Chapter 41 Renal replacement therapy
When acute renal failure (ARF) is severe, resolution can take several days or weeks. During this time, the kidneys cannot maintain homeostasis of fluid, potassium, metabolic acid and waste products. Life-threatening complications frequently develop. In these patients, extracorporeal techniques of blood purification must be applied to prevent such complications. Such techniques, broadly named renal replacement therapy (RRT), include continuous haemofiltration (HF), intermittent haemodialysis (IHD) and peritoneal dialysis (PD), each with its technical variations. All of these techniques rely on the principle of removing unwanted solutes and water through a semipermeable membrane. Such membrane is either biological (peritoneum) or artificial (haemodialysis or HF membranes), and each offers several advantages, disadvantages and limitations.
PRINCIPLES
The principles of RRT have been extensively studied and described.1–3 This is a summary of some aspects which are relevant to the critical care physician.
WATER REMOVAL
SOLUTE REMOVAL
The removal of unwanted solute can be achieved by:
INDICATIONS FOR RENAL REPLACEMENT THERAPY
In the critically ill patient, RRT should be initiated early, prior to the development of complications. Fear of early dialysis stems from the adverse effects of conventional IHD with cuprophane membranes, especially haemodynamic instability, and from the risks and limitations of continuous or intermittent PD.4–5 However, continuous RRT (CRRT)6,7 or slow low-efficiency daily dialysis (SLEDD)8 minimises these effects. The criteria for the initiation of RRT in patients with chronic renal failure may be inappropriate in the critically ill.9,10 A set of modern criteria for the initiation of RRT in the intensive care unit (ICU) is presented in Table 41.1.
Table 41.1 Modern criteria for the initiation of renal replacement therapy (RRT) in the intensive care unit*
| Oliguria (urine output < 200 ml/12 h) |
| Anuria (urine output: 0–50 ml/12 h) |
| [Urea] > 35 mmol/l |
| [Creatinine] > 400 μmol/l |
| [K+] > 6.5 mmol/l or rapidly rising† |
| Pulmonary oedema unresponsive to diuretics |
| Uncompensated metabolic acidosis (pH < 7.1) |
| [Na+] < 110 and > 160 mmol/l |
| Temperature > 40oC |
| Uraemic complications (encephalopathy/myopathy/neuropathy/pericarditis) |
| Overdose with a dialysable toxin (e.g. lithium) |
* If one criterion is present, RRT should be considered. If two criteria are simultaneously present, RRT is strongly recommended.
† Please be aware of differences between plasma versus serum measurement in your laboratory.
With IHD, CRRT or SLEDD there are limited data on what is ‘adequate’ intensity of dialysis. However, this concept should include maintenance of homeostasis at all levels,10 and better uraemic control may translate into better survival.11,12 An appropriate target urea might be 15–25 mmol/l, with a protein intake around 1.5 g/kg per day. This can be easily achieved using CRRT at urea clearances of 35–45 l/day depending on patient size and catabolic rate. If intermittent therapy is used, daily and extended treatment as described with SLEDD becomes desirable.13
MODE OF RENAL REPLACEMENT THERAPY
There is a great deal of controversy as to which mode of RRT is ‘best’ in the ICU, due to the lack of randomised controlled trials comparing different techniques. In their absence, techniques of RRT may be judged on the basis of the following criteria:
CRRT and SLEDD offer many advantages over PD and conventional IHD (3–4 h/day, 3–4 times/week),13 and whereas CRRT is almost exclusively used in some centres,14 only a minority of American ICU patients receive CRRT. Some salient aspects of CRRT, IHD and PD require discussion.
CONTINUOUS RENAL REPLACEMENT THERAPY
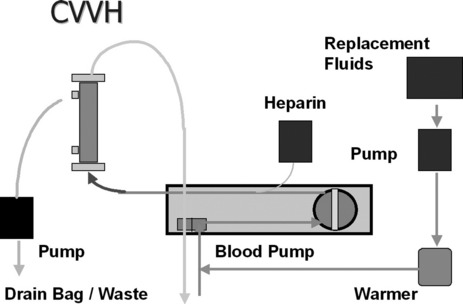
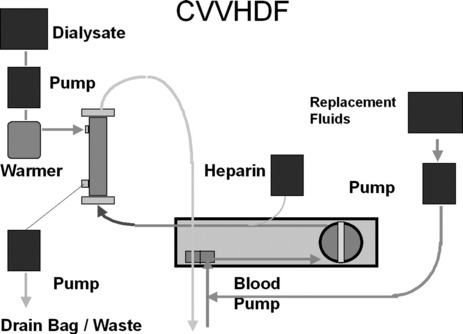
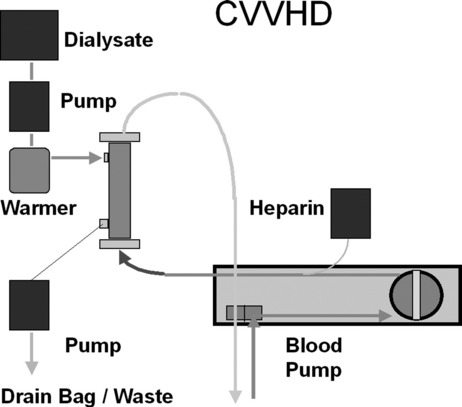
First described in 1977, CRRT has undergone several technical modifications. Initially, it was performed as an arteriovenous therapy (continuous arteriovenous HF: CAVH). The need to cannulate an artery, however, is associated with 15–20% morbidity. Accordingly, double-lumen catheters and peristaltic blood pumps have come into use (continuous venovenous HF: CVVH) with or without control of ultrafiltration rate. Ultrafiltration rates of 2 l/h yield urea clearances > 30 ml/min. Diagrams illustrating typical HF circuits are presented in Figures 41.1–41.3.
No matter what technique is used, the following outcomes are predictable:
DOSE OF CRRT AND CIRCUIT ANTICOAGULATION
The optimal dose (expressed as effective effluent per kilogram per hour) of CRRT remains unknown. Several studies suggest that higher dose may translate into better outcome.12,15 However such studies have been single-centre in nature and require confirmation in multicentre randomised controlled trials. Such trials are now under way (see below).16 It seems wise to wait for the results of larger studies before making any changes to the typical dose of approximately 25 ml/kg per hour currently delivered in Australia and New Zealand.
The flow of blood through an extracorporeal circuit causes activation of the coagulation cascade and promotes clotting of the filter and circuit itself. In order to delay such clotting and achieve acceptable operational lives (approximately 24 hours) for the circuit, anticoagulants are frequently used.17 However, circuit anticoagulation increases risk of bleeding. Therefore, the risks and benefits of more or less intense anticoagulation and alternative strategies (Table 41.2) must be considered.
Table 41.2 Strategies for circuit anticoagulation during continuous renal replacement therapy
| No anticoagulation |
| Low-dose prefilter heparin (< 500 IU/h) |
| Medium-dose prefilter heparin (500–1000 IU/h) |
| Full heparinisation |
| Regional anticoagulation (prefilter heparin and postfilter protamine, usually at a 100 IU:1 mg ratio) |
| Regional citrate anticoagulation (prefilter citrate and postfilter calcium – special calcium-free dialysate needed) |
| Low-molecular-weight heparin |
| Prostacyclin |
| Heparinoids |
| Serine proteinase inhibitors (nafamostat mesylate) |
In the vast majority of patients, low-dose heparin (< 500 IU/h) is sufficient to achieve adequate filter life, is easy and cheap to administer and has almost no effect on the patient’s coagulation tests. In some patients, a higher dose is necessary. In others (pulmonary embolism, myocardial ischaemia), full heparinisation may actually be concomitantly indicated. Regional citrate anticoagulation is very effective but requires a special dialysate or replacement fluid.18 Regional heparin/protamine anticoagulation is also somewhat complex but may be useful if frequent filter clotting occurs and further anticoagulation of the patient is considered dangerous. Low-molecular-weight heparin is also easy to give but more expensive. Its dose must be adjusted for the loss of renal function. Heparinoids and prostacyclin may be useful if the patient has developed heparin-induced thrombocytopoenia and thrombosis. Finally, in perhaps 10–20% of patients anticoagulation is best avoided because of endogenous coagulopathy or recent surgery. In such patients adequate filter life can be achieved provided that blood flow is kept at about 200 ml/min and vascular access is reliable.19
CRRT TECHNOLOGY
The increasing use of venovenous CRRT has led to the development of a field of CRRT technology which offers different kinds of machines to facilitate its performance.20 Some understanding of these devices is important to the successful implementation of CRRT in any ICU. One can a have simple blood pump with safety features (air bubble trap and pressure alarms) and use widely available volumetric pumps to control replacement or dialysate flow and effluent flow. Such adaptive technology is inexpensive, but is not user-friendly. Also, volumetric pumps have an inherent inaccuracy of about 5%, which, in a system exchanging up to 50 l/day, can cause problems. Various manufacturers have now produced custom-made machines for HF. These machines are safer and have much more sophisticated pump control systems, alarms and graphic displays. They are much more user-friendly, especially with the set-up procedure.
The choice of membrane is also a matter of controversy. There are no controlled studies to show that one confers a clinical advantage over the others. The AN 69 is the most commonly used CRRT membrane in Australia. The issue of membrane size is also controversial as no controlled studies have compared different membrane surface sizes. If high-volume haemofiltration is planned, however, the membrane surface needs to be in the 1.6–2 m2 range.
INTERMITTENT HAEMODIALYSIS
Vascular access is typically by double-lumen catheter, as in continuous HF. The circuit is also the same. Countercurrent dialysate flow is used as in CVVHD. The major differences are that standard IHD uses high dialysate flows (300–400 ml/min), generates dialysate by mixing purified water and concentrate and treatment is applied for short periods of time (3–4 hours), usually every second day. These differences have important implications. Firstly, volume has to be removed over a short period of time and this may cause hypotension. Repeated hypotensive episodes may delay renal recovery.4 Secondly, solute removal is episodic. This translates into inferior uraemic control21 and acid–base control. Limited fluid and uraemic control imposes unnecessary limitations on nutritional support. Furthermore, rapid solute shifts increase brain water content and raise intracranial pressure.22 Finally, much controversy has surrounded the issue of membrane bioincompatibility. Standard low-flux dialysing membranes made of cuprophane are known to trigger the activation of several inflammatory pathways, when compared to high-flux synthetic membranes (also used for continuous HF). It is possible that such proinflammatory effect contributes to further renal damage and delays recovery or even affects mortality. Given the minimal difference in cost between biocompatible and bioincompatible low-flux membranes, biocompatible membranes (polysulfone) are now preferred.
The limitations of applying ‘standard’ IHD to the treatment of ARF9 has led to the development of new approaches (so-called ‘hybrid techniques’) such as SLEDD.13 These techniques seek to adapt IHD to the clinical circumstances and thereby increase its tolerance and its clearances.
PERITONEAL DIALYSIS
This technique is now uncommonly used in the treatment of adult ARF in developed countries.14 However, it may be an adequate technique in developing countries or in children where the peritoneal membrane has a greater relative surface or alternatives are considered too expensive, too invasive, or are not available. Typically access is by the insertion of an intraperitoneal catheter. Glucose-rich dialysate is then inserted into the peritoneal cavity and acts as the ‘dialysate’. After a given ‘dwell time’ it is removed and discarded with the extra fluid and toxins that have moved from the blood vessels of the peritoneum to the dialysate fluid. Machines are also available which deliver and remove dialysate at higher flows, providing intermittent treatment or higher solute clearances. Several major shortcomings make PD relatively unsuited to the treatment of adult ARF:
There have not been any reports of the sole use of PD for the treatment of adult patients with ARF in the last 15 years. A randomised trial comparing PD to CVVH found that PD was associated with increased mortality.23
OTHER BLOOD PURIFICATION TECHNIQUES
PLASMAPHERESIS OR PLASMA EXCHANGE
With this technique, plasma is removed from the patient and exchanged with fresh frozen plasma (FFP) and a mixture of colloid and crystalloid solutions. This technique can also be performed in an ICU familiar with CRRT techniques. A plasmafilter (a filter that allows the passage of molecules up to 500 kD) instead of a haemofilter is inserted in the CVVH circuit, and the filtrate (plasma) is discarded. Plasmapheresis can also be performed with special machines using the principles of centrifugation. The differences, if any, between centrifugation and filtration technology are unclear. Replacement (postfilter) will occur as in CVVH using, for example, a 50/50 combination of FFP and albumin. Plasmapheresis has been shown to be effective treatment for thrombotic thrombocytopenic purpura and for several diseases mediated by abnormal antibodies (Guillain–Barré syndrome, cryoglobulinaemia, myasthenia gravis, Goodpasture’s syndrome) in which antibody removal appears desirable. Its role in the treatment of sepsis remains uncertain.24
BLOOD PURIFICATION TECHNOLOGY OUTSIDE ARF
There is growing interest in the possibility that blood purification may provide a clinically significant benefit in patients with severe sepsis/septic shock by removing circulating ‘mediators’. A variety of techniques, including plasmapheresis, high-volume HF, very-high-volume HF, coupled plasma filtration adsorption24–27 and large-pore HF,27 are being studied in animals and in phase I/II studies in humans. Initial experiments support the need to continue exploring this therapeutic option. However, no suitably powered randomised controlled trials have yet been reported. Also, blood purification technology in combination with bioreactors containing either human or porcine liver cells is under active investigations as a form of artificial liver support for patients with fulminant liver failure or for patients with acute-on-chronic liver failure. Albumin-based dialysis has been developed to deal with protein-bound toxins in patients with liver failure. This system, known as MARS (molecular adsorption recirculating system), has shown benefits in patients with elevated intracranial pressure and/or acute-on-chronic liver failure, but not in patients with fulminant liver failure.28
DRUG PRESCRIPTION DURING DIALYTIC THERAPY
ARF and RRT profoundly affect drug clearance. A comprehensive description of changes in drug dosage according to the technique of RRT, residual creatinine clearance and other determinants of pharmacodynamics is beyond the scope of this chapter and can be found in specialist texts.29 Table 41.3 provides general guidelines for the prescription of drugs commonly used in the ICU.
| Drug | CRRT | IHD |
|---|---|---|
| Aminoglycosides | Normal dose q 36 h | 50% normal dose q 48 h – 2/3 redose after IHD |
| Cefotaxime or ceftazidime | 1 g q 8–12 h | 1 g q 12–24 h after IHD |
| Imipenem | 500 mg q 8 h | 250 mg q 8 h and after IHD |
| Meropenem | 500 mg q 8 h | 250 mg q 8 h and after IHD |
| Metronidazole | 500 mg q 8 h | 250 mg q 8 h and after IHD |
| Co-trimoxazole | Normal dose q 18 h | Normal dose q 24 h after IHD |
| Amoxicillin | 500 mg q 8 h | 500 mg daily and after IHD |
| Vancomycin | 1 g q 24 h | 1 g q 96–120 h |
| Piperacillin | 3–4 g q 6 h | 3–4 g q 8 h and after IHD |
| Ticarcillin | 1–2 g q 8 h | 1–2 g q 12 h and after IHD |
| Ciprofloxacin | 200 mg q 12 h | 200 mg q 24 h and after IHD |
| Fluconazole | 200 mg q 24 h | 200 mg q 48 h and after IHD |
| Acyclovir | 3.5 mg/kg q 24 h | 2.5 mg/kg per day and after IHD |
| Gancyclovir | 5 mg/kg per day | 5 mg/kg per 48 h and after IHD |
| Amphotericin B | Normal dose | Normal dose |
| Liposomal amphotericin | Normal dose | Normal dose |
| Ceftriaxone | Normal dose | Normal dose |
| Erythomycin | Normal dose | Normal dose |
| Milrinone | Titrate to effect | Titrate to effect |
| Amrinone | Titrate to effect | Titrate to effect |
| Catecholamines | Titrate to effect | Titrate to effect |
| Ampicillin | 500 mg q 8-hourly | 500 mg daily and after IHD |
CRRT, continuous renal replacement therapy; IHD, intermittent haemodialysis.
* The above values represent approximations and should be used as a general guide only. Critically ill patients have markedly abnormal volumes of distribution for these agents which will affect dosage. CRRT is conducted at variable levels of intensity in different units, also requiring adjustment. The values reported here relate to continuous venovenous haemofiltration at 2 l/h of ultrafiltration. Vancomycin is poorly removed by continuous venovenous haemofiltration. IHD may also differ from unit to unit. The values reported here relate to standard IHD with low-flux membranes for 3–4 hours every second day.
SUMMARY
The field of RRT has undergone remarkable changes over the last 10 years and is continuing to evolve rapidly. Technology is being improved to facilitate clinical application and new areas of research are developing. CRRT is now firmly established throughout the world as perhaps the most commonly used form of RRT. Conventional dialysis, however, which was slowly losing ground, is reappearing in the form of extended, slow-efficiency treatment, especially in the USA. Two large phase IV trials (> 1000 patients) are under way in the USA and in Australia/New Zealand to compare different dialytic strategies (IHD or SLEDD or CRRT) or to define the optimal dose of CRRT and the results should be available in 2008. In the meantime the use of novel membranes, of sorbents and of different intensities of treatment is being explored in the area of sepsis management and liver support. Intensivists need to keep abreast of this rapid evolution if they are to offer their patients the best of care.
1 Sargent J, Gotch F. Principles and biophysics of dialysis. In: Maher J, editor. Replacement of Renal Function by Dialysis. Dordrecht: Kluwer Academic; 1989:87-102.
2 Henderson L. Biophysics of ultrafiltration and hemofiltration. In: Maher J, editor. Replacement of Renal Function by Dialysis. Dordrecht: Kluwer Academic; 1989:300-332.
3 Nolph KD. Peritoneal dialysis. In: Brenner BM, Rector FC, editors. The Kidney. Philadelphia: WB Saunders; 1986:1791-1845.
4 Conger JD. Does hemodialysis delay recovery from acute renal failure? Semin Dial. 1990;3:145-146.
5 Howdieshell TR, Blalock WE, Bowen PA, et al. Management of post-traumatic acute renal failure with peritoneal dialysis. Am Surg. 1992;58:378-382.
6 Bellomo R, Boyce N. Continuous venovenous hemodiafiltration compared with conventional dialysis in critically ill patients with acute renal failure. ASAIO J. 1993;39:M794-797.
7 Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intens Care Med. 1999;25:805-881.
8 Chatoth DK, Shaver MJ, Marshall MR, et al. Daily 12-hour sustained low-efficiency hemodialysis (SLED) for the treatment of critically ill patients with acute renal failure: initial experience. Blood Purif. 17, 1999. abstract 16
9 Paganini EP. Dialysis is not dialysis is not dialysis! Acute dialysis is different and needs help!. Am J Kidney Dis. 1998;32:832-833.
10 Bellomo R, Ronco C. Adequacy of dialysis in the acute renal failure of the critically ill: the case for continuous therapies. Int J Artif Organs. 1996;19:129-142.
11 Kanagasundaram NS, Paganini EP. Critical care dialysis – a Gordian knot (but is untying the right approach?). Nephrol Dial Transplant. 1999;14:2590-2594.
12 Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet. 2000;356:26-30.
13 Marshall MR, Golper TA, Shaver MJ, et al. Hybrid renal replacement modalities for the critically ill. Contrib Nephrol. 2001;132:252-257.
14 Cole L, Bellomo R, Silvester W, et al. A prospective, multicenter study of the epidemiology, management and outcome of severe acute renal failure in a ‘closed’ ICU system. Am J Respir Crit Care Med. 2000;162:191-196.
15 Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312-1317.
16 Bellomo R. Do we know the optimal dose for renal replacement therapy in the intensive care unit? Kidney Int. 2006;70:1202-1204.
17 Mehta R, Dobos GJ, Ward DM. Anticoagulation procedures in continuous renal replacement. Semin Dial. 1992;5:61-68.
18 Naka T, Egi M, Bellomo R, et al. Low-dose citrate continuous veno-venous hemofiltration and acid–base balance. Int J Artif Organs. 2005;28:222-228.
19 Tan HK, Baldwin I, Bellomo R. Hemofiltration without anticoagulation in high-risk patients. Intens Care Med. 2000;26:1652-1657.
20 Ronco C, Brendolan A, Bellomo R. Current technology for continuous renal replacement therapies. In: Ronco C, Bellomo R, editors. Critical Care Nephrology. Dordrecht: Kluwer Academic; 1998:1327-1334.
21 Macias WL, Clark WR. Azotemia control by extracorporeal therapy in patients with acute renal failure. N Horizons. 1995;3:688-693.
22 Davenport A. The management of renal failure in patients at risk of cerebral edema/hypoxia. N Horizons. 1995;3:717-724.
23 Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895-902.
24 Reeves JH, Butt WW, Shann F, et al. Continuous plasmafiltration in sepsis syndrome. Crit Care Med. 1999;27:2096-2104.
25 Bellomo R, Baldwin I, Ronco C. High-volume hemofiltration. Contrib Nephrol. 2001;132:375-382.
26 Brendolan A, Bellomo R, Tetta C, et al. Coupled plasma filtration adsorption in the treatment of septic shock. Contrib Nephrol. 2001;132:383-391.
27 Uchino S, Bellomo R, Morimatsu H, et al. Cytokine dialysis: an ex-vivo study. ASAIO J. 2002;48:650-653.
28 Mitzner SR, Stange J, Klammt S, et al. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol. 2001;12:S75-82.
29 Buckmaster J, Davies AR. Guidelines for drug dosing during continuous renal replacement therapies. In: Ronco C, Bellomo R, editors. Critical Care Nephrology. Dordrecht: Kluwer Academic; 1998:1327-1334.