Chapter 14 Reproductive Disorders in the Adolescent Patient
MENSTRUAL DISORDERS
Dysmenorrhea
In adolescents, the incidence of dysmenorrhea can be quite high and is often underreported or ignored by healthcare providers.1–3 In a survey of 2699 normal adolescents, the prevalence of dysmenorrhea was 59.7%.2 The authors noted that the prevalence of dysmenorrhea increased with age from 39% at age 12 to 72% at age 17. In a large Swedish epidemiologic study on adolescents with dysmenorrhea, 15% of responders reported disrupted daily activity secondary to severe dysmenorrhea.1
Primary and secondary dysmenorrhea must be differentiated because management may vary significantly (Table 14-1). Primary dysmenorrhea is defined as menstrual pain that is not associated with a specific underlying pathology. Secondary dysmenorrhea is menstrual-associated pain that is due to an underlying pathologic process. History and pelvic examination are used to rule out secondary dysmenorrhea. Primary dysmenorrhea requires the initiation of ovulatory cycles. Thus, primary dysmenorrhea will present typically within the first 12 to 24 months after menarche.
Table 14-1 The Gynecologic Differential Diagnosis of Pain with Menses
| Primary | Prostaglandin production abnormalities |
| Secondary |
The onset, duration, and intensity of pain should be recorded. Cramps commonly begin on the first day of menses. The median duration of dysmenorrhea is 2 days.4 Typically, pain diminishes after the third day of bleeding. The patient may report associated symptoms of nausea, vomiting, low back pain, headache, diarrhea, dizziness, and occasionally fainting.5
The severity of dysmenorrhea appears to increase with the duration of menstruation.6 Certain features of the personal history may point the investigation in a particular direction. For example, a family history of endometriosis may increase the level of suspicion for this disease. The onset of pain with menarche raises the possibility of an obstructive müllerian anomaly.
Pathogenesis of Dysmenorrhea
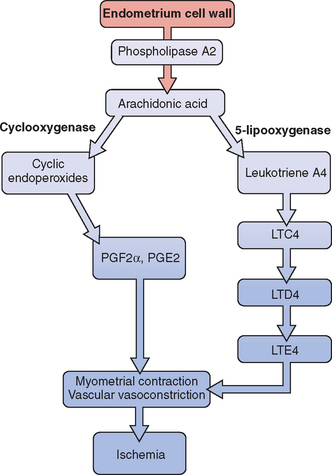
Release of prostaglandin F2α (PGF2α) from the secretory endometrium leads to myometrial contractions and subsequently primary dysmenorrhea (Fig. 14-1). Multiple studies have documented increased levels of PGF2α in the menstrual fluid and endometrial tissue of women who are dysmenorrheic versus eumenorrheic women.7,8 Release of prostaglandins into the circulation, typically within the first 48 hours from initiation of menses, accounts for some of the associated symptoms of nausea, headache, vomiting, and diarrhea.
The uterus can produce and metabolize leukotrienes (LT). Higher leukotriene levels are present in the myometrium and endometrium of adult women with dysmenorrhea.9 Levels of LTC4 and LTD4 have been found to be significantly higher in the menstrual blood of women with primary dysmenorrhea than in those without.10 Thus, these potent vasoconstrictors and inflammatory mediators may play a role in producing the symptom of dysmenorrhea.
Treatment
Many adolescents self-medicate, but it is typically in subtherapeutic doses. Lower doses of over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) have been used in conjunction with other modes of treatment such as local heat therapy, exercise, and herbal therapy. NSAIDs reduce prostaglandin production by inhibiting cyclo-oxygenase. Multiple studies have shown that NSAIDs reduce the severity of symptoms associated with dysmenorrhea.11 A clear advantage of one NSAID over another has not been demonstrated.
If NSAIDs are unsuccessful in the management of symptoms, oral contraceptives are recommended as the next tier of therapy. By decreasing endometrial growth, oral contraceptives limit the production of prostaglandins and leukotrienes. Indeed, lower levels of prostaglandins have been reported in the menstrual fluid of women on oral contraceptives compared with patients with dysmenorrhea who were not taking these drugs.7
There is clear evidence that adolescents taking oral contraceptives have reduced rates of dysmenorrhea.12 Because depot medroxyprogesterone acetate (DMPA) inhibits ovulation and causes endometrial atrophy, it may be used to decrease dysmenorrhea symptoms in adolescents. However, the possible side effects—including osteoporosis—may limit its long-term use in adolescents.
Dietary changes may impact dysmenorrhea. In a prospective, randomized, double-blind crossover study, 42 adolescents between the ages of 15 and 18 were given placebo or fish oil for 2 months.13 The results showed a significant reduction in dysmenorrhea in those who received the fish oil supplementation. This study seems to suggest that supplementation with omega-3 fatty acids may alleviate dysmenorrhea in adolescents. It has been suggested that omega-3 fatty acids prevent buildup of the more potent prostaglandins and leukotrienes that are associated with vasoconstriction and myometrial contractions.
Polycystic Ovary Syndrome
Irregular menstrual cycles, an important characteristic of polycystic ovary syndrome (PCOS), are frequently encountered during the first couple years of menstruation.14 Most of these menstrual abnormalities normalize in the first 2 years after menarche, but careful follow-up and examination are necessary. In one study, half of adolescent girls between the ages of 14 and 16 with oligomenorrhea still had this menstrual problem at age 18.15
Polycystic ovary syndrome is the most common reproductive endocrine abnormality associated with irregular periods in adult women of reproductive age. Because this syndrome starts at puberty, adolescents must be monitored closely for signs of this disorder because early intervention may have a beneficial long-term effect.16 Although subtle signs and symptoms may exist during childhood, the diagnosis is typically deferred until puberty when patients present with irregular menstruation. During the peripubertal period, the hypothalamic-pituitary-ovarian (HPO) axis is relatively immature, which can lead to anovulatory cycles and make it difficult to diagnose PCOS during this time.
Menarche usually occurs at a normal age in adolescents with PCOS. But data suggest that premature pubarche may be associated with development of PCOS.17–19 In a group of postpubertal adolescents with premature pubarche, 45% of the patients showed evidence of functional ovarian hyperandrogenism.17 In a study comparing 98 children with premature pubarche to Tanner stage and bone age-matched controls, mean serum insulin levels were elevated in all participants with premature pubarche for all Tanner stages. Hyperinsulinemia was noted in all participants with premature pubarche, implying that insulin resistance was present during childhood.20
Signs and Symptoms
According to the Rotterdam European Society of Human Reproduction (ESHRE)/American Society for Reproductive Medicine (ASRM)-sponsored PCOS consensus workshop, two of the following three criteria must be present in order to diagnose PCOS: irregular periods, polycystic ovaries on ultrasound, and physical or biochemical evidence of excess androgens.16 Other etiologies, such as late-onset congenital adrenal hyperplasia (CAH), hyperprolactinemia, or androgen-secreting tumors, which may lead to hyperandrogenism, must be excluded.
The most common presenting complaint of adolescents with PCOS is oligomenorrhea followed by episodes of menorrhagia. The presence of oligomenorrhea at age 15 was shown to be a better predictor of oligomenorrhea at age 18 than high testosterone levels, luteinizing hormone (LH) concentration, or polycystic ovaries at ultrasound.14
Hirsutism in adolescents is most commonly caused by PCOS.21 Other skin manifestations of hyperandrogenism include acne and alopecia. Acanthosis nigricans and skin tags may be seen secondary to the accompanying hyperinsulinemia. Girls with PCOS had higher LH and androgen concentrations than controls.22 The same findings have been reported in women with PCOS. Increased LH pulsatility, elevated LH/follicle stimulating hormone (FSH) ratios, and increased ovarian volume have been described in girls with PCOS.23
During childhood, obesity is the prime cause of insulin resistance.24 However, PCOS in adolescents (and women as well) bestows a risk for hyperinsulinemia independent of weight and body composition. Euglycemic clamp studies in two groups of obese 12-year-old girls with and without PCOS showed that fasting plasma glucose levels in the PCOS group were higher than those in the non-PCOS group. Insulin sensitivity was decreased by 50% in the PCOS group compared with the non-PCOS group.25 This metabolic abnormality is a precursor to type 2 diabetes mellitus and is generally detected early in the course of PCOS. Indeed, a 33% rate of impaired glucose tolerance is noted in obese adolescents with PCOS.26 Hyperinsulinemia has also been detected in lean oligomenorrheic and hyperandrogenic adolescents.27 Thus, lean PCOS adolescents are also at increased risk of developing type 2 diabetes mellitus.
Depression has been detected in one half of women with PCOS. The symptoms of hirsutism, obesity, acne, and alopecia place significant emotional stress on the adolescent during a time when she is particularly vulnerable. Because social acceptance is so important during adolescence, the girl who physically looks different from her peers is more likely to feel social anxiety.28–30
It is important to obtain a complete family history of significant endocrine problems. A family history of PCOS appears to be a significant risk factor for the development of PCOS. The rates of PCOS in premenopausal mothers and sisters of patients with PCOS are 35% and 40%, respectively.31 The patient is questioned regarding the existence of PCOS, male alopecia, female hyperandrogenism, menstrual irregularities, diabetes, obesity, and infertility on either her mother’s or father’s side of the family.
Laboratory Evaluation
Endocrinologic evaluation in the adolescent is the same as that in adults and includes assessment of serum androgens as well as exclusion of other endocrine disorders associated with similar presentations such as late-onset CAH and hyperprolactinemia. Details of this investigation are discussed in detail in Chapter 15. Periodically, an oral glucose tolerance test may be necessary to assess for impaired glucose tolerance—especially in obese adolescents. Although ultrasound of the pelvis may be performed instead of a pelvic examination in the apprehensive young patient, it is not absolutely necessary.
Treatment
Oral contraceptives not only regulate menses, but also decrease hirsutism and acne. By inhibiting gonadotropin secretion and increasing sex hormone-binding globulin, oral contraceptives decrease the amount of free testosterone, with a subsequent beneficial effect on the hair follicle. Contrary to what most adolescents believe, oral contraceptives do not increase body weight or fat in teenagers with PCOS.32 However, there is some concern that they may increase insulin resistance in a group already at risk for hyperinsulinemia.33
Antiandrogens such as spironolactone are quite effective in diminishing the symptoms of hirsutism and acne. A decrease in the width of the hair shaft and amount of sexual hair growth is noted at doses up to 200 mg/day. Menstrual abnormalities may occur with use of spironolactone; thus, concomitant use of oral contraceptives is commonly recommended. Hair growth changes may not be evident for 6 to 9 months. But once the rate of hair growth is decreased, she may proceed with use of cosmetic agents such as electrolysis and laser therapy. Eflornithine cream, which inhibits ornithine decarboxylase—an enzyme within the hair follicle—can slow hair growth.34 It may be applied twice a day to the affected area, but as with spironolactone, it may take several months to see any improvement in hair growth.
The role of insulin-sensitizing agents in the management of PCOS in adolescents is not clear. Like adults, many PCOS adolescents have insulin resistance and are at increased risk for developing diabetes and cardiovascular disease. But the first line of therapy to normalize the abnormal metabolic profile of such patients is lifestyle modification, which includes exercise and diet. Lifestyle modification in adolescent girls is especially important because behavioral changes during puberty may have long-lasting effects. Lifestyle modification may be even more beneficial than metformin therapy in the prevention of diabetes.35 In addition, data documenting the safety of metformin use and long-term outcome in adolescents are lacking. Thus, it may be difficult to justify lifelong use of metformin in this population.
There is good evidence, however, that metformin can normalize abnormal metabolic parameters both in obese and nonobese adolescents. A study of 15 obese adolescents with PCOS and impaired glucose tolerance who were given metformin 850 mg twice a day showed that the therapy decreased hepatic and peripheral insulin resistance, improved glucose tolerance, and decreased free and total testosterone levels.36 When metformin was given to nonobese adolescents, an improvement in hirsutism, free androgen index, lipid profile, and insulin parameters was again noted.37 Unfortunately, all improvements in lipid profile, hyperandrogenism, menstruation, and hyperinsulinemia are reversed within 3 months of termination of metformin.37,38
Abnormal Uterine Bleeding
Abnormal uterine bleeding (AUB) is very common in girls for the first 1 to 2 years after menarche. It is defined as excessive, prolonged, or irregular bleeding in the absence of structural lesions of the uterus. In a study evaluating the menses of over 5000 adolescents, the incidence of irregular menstrual cycles was 43% during the first year of menses and 20% in the sixth year.3 Thus, most adolescents eventually experience normal menses, but in the longest follow-up study on record, 5% continued to have severe episodes of anovulatory bleeding in adult life.39
Etiology
Because AUB is a diagnosis of exclusion, other possible causes of vaginal bleeding must be considered. The differential diagnosis of AUB is vast; a summary is given in Table 14-2. A more detailed review is given in Chapter 21. In most instances, the cause of anovulation remains unknown in adolescents. Chronic illnesses such as liver cirrhosis and renal failure may contribute to anovulation and subsequent AUB. Other circumstances that can lead to anovulation include eating disorders, weight changes, and vigorous athletics. Because it may be difficult to obtain a correct sexual history from a teenager, pregnancy complications such as a missed abortion must be considered. Although local disorders such as leiomyomas are uncommon, others such as cervicitis are more common and present as prolonged vaginal bleeding.
Table 14-2 Differential Diagnosis of Abnormal Uterine Bleeding
| Pregnancy-related disorders |
| Disorders of blood hemostasis |
| Chronic illness |
| Sexually transmitted disease |
| Endocrine disorders |
| Medication |
| Local gynecologic abnormalities |
Teenagers presenting with ovulatory AUB (menorrhagia) must be assessed for bleeding disorders. In one retrospective study, a primary coagulation defect was found in 19% of patients who were admitted to the hospital with acute menorrhagia. The prevalence of bleeding disorders rose to 50% in the subgroup of girls with menarchal menorrhagia.40 More recent studies in which the bleeding disorder was more likely to have been detected during premenarche showed a much lower rate of newly diagnosed coagulopathy.41,42 Only 3% of patients admitted for menorrhagia in these studies had a newly diagnosed coagulation disorder. On the other hand, patients hospitalized with hematologic disorders that require chemotherapy may develop severe vaginal bleeding.
Evaluation
The laboratory evaluation in teens with excess vaginal bleeding initially includes a pregnancy test and complete blood count with platelets. The complete blood count can be used to evaluate the severity of the bleeding and as a guideline to determine the urgency of care. In addition, it may help rule out some major hematologic problems. If a bleeding disorder is suspected, a bleeding time, a prothrombin time, and activated partial thromboplastin time as well as a screening test for Von Willebrand disease should be ordered. Details of these are given in Chapter 21. Patients should be evaluated for potential endocrine disorders such as thyroid disease and pituitary adenomas.
Treatment
There are data to support the use of NSAIDs to decrease the amount of blood loss.43,44 This is especially the case in patients with ovulatory AUB (menorrhagia). Patients presenting with mild anemia frequently experience moderate to heavy menses every 2 to 3 weeks. In such cases, oral contraceptives or progesterone may be used to control the bleeding. Use of medroxyprogesterone acetate or norethindrone acetate for 10 to 14 days can help stabilize the endometrium followed by global shedding and controlled period. These agents may not be successful in cases where the bleeding has been heavy and prolonged. In such cases, the endometrium usually is very thin and some amount of estrogen is necessary to heal the lining. Thus, monophasic combined oral contraceptives may be more effective. Although 10 to 14 days of cyclic progestin therapy is adequate for anovulatory AUB, luteal phase progestin therapy is less effective for ovulatory AUB. Progestin therapy for 21 days of the cycle (day 5 to day 26, for example) is more effective.
Treatment of Severe Anemia
Adolescents whose initial hemoglobin level is less than 7 g/dL and are bleeding actively and heavily may need to be admitted for blood transfusion. A coagulation panel must be assessed prior to any blood transfusion or hormonal manipulation. If deemed clinically stable, she may be initiated on oral contraceptives. However, if she is severely hemorrhaging or unable to take medications orally, she can be given intravenous conjugated estrogens (Premarin) 25 mg every 4 hours for 24 hours or less if she stops bleeding.45 Once the bleeding has decreased or stopped, the patient should be given a combination oral contraceptive or progesterone.
In instances where high-dose estrogen therapy is contraindicated, high doses of progesterone such as norethindrone and medroxyprogesterone acetate may be used. Gonadotropin-releasing hormone agonists have also been used to manage AUB in patients who are unresponsive to hormonal management or have contraindications to their use. Antifibrinolytic agents have been used successfully to manage menorrhagia. However, there is minimal data on their use for this indication in adolescents. In one comparative study in adults, mefenamic acid decreased blood loss by 20% and tranexamic acid reduced blood loss by 54%.46 Its use in adolescents has been reserved for patients with organic pathology in whom hormonal therapy has failed.
Dilatation and curettage and hysteroscopy are indicated only when the patient is unresponsive to medical therapy—they are the last line of evaluation and therapy for teenagers. A report of successful endometrial ablation on a teen with severe uterine bleeding complicated by end-stage renal disease exemplifies the critical nature of some patients.47 However, this procedure is only considered an alternative to hysterectomy. Other medical treatments that are used in adults have been sporadically used in adolescent girls (see Chapter 21).
Long Term Outcome
Continued follow-up is mandatory in adolescents experiencing AUB, as illustrated by the results of a 25-year follow-up study.39 This study was performed before hormonal therapy was an option. However, it is quite revealing that the change to normal menses was greatest in the first 2 years of the study. By 4 years, 50% still had AUB. No patient developed normal periods after 10 years of persistent abnormal bleeding. More than half of these patients presented with infertility. Hence, follow-up provides an opportunity to either reassure the adolescent as she normalizes or to educate her about the need for continued medical therapy and potential options for the future.
AMENORRHEA
Primary amenorrhea is defined as the absence of any menstrual flow by age 15 if the patient has developed secondary sexual characteristics or within 5 years of breast development. Secondary amenorrhea is defined as the absence of menstruation for 6 months in an adolescent who has previously established menstruation or the absence of menstruation for a minimum of 3 previous cycle intervals. Although these terms provide information regarding the menstrual cycle, they do not help exclude various diagnoses. Amenorrhea is discussed in detail in Chapter 16. However, an overview is given here to address the differential diagnosis in an adolescent girl who has gone through puberty normally but presents without a period. These patients typically enter the healthcare system through a gynecologist. Patients may have amenorrhea associated with a pubertal disorder. This topic is covered in a separate chapter (see Chapter 11).
Amenorrhea without Pubertal Delay
The differential diagnosis of the adolescent presenting with normal secondary sexual characteristics and amenorrhea is vast (Table 14-3). Hence, only the most common ones are discussed here. Pregnancy must be excluded first. The adolescent is questioned and assessed for signs of hirsutism, galactorrhea, weight changes, dietary changes, changes in exercise patterns, chronic illness, visual changes, and headaches.
Table 14-3 Differential Diagnosis of Amenorrhea without Pubertal Delay
| Congenital anomalies of the genital tract |
Imperforate Hymen
This anomaly results from incomplete canalization of the hymen (Fig. 14-2). It is most commonly detected during adolescence. It may be diagnosed in utero or during the neonatal period.48 The incidence of this anomaly is approximately 0.1% of term infant girls, and it is believed to occur sporadically. However, there have been rare reports of familial occurrence of imperforate hymen.49,50 Although a common mode of inheritance has not been identified, it seems prudent to obtain a family history of reproductive tract defects.
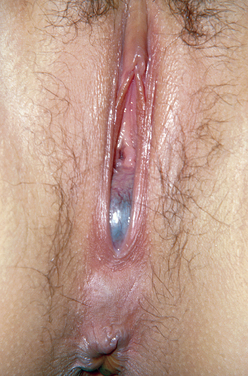
Figure 14-2 In this photograph, a bulging bluish membrane is easily seen that represents an imperforate hymen.
When detected during infancy, the imperforate hymen presents as a mucocele. It is typically asymptomatic and evident on inspection of the external genitalia. In some instances, the hydrocolpos is large enough to cause urinary tract obstruction and urine retention. In such cases, surgical decompression of the hydrocolpos is necessary. Urinary tract infections have been reported in patients with mucoceles and may potentially lead to formation of a pyocolpos.51
Treatment
Long-term studies are few. Fifteen patients with imperforate hymen were evaluated over a period of 14 years.52 Although half of the patients expressed concern about their future fertility, the 11 patients who had become sexually active at the time of the questionnaire denied any sexual dysfunction. On examination, all 15 patients had normal external genitalia and no difficulty with micturition or defecation. In a similar study, Rock53 assessed the reproductive outcome of 22 women who underwent repair of their imperforate hymen. Of the 15 women who attempted pregnancy, 86% conceived.
Androgen Insensitivity Syndrome
Complete androgen insensitivity syndrome is the third most common cause of primary amenorrhea after gonadal dysgenesis and müllerian agenesis. The incidence of androgen insensitivity syndrome is between 1/40,000 and 1/99,000; it was first reported in the literature by Morris in 1953.54,55 Such adolescents have a 46,XY karyotype. In 85% to 90% of cases of androgen insensitivity syndrome, a mutation may be found in the androgen receptor gene.56 This mutation is located on the X chromosome at Xq11-1257 and leads to a continuum of virilization disorders ranging from mild androgen insensitivity syndrome to the complete form. Over 300 mutations in the X-linked androgen receptor gene have been identified.58
The inability to recognize androgens leads to development of female external genitalia in these patients. However, due to the existence of the testes and production of müllerian inhibiting substance (MIS), the müllerian system usually regresses.59,60 These patients have normal-appearing female external genitalia associated with a blind-ending vaginal pouch. Although most patients are diagnosed after puberty and on occasion at birth or during infancy, this diagnosis is encountered when a baby girl with no evidence of sexual ambiguity presents with unilateral or bilateral inguinal hernias.
Treatment
Such patients typically have a blind-ending vaginal pouch. Thus, vaginal lengthening may be necessary through serial dilatation of the pouch. Surgery is typically not indicated. In one long-term study performed at Johns Hopkins University, 14 women with complete androgen insensitivity syndrome were followed for decades.62 Their ages ranged from mid-20s to mid-60s. More than half of these women did not require lengthening of the vagina. All the women were satisfied with their female gender, 71% reported satisfaction with their sexual function, and 77% experienced orgasm. More than half of the women exhibited a limited amount of knowledge about androgen insensitivity syndrome. But only half of them were dissatisfied with this limited knowledge. This study stresses the importance of continued counseling and support for adolescents as they progress into adulthood.
EATING DISORDERS
The incidence of eating disorders peaks during the early teens around the time of menarche and also in the late teens when the adolescent is about to leave home for college or a new job. Eating disorders can be categorized into anorexia nervosa, bulimia nervosa, and eating disorder not otherwise specified (Table 14-4). Adolescents with anorexia nervosa have a fear of becoming fat and a distorted body image and have experienced extreme weight loss or lack of weight gain. Anorexia nervosa affects 1% of adolescents, the majority of whom are females.63 Patients with bulimia may have normal weight or be overweight. They consume or “binge” large amounts of food at least twice a week for a period of at least 3 months. This is typically followed by purging via self-induced vomiting, use of laxatives, or hyperexercising. It is estimated that 1% to 5% of high school females and as many as 19% of college girls have bulimia.64
Adapted from American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Washington, D.C., American Psychiatric Association, 1994, pp. 539-550.
The incidence of eating disorder is dependent on tests used to assess the problem. When the modified eating attitudes test was administered, the rate of eating disorder among girls who came to the hospital or endocrinology clinic was 17% compared with a rate of 7% in those presenting for health maintenance visits in the same institution.65 Female athletes are particularly vulnerable to eating disorders. Unhealthy eating behavior has been reported in up to 62% of college gymnasts.66 Many employ unhealthy behaviors to control their weight, including the use of diet pills, diuretics and laxatives, binging, and self-induced vomiting. The female athletic triad is the combination of amenorrhea, eating disorder, and osteopenia. To improve her athletic performance, the patient attempts to lose weight. However, with time, the desire to lose weight eventually becomes the primary goal even at the expense of her athletic performance.
Signs and Symptoms
The signs and symptoms of anorexia nervosa and bulimia are noted in Tables 14-5 and 14-6. Menstrual abnormalities such as amenorrhea and oligomenorrhea are the usual presenting symptoms to the gynecologist. A common symptom in such patients is constipation, which may be accompanied by abdominal pain. The methods by which patients attempt to control their weight may contribute to their symptoms. Thus, cardiac arrhythmias and electrolyte abnormalities are noted in patients who lose weight very quickly via severe purging and binging and use of laxatives and diuretics. Chronic problems of binge eating and purging include enlargement of the parotid gland, mouth sores, eroded dental enamel, and osteoporosis.
Table 14-6 Symptoms and Signs of Bulimia Nervosa
Evaluation
The evaluation of a patient with an eating disorder is described in Table 14-7. An attempt is made to determine maximum and minimum weight and the patient’s ideal body weight. Screening questions such as stress associated with sports, family, and school must be asked. To determine eating attitudes, a screening test for eating disorder should be performed. Concern by family members or peers about an eating disorder should raise a red flag. Other behaviors that may be suspicious are isolation from friends, recent changes in dietary habits, change in bowel habits, and frequent trips to the bathroom after meals. The physical examination should focus on measuring the patient’s height and weight, orthostatic blood pressure, and pulse.
| Document eating attitudes |
The endocrine abnormalities noted in patients with anorexia nervosa include low estradiol and gonadotropin levels, high cortisol levels, and normal prolactin levels.67 Although thyroid-stimulating hormone (TSH) and free thyroxine (T4) levels are normal, the reverse T3 is high and T3 levels are low. In adolescents with anorexia nervosa, LH regresses to prepubertal levels at the nadir of the weight loss.68 Finally, the leptin level is low in patients with anorexia nervosa and is predictive of amenorrhea in underweight women. Although these hormonal abnormalities normalize with weight gain, some patients still remain amenorrheic.
Complications
A well-known complication of anorexia nervosa is bone loss.69 Adolescence is a critical period for bone accretion because over half of the entire bone mass is achieved during the teen years. In a study of 50 adolescents with anorexia, 90% had reduction in bone mass if they had amenorrhea lasting for more than 6 months. Those who developed anorexia before menarche had lower bone mass than those who developed the disease after menarche. In addition, the premenarchal girls with anorexia were followed for 3 years, and no increase in bone mass was noted, which suggests that the insult was potentially permanent.70
Management
Weight restoration is essential to allow spontaneous resumption of menses.71 Increase in body weight and resumption of menses are usually associated with increase in bone mineral density.72,73 However, it is not clear whether this bone loss is completely reversible.74 The role of estrogen therapy in the treatment of osteopenia in adolescents with eating disorders is unclear and has less benefit if there is little weight gain.75 In a randomized clinical trial of 48 women, no significant change was noted in the bone mineral density of the group that received estrogen compared with the control group. However, a subset of the estrogen group with an initial body weight that was 70% less than their ideal weight had a 4% increase in mean bone mineral density, whereas the controls of comparable low initial body weight had a 20% decrease in bone mineral density. This study suggested that estrogen therapy might benefit a subset of very low-weight women with anorexia nervosa.76
Although full recovery in regards to weight, development, menstruation, and normal eating behavior occurs in 50% to 70% of treated adolescents,77,78 physical and mental disorders are noted into adulthood.79 These include anxiety disorders, cardiovascular symptoms, chronic pain, and chronic fatigue and depressive disorders. These findings suggest that not only is increased recognition and identification of eating disorders in adolescents of utmost importance, but also once detected, patients should be directed toward specialized treatment programs.
OVARIAN TORSION
Incidence
Ovarian torsion causes venous congestion, intraovarian hemorrhage, and eventual tissue necrosis. It is encountered in 3% of all emergent gynecologic surgery.80 The true incidence of ovarian torsion is unknown in children and adolescents. However, when the pathology of 140 girls younger than age 21 (median age, 15) presenting with a pelvic mass was reviewed in one institution in Louisville, Ky., 17.8% of the patients had ovarian torsion.81 A review of a children’s hospital 15-year experience with surgical management of ovarian masses in 102 young children (mean age, 9.8 ± 5.5 years) showed that nearly one third of their patients had ovarian torsion.82
Etiology
There are two main risk factors for ovarian torsion: prior existence of an ovarian mass or cyst and prior pelvic surgeries. The most common pathologies are ovarian cysts, benign teratomas, and serous cystadenomas.83 Normal ovaries have also been noted, and torsion in such instances may be secondary to increased mobility of the adnexa secondary to an elongated utero-ovarian ligament or long fallopian tube and mesosalpinx.84,85 Malignant lesions are not a common cause of ovarian torsion in the adolescent population.81,82
Signs and Symptoms
Typical presenting complaints may include sudden onset, sharp and stabbing pain, or colicky pelvic/abdominal pain radiating to flank, back, or groin. The pain is usually associated with nausea and vomiting that waxes and wanes.83 Peritoneal signs of rebound and guarding are very late signs and thus are rare at presentation. The patient may or may not have fever and leukocytosis depending on the timing of presentation.
A high index of suspicion remains the most valuable tool for making this diagnosis. Patients with ovarian torsion have a delayed diagnosis as a result of failure to consider the possibility of ovarian torsion at presentation. In a review of 87 cases of confirmed ovarian torsion in two hospitals, ovarian torsion was considered in the differential diagnosis in only 47% of the cases.83
Imaging
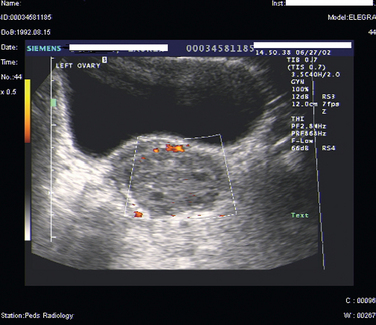
Ultrasound of the pelvis is the primary imaging modality used to evaluate patients with suspected ovarian torsion, but magnetic resonance imaging (MRI) may also be useful (Figs. 14-3 and 14-4). The ovarian ultrasound image may be very nonspecific and must be differentiated from an ovarian cyst or an ovarian mass. The sonographic features of a twisted ovary include a complex, solid or cystic central ovarian mass associated with multiple peripheral small cysts.86 This appearance reflects congestion of the ovary and is considered to be moderately sensitive but highly specific for torsion.87 The presence of these findings (ovarian mass and peripheral small cysts) in a study of 41 girls ages 3 to 13 was associated with a sensitivity of 64% but a specificity of 97%.88 However, these findings are not always observed because the appearance of the ovary may be related to the duration and degree of the torsion.
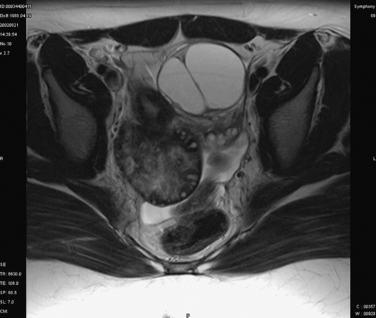
Figure 14-4 Magnetic resonance image of the pelvis showing an edematous ovary suggestive of torsion.
Color Doppler ultrasound to detect the absence of blood flow to the ovary is another modality commonly used to assess ovarian torsion. Lack of flow to the ovary should indicate torsion. On the other hand, absence of internal ovarian flow may be nonspecific and may be found in cystic ovarian lesions as well as in twisted ovaries.89 There are conflicting data as to the utility of this modality. Normal Doppler flow to the ovaries does not exclude torsion, and Doppler flow was noted to be present in 60% of surgically confirmed twisted ovaries.90,91 Intermittent torsion or incomplete vascular occlusion may account for the continued flow to the ovary. Technical difficulties may also lead to false-negative results.92
In adolescents in whom trans-abdominal ultrasonography may be the more common means of assessing the ovary, it is likely that the twisted vascular pedicle may not even be appreciated. Doppler ultrasound may potentially be useful in predicting the viability of the ovarian tissue. A report that showed both arterial and venous flow in 16 of 28 surgically confirmed twisted ovaries also demonstrated that 15 of these ovaries were viable.90 Thus, in conjunction with the findings at the time of surgery, Doppler flow to the ovaries may be able to assist with the decision to proceed with conservative surgery versus oophorectomy.
Management
Once the diagnosis of ovarian torsion is made, the time to surgery should be minimized to maximize ovarian viability (Fig. 14-5). However, in one review, only 26 of 87 patients had surgery within 24 hours of presentation. The mean time from presentation to surgery was 5.8 days.83 The goal of surgery is “detorsion” and possible salvage of the ovary.93,94 After the ovary is untwisted, it is assessed for signs of reperfusion. In a review of 58 women who had laparoscopic detorsion of their ovaries, 54 of the 58 (93%) ovaries showed sonographic evidence of ovarian function by 3 months postsurgery.95 Thus, although ovaries were noted to be necrotic or ischemic at the time of surgery, ovarian function is conserved. After detorsion of the blue/black ovary and establishment of reperfusion, the ovary should be assessed for signs of viability. Mild postoperative febrile morbidity may be noted. It may take several months for the size of the ovary to decrease to normal.96
During surgery, an attempt should be made to find the cyst or mass that may have caused the torsion. However, edematous, friable ovarian tissue may make it difficult to find the mass. All cases of detorsion should be followed up with ultrasound imaging of the ovaries. Given the low malignancy potential in adolescents, a persistent mass may warrant follow-up laparoscopy for cystectomy.
Ovariopexy
The role of ovariopexy is not clearly defined by the literature.97,98 Ovariopexy seems to be indicated in cases of repeat or bilateral ovarian torsion, prior loss of adnexa, or abnormally elongated utero-ovarian ligament. This decision has typically been left to the surgeon’s discretion. It would seem likely that ovariopexy could reduce the risk of future ovarian torsion, but there are no data to support this supposition. Several methods of ovariopexy have been described. They include fixation to the pelvic sidewall or attachment to the round ligament or the posterior aspect of the uterus. Shortening of the utero-ovarian ligament by placing a running stitch through the elongated utero-ovarian ligament may maintain the ovary in the most anatomically correct position.99
CHRONIC PAIN DISORDERS
The incidence of recurrent abdominal pain in children and adolescents is estimated at 10% of school-age children.100 In one study, organic causes were identified in 1 of 20 patients.101 In recent years, an increasing number of teenagers have had chronic pelvic pain from a variety of organic causes.102
The etiology of recurrent abdominal pain can be secondary to gynecologic or nongynecologic causes. The rare and the not-so-rare causes are discussed in Table 14-8. Rare causes of abdominal pain include appendiceal colic, Henoch-Schönlein-purpura, and chronic pancreatitis.
| Gynecologic causes |
Before 1980, many adolescents with chronic pelvic pain underwent laparotomy.103 However, the modern multidisciplinary approach used in the investigation and management of chronic pelvic pain rarely resorts to laparotomy. The team should consist of representatives from gynecology, gastroenterology, urology, physical therapy, psychology, and/or psychiatry for assessment and treatment.
Endometriosis in the Adolescent
Multiple studies have confirmed the existence of endometriosis in adolescents. The incidence ranges from 38% to 73%.104–106 Although rare cases of premenarchal endometriosis have been described, the mean age of presentation is 15.9 years. Adolescents can present with both cyclic and acyclic pain. Other presenting symptoms are dysmenorrhea, irregular periods, dyspareunia, abdominal pain, nausea, constipation, and diarrhea.
The physical examination usually fails to reveal an underlying pathology. In most series, pelvic tenderness is noted on examination. Diffuse or localized pelvic tenderness is noted in most patients. The likelihood of finding and palpating an endometrioma is small in adolescents. Few patients will have an adnexal mass.107 Hence, neither the physical examination nor the history is very helpful in identifying this diagnosis. The history and physical examination are performed to primarily rule out other causes of chronic pelvic and abdominal pain.
Thus, the primary means of detecting endometriosis in the adolescent is laparoscopy. Once the patient has failed to respond to NSAIDs and oral contraceptives, laparoscopy for assessment of the pelvis may be indicated. Low-stage disease is found in the majority of cases. But as in adults, the stage of disease is not a good indicator of the degree of pain experienced. The distribution of severity of disease is typically weighted to the early stages. In one study, stage I endometriosis was seen in 80% of patients and stage II in 12% of patients.106 Stage III and stage IV disease was seen in only 6% and 2% of their subjects, respectively, and these patients were more likely to have obstructive müllerian anomalies. Relief of the obstruction leads to regression of these endometriotic lesions and pain.108 Hence, extensive ablation and excision of these lesions may not be indicated in this subgroup of patients.
The laparoscopist must be aware of the unique aspects of endometriosis in the adolescent. The lesions noted in the adolescent are atypical and may appear as clear papules, red lesions, flamelike lesions, white lesions, and glandular lesions. Clearly, the appearance of endometriosis changes as the woman ages. To help identify clear lesions, Laufer described the technique of filling the pelvis with fluid and assessing the pelvic sidewalls laparoscopically “under water.”109 Subtle endometriotic lesions have been reported in 40% to 55% of patients.110,111 Hence, a biopsy of atypical lesions may be warranted to help confirm the diagnosis.
The goal of therapy in such patients is control of pain and preservation of fertility. Due to the long-term nature of the disease, medical therapy is the mainstay of treatment. Surgical therapy is used primarily for diagnosis and occasionally for extirpation of disease. The response to removal of disease via excision or ablation of lesions in stage I patients (seen primarily in adolescents) has been reported to be relatively poor.112 As in adults, agents used for pain control in adolescents include oral contraceptives, NSAIDs, progestational agents, and GnRH agonists. Although danazol improves pain, its androgenic side effects are intolerable to the adolescent considering long-term therapy.113 Concomitant symptoms, such as constipation and irritable bowel syndrome, should be treated aggressively and the adolescent seen every 3 to 6 months. This type of follow-up will be reassuring to the patient and her family. Questions regarding the long-term nature of the disease and fertility issues should be addressed. Free and open discussion will also provide the opportunity to educate and empower the patient and help her avoid the need for repetitive surgery.
Pelvic Inflammatory Disease
A high index of suspicion must be maintained for pelvic inflammatory disease (PID) in the adolescent population. Most clinicians will use laparoscopy to definitively diagnose PID in adolescents if they have recurrent symptoms and cultures are negative.114 In addition, laparoscopy is indicated if symptoms of pelvic pain persist despite treatment of culture-proven PID. An empathic approach must be maintained in both relieving pain and dealing honestly with the patient’s questions regarding future fertility.
Müllerian Anomalies
Müllerian anomalies are more commonly the cause of pelvic pain and dysmenorrhea in adolescents than adults. Hence, it is mandatory to confirm normal müllerian anatomy either via pelvic examination or imaging in the adolescent presenting with pain to the gynecologist. These anomalies are discussed in detail in Chapters 12 and 51.
Lactose Malabsorption
A nongynecologic cause of chronic abdominal pain is lactose malabsorption. At least one study identified lactose malabsorption in 24% of patients ages 6 to 18 with recurring abdominal pain.115 Presenting symptoms are increased flatus, diarrhea, bloating, and abdominal pain. An inquiry regarding gastrointestinal symptoms should be routine during the history and physical examination of an adolescent presenting with pelvic/abdominal pain. If the level of suspicion is high for gastrointestinal causes, an evaluation should be requested from a pediatric gastroenterologist. Lactose malabsorption can usually be identified by an abnormal breath hydrogen test.
Chronic Constipation
This is marked by a history of infrequent bowel movements or straining with bowel movements. A careful physical examination, including a rectal examination, may also point to the diagnosis. Irregular bowel movements may also accompany other disorders such as endometriosis. A double-blind, randomized study showed that the use of empirical fiber decreased the frequency of recurrent abdominal pain, possibly due to alterations in colonic transit time.116
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) may also be a cause of recurrent abdominal pain. It affects 14% of high school students and 6% of students in earlier years.117 Symptoms include alternating constipation and diarrhea, abdominal pain, frequent bowel movements, straining, bloating, and a sense of incomplete evacuation. Children who suffer from depression, headaches, and anxiety seem more prone to IBS.118 A recent study measured rectal sensation and rectal contractile response to a meal in eight children with IBS and concluded that they had a significantly lower threshold response for pain and a disturbed contractile response to a meal.119 They further concluded that sensory and motor abnormalities may play a pathologic role in childhood IBS. Dietary therapy includes adding extra fiber and may provide symptomatic relief.
Inflammatory Bowel Disease
One quarter of cases of inflammatory bowel disease (IBD) are diagnosed during childhood or adolescence.120 Many patients with IBD have a genetic predisposition. A relatively small percentage of patients with Crohn’s disease present with isolated abdominal pain.121 Concomitant symptoms can include weight loss, anorexia, diarrhea, and fever. An increased sedimentation rate and abnormal complete blood count may suggest the presence of Crohn’s disease. All patients with recurrent abdominal pain should be checked for occult blood via rectal examination, and both the perianal and vulvar area must be examined carefully. Any patient with nocturnal bowel movements, tenesmus, fecal urgency, heme-positive stool, and abdominal pain relieved by loose bowel movements must be investigated for IBD. A diagnosis is usually made by a colonoscopy and biopsy.
Abdominal Wall Trigger Points
Over 90% of patients with chronic pelvic pain have been found to have abdominal wall trigger points.122 By tensing the rectus abdominis muscles, trigger points may be better elucidated.123 These tender abdominal or pelvic muscles can be easily treated in pain therapy units by local infiltration or by a physical therapist who is well versed in pelvic floor therapy. The therapist can be useful in teaching the patient exercises that can help reduce the pain and supporting patient education efforts (e.g., explaining that the disease is not in the pelvis).
1 Andresch B, Milsom I. An epidimiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144:655-660.
2 Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68:661-664.
3 Widholm O, Kantero RL. A statistical analysis of the menstrual patterns of 8000 Finnish girls and their mothers. Acta Obst Gyn Scan. 1971;14(Suppl):1-36.
4 Harlow SD, Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. BJOG. 1996;103:1134-1142.
5 Sultan C, Jeandel C, Paris F, Trimeche S. Adolescent dysmenorrhea. Endocr Dev. 2004;7:140-147.
6 Balbi C, Musone R, Menditto A, et al. Influence of menstrual factors and dietary habits on menstrual pain in adolescence age. Eur J Obstet Gynecol Reprod Biol. 2000;91:143-148.
7 Chan WY, Dawood MY. Prostaglandin levels in menstrual fluid of nondysmenorrheic and of dysmenorrheic subjects with and without oral contraceptive or ibuprofen therapy. Adv Prostaglandin Thromboxane Res. 1980;8:1443-1447.
8 Lundstrom V, Green K. Endogenous levels of prostaglandin F2α and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. Am J Obstet Gynecol. 1978;130:640-646.
9 Rees MC, DiMarzo V, Tippins JR, et al. Leukotriene release by endometrium and myometrium throughout the menstrual cycle in dysmenorrhoea and menorrhagia. J Endocrinol. 1987;113:291-295.
10 Nigam S, Benedetto C, Zonca M, et al. Increased concentrations of eicosanoids and platelet-activating factor in menstrual blood from women with primary dysmenorrhea. Eicosanoids. 1991;4:137-141.
11 Marjoribanks J, Proctor ML, Farquhar C. Nonsteroidal anti-inflammatory drugs for primary dysmenorrhoea. Cochrane Database System Rev. 2006:3. CD001751.
12 Robinson JC, Plichta S, Weisman CS, et al. Dysmenorrhea and use of oral contraceptives in adolescent women attending a family planning clinic. Am J Obstet Gynecol. 1992;166:578-583.
13 Harel Z, Biro FM, Kotenhahn RK. Supplementation with omega-3 fatty acids in the management of dysmenorrhea in adolescents. Am J Obstet Gynecol. 1996;174:1335-1338.
14 Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
15 van Hooff MH, Voorhorst FJ, Kaptein MB, et al. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod. 2004;19:383-392.
16 Salmi DJ, Zisser HC, Jovanovic L. Screening for and treatment of polycystic ovary syndrome in teenagers. Exp Biol Med (Maywood). 2004;229:369-377.
17 Ibanez L, Potau N, Virdis R, et al. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: Increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1599-1603.
18 Ibanez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671-696.
19 Kent SC, Legro RS. Polycystic ovary syndrome in adolescents. Adolesc Med. 2002;13:73-88.
20 Ibanez L, Potau N, Zampolli M, et al. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab. 1997;82:2283-2288.
21 Plouffe LJr. Disorders of excessive hair growth in the adolescent. Obstet Gynecol Clin North Am. 2000;27:79-99.
22 van Hooff MH, Voorhorst FJ, Kaptein MB, et al. Polycystic ovaries in adolescents and the relationship with menstrual cycle patterns, luteinizing hormone, androgens, and insulin. Fertil Steril. 2000;74:49-58.
23 Apter D, Butzow T, Laughlin GA, Yen SSC. Metabolic features of polycystic ovary syndrome are found in adolescent girls with hyperandrogenism. J Clin Endocrinol Metab. 1995;80:2966-2973.
24 Caprio S. Insulin resistance in childhood obesity. J Pediatr Endocrinol. 2002;15(Suppl 1):487-492.
25 Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38-44.
26 Palmert MR, Gordon CM, Kartashov AI, et al. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017-1023.
27 Ibanez L, Valls C, Ferrer A, et al. Sensitization to insulin induces ovulation in nonobese adolescents with anovulatory hyperandrogenism. J Clin Endocrinol Metab. 2001;86:3595-3598.
28 Barth JH, Catalan J, Cherry CA, Day A. Psychological morbidity in women referred for treatment of hirsutism. J Psychosom Res. 1993;37:615-619.
29 Rasgon NL, Rao RC, Hwang S, et al. Depression in women with polycystic ovary syndrome: Clinical and biochemical correlates. J Affect Disord. 2003;74:299-304.
30 Sonino N, Fava GA, Mani E, et al. Quality of life of hirsute women. Postgrad Med J. 1993;69:186-189.
31 Kahsar-Miller MD, Nixon C, Boots LR, et al. Prevalence of polycystic ovary syndrome (PCOS) in first degree relatives of patients with PCOS. Fertil Steril. 2001;75:53-58.
32 Lloyd T, Lin HM, Matthews AE, et al. Oral contraceptive use by teenage women does not affect body composition. Obstet Gynecol. 2002;100:235-239.
33 Morin-Papunen LC, Vauhkonen I, Koivunen RM, et al. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2000;85:3161-3168.
34 Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-201.
35 Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2002;346:393-403.
36 Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: Amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555-1559.
37 Ibanez L, Valls C, Potau N, et al. Sensitization to insulin in adolescent girls to normalize hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism after precocious pubarche. J Clin Endocrinol Metab. 2000;85:3526-3530.
38 Ibanez L, Valls C, Marcos MV, et al. Insulin sensitization for girls with precocious pubarche and with risk for polycystic ovary syndrome: Effects of prepubertal initiation and postpubertal discontinuation of metformin treatment. J Clin Endocrinol Metab. 2004;89:4331-4337.
39 Southam AL, Richart RM. The prognosis for adolescents with menstrual abnormalities. Am J Obstet Gynecol. 1966;94:637-645.
40 Claessens EA, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
41 Falcone T, Desjardins C, Bourque J, et al. Dysfunctional uterine bleeding in adolescents. J Reprod Med. 1994;39:761-764.
42 Smith YR, Quint EH, Hertzberg RB. Menorrhagia in adolescents requiring hospitalization. J Pediatr Adolesc Gynecol. 1998;11:13-15.
43 Anderson AB, Haynes PJ, Guillebaud J, Turnbull AC. Reduction of menstrual blood loss by prostaglandin-synthetase inhibitors. Lancet. 1976;1:774-776.
44 Fraser IS, Pearse C, Shearman RP, et al. Efficacy of mefenamic acid in patients with a complaint of menorrhagia. Obstet Gynecol. 1981;58:543-551.
45 DeVore GR, Owens O, Kase N. Use of intravenous premarin in the treatment of dysfunctional uterine bleeding—a double-blind randomized control study. Obstet Gynecol. 1982;59:285-291.
46 Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: Randomised controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ. 1996;313:579-582.
47 Zurawin RK, Pramanik S. Endometrial balloon ablation as a therapy for intractable uterine bleeding in an adolescent. J Pediatr Adolesc Gynecol. 2001;14:119-121.
48 Messina M, Severi FM, Bocchi C, et al. Voluminous perinatal pelvic mass: A case of congenital hydrometrocolpos. J Matern Fetal Neonat Med. 2004;15:135-137.
49 Stelling JR, Gray MR, Davis AJ, et al. Dominant transmission of imperforate hymen. Fertil Steril. 2000;74:1241-1244.
50 Usta IM, Awwad JT, Usta JA, et al. Imperforate hymen: Report of an unusual familial occurrence. Obstet Gynecol. 1993;82(Suppl):655-656.
51 Brevetti LS, Kimura K, Brevetti GR, et al. Pyocolpos: Diagnosis and treatment. J Pediatr Surg. 1997;32:110-111.
52 Liang CC, Chang SD, Soong YK. Long-term follow-up of women who underwent surgical correction for imperforate hymen. Arch Gynecol Obstet. 2003;269:5-8.
53 Rock JA, Zacur HA, Dlugi AM, et al. Pregnancy success following surgical correction of imperforate hymen and complete transverse vaginal septum. Obstet Gynecol. 1982;59:448-451. Available from http://www.refworks.com.
54 Boehmer AL, Brinkmann O, Bruggenwirth H, et al. Genotype versus phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab. 2001;86:4151-4160.
55 Morris JM. The syndrome of testicular feminization in male pseudohermaphroditism. Am J Obstet Gynecol. 1953;65:1192-1211.
56 Sultan C, Lumbroso S, Paris F, et al. Disorders of androgen action. Semin Reprod Med. 2002;20:217-228.
57 Brown CJ, Goss SJ, Lubahn DB, et al. Androgen receptor locus on the human X chromosome: Regional localization to Xq11-12 and description of a DNA polymorphism. Am J Hum Genet. 1989;44:264-269.
58 Gottlieb B, Lehvaslaiho H, Beitel LK, et al. The androgen receptor gene mutations database. Nucleic Acids Res. 1998;26:234-238.
59 Damiani D, Mascolli MA, Almeida MJ, et al. Persistence of müllerian remnants in complete androgen insensitivity syndrome. J Pediatr Endocrinol Metab. 2002;15:1553-1556.
60 Oka M, Katabuchi H, Munemura M, et al. An unusual case of male pseudohermaphroditism: Complete testicular feminization associated with incomplete differentiation of the müllerian duct. Fertil Steril. 1984;41:154-156.
61 Speroff L, Fritz MA. Amenorrhea. In: Speroff L, Fritz MA, editors. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004:401-463.
62 Wisniewski AB, Migeon CJ. Long-term perspectives for 46,XY patients affected by complete androgen insensitivity syndrome or congenital micropenis. Semin Reprod Med. 2002;20:297-304.
63 Lucas AR. The eating disorder “epidemic”: More apparent than real? Pediatr Ann. 1992;21:746-751.
64 Rome ES. Eating disorders. Obstet Gynecol Clin North Am. 2003;30:353-377.
65 Rome ES, Imrie RK, Rybicki LA, Gidwani G. Prevalence of abnormal eating attitudes and behaviors in hospital-based primary and tertiary care clinics: A window of opportunity? J Pediatr Adolesc Gynecol. 1996;9:133-138.
66 Rosen LW, Hough DO. Pathogenic weight-control behaviors of female college gymnasts. Physician Sportsmed. 1988;16:140-146.
67 Stoving RK, Hangaard J, Hansen-Nord M, Hagen C. A review of endocrine changes in anorexia nervosa. J Psychiatr Res. 1999;33:139-152.
68 Boyar RM, Katz J, Finkelstein JW, et al. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. NEJM. 1974;291:861-865.
69 Ayers JW, Gidwani GP, Schmidt IM, Gross M. Osteopenia in hypoestrogenic young women with anorexia nervosa. Fertil Steril. 1984;41:224-228.
70 Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
71 Golden NH, Jacobson MS, Schebendach J, et al. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med. 1997;151:16-21.
72 Drinkwater BL, Bruemner B, Chesnut CH3rd. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
73 Drinkwater BL, Nilson K, Ott S, Chesnut CH3rd. Bone mineral density after resumption of menses in amenorrheic athletes. JAMA. 1986;256:380-382.
74 Bachrach LK, Guido D, Katzman D, et al. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86:440-447.
75 Kreipe RE, Hicks DG, Rosier RN, Puzas JE. Preliminary findings on the effects of sex hormones on bone metabolism in anorexia nervosa. J Adolesc Health. 1993;14:319-324.
76 Klibanski A, Biller BM, Schoenfeld DA, et al. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
77 Strober M, Freeman R, Morrell W. Atypical anorexia nervosa: Separation from typical cases in course and outcome in a long-term prospective study. Int J Eat Disord. 1999;25:135-142.
78 Yager J, Andersen AE. Clinical practice. Anorexia nervosa. NEJM. 2005;353:1481-1488.
79 Johnson JG, Cohen P, Kasen S, Brook JS. Eating disorders during adolescence and the risk for physical and mental disorders during early adulthood. Arch Gen Psychiatry. 2002;59:545-552.
80 Hibbard LT. Adnexal torsion. Am J Obstet Gynecol. 1985;152:456-461.
81 Templeman C, Fallat ME, Blinchevsky A, Hertweck SP. Noninflammatory ovarian masses in girls and young women. Obstet Gynecol. 2000;96:229-233.
82 Cass DL, Hawkins E, Brandt ML, et al. Surgery for ovarian masses in infants, children, and adolescents: 102 consecutive patients treated in a 15-year period. J Pediatr Surg. 2001;36:693-699.
83 Houry D, Abbott JT. Ovarian torsion: A fifteen-year review. Ann Emerg Med. 2001;38:156-159.
84 Buss JG, Lee RA. Sequential torsion of the uterine adnexa. Mayo Clin Proc. 1987;62:623-625.
85 Prasad M, Bone CD, Arafat Q. Torsion of a normal adnexum in an adolescent. J Obstet Gynaecol. 2002;22:454-455.
86 Graif M, Shalev J, Strauss S, et al. Torsion of the ovary: Sonographic features. Am J Roentgenol. 1984;143:1331-1334.
87 Hurh PJ, Meyer JS, Shaaban A. Ultrasound of a torsed ovary: Characteristic gray-scale appearance despite normal arterial and venous flow on Doppler. Pediatr Radiol. 2002;32:586-588.
88 Graif M, Itzchak Y. Sonographic evaluation of ovarian torsion in childhood and adolescence. Am J Roentgenol. 1988;150:647-649.
89 Quillin SP, Siegel MJ. Transabdominal color Doppler ultrasonography of the painful adolescent ovary. J Ultrasound Med. 1994;13:549-555.
90 Lee EJ, Kwon HC, Joo HJ, et al. Diagnosis of ovarian torsion with color Doppler sonography: Depiction of twisted vascular pedicle. J Ultrasound Med. 1998;17:83-89.
91 Pena JE, Ufberg D, Cooney N, Denis AL. Usefulness of Doppler sonography in the diagnosis of ovarian torsion. Fertil Steril. 2000;73:1047-1050.
92 Hamper UM, DeJong MR, Caskey CI, Sheth S. Power Doppler imaging: Clinical experience and correlation with color Doppler US and other imaging modalities. Radiographics. 1997;17:499-513.
93 Zweizig S, Perron J, Grubb D, Mishell DRJr. Conservative management of adnexal torsion. Am J Obstet Gynecol. 1993;168:1791-1795.
94 Oelsner G, Bider D, Goldenberg M, et al. Long-term follow-up of the twisted ischemic adnexa managed by detorsion. Fertil Steril. 1993;60:976-979.
95 Cohen SB, Oelsner G, Seidman DS, et al. Laparoscopic detorsion allows sparing of the twisted ischemic adnexa. J Am Assoc Gynecol Laparosc. 1999;6:139-143.
96 Templeman C, Hertweck SP, Fallat ME. The clinical course of unresected ovarian torsion. J Pediatr Surg. 2000;35:1385-1387.
97 Grunewald B, Keating J, Brown S. Asynchronous ovarian torsion—the case for prophylactic oophoropexy. Postgrad Med J. 1993;69:318-319.
98 Crouch NS, Gyampoh B, Cutner AS, Creighton SM. Ovarian torsion: To pex or not to pex? Case report and review of the literature. J Pediatr Adolesc Gynecol. 2003;16:381-384.
99 Germain M, Rarick T, Robins E. Management of intermittent ovarian torsion by laparoscopic oophoropexy. Obstet Gynecol. 1996;88:715-717.
100 Wyllie R, Kay M. Causes of recurrent abdominal pain. Clin Pediatr (Phila). 1993;32:369-371.
101 Apply J, Nash N. RAP-field survey of disease in childhood. Arch Dis Child. 1958;33:165.
102 Poole SR. Recurrent abdominal pain in childhood and adolescence. Am Fam Physician. 1984;30:131-137.
103 Goldstein DP, deCholnoky C, Leventhal JM, Emans SJ. New insights into the old problem of chronic pelvic pain. J Pediatr Surg. 1979;14:675-680.
104 Vercellini P, Fedele L, Arcaini L, et al. Laparoscopy in the diagnosis of chronic pelvic pain in adolescent women. J Reprod Med. 1989;34:827-830.
105 Laufer MR, Goitein L, Bush M, et al. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J Pediatr Adolesc Gynecol. 1997;10:199-202.
106 Reese KA, Reddy S, Rock JA. Endometriosis in an adolescent population: The Emory experience. J Pediatr Adolesc Gynecol. 1996;9:125-128.
107 Chatman DL, Ward AB. Endometriosis in adolescents. J Reprod Med. 1982;27:156-160.
108 Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
109 Laufer MR. Identification of clear vesicular lesions of atypical endometriosis: A new technique. Fertil Steril. 1997;68:739-740.
110 Redwine DB. Age-related evolution in color appearance of endometriosis. Fertil Steril. 1987;48:1062-1063.
111 Stripling MC, Martin DC, Chatman DL, et al. Subtle appearance of pelvic endometriosis. Fertil Steril. 1988;49:427-431.
112 Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696-700.
113 Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database System Rev. 2006. CD000068.
114 Rome ES, Moszczenski SA, Craighill M, et al. A clinical pathway for pelvic inflammatory disease for use on an inpatient service. Clin Perform Qual Health Care. 1995;3:185-196.
115 Webster RB, DiPalma JA, Gremse DA. Lactose maldigestion and recurrent abdominal pain in children. Dig Dis Sci. 1995;40:1506-1510.
116 Feldman W, McGrath P, Hodgson C, et al. The use of dietary fiber in the management of simple, childhood, idiopathic, recurrent, abdominal pain. Results in a prospective, double-blind, randomized, controlled trial. Am J Dis Child. 1985;139:1216-1218.
117 Hyams JS, Burke G, Davis PM, et al. Abdominal pain and irritable bowel syndrome in adolescents: A community-based study. J Pediatr. 1996;129:220-226.
118 Hyams JS, Treem WR, Justinich CJ, et al. Characterization of symptoms in children with recurrent abdominal pain: Resemblance to irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 1995;20:209-214.
119 Van Ginkel R, Voskuijl WP, Benninga MA, et al. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology. 2001;120:31-38.
120 Hait L, Bousvaros A, Grand R. Pediatric inflammatory bowel disease: What children can teach adults. Inflam Bowel Dis. 2005;11:519-527.
121 Wyllie R, Sarigol S. The treatment of inflammatory bowel disease in children. Clin Pediatr (Phila). 1998;37:421-425.
122 Slocumb JC. Neurological factors in chronic pelvic pain: Trigger points and the abdominal pelvic pain syndrome. Am J Obstet Gynecol. 1984;149:536-543.
123 Thomson WH, Dawes RF, Carter SS. Abdominal wall tenderness: A useful sign in chronic abdominal pain. Br J Surg. 1991;78:223-225.