of a specific vascular endothelial growth factor in fetal bovine retina. Exp Cell Res. 1987;169:287–295.
47 Stitt AW, Simpson DA, Boocock C, et al. Expression of vascular endothelial growth factor (VEGF) and its receptor is regulated in eyes with intra-ocular tumours. J Pathol. 1998;186:306–312.
48 Plate KH, Breier G, Welch HA, et al. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848.
49 Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845.
50 Vinores SA, Kuchle M, Mahlow J, et al. Blood–ocular barrier breakdown in eyes with ocular melanoma. A potential role for vascular endothelial growth factor/vascular permeability factor. Am J Pathol. 1995;147:1289–1297.
51 Goto F, Goto K, Weindel K, et al. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993;69:508–517.
52 Pepper MS, Ferrara N, Orci L, et al. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831.
53 Asahara T, Bauters C, Zheng LP, et al. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:365–371.
54 Smoleroff JW, Agatston SA. Metastatic carcinoma of the retina: report of a case, with pathologic observations. Arch Ophthalmol. 1934;12:359–365.
55 Uhler EM. Metastatic malignant melanoma of the retina. Am J Ophthalmol. 1940;23:158–162.
56 Kennedy RJ, Rummel WD, McCarthy JL, et al. Metastatic carcinoma of the retina. Report of a case and the pathologic findings. Arch Ophthalmol. 1958;60:12–18.
57 Duke JR, Walsh FB. Metastatic carcinoma to the retina. Am J Ophthalmol. 1959;47:44–48.
58 Liddicoat DA, Wolter JR, Wilkinson WC. Retinal metastasis of malignant melanoblastoma: a case report. Am J Ophthalmol. 1959;48:172–177.
59 Riffenburgh RS. Metastatic malignant melanoma to the retina. Arch Ophthalmol. 1961;66:487–489.
60 Koenig RP, Johnson DL, Monahan RH. Bronchogenic carcinoma with metastases to the retina. Am J Ophthalmol. 1963;56:827–829.
61 Flindall RJ, Fleming KO. Metastatic tumour of the retina. Can J Ophthalmol. 1967;2:130–132.
62 Klein R, Nicholson DH, Luxenberg MN. Retinal metastasis from squamous cell carcinoma of the lung. Am J Ophthalmol. 1977;83:358–361.
63 Young SE, Cruciger M, Lukeman J. Metastatic carcinoma to the retina: case report. Ophthalmology. 1979;86:1350–1354.
64 Robertson DM, Wilkinson CP, Murray J, et al. Metastatic tumor to the retina and vitreous cavity from primary melanoma of the skin: treatment with systemic and subconjunctival chemotherapy. Ophthalmology. 1981;88:1296–1301.
65 Letson AD, Davidorf EH. Bilateral retinal metastases from cutaneous malignant melanoma. Arch Ophthalmol. 1982;100:605–607.
66 de Bustros S, Augsburger JJ, Shields JA, et al. Intraocular metastases from cutaneous malignant melanoma. Arch Ophthalmol. 1985;103:937–940.
67 Takagi T, Yamaguchi T, Mizoguchi T, et al. A case of metastatic optic nerve head and retinal carcinoma with vitreous seeds. Ophthalmologica. 1989;199:123–126.
68 Eagle Jr RC. Carcinomatous retinitis. Presentation to the Eastern Ophthalmic Pathology Society, Hilton Head, SC, 1988.
69 Best SJ, Taylor W, Allen JP. Metastatic cutaneous malignant melanoma of the vitreous and retina. Aust NZ J Ophthalmol. 1990;18:397–400.
70 Leys AM, VanEyck LM, Nuttin BJ, et al. Metastatic carcinoma to the retina. Clinicopathologic findings in two cases. Arch Ophthalmol. 1990;108:1448–1452.
71 Striebel-Gerecke SU, Messmer EP, Landolt U. Retinale und vitreale metastase eines kleinzelligen bronchuskarizinoms. Klin Monatsbl Augenheilkd. 1992;200:535–536.
72 Tachinami K, Katayama T, Takeda N, et al. A case of metastatic carcinoma to the retina. Nippon Ganka Gakkai Zasshi. 1992;96:1336–1340.
73 Balestrazzi E, Blasi MA, Marullo M, et al. Local excision of retinal metastasis from cutaneous melanoma. Eur J Ophthalmol. 1995;5:149–154.
74 Spraul CW, Lang GE, Grossniklaus HE, et al. Metastatic adenocarcinoma to the retina in a patient with Muir–Torre syndrome. Am J Ophthalmol. 1995;120:248–250.
75 Spraul CW, Martin DF, Hagler WS, et al. Cytology of metastatic cutaneous melanoma to the vitreous and retina. Retina. 1996;16:328–332.
76 Cangiarella JF, Suhrland MJ, Cajigas A, et al. Esophageal carcinoma metastatic to the retina. Diagnosis of a case by cytologic examination of intraocular vitreous washings. Acta Cytol. 1996;40:995–998.
77 Gunduz K, Shields JA, Shields CL, et al. Cutaneous melanoma metastatic to the vitreous cavity. Ophthalmologica. 1998;105:600–605.
78 Spadea L, Bisti S, Colucci S, et al. Normal EOG values in intraretinal metastasis from cutaneous melanoma: a case report. Doc Ophthalmol. 1999;96:305–309.
79 Soheilian M, Mirbabai F, Shahsavari M, et al. Metastatic cutaneous melanoma to the vitreous cavity masquerading as intermediate uveitis. Eur J Ophthalmol. 2002;12:324–327.
80 Hutchison BM, McAllister IL, Barry CJ. Bowel carcinoma metastatic to the retina. Clin Experiment Ophthalmol. 2001;29:438–439.
81 Truong SN, Fern CM, Costa DL, et al. Metastatic breast carcinoma to the retina: optical coherence tomography findings. Retina. 2002;22:813–815.
82 Zografos L, Ducrey N, Beati D, et al. Metastatic melanoma in the eye and orbit. Ophthalmology. 2003;110:2245–2256.
83 Saornil MA, Blanco G, Sarasa JL, et al. Isolated metastasis of gastric adenocarcinoma to the retina: first presentation of systemic disease. Acta Ophthalmol Scand. 2004;82:86–88.
84 Zografos L, Mirimanoff RO, Angeletti CA, et al. Systemic melanoma metastatic to the retina and vitreous. Ophthalmologica. 2004;218:424–433.
85 Apte RS, Dibernardo C, Pearlman JR, et al. Retinal metastasis presenting as a retinal hemorrhage in a patient with adenocarcinoma of the cecum. Arch Ophthalmol. 2005;123:850–853.
86 Sirimaharaj M, Hunyor AP, Chan WC, et al. Unusual ocular metastasis from breast cancer. Clin Experiment Ophthalmol. 2006;34:74–76.
87 Rundle P, Rennie I. Photodynamic therapy for solitary retinal metastasis from breast carcinoma. Eye (Lond). 2006;20:1410–1412.
88 Kim CY, Ha CW, Lee SC. Vitreous and retinal metastasis from gastric cancer. Eur J Ophthalmol. 2010;20:615–617.
89 Nussenblatt RB, Whitcup SM. Uveitis, fundamentals and clinical practice, 3rd ed. Philadelphia: Mosby; 2004.
90 Holland GN. Executive committee of the American Uveitis Society: Standard diagnostic criteria, for the acute retinal necrosis syndrome. Am J Ophthalmol. 1994;117:663–666.
91 Char DH. Clinical ocular oncology, 2nd ed. Philadelphia: Lippincott-Raven; 1997. p. 103–22, 159–60
92 Shields JA, Shields CL, Ehya H, et al. Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick lecture. Ophthalmology. 1993;100:1677–1684.
93 Augsburger JJ. Fine needle aspiration biopsy of suspected metastatic cancers to the posterior uvea. Trans Am Ophthalmol Soc. 1988;86:499–560.
94 Shields CL, Shields JA, De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–245.
95 Shields CL, Shields JA, De Potter P, et al. Plaque radiotherapy for the management of uveal metastasis. Arch Ophthalmol. 1997;115:203–209.