Respiratory Muscle Training
The capacity of the respiratory muscle pump is vital for the movement of air to the level of gas exchange in the respiratory system. Impairment of the respiratory pump compromises ventilation, gas exchange, and tissue respiration. The respiratory muscles drive ventilation. In conditions where the load on the respiratory muscles is increased or the capacity of the respiratory muscles is decreased, muscle weakness can occur. Breathing pattern (i.e., rapid shallow breathing) adapts, however, to help counter respiratory muscle fatigue.1 Although this adaptation decreases the effectiveness of gas exchange, a breathing pattern with a lower tidal volume and higher breathing rate helps minimize respiratory muscle fatigue.
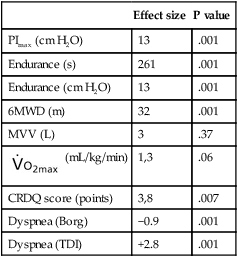
Although the adaptation in breathing pattern affects the effectiveness of gas exchange, prevention of muscle fatigue and respiratory arrest requires modification of the breathing pattern (i.e., there is a decrease in tidal volume and an increase in breathing rate). Figure 26-1 illustrates this adaptation in patients at risk for respiratory failure. Respiratory muscle weakness increases the relative load for breathing (inspiratory pressure/maximum inspiratory pressure [PI/PImax] ratio, see Figure 26-1), and this can lead to clinical consequences such as dyspnea, impaired exercise performance, ineffective coughing, respiratory insufficiency, weaning failure, and death. Dysfunction of the respiratory muscles is observed in several conditions such as chronic obstructive pulmonary disease, asthma, cystic fibrosis, neuromuscular disease including spinal cord injury, congestive heart failure, and critical illness. This chapter describes the clinical relevance of respiratory muscle weakness, the assessment and testing of respiratory muscle function, and the application and effectiveness of respiratory muscle training for the conditions above and its role in weaning some patients from mechanical ventilation.
Respiratory Muscle Assessment
Respiratory Muscle Strength Testing
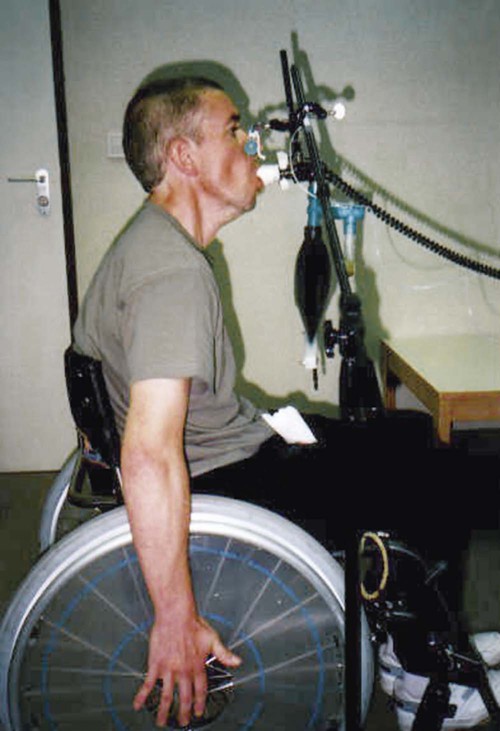
Clinically, respiratory muscle strength is measured as PImax and maximum expiratory pressure (PEmax). These pressures are measured using a small cylinder that fits in the patient’s mouth with a circular mouthpiece (Figure 26-2). A small leak in the cylinder (2 mm diameter and 15 mm length) prevents high pressures that could be caused by the contraction of the cheek muscles.2 Standardizing the lung volume at which the pressures are measured is crucial.3 To prevent chest wall and lung recoil pressures from contributing to the pressure generated by the inspiratory muscles, measurements are recorded at functional residual capacity (FRC). This lung volume, however, is difficult to standardize. In clinical practice, PImax is measured from residual volume, whereas PEmax is measured at total lung capacity (TLC). At least five repetitions should be performed. Respiratory muscle testing is described in detail in the American Thoracic Society/European Respiratory Society position statement (2002).4
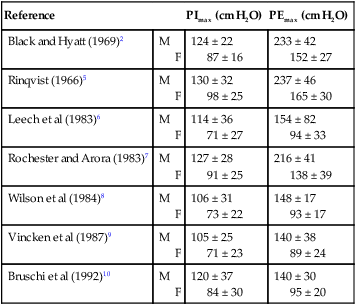
Several groups of investigators have published norms for PImax and PEmax (Table 26-1).2,7,8 Regardless of the norms used, the standard deviation is typically large. Therefore weakness is not easy to define.11 Inspiratory weakness is defined as a PImax lower than 50% of predicted12 in the presence of clinical signs (e.g., dyspnea, impaired cough, and orthopnea) consistent with reduced respiratory muscle strength. Other methods, such as the sniff maneuver, have been developed as tools to quantify global respiratory muscle function.13 The results of the sniff maneuver have been reported to be highly reliable in children with neuromuscular disease. More invasive methods such as electric or magnetic diaphragm stimulation can provide more accurate and detailed information on diaphragmatic function14 and are useful in the diagnosis of diaphragmatic paresis. For most clinical applications, however, the assessment of inspiratory and expiratory mouth pressures is sufficient.
Table 26-1
Reference Values for Maximal Inspiratory and Expiratory Mouth Pressures in Healthy Adults
| Reference | PImax (cm H2O) | PEmax (cm H2O) | |
| Black and Hyatt (1969)2 | M F |
124 ± 22 87 ± 16 |
233 ± 42 152 ± 27 |
| Rinqvist (1966)5 | M F |
130 ± 32 98 ± 25 |
237 ± 46 165 ± 30 |
| Leech et al (1983)6 | M F |
114 ± 36 71 ± 27 |
154 ± 82 94 ± 33 |
| Rochester and Arora (1983)7 | M F |
127 ± 28 91 ± 25 |
216 ± 41 138 ± 39 |
| Wilson et al (1984)8 | M F |
106 ± 31 73 ± 22 |
148 ± 17 93 ± 17 |
| Vincken et al (1987)9 | M F |
105 ± 25 71 ± 23 |
140 ± 38 89 ± 24 |
| Bruschi et al (1992)10 | M F |
120 ± 37 84 ± 30 |
140 ± 30 95 ± 20 |
Respiratory Muscle Endurance Testing
Several tests for assessing respiratory muscle endurance capacity have been described. Most commonly the patient breathes against a submaximal inspiratory load (60% to 75% PImax) for as long as possible.15,16 This test can detect changes in inspiratory muscle endurance after training. Incremental threshold loading, breathing against an incremental load (approximately 5 cm H2O) every 2 minutes, is also reproducible.17 The highest load that can be sustained for two minutes is termed the sustainable pressure and is expressed as a percentage of the maximal load.17,18 Healthy people can usually sustain 70% of the PImax for 2 minutes.18 Johnson and colleagues17 reported large intra-individual variations in this percentage, which tends to decrease with age. The sustainable pressure has been shown to be less than the maximal inspiratory and expiratory pressures in individuals with chronic obstructive pulmonary disease (COPD).19 A third method20–22 is a repetitive maximal inspiratory or expiratory maneuver performed against an occluded airway with a well-defined contraction duration (10 seconds) and relaxation time (5 seconds). The relative decline in maximal pressure after 18 contractions is a measure of the endurance capacity of the respiratory muscles.
Respiratory Muscle Training Methods
Comparable to muscle training of the peripheral skeletal muscles, training of the respiratory muscles aims to improve specific characteristics of inspiratory or expiratory muscle contraction force, endurance, or velocity (Table 26-2).23–25
Table 26-2
| IMT | RMET (Voluntary Isocapnic Hyperpnea) | |
| Type | Strength | Endurance |
| Duration | 15 minutes, twice daily, | 30 minutes, 6-12 weeks |
| Frequency | 5-7x per week | 5x per week |
| Intensity | 30-50%PImax with individual incremental load, weekly control of load/technique | VE = 50-60% MVV, breathing frequency 50-60/min |
Inspiratory Muscle Training
Because the inspiratory muscles are mainly involved in repetitive contractions with low intensity, training strategies emphasize enhancing inspiratory muscle endurance. Two types of training (i.e. threshold loading [Figure 26-3] and normocapnic hyperpnea [NCH] [Figure 26-4]) are currently practiced. During NCH, the patient is asked to breathe at a high proportion (>60%) of the maximal voluntary ventilation for 15 to 20 minutes.23,26 In the past, the equipment used for this type of training was complicated, but recently a simpler, partial rebreathing system has been developed.27

During inspiratory resistive breathing the patient inhales through a mouthpiece with an adapter fitted with an adjustable diameter. This resistance is flow-dependent. Adequate training intensity is achieved only with feedback of the target pressure if flow and pressure are tightly coupled.28 Recently, a flow-independent resistance has been developed, the so-called threshold loading valve, a valve that opens at a critical pressure.29,30 Training intensity varies across studies, from as high as maximal sustained inspiratory (Müller) maneuvers31 to 50% to 80% PImax16,32–34 to a low intensity of 30% PImax.34–37 All these studies carefully controlled the target pressure or the threshold loading during training.30 Threshold loading has the advantage of being independent of inspiratory flow rate30 but requires a build-up of negative pressure before flow is initiated; hence it is inertive in nature. The fact that threshold loading enhances velocity of inspiratory muscle shortening38 could provide additional benefit because this shortens inspiratory time and increases time for exhalation and relaxation. Increased relaxation time may prevent the development of muscle fatigue in patients who are at risk. In addition, shortening inspiratory time will increase expiratory time, thus reducing dynamic hyperinflation, which is an important advantage in patients with COPD.
Expiratory Muscle Training
Explosive expiratory maneuvers and low-intensity contractions of the abdominal muscles are similar to those involved in coughing, the Valsalva maneuver, and exercise. Consequently, training parameters for expiratory muscle training can be either high-intensity strength training or moderate- to low-intensity endurance training. An example of the parameters for endurance expiratory muscle training is 30 minutes of continuous training at 15% to 45% of PEmax.39 An example of strength training parameters is 15 Valsalva maneuvers at 60% of PEmax.40 Both training programs can be implemented using expiratory resistance at the mouth, such as threshold loading.40,41
Respiratory Muscle Training in Health
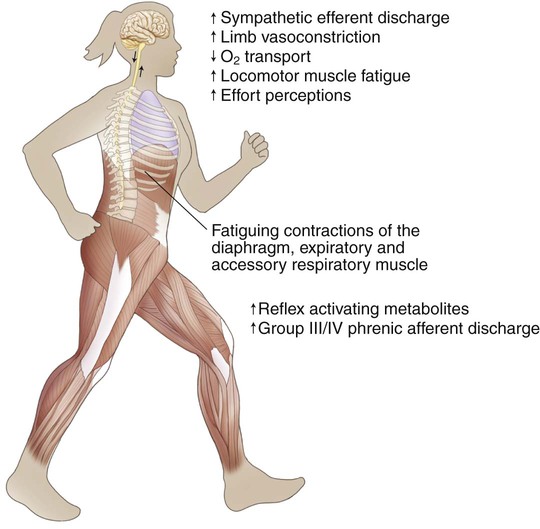
Diaphragm fatigue can occur during sustained high-intensity exercise.42,43 Several studies have shown that unloading respiratory muscles (by using low-density gas mixtures or mechanical ventilation) can lead to an increase in exercise performance.44,45 In athletes, heavy (but not moderate) respiratory muscle work has an impact on whole body exercise performance by affecting limb blood flow and limb muscle fatigue during heavy exercise.46,47 One mechanism to explain these improvements in exercise performance is the “respiratory muscle metaboreflex” (Figure 26-5).47 Therefore a rational basis exists for improving the strength and endurance of the respiratory muscles in order to improve exercise performance. In 14 competitive rowers, inspiratory muscle training (IMT) significantly improved PImax and performance on a 5000-meter trial compared with a placebo control group.48 A small but significant decrease in swimming time needed to cover 100 meters and 200 meters was observed after 6 weeks of IMT compared with a placebo control group.49 After following 5 weeks of respiratory muscle endurance training (RMET), nine competitive cyclists had a 25 seconds (+2%) improvement on an 8-km trial. No differences were founded in peak work rate or maximal exercise capacity (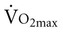 ).50 Leddy and colleagues (2007)51 reported a 4% decrease in time on a 4-mile run after 4 weeks of RMET. All these small improvements in exercise performance are also practical and relevant since small differences in athletes’ performance can be the difference between winning and losing a race. On the other hand, several studies in endurance athletes observed no differences in maximal exercise capacity, other than an increase in respiratory muscle strength and endurance.52,53
).50 Leddy and colleagues (2007)51 reported a 4% decrease in time on a 4-mile run after 4 weeks of RMET. All these small improvements in exercise performance are also practical and relevant since small differences in athletes’ performance can be the difference between winning and losing a race. On the other hand, several studies in endurance athletes observed no differences in maximal exercise capacity, other than an increase in respiratory muscle strength and endurance.52,53
Respiratory Muscle Training in Disease
Chronic Obstructive Pulmonary Disease
Respiratory muscle weakness is often observed in patients with chronic obstructive pulmonary disease (COPD).54–58 Adaptation of the diaphragm results in greater fatigue resistance and improved muscle function.59,60 The diaphragm is remodeled in part by the transformation of fast fiber types to slow fiber types.61,62 Despite these adaptations, both functional inspiratory muscle strength55 and endurance56 remain compromised in patients with COPD and contribute to hypercapnia,63 dyspnea,64,65 nocturnal oxygen desaturation,33 and reduced walking distance.66 During exercise, patients with COPD use a larger proportion of their maximal inspiratory pressure than healthy people.67 This pattern of breathing is closely related to dyspnea during exercise67 and may induce respiratory muscle fatigue and probably limits exercise capacity.
Another mechanism involved in the potential role of respiratory muscles in exercise limitation is the respiratory muscle metaboreflex (see Figure 26-5). Inspiratory muscles should become more fatigue-resistant by increasing their work capacity and should consume a smaller fraction of the total cardiac output during exercise, which in turn, should be available for the working muscles during exercise. In patients with COPD, diaphragmatic fatigue after exercise has been observed in only a small number of patients.11,68 However, this might be related to the insufficient length of the exercise protocols and the absence of inspiratory muscle weakness in the subjects evaluated. Improved limb muscle blood flow after IMT has been observed in patients with chronic heart failure69–71 but has not yet been studied in patients with COPD. Reductions in dynamic hyperinflation and dyspnea during exercise after IMT have also been proposed as mechanisms linking inspiratory muscle capacity to whole body endurance capacity; thus further study is needed.72
Compelling evidence suggests that breathing against an inspiratory load increases both maximal inspiratory pressure16,28,32–37,73 and endurance of the inspiratory muscles.28,33,34,73 Respiratory muscle training has been reported to produce hypertrophy of predominantly type I and IIA fibers in the diaphragms of hamsters.74,75 The proportion of type I fibers and size of type II fibers in the external intercostals after IMT has been recently reported to increase in a study of patients with COPD.76 Dyspnea36,37,77 and nocturnal desaturation time33 also decreased, while exercise performance tended to improve (Table 26-3).78,79 In addition to exercise training, IMT can improve exercise capacity more than exercise training alone.16,32,35,37 Based on systematic reviews with meta-analyses, IMT can improve inspiratory muscle strength and endurance, functional exercise capacity, and quality of life while also decreasing dyspnea.78,79 The addition of IMT to a general exercise training program improved PImax and tended to improve the exercise performance of patients with inspiratory muscle weakness (PImax < 60 cm H2O).78,79 Long-term effects were shown for exercise performance and dyspnea in patients with COPD who continued performing their training program three times per week at high training intensity (60% PImax).80 NCH was applied in one randomized controlled trial and resulted in enhanced respiratory muscle endurance and exercise capacity as well as quality of life in patients with COPD.26 Inspiratory muscle endurance training was shown to be less effective than respiratory muscle strength training.79
Table 26-3
Effect Size of Inspiratory Muscle Training Based on Meta-Analysis (n = 32)
| Effect size | P value | |
| PImax (cm H2O) | 13 | .001 |
| Endurance (s) | 261 | .001 |
| Endurance (cm H2O) | 13 | .001 |
| 6MWD (m) | 32 | .001 |
| MVV (L) | 3 | .37 |
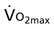 (mL/kg/min) (mL/kg/min) |
1,3 | .06 |
| CRDQ score (points) | 3,8 | .007 |
| Dyspnea (Borg) | −0.9 | .001 |
| Dyspnea (TDI) | +2.8 | .001 |
From Gosselink R, De Vos J, van den Heuvel SP, et al: Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur Respir J 2011;37:416-425, 2011.
Asthma
Respiratory muscle weakness is not frequently observed in patients with asthma.80–82 Although some studies report a moderate reduction in inspiratory muscle strength,81 other studies have not found inspiratory muscle strength to be affected.20,56 Inspiratory muscle endurance has been shown to be increased in people with asthma20 but reduced in those who are steroid dependent.56 In patients with asthma, dyspnea has been observed to be greater in women than in men, resulting in their greater need for beta-2 agonists. This may reflect sex differences in inspiratory muscle strength.80 IMT has been studied in people with asthma, and evidence from randomized trials supports that, in addition to improvements in inspiratory muscle strength, dyspnea and beta-2 agonist use was reduced in those with high dyspnea and high use of beta-2 agonists,83 especially among women.80 A Cochrane review, however, concluded that the evidence supporting a role for IMT for people with asthma was inconclusive.84
Cystic Fibrosis
Reports on the inspiratory muscle strength in people with cystic fibrosis (CF) have also been contradictory. Some have shown that inspiratory muscle strength is decreased,85–88 whereas others have shown that it is normal89,90 and others have shown it is supranormal.91–93 The discrepancy across studies can be attributed to methodological inconsistencies such as age, degree of pulmonary dysfunction, nutritional status, and the assessment of PImax. Severity of disease, nutritional depletion, and systemic inflammation have been considered contributors to respiratory muscle weakness in people with CF.94 On the other hand, normal or supranormal PImax in the CF population can be explained by the chronic increase of respiratory workload due to the combination of progressive airflow obstruction, hyperinflation, and airway resistance, which could produce a training effect.89,95–97
Given the similarities in abnormal lung mechanics for people with CF and with COPD, the rationale to indicate IMT for patients with CF is based on the benefits observed with this intervention in patients with COPD. There are few reports on IMT in patients with CF.91,95,96,98,99 The modalities of IMT used in these patients are similar to those described for other patients with chronic obstructive diseases such as COPD and asthma. Early studies described the use of the NCH98 and inspiratory resistive training91 modalities. Recent studies, however, have used threshold loading.95,96,99 As yet, there is no consensus by experts regarding the optimal training intensity for patients with CF. Even though training at low intensity (40% PImax) improves inspiratory endurance,95 high-intensity training (80% PImax) can further augment inspiratory muscle function, pulmonary function, and exercise capacity.96
Recently, two meta-analyses have been published about the effects of inspiratory muscle training in patients with CF.100,101 Both systematic reviews point out a scarcity of randomized controlled trials and a huge difference among inspiratory muscle training protocols. Therefore evidence is weak supporting IMT as a means of improving inspiratory muscle function in children, adolescents, and adults with CF. In addition, the benefit of IMT on dyspnea, exercise capacity, and quality of life for these populations remains unclear.
Quadriplegia
Several studies have reported that inspiratory or expiratory muscle training is a means of enhancing inspiratory or expiratory muscle function in individuals with quadriplegia, reducing dyspnea, and improving exercise performance.102–105 In one systematic review, the investigators concluded that respiratory muscle training (RMT) tended to improve expiratory muscle strength, vital capacity, and residual volume. Insufficient data were available to make conclusions concerning the effects of RMT on inspiratory muscle strength, respiratory muscle endurance, quality of life, exercise performance, and respiratory complications.105 A randomized controlled trial showed that NCH training in patients with spinal cord injury improved respiratory muscle strength and endurance. Further, respiratory complications occurred less frequently after training.105
Neurological and Neuromuscular Disease
The mechanisms underlying respiratory muscle dysfunction in patients with neuromuscular disease (NMD) are complex and dependent on the particular disease and its stage. In patients with amyotrophic lateral sclerosis (ALS), involvement of inspiratory muscle weakness is prominent and underlies ventilatory insufficiency and symptoms, while expiratory muscle weakness is mainly present in symptomatic patients and affects cough.106 Progressive respiratory muscle weakness has been described in Duchenne muscular dystrophy (DMD). A recent study has confirmed a progressive impairment of the diaphragm compensated by an increase in the contribution of the ribcage to tidal volume.107 Patients with Parkinson syndrome can exhibit respiratory muscle weakness even if the severity is mild, but the syndrome is less associated with dyspnea.108 The weakness is associated with impaired exercise performance while quality of life and daily living activities appear unaffected.109 Respiratory muscle function is affected in patients with acute ischemic stroke but it is not related to dyspnea.110 Diaphragm contractility is affected on the contralateral side, which may be related to an increased incidence of pneumonia.111 In addition, acute ischemic stroke has been related to expiratory muscle weakness and reduced peak flow rate, which may determine cough inefficiency.112 Besides diminished respiratory strength and endurance, patients with generalized myasthenia gravis are more dyspneic than are control subjects.113
The indications for IMT in NMD are based on the benefits of strengthening the respiratory muscles, which may delay pulmonary complications and the onset of respiratory failure.114 Studies have reported improved respiratory muscle function after IMT in various types of NMD.115–117 Inspiratory muscle training (60% of sniff nasal inspiratory pressure, for 10 minutes, 3 times a day, 7 days a week, for 12 weeks) resulted in strengthening of the inspiratory muscles and also delayed the decline in respiratory function in patients with ALS.118 Because of controversy about the benefits of respiratory muscle training in patients with DMD, either improvement or insignificant changes in respiratory muscle function, a recent statement of American Thoracic Society does not completely recommend this intervention for these patients.4
In studies that reported improvement in respiratory muscle function, the inspiratory resistance adjustments varied from 30%117 to 70%-80% PImax119 and the period of training from 6 weeks to 24 months. An additional benefit was reduced perception of respiratory load, which could translate into reduced dyspnea.117 In patients with Parkinson syndrome, IMT increased both strength and endurance of the respiratory muscles and reduced the perception of dyspnea.120 One study has described the benefits of expiratory muscle training for patients with Parkinson disease.121 An improvement in voluntary cough and swallowing was observed after training the expiratory muscles, which consisted of five sets of five breaths against 75% PEmax for 4 weeks. However, whether these benefits can prevent aspiration in these patients remains unclear. In patients with subacute stroke, IMT improved respiratory muscle function, exercise capacity, and quality of life.122 In summary, the protocol consisted of two 15-minute sessions, starting with a load of 40% PImax and going up to 60% PImax (threshold loading) for six times a week for 6 weeks. Patients with myasthenia gravis have showed improvement in respiratory muscle function and also in dyspnea symptoms after inspiratory123,124 and inspiratory plus expiratory muscle training.125 For patients with multiple sclerosis, IMT has been recommended by Klefbeck and colleagues (2003)126 as an additional intervention to physical training. In that study, patients breathed daily for 30 times through an inspiratory resistance of 40%-60% PImax imposed by the threshold loading over a 10-week period. Although inspiratory muscle function is commonly affected in neuromuscular diseases, expiratory muscle strength is preferentially impaired in patients with multiple sclerosis40 and it is inversely related to their disability status;127 thus EMT has been shown to be beneficial for patients with multiple sclerosis.40,128
An association exists between training intensity and improvement of respiratory muscle capacity.116 Patients with NMD who have more than 25% of predicted pulmonary function can respond to training.31,117 Patients with severe impairment of ventilatory function, however, may show less improvement in respiratory strength because they are less able to achieve the critical training intensity, respiratory muscle weakness may predispose their muscle to damage rather than benefit from training, and their muscle mass may be insufficient. In the long term, the progression of most neuromuscular diseases affecting muscle probably impedes an optimal training effect.
Chronic Heart Failure
Although impairment of cardiac function is the principal factor associated with reduced functional capacity in people with chronic heart failure (CHF), abnormal respiratory muscle function plays an important role in premature exercise termination and contributes to sensory consequences, especially breathlessness on exertion. Exertional dyspnea can result from respiratory muscle weakness, increased respiratory muscles activity, or both.64
Reduced respiratory muscle strength in patients with CHF has been shown both with volitional129–131 and nonvolitional (twitch transdiaphragmatic pressure)132 measurements. Changes in pulmonary function secondary to ventricular dysfunction, increased left ventricular size, chronic pulmonary congestion, reduced lung compliance, and increased airway resistance133 result in an altered breathing pattern in which patients breathe near residual volume at rest and experience limitations related to expiratory flow with minimal exercise.134 Therefore the ventilatory muscles are subjected to increased work of breathing (WOB),133,135 which may lead to respiratory muscle fatigue. As a positive consequence of the high WOB in people with heart failure, however, the diaphragm exhibits a predominance of slow myosin heavy chain isoforms with an increase in oxidative enzymes and a decrease in glycolytic capacity.136 These adaptations are similar to those observed after endurance training of the limb muscles in healthy people. These observations are consistent with the well-preserved endurance of the inspiratory muscles when expressed as a function of endurance time, as well as the inspiratory muscle load-to-capacity ratio in patients with CHF.137
The effects of inspiratory muscle training in patients with CHF have been well documented.44,70,138–144 No consensus exists in the literature related to types of training, intensity, and duration of IMT, which may explain the range in responses of patients with CHF to IMT. Based on the literature, the usual intensity is at least 30% of PImax,44,70,138,142,144 but an intensity of 60% of PImax143 has also been used. Although some studies used IMT three times per week,138,139,141,143 others trained the patients either six140 or seven times per week.70,144 The duration of each session varied between 10 and 30 minutes, and sessions were performed over 6 to 12 weeks.
IMT for people who have CHF increases inspiratory strength44,70,138–140,142–144 and endurance,138,140–143 increases maximal exercise capacity as reflected by improved exercise duration141 or oxygen consumption,70,142,143 increases submaximal exercise capacity evaluated by the 12-minute walking test140 and 6-minute walking distance,70,138,140–143 and improves both dyspnea139–143 and quality of life.70,143 Recently, IMT has been reported to improve oxygen uptake efficiency in patients with CHF and inspiratory muscle weakness (PImax < 70% of predicted).145 In addition, 4 weeks of IMT led to an improvement of limb blood flow during exercise.69 It has been speculated that IMT reduces inspiratory muscle overload and, in turn, peripheral muscle vasoconstriction, a contributor to exercise limitation in these patients.146
Weaning Failure
The majority (75% to 80%) of patients who are mechanically ventilated can be weaned to breathe spontaneously without difficulty.147 Patients who experience difficulty weaning have poorer prognoses, including increased mortality and prolonged intensive care unit (ICU) stay and rehabilitation. Several factors contribute to weaning failure, such as the underlying disease, age, muscle weakness, malnutrition, hypoxemia and hypercapnia, and medications (e.g., corticosteroids and muscle relaxants). The method of weaning affects the outcome.147 Current expert opinion supports that familiarity and skill are the most important determinants of the choice of weaning. The specific method seems less important. Weaning based on bedside assessment and the use of standardized protocols is effective. Daily screening of respiratory function and trials of spontaneous breathing result in shortening of the period of mechanical ventilation, thus reducing the complications and costs associated with an ICU stay.148–152 A spontaneous breathing trial can be used to assess readiness for extubation with the performance of serial measures such as tidal volume, respiratory rate, maximal inspiratory airway pressure, and the rapid shallow breathing index.153 Early detection of worsening clinical signs such as distress, airway obstruction, and paradoxical chest wall motion, ensures that serious problems are prevented. Airway patency and protection (i.e., an effective cough mechanism) should be assessed before the commencement of weaning. Peak cough flow is a useful parameter to predict successful weaning in patients with neuromuscular disease or spinal cord injury when extubation is anticipated.154 A rating of “airway care score” has been developed based on the quality of the patient’s cough during airway suctioning, the absence of excessive secretions, and the frequency of airway suctioning.150 These therapist-driven protocols are action plans based on consensus and expert opinion. Physical therapists contribute to the assessment and treatment of patients who have difficulty being weaned from mechanical ventilation. Noninvasive ventilation can facilitate weaning,155 prevent postextubation failure in patients at risk,156 and reduce ICU costs.157 In addition, there is accumulating evidence that weaning problems are associated with failure of the respiratory muscles to resume ventilation.158 Inspiratory muscle training might be beneficial in patients who fail to wean. Uncontrolled trials of inspiratory muscle training (IMT, threshold loading; see Figure 26-3) observed an improvement in inspiratory muscle function and a reduction in duration of mechanical ventilation and weaning time.159–162 Interim analysis of a randomized controlled trial comparing inspiratory muscle training at moderate intensity (≈50% PImax) with sham training in patients with weaning failure showed that a larger proportion of the training group (76%) could be weaned compared with the sham group (35%).163 The addition of IMT in the management of acute critically ill patients from the beginning of mechanical ventilation neither reduced weaning time nor the reintubation rate.164
Biofeedback of the pattern of breathing to the patient can also enhance the weaning process of patients receiving long-term mechanical ventilation.165 Voice and touch may be used to augment weaning success either by stimulation to improve ventilatory drive or by reducing anxiety.166 Environmental influences, such as ambulating with a portable ventilator, have been shown to benefit attitudes and outlooks in long-term ventilator-dependent patients.167

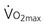 , maximal oxygen uptake capacity; CRDQ, chronic respiratory disease questionnaire; Borg, scale of breathlessness; TDI, total dyspnea index.
, maximal oxygen uptake capacity; CRDQ, chronic respiratory disease questionnaire; Borg, scale of breathlessness; TDI, total dyspnea index.