Chapter 25 Restless Legs Syndrome in Medical Disorders
Restless legs syndrome (RLS) has been associated with multiple medical conditions in addition to its association with the neurological conditions discussed in Chapter 26. The most noteworthy of these medical conditions are end-stage renal disease (ESRD) and medical conditions that produces iron deficiency (ID). The principal features that link these disorders to RLS are (1) higher rates of RLS in these disorders than those seen in the general population, (2) temporal association of the onset of RLS to the presence of these disease processes, and (3) resolution of the RLS symptoms with treatment of the precipitating condition. For these reasons, RLS associated with either ESRD or ID is defined in “secondary” RLS. However, it should be made clear that the pathophysiology of both primary and secondary RLS is not clearly understood and that those with secondary RLS may share some or all of the various underlying bases for the symptoms occurring in “idiopathic,” or primary, RLS. Similarly, those with RLS associated with a particular medical condition may not form a homogeneous etiological entity. Nevertheless, subtyping of diseases by associated medical conditions is one of the potential means by which more homogeneous groups can be created with which to understand underlying mechanisms of disease development and progress. This chapter focuses first on ESRD and then on the broader concept of the relation of ID to RLS and in particular the occurrence of RLS in medical conditions causing ID. We conclude with a discussion of the relationship between RLS and rheumatic conditions and diabetes.
Ekbom1 first recognized the association between RLS and ID more than 50 years ago. More recent work has identified ID as a risk factor for RLS, related to poor nutrition, blood donation, and medical procedures that produce blood loss or hemolysis. Furthermore, ID may be a common thread tying together essentially many of the seemingly diverse secondary causes of RLS other than those that are primarily neurological, such as neuropathy. Given the significance of ID in RLS and our inability to measure central nervous system iron on a clinical basis, the criteria for determining ID also need to be adjusted to a level of ferritin of less than 45 to 50 µg/L or percentage transferrin saturation of less than 16%. Iron treatment should be considered for all RLS patients who are iron deficient (see Chapter 34). Both ESRD and ID-related RLS provide an opportunity to better understand the pathophysiology of all RLS.
Restless Legs Syndrome in End-Stage Renal Disease
The high prevalence and severe nature of RLS in ESRD potentially provide some tantalizing clues as to the pathogenesis of RLS. ESRD is generally defined as the irreversible loss of renal function, to a creatinine clearance of less than 15 ml/min per 1.73 m2. It is present in roughly 400,000 adults in the United States, with an incidence of 260 cases per million per year in whites and 840 per million per year in African Americans. The incidence of ESRD is increasing by 6% per year, probably as a result of the increasing prevalence of diabetes, which accounts for nearly half of all cases of ESRD.2 Unfortunately, the long-term prognosis for ESRD is poor, with mortality rates of roughly 20% per year. Over 80% of individuals with ESRD are on hemodialysis, although some people use peritoneal dialysis and others receive transplanted kidneys, often after a period of receiving one of the types of dialysis.
Roughly 60% to 80% of patients with ESRD describe difficulty with nocturnal sleep quality and quantity and/or excessive daytime sleepiness.3 Multiple causes for sleep disturbance are present in those with ESRD. Depressive and anxiety disorders, poor sleep hygiene (e.g., daytime napping), pain, pruritis, symptoms of heart disease (nocturnal dyspnea or chest pain), and symptoms of diabetes (neuropathic pain, fluctuations in blood sugar) are all common in ESRD, and all may contribute to difficulties with nocturnal sleep. Among primary sleep disorders, central and obstructive sleep apneas and RLS are commonly observed at higher rates than in the general population.3
Prevalence and Characteristics of Restless Legs Syndrome in End-Stage Renal Disease
In 1966, Callaghan4 first described five patients with classic RLS in ESRD, which he thought was related to subclinical neuropathy. He also described RLS in the upper limbs and leg movements during sleep in two of these patients, both of which are common associated symptoms of RLS. A number of studies have reported the association of ESRD and RLS and have further clarified the prevalence, characteristics, and consequences of the disorder in this population.
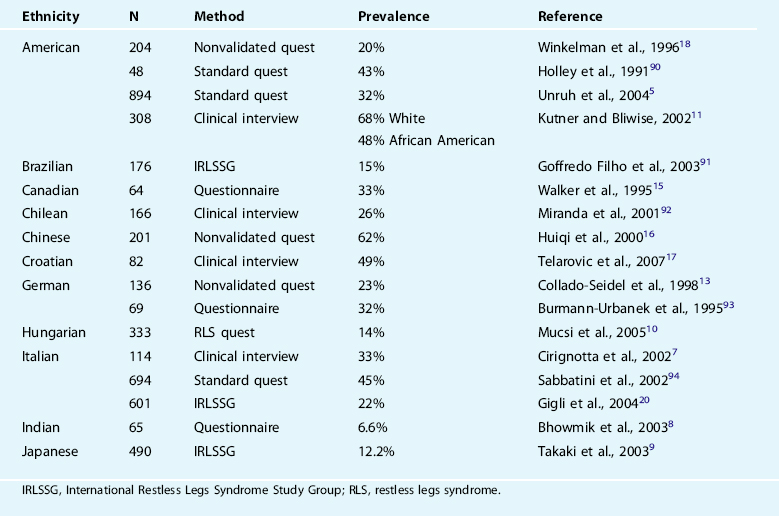
Estimates of the prevalence of RLS in ESRD vary between 15% and 70% in the published studies (Table 25-1). A number of potential factors contribute to this variability, including the method of patient recruitment, method of diagnosing RLS (questionnaire versus interview), the ethnicity of the population, adequacy of the anemia treatment, use of intravenous iron in treatments, and the severity of RLS required for a diagnosis. There does not appear to be any difference between rates of RLS in those receiving hemodialysis from those getting peritoneal dialysis.5 There are no data on individuals with acute renal failure or on rates of RLS before dialysis dependence.
The RLS phenotype in ESRD appears to be very similar to that of primary RLS. There is some suggestion, from studies including both ESRD-related and primary RLS, that the daytime symptoms and nocturnal sleep disruption are more severe in the former and in particular the leg movements in sleep may be more pronounced.6 Nevertheless, diagnosis of RLS in ESRD is complicated by comorbid disease commonly observed in those with ESRD, particularly peripheral neuropathy. For instance, one Italian study demonstrated that a questionnaire similar to that usually used as a screening tool for RLS had modest specificity (81.6%) with low sensitivity (44.7%), giving a low positive predictive value of 47.4% in identifying ESRD patients with RLS.7 Less than half of the patients identified as having RLS by the questionnaire actually had the disorder. This raises some uncertainty about the true prevalence of RLS in dialysis, but even with these limits the best estimates indicate a very high RLS prevalence.
Dramatic ethnic variation has been reported for prevalence of ESRD-related RLS, with northern Europeans generally demonstrating higher rates than those reported in India,8 Japan,9 and Hungary.10 However, the wide variability in prevalence rates found across all ethnicities in Table 25-1 casts doubt on the validity of the often reported regional or ethnic differences in RLS prevalence. Similarly, it is unclear whether African American patients with ESRD have lower11 or the same12 rates as whites living in the same areas.
Etiology of Restless Legs Syndrome in End-Stage Renal Disease
The pathogenesis of RLS in ESRD is largely unknown, although treatment studies suggest that iron and anemia status are significant factors. A number of studies have assessed large groups of such patients and compared a variety of variables in ESRD patients with and without RLS, looking for factors that might distinguish the two groups. The majority of such analyses have been negative. Although Collado-Seidel and colleagues13 found higher parathyroid hormone (PTH) levels in ESRD patients with RLS than in those without RLS, Stepaniski and colleagues14 found the opposite result, with lower PTH levels in ESRD-related RLS patients, suggesting that this finding and that by Collado-Seidel and colleagues were probably type 1 errors resulting from inadequate statistical correction for the number of factors evaluated. Walker and colleagues15 found that BUN and creatinine levels were higher in those with RLS and ESRD, but again these results did not use statistics with adequate control for the multiple comparisons and the elevation in creatinine levels did not meet the usual criteria for statistical significance (p >.05). These results, not surprisingly, have not been confirmed in other studies. Similarly, some authors have found that duration of dialysis dependence or number of dialysis sessions predicts RLS in ESRD, whereas others have not.16,17 This disagreement cannot be explained by the problem of not correcting for multiple comparisons, but there are significant confounding factors affecting dialysis duration that likely differ between dialysis programs. Of note is that most studies have not found a consistent relationship between ESRD-related RLS and other common predictors of RLS in the general population such as age, gender, and iron indices.10,15,18,19 Similarly, although some authors have found a higher prevalence of (only) severe RLS in diabetics with ESRD,5,16 others have not.18,20 One study found an increased history of use of calcium channel blockers.21
Given the inconsistency and negative findings of the majority of investigations into correlates of RLS in ESRD, our understanding of the pathogenesis of RLS in this condition is limited to treatment studies. Extrapolation of theories regarding primary RLS pathophysiology to ESRD-related RLS seems reasonable, although little work has been performed testing such theories. For instance, no neuroimaging or neuroendocrine assessments of dopamine function have been performed in ESRD-related RLS. Although rates of familial RLS in those with ESRD appear to be similar to those in the general population,22 genetic studies, which have been of value in defining chromosomal markers in familial primary RLS, may still help define an underlying genetic predisposition to develop ESRD-related RLS.
Finally, although normalization of the hematocrit with supplemental erythropoietin has been shown to reduce the PLM index during sleep,22 serum iron measures do not predict those patients with ESRD who develop RLS.15,18 However, some of the usual serum measures of iron status (e.g., ferritin) are distorted by the disease process in ESRD. Alternative evaluations available for iron as significant factors in the pathogenesis of RLS are the clinical responses to aggressive iron treatment (combined with erythropoietin) and the post-transplantation status. Fortunately, here we have better data. First, there is a blinded, placebo-controlledation study of intravenous iron administration that showed effective an dramatic reduction in RLS symptoms,23 even though iron markers were normal before intravenous iron treatment. Second, there is and anecdotal impression that rates of RLS in ESRD have decreased since the widespread adoption of supplemental intravenous iron administration combined with erythropoietin. Third, RLS rarely occurs among post-transplantation patients, but the small number with RLS have significantly lower hemoglobin levels and more ID than those without RLS.24 Further work in this area, in particular with assessment of central nervous system indices of iron storage and function, will be important. At this point, iron insufficiency is the only putative cause of RLS in ESRD that has some experimental support.
Restless Legs Syndrome Morbidity in End-Stage Renal Disease
Although it is not a criterion for RLS, sleep disturbance is one of the most salient symptoms of RLS, and the one that often drives patients to seek treatment. All studies examining self-reported sleep in patients with RLS in ESRD have demonstrated severe impairments in sleep, including difficulty falling asleep, frequent nocturnal awakenings, early morning awakening, and shortened total sleep time, with some data suggesting worsened sleep with increasing severity of RLS.3 Studies using polysomnography to assess sleep in ESRD patients with RLS have generally shown prolonged sleep latency, increases in the percentage of light sleep (stages I and II), and reductions in total sleep time and sleep efficiency,22,25 which is similar to the sleep of RLS patients in general.25 ESRD patients with RLS also consistently take more hypnotic medications than those without RLS.5,14,16,17
The sleep disturbance in these patients is certainly a result of the sensory dysesthesia and motor restlessness present during wake but may also be related to periodic leg movements of sleep (PLMS), which are present in the majority of RLS patients but also in some patients with ESRD without RLS. In those with ESRD and RLS, the index of PLM per hour of sleep has been noted to be higher than in idiopathic RLS and to be inversely correlated with reports of subjective sleep quality,24 as well as to quality of life.26
Excessive daytime sleepiness (EDS) is present in 30% to 50% of patients with ESRD, by either physiologic (Multiple Sleep Latency Test [MSLT]) or subjective (Epworth Sleepiness Scale [ESS]) measurement.27,28 Although other features of ESRD, such as blood urea nitrogen,22 and obstructive sleep apnea, certainly relate to this finding, it appears that RLS and/or PLMS is closely associated to patients’ EDS. Both Hui and coworkers29 and Gigli and coworkers20 found an association between ESRD patients with RLS and excessive daytime sleepiness, the latter reporting that scores on the ESS greater than 9 were over five times (16% versus 3%) as likely for those patients reporting RLS as for those without these symptoms. Nevertheless, the average ESS values reported (of ≅4) are certainly within the normal range. PLMS may also relate to daytime sleepiness. Hanly and coworkers28 investigated 24 patients with ESRD and found that the PLM index was significantly and substantially higher in those with EDS than those without EDS (57 versus 6 per hour of sleep), whereas multiple indices of sleep apnea severity were not significantly associated with measures of daytime sleepiness. Stepanski and coworkers,14 in a smaller group of ESRD patients without obstructive sleep apnea, found similar results.
Compliance with hemodialysis (hemodialysis) treatments, which requires relative immobility for 4 hours, 3 times per week, is suboptimal for the general group of patients with ESRD. Roughly 8% of patients in the United States miss at least one hemodialysis session per month, and nearly 20% shorten a session by at least 10 minutes.30 However, for those with RLS, adherence to hemodialysis is one of the most frustrating and uncomfortable aspects of their life. Winkelman and coworkers18 found that reported premature discontinuation of dialysis was substantially more likely in patients with ESRD-related RLS compared with those without RLS (p <.0001). Nonadherence with the dialysis prescription has been associated with a variety of poor intermediate outcomes (e.g., hospitalization), as well as with premature mortality, the latter being 14% to 30% more likely with just one skipped session per month.24,26,30–32
The studies with an adequate sample size (more than 10 RLS patients) have shown that multiple features of quality of life are worse in patients with ESRD-related RLS than in those without RLS.5,26 Unruh’s5 epidemiologic study of 145 patients with RLS found that mental health composite scores and vitality were substantially worse in those with RLS, even after controlling for sleep quality. Mucsi and associates33 also found that a kidney disease–specific quality of life was impaired in those with RLS, compared with those without RLS, even after controlling for sleep quality.
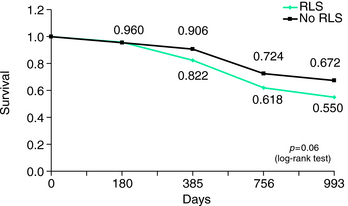
The two reports of increased mortality in ESRD-related RLS and a similar finding in ESRD patients with PLMS are possibly the most important and surprising findings related to consequences of RLS. Winkelman and associates18 first reported a significantly higher mortality rate (odds ratio = 1.85) at 2-year follow-up in ESRD patients with at least moderate RLS compared with those without RLS (total N = 204), which remained significant even after controlling for age, gender, and duration of dialysis (p <.02) (Fig. 25-1). Unruh and associates5 replicated this finding in a study of 894 patients with ESRD, even after controlling for multiple potential confounds including coincident disease burden, although the increased mortality risk was present only for those with severe RLS (hazard ratio = 1.39). Benz and associates34 also found elevated mortality rates in those ESRD patients with elevated rates of PLMS by overnight polysomography.
Work-up of the End-Stage Renal Disease Patient With Restless Legs Syndrome
Once the diagnosis of RLS is made in a patient with ESRD, an evaluation for potential underlying causes and/or important comorbidities is recommended. The primary reversible causes of RLS in this population are ID and medications. Iron deficiency is common in patients on hemodialysis and is believed to be related to chronic phlebotomy, dialysis-related blood loss, and hemolysis.35 It may have become more pronounced since the introduction of erythropoietin administration. Its importance is underscored by its association as an independent predictor of dialysis sign-offs.18 Iron deficiency is assessed in different ways in patients with ESRD than in those with primary RLS, given the unreliability of a ferritin determination as a reflection of underlying iron status, due to its role as an acute phase reactant. For this reason, morning fasting serum iron and transferrin saturation are commonly used.36
A variety of medications can exacerbate RLS, including serotonergic antidepressants,37 dopaminergic antagonists,38 and withdrawal from opiates.39 Avoidance of the former medications when possible, and avoidance of daytime use of short-acting opioids may reduce the severity or prevent the appearance of RLS altogether.
Treatment of Restless Legs Syndrome in End-Stage Renal Disease
Four trials using L-dopa have been published for ESRD-related RLS.40–43 Three were crossover trials—one with an active comparator, comparing L-dopa SR with ropinirole, and the other two against placebo. The other trial was an open-label retrospective comparison of L-dopa against gabapentin. One crossover trial was open label, and the placebo comparisons were double blinded. All studies were small, with only 41 patients receiving L-dopa in all four studies combined. Differing outcome measures were used in the studies to assess RLS severity (none of them used the IRLSSG severity scale). Three of the four studies found an overall reduction in RLS severity of 18% to 34%, using L-dopa doses of 146 to 190 mg, given once per night. The fourth study, comparing L-dopa with placebo, which did not find a treatment difference, used only 100 mg of L-dopa SR. Substantial improvements in sleep were observed in three of four studies with L-dopa. In the study using L-dopa SR, total sleep time was improved 41% (from 4.1 ± 1.7 to 5.8 ± 1.6 hours) but was still significantly less than that obtained with ropinirole (7.9 ± 1.0). Again, in the study using L-dopa SR 100 mg, no differences in sleep duration or latency were observed. In the short-acting L-dopa study employing polysomnography, the PLM index was significantly decreased in the first 4 hours of the night (from 119 ± 56 to 69 ± 61), but had much less effect in the final half of the night, whereas the study using the low-dose SR, the PLM index was decreased throughout the night. L-Dopa was generally well tolerated in these studies, with only one patient withdrawing due to side effects (vomiting).
The dopaminergic agonist ropinirole demonstrated remarkable efficacy in an open-label crossover trial versus L-dopa (noted previously) in 11 patients with ESRD-related RLS.42 Dosages ranged from 0.25 to 2.0 mg, with a mean of 1.45 mg, given 2 hours before bedtime. Self-reported severity with the RLS-6 decreased nearly 75%, sleep duration increased 88% (to nearly 8 hours), patient-rated CGI mean improvement was 2.7 (between much improved and very much improved), and four patients reported elimination of all RLS symptoms. No adverse events were reported. Less than 10% of ropinirole is excreted as unchanged drug in the urine, the drug is almost entirely inactivated by metabolism in the liver, and none of the major circulating metabolites have pharmacological activity, thus decreasing the potential toxicity of this agent in ESRD.
Gabapentin has also shown promising efficacy in ESRD-related RLS in one placebo-controlled double-blinded crossover trial44 and one open-label comparison with L-dopa.41 In the crossover study, 12 of 16 patients responded to 200 to 300 mg of gabapentin, whereas only 2 of 16 responded to placebo. Two subjects dropped out due to gabapentin-related lethargy. Gabapentin is not metabolized and is eliminated exclusively by renal excretion. Gabapentin serum concentrations are decreased roughly by one half by 4 hours of dialysis.45 Thus, when gabapentin is administered to anuric patients, it is recommended that it be given only after dialysis treatments, which will then replace the drug eliminated during the hemodialysis treatment and maintain steady-state concentrations as if it were being administered TID or QID.
In one short crossover trial,46 the α1-antagonist clonidine (at an average dose of 0.075 mg BID) was superior to placebo in reducing the symptoms of RLS in patients with ESRD. Eight of 10 patients had complete relief of RLS symptoms in the clonidine group versus only 1 of 10 for placebo. However, clonidine is excreted by the kidneys, and thus the half-life is substantially lengthened and serum levels increased in those with ESRD.
In a patient with low serum iron, either the oral or intravenous route is used as supplemental sources. For oral iron, Fe2+ 150 to 200 mg of elemental iron per day is recommended to keep hemoglobin at 11 to 12 g/dl, ferritin greater than 100, transferrin saturation greater than 20%, and/or reticulocyte hemoglobin content (CHr) greater than 29 pg. Intravenous iron is often given at the time of dialysis sessions, and 1 g is generally administered over 10 dialysis sessions when poor absorption or side effects of oral iron occur.47
A careful double-blind placebo-controlled treatment trial of intravenous iron in ESRD-related RLS found a significantly larger therapeutic effect of 1000 mg than with placebo.23 The treatment benefit was present 1 week after infusion, was maximal at 2 weeks, and had lost significance by 4 weeks. No excess adverse effects were observed in the intravenous iron group.
Improvement in symptoms of RLS in patients with ESRD after kidney transplantation was first noted in 1986 in a single case.48 More recently, this has been confirmed in larger case series.49,50 Winkelman and colleagues18 described 11 patients with ESRD and RLS whose kidney transplantation produce rapid (within 1 to 21 days) relief of RLS. In some patients, failure of the transplant led to reappearance of RLS, and in one patient, retransplantation again led to relief of symptoms. Molnar and colleagues50 replicated these findings in a cross-sectional assessment of 816 transplanted patients. They found that estimated glomerular filtration rate predicted reappearance of RLS, with declining kidney function predicting symptoms, such that 2% who had a glomerular filtration rate greater than 60 ml/min/1.73 m2 had RLS, whereas 24% of those with a glomerular filtration rate of less than 60 ml/min/1.73 m2 had RLS.
Conditions With Iron Deficiency and Restless Legs Syndrome
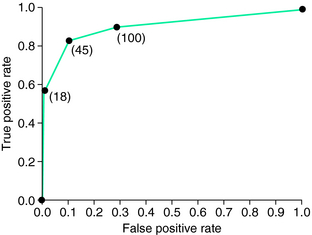
There is increasing evidence that there is a relationship between ID and RLS. In fact, ID may be a major cause of all secondary RLS not associated with neurologic diseases, including ESRD and pregnancy. The patient with ID may not have anemia. Once anemia is present, the marrow iron stores are zero and the ferritin level is often less than 10 µg/L,51 but marrow iron stores may be zero even with ferritin levels as high as 100 µg/L. O’Keeffe52,53 noted the values for normal serum ferritin are usually set by population samples ignoring the biological issues of relation between serum ferritin and bone marrow iron status. When Guyatt and associates54 evaluated the standard receiver-operator characteristic (ROC) curve to assess prediction of bone marrow ID from serum ferritin, they found 45 µg/L to be the optimal criterion (Fig. 25-2). These data were obtained in a population with suspected ID, which of course alters the usual ROC curve, but RLS patients can be assumed to represent a population with increased risk of ID. Thus, it becomes appropriate to apply these criteria to all clinical management of RLS. Moreover, both ferritin and percent transferrin saturation should be used to evaluate iron status in RLS. A low value in either one indicates probable ID for an RLS patient: that is, ferritin less than 50 μ/L or transferrin saturation less than 16% indicates probable ID.
Several lines of research demonstrate the importance of iron for RLS. Research data indicate that a major cause of RLS may be a decreased level of availability and acquisition of iron in the basal ganglia (substantia nigra and putamen), adversely affecting dopamine synthesis.55,56 Some other studies have found that there is a relationship between the concentration of serum ferritin and the severity of the symptoms.57,58 It is also hypothesized, in one study, that there is a defect in transport of iron into the brain leading to reduced brain iron. This study suggests that RLS is a disorder of blood-brain barrier iron transport.59
A causal relationship between iron metabolism and RLS would also explain the circadian pattern seen in the symptomatology of RLS, as there is a drop in iron availability at night, with a 30% to 50% drop in serum iron concentration.60
Studies have also investigated if iron supplement is an effective treatment of RLS. In two studies where oral iron was given to those with reduce serum ferritin (below 45 µg/L in one61 and below 75 µg/L in the other62) both showed reduced RLS symptoms with the treatment. In a poorly designed study that did not control for low serum ferritin there was no benefit for oral iron treatment,63 but in this study the serum ferritin values were high enough that there would be little, if any, absorption of the oral iron and thus any observed treatment benefit would have been the same as the placebo treatment. Intravenous iron has been reported to be effective in treatment in two studies.64,65 However, there is a need for more data on trials with oral or intravenous iron in RLS, using a randomized placebo-controlled protocol.
The first report in the literature on a claimed relationship between ID and restlessness in the legs was written by an anonymous author as a letter to the editor in the British Medical Journal in 1946.66 This short document is also informative about how this patient experienced her symptoms, particularly in relation to her pregnancies, and is quoted in full below:
I am writing this in case such a line of treatment would help other cases. The diagnosis of the anemia was arrived at accidentally. I was in good general health. Having “jittery legs“ sounds a stupid form of complaint, but it can be a real burden physically, mentally, and socially, and like chilblains is one of the things for which the medical profession offers sympathy but little relief.-I am, etc.,
The lack of awareness of RLS was certainly bigger at that time, only 1 year after Ekbom published his thesis “Restless Legs—A Clinical Study of a Hitherto Overlooked Disease.”1 Accordingly, there was no therapy known for physicians to offer these patients until many years later. In his 1945 thesis, Ekbom reports three patients with RLS who also had anemia, but he did not recognize the relationship between ID and RLS. Therefore, 10 years later, he alluded to the “Doctor’s Wife” as the first person in the literature who recognized the relationship between iron and restless legs.67
In 1953, Nordlander64 published a sample of 22 patients with restless legs, who received one or two intravenous injections of iron, which proved very effective, regardless of whether the patient had anemia or ID. In the same paper, he reported that he observed three cases of anemia from different causes (bleeding gastric ulcer, chronic nephritis, and leukemia) where restless legs developed in parallel with the anemia and disappeared when the anemia was cured by blood transfusions. In the patient with chronic nephritis, this parallelism was repeated several times.64
In 1955, Ekbom67 reported on two men with malignancies (cancer in the urinary bladder and cancer in the gut) who had restless legs. In both of these cases, the main reason for them to see a physician was restlessness in their legs, which had a severe negative impact on their sleep. At examination, anemia and ID were observed. The symptoms of restless legs were improved in these two cases with intravenous injection of iron. In the same paper, Ekbom reported 34 cases (25 female, 9 male) with RLS, where 13 (38%) had low serum iron values, all below the lower limit of normal.
Due to these findings, together with the observations of the cancer cases, Ekbom claimed that there often is reason for the physician to ask for symptoms of restless legs in patients presenting with anemia and/or ID and to perform analyses of iron status in RLS cases. If ID is diagnosed, its cause should always be pursued, at least in males.67
In a population study of individuals 25 years old or older, all persons with a suspicion of ID were questioned in 1964 by Aspenstrom68 about the compulsory phagomania called pica and about symptoms of restless legs. Pica was almost never mentioned spontaneously, so this looks like the situation with RLS subjects! During 1 month, 80 cases of verified ID were found. Two thirds (42 of 64) of the women and 25% of the men admitted the presence of typical pica. Restless legs were reported in 27% of the women and in 25% of the men. Thus, in this study, pica was found to be the most common specific complaint in female ID, but restless legs was a complaint in nearly half of the female subjects who were examined. Restless legs, particularly in women, blood donors, and gastrectomized persons, should probably be initially treated with iron according to the author.68
In 1966, Karl Ekbom Jr.69 approached 317 patients during a follow-up examination 7 to 9 years after partial gastrectomy by the Billroth I or II method for gastric or duodenal ulcer. Restless legs syndrome had developed after the operation in 40 cases (12.6%).
Regular blood donation may constitute a significant cause of ID, especially in female donors.70 The recommended intake of iron to substitute for iron loss after one blood donation is 2000 mg. This recommendation is based on the fact that there is a loss of iron of 200 to 250 mg at each blood donation and that the uptake from the gastrointestinal tract of the iron taken orally is 10%. Thus, if the mean intake of iron is much lower than what is recommended after each donation, net losses of iron occur over several years and may lead to a substantial negative impact on iron metabolism in the human body.
Ekbom71 was the first to report two cases of blood donors, both without anemia, but with low serum iron values, with concomitant RLS. Silber and Richardson72 and Kryger and colleagues73 have also reported on cases series with repeated blood donations, and it was hypothesized that their RLS was a result of their ID, due to an insufficient iron substitution after blood donations.
In a Swedish survey, 946 consecutive blood donors ages 18 to 64 years were assessed with use of a questionnaire.74 Red blood cell distribution width (RDW) was also recorded, a value of blood cell production that increases in relation to ID. RLS affected 14.7% of the male blood donors and 24.7%, of the female blood donors. The mean intake of iron among the blood donors after each blood donation was only 781 mg, thus much lower than recommended. Among the women, 7.4% presented with an RDW greater than 14.5%, which strongly indicates ID. In this group of women, 37.5% were affected by RLS. The female RLS sufferers were more affected than the female non-RLS subjects with both problems initiating and maintaining sleep, and were also more unrefreshed on awakening. Although the intake of iron was higher among women than among men, the women showed a higher frequency of RLS than the men. Moreover, a relationship between ID and RLS was only shown among the female blood donors. It appears that female blood donors have a gender-based susceptibility for ID.
For those who have RLS, blood donation may necessitate a greater iron intake to attain even higher ferritin levels. This recommendation was made by Ekbom to RLS subjects donating blood as early as 1956!71
In the course of routine patient care, Tings and collaborators75 in Goettingen, Germany, noted that numerous LA patients also had RLS. This observation prompted them to explore the occurrence of RLS in a group of LA patients.
Of 25 LA patients, 12 (48%) were found to have RLS. One patient already had RLS before the LA procedure was initiated. The other 11 patients developed RLS during regular LA treatments. Of the affected patients, 5 (42%) had a positive family history for RLS. Laboratory investigation showed that 11 of 12 patients with RLS had ferritin levels below, or at the lower limit of, the normal range, indicating a depletion of body iron stores. Consistent with this finding, transferrin levels were elevated or in the upper range of normal in these patients. However, there were no significant differences in the iron-binding parameters between the LA patients both with and without concomitant RLS. These data corroborate the data on RLS in Swedish blood donors,74 that depletion of iron may be sufficient to induce RLS only in susceptible individuals. These data give further support to the hypothesis that ID is primarily a trigger for RLS in patients who have a genetic predisposition for the syndrome.
Summary
There is growing evidence showing that iron insufficiency is involved in the pathophysiology of restless legs. It is hypothesized that iron insufficiency might be the second most common etiology of RLS after heredity. However, because central nervous system ID appears to be marked particularly in early-onset RLS patients, it is open for speculation as to whether iron might also play a major role in the genetics of RLS, as the end result of the genetic error leading to RLS may be due to a defect in uptake or a disordered metabolism of iron in the central nervous system. The report of an association between RLS and the NOS-1 gene on chromosome 1276 would be a possible example of such a genetic relationship given its relation to iron signaling for the regulation of DMT1.77
Rheumatoid Arthritis
Rheumatoid arthritis is another medical condition associated with anemia and iron status. The relation to RLS has not, however, been well established. Salih and colleagues78 in a survey of arthritis patients, reported an RLS prevalence of 25% for rheumatoid arthritis compared with 4% for osteoarthritis. These two conditions share some symptom overlap, but included in the differences between them is the relation to iron. Rheumatoid arthritis commonly occurs with anemia and iron compromise not generally seen with osteoarthritis. Györfi and colleagues79 examined the potential relations between iron status and RLS. They found in a small group of 19 rheumatoid arthritis patients that 9 (47%) had RLS, and these had a statistically significant elevation in serum transferrin receptor indicating an ID. These small studies hardly suffice to demonstrate a connection between rheumatoid arthritis, iron status, and RLS, but they are consistent with the body of literature suggesting that when ID occurs with any medical condition it increases the risk of RLS. Future studies of associations between RLS and both arthritis and other pain conditions need to be done with evaluation of the iron status of the patients.
An increased frequency of RLS has been suggested in additional rheumatic autoimmune conditions such as fibromyalgia,80,81 scleroderma,82 and Sjögren’s syndrome.83
It should be noted that even if RLS is associated with these conditions, they are sufficiently uncommon and do not manifest with RLS, so that routine screening of RLS patients for these conditions is not useful.84
Diabetes
Some epidemiologic studies85,86 and clinical series87 have found a higher frequency of RLS in diabetics. More compelling evidence has emerged from studies of diabetic clinical populations,88 including a case-control study89 that showed a three-fold increase in diabetics compared with other endocrinology controls (17.7% versus 5.5%). One factor that has been highlighted in recent studies was the presence of diabetic neuropathy as a risk factor for RLS in diabetic populations.89 The likelihood of this risk factor is also supported by a study of patients with diabetic neuropathy who had an RLS frequency of 33%.89
1. Ekbom KA. Restless legs. Acta Med Scand. 1945;158(suppl):1-123.
2. McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med Clin North Am. 2005;89:419-445.
3. Parker KP. Sleep disturbances in dialysis patients. Sleep Med Rev. 2003;2:131-143.
4. Callaghan N. Restless legs syndrome in uremic neuropathy. Neurology. 1966;16:359-361.
5. Unruh ML, Levey AS, D’Ambrosio C, et al. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Restless legs symptoms among incident dialysis patients: Association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43:900-909.
6. Wetter TC, Stiasny K, Kohnen R, et al. Polysomnographic sleep measures in patients with uremic and idiopathic restless legs syndrome. Mov Disord. 1998;13:820-824.
7. Cirignotta F, Mondini S, Santoro A, et al. Reliability of a questionnaire screening restless legs syndrome in patients on chronic dialysis. Am J Kidney Dis. 2002;40:302-306.
8. Bhowmik D, Bhatia M, Gupta S, et al. Restless legs syndrome in hemodialysis patients in India: A case controlled study. Sleep Med. 2003;4:143-146.
9. Takaki J, Nishi T, Nangaku M, et al. Clinical and psychological aspects of restless legs syndrome in uremic patients on hemodialysis. Am J Kidney Dis. 2003;41:833-839.
10. Musci I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20:571-577.
11. Kutner NG, Bliwise DL. Restless legs complaint in African-American and Caucasian hemodialysis patients. Sleep Med. 2002;3:497-500.
12. Unruh M, Miskulin D, Yan G, et al; HEMO, Study Group. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int. 2004;65:1482-1491.
13. Collado-Seidel V, Kohnen R, Samtleben W, et al. Clinical and biochemical findings in uremic patients with and without restless legs syndrome. Am J Kidney Dis. 1998;31:324-328.
14. Stepanski E, Faber M, Zorick F, et al. Sleep disorders in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1995;6:192-197.
15. Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995;26:751-756.
16. Huiqi Q, Shan L, Mingcai Q. Restless legs syndrome (RLS) in uremic patients is related to the frequency of hemodialysis sessions. Nephron. 2000;86:540.
17. Telarovic S, Relja M, Trkulja V. Restless legs syndrome in hemodialysis patients: association with calcium antagonists. A preliminary report. Eur Neurol. 2007;58:166-169.
18. Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996;28:372-378.
19. Roger SD, Chen SC, Lawrence S, et al. Pseudomonas putrefaciens bacteraemia in a peritoneal dialysis patient. Nephrol Dial Transplant. 1991;6:73.
20. Gigli GL, Adorati M, Dolso P, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5:309-315.
21. Benz RL, Pressman MR, Hovick ET, et al. A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (The SLEEPO study). Am J Kidney Dis. 1999;34:1089-1095.
22. Winkelmann J, Wetter TC, Collado-Seidel V, et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep. 2000;23:597-602.
23. Sloand JA, Shelly MA, Feigin A, et al. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis. 2004;43:663-670.
24. Molnar MZ, Novak M, Ambrus C, et al. Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis. 2005;45:388-396.
25. Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907-914.
26. Rijsman RM, de Weerd AW, Stam CJ, et al. Periodic limb movement disorder and restless legs syndrome in dialysis patients. Nephrology (Carlton). 2004;9:353-361.
27. Parker KP, Bliwise DL, Bailey JL, et al. Daytime sleepiness in stable hemodialysis patients. Am J Kidney Dis. 2003;41:394-402.
28. Hanly PJ, Gabor JY, Chan C, et al. Daytime sleepiness in patients with CRF: Impact of nocturnal hemodialysis. Am J Kidney Dis. 2003;41:403-410.
29. Hui DS, Wong TY, Li TS, et al. Prevalence of sleep disturbances in Chinese patients with end stage renal failure on maintenance hemodialysis. Med Sci Monit. 2002;8:CR331-CR336.
30. Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: Associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254-262.
31. Held PJ, Port FK, Wolfe RA, et al. The dose of hemodialysis and patient mortality. Kidney Int. 1996;50:550-556.
32. Leggat JEJr, Orzol SM, Hulbert-Shearon TE, et al. Noncompliance in hemodialysis: predictors and survival analysis. Am J Kidney Dis. 1998;32:139-145.
33. Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005. Epub Jan 25
34. Benz RL, Pressman MR, Hovick ET, et al. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35:1052-1060.
35. Fishbane S, Mittal SK, Maesaka JK. Beneficial effects of iron therapy in renal failure patients on hemodialysis. Kidney Int Suppl. 1999;69:S67-S70.
36. Bickford AK. Evaluation and treatment of iron deficiency in patients with kidney disease. Nutr Clin Care. 2002;5:225-230.
37. Hargrave R, Beckley DJ. Restless leg syndrome exacerbated by sertraline. Psychosomatics. 1998;39:177-178.
38. Winkelmann J, Trenkwalder C. Pathophysiology of restless-legs syndrome. Review of current research. Nervenarzt. 2001;72:100-107.
39. Freye E, Levy JV, Partecke L. Use of gabapentin for attenuation of symptoms following rapid opiate detoxification (ROD)—correlation with neurophysiological parameters. Neurophysiol Clin. 2004;34:81-89.
40. Trenkwalder C, Stiasny K, Pollmacher T, et al. L-dopa therapy of uremic and idiopathic restless legs syndrome: A double-blind, crossover trial. Sleep. 1995;18:681-688.
41. Micozkadioglu H, Ozdemir FN, Kut A, et al. Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: An open-label study. Ren Fail. 2004;26:393-397.
42. Pellecchia MT, Vitale C, Sabatini M, et al. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis: An open randomized crossover trial versus levodopa sustained release. Clin Neuropharmacol. 2004;27:178-181.
43. Walker SL, Fine A, Kryger MH. L-DOPA/carbidopa for nocturnal movement disorders in uremia. Sleep. 1996;19:214-218.
44. Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001;38:104-108.
45. Wong MO, Eldon MA, Keane WF, et al. Disposition of gabapentin in anuric subjects on hemodialysis. J Clin Pharmacol. 1995;35:622-626.
46. Ausserwinkler M, Schmidt P. Successful clonidine treatment of restless leg syndrome in chronic kidney insufficiency. Schweiz Med Wochenschr. 1989;119:184-186.
47. National Kidney Foundation. NKF-DOQ1 Clininal Practice Guidelines for Vascular Access. New York: National Kidney Foundation; 2001.
48. Yasuda T, Nishimura A, Katsuki Y, et al. Restless legs syndrome treated successfully by kidney transplantation—A case report. Clin Transpl. 138, 1986.
49. Winkelmann J, Stautner A, Samtleben W, et al. Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Mov Disord. 2002;17:1072-1076.
50. Molnar MZ, Novak M, Ambrus C, et al. Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis. 2005;45:388-396.
51. Brittenham GM. Disorders of iron metabolism: Iron deficiency and overload. In: Hoffman R, Benz EJ, Shattil SJ, et al, editors. Hematology: Basic Principles and Practice. New York,: Churchill Livingstone; 2000:397-428.
52. O’Keeffe ST. Iron deficiency with normal ferritin levels in restless legs syndrome. Sleep Med. 2005;6:281-282.
53. O’Keeffe ST. Secondary causes of restless legs syndrome in older people. Age Ageing. 2005;34:349-352.
54. Guyatt GH, Patterson C, Ali M, et al. Diagnosis of iron-deficiency anemia in the elderly. Am J Med. 1990;88:205-209.
55. Allen RP, Barker PB, Wehrl F, et al. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263-265.
56. Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired iron acquisition in restless legs syndrome. Neurology. 2003;61:304-309.
57. O’Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age Ageing. 1994;23:200-203.
58. Sun ER, Chen CA, Ho G, et al. Iron and the restless legs syndrome. Sleep. 1998;21:371-377.
59. Earley CJ, Connor JR, Beard JL, et al. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698-1700.
60. Scales WE, Vander AJ, Brown MB, et al. Human circadian rhythms in temperature, trace metals, and blood variables. J Appl Physiol. 1988;65:1840-1846.
61. O’Keeffe ST, Noel J, Lavan JN. Restless legs syndrome in the elderly. Postgrad Med J. 1993;69:701-703.
62. Wang Y, Mysliviec V, Fischer C, et al. Efficacy of iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo controlled study. Association of Professional Sleep Societies. Sleep. 2006:A273.
63. Davis BJ, Rajput A, Rajput ML, et al. A randomized, double-blind placebo-controlled trial of iron in restless legs syndrome. Eur Neurol. 2000;43:70-75.
64. Nordlander NB. Therapy in restless legs. Acta Med Scand. 1953;145:453-457.
65. Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231-235.
66. A doctor’s wife [letter]. Br Med J. 1946:134.
67. Ekbom KA. Restless legs as an early symptom of cancer [in Swedish]. Lakartidningen. 1955;52:1875.
68. Aspenstrom G. Pica and restless legs in iron deficiency [in Swedish]. Lakartidningen. 1964;61:1174-1177.
69. Ekbom K. Restless legs syndrome after partial gastrectomy. Acta Neurol Scand. 1966;42:79-89.
70. Cancado RD, Chiattone CS, Alonso FF, et al. Iron deficiency in blood donors. Sao Paulo Med J. 2001;119:132-134.
71. Ekbom KA. Restless legs among blood donors [in Swedish]. Lakartidningen. 1956;53:3098.
72. Silber MH, Richardson JW. Multiple blood donations associated with iron deficiency in patients with restless legs syndrome. Mayo Clin Proc. 2003;78:52-54.
73. Kryger MH, Shepertycky M, Foerster J, et al. Sleep disorders in repeat blood donors. Sleep. 2003;26:625-626.
74. Ulfberg J, Nystrom B. Restless legs syndrome in blood donors. Sleep Med. 2004;5:115-118.
75. Tings T, Schettler V, Canelo M, et al. Impact of regular LDL apheresis on the development of restless legs syndrome. Mov Disord. 2004;19:1072-1075.
76. Winkelmann J, Lichtner P, Schormair B, et al. Variants in the neuronal nitric oxide synthase (nNOS, NOS1) gene are associated with restless legs syndrome [abstract]. Mov Disord. 8, 2007.
77. Cheah JH, Kim SF, Hester LD, et al. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase dexras1. Neuron. 2006;51:431-440.
78. Salih AM, Gray RE, Mills KR, et al. A clinical, serological and neurophysiological study of restless legs syndrome in rheumatoid arthritis. Br J Rheumatol. 1994;33:60-63.
79. Györfi M, Szakács Z, Koumlves P. Restless legs syndrome and serum transferrin receptor and ferritin levels in patients with rheumatoid arthritis. Sleep. 2003;26:A334.
80. Yunus MB, Aldag JC. Restless legs syndrome and leg cramps in fibromyalgia syndrome: A controlled study. BMJ. 1996;312:1339.
81. Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21:481-497.
82. Prado GF, Allen RP, Trevisani VM, et al. Sleep disruption in systemic sclerosis (scleroderma) patients: Clinical and polysomnographic findings. Sleep Med. 2002;3:341-345.
83. Gudbjornsson B, Broman JE, Hetta J, et al. Sleep disturbances in patients with primary Sjogren’s syndrome. Br J Rheumatol. 1993;32:1072-1076.
84. Ondo W, Tan EK, Mansoor J. Rheumatologic serologies in secondary restless legs syndrome. Mov Disord. 2000;15:321-323.
85. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137-2141.
86. Phillips B, Hening W, Britz P, et al. Prevalence and correlates of restless legs syndrome: Results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76-80.
87. Skomro RP, Ludwig S, Salamon E, et al. Sleep complaints and restless legs syndrome in adult type. 2 diabetics. Sleep Med. 2001;2:417-422.
88. Lopes LA, Lins C, de M, Adeodato VG, et al. Restless legs syndrome and quality of sleep in type. 2 diabetes. Diabetes Care. 2005;28:2633-2636.
89. Merlino G, Fratticci L, Valente M, et al. Association of restless legs syndrome in type. 2 diabetes: A case-control study. Sleep. 2007;30:866-871.
90. Holley JL, Nespor S, Rault R. Characterizing sleep disorders in chronic hemodialysis patients. ASAIO Trans. 1991;37:M456-M457.
91. Goffredo Filho GS, Gorini CC, Purysko AS, et al. Restless legs syndrome in patients on chronic hemodialysis in a Brazilian city: Frequency, biochemical findings and comorbidities. Arq Neuropsiquiatr. 2003;61(3B):723-727.
92. Miranda M, Araya F, Castillo JL, et al. (Restless legs syndrome: A clinical study in adult general population and in uremic patients). Rev Med Chil. 2001;129:179-186.
93. Burmann-Urbanek M, Sanner B, Laschewski F, et al. (Sleep disorders in patients with dialysis-dependent renal failure). Pneumologie. 1995;49(supp. 1):158-160.
94. Sabbatini M, Minale B, Crispo A, et al. Insomnia in maintenance haemodialysis patients. Nephrol Dial Transplant. 2002;17:852-856.