Chapter 529 Renal Failure
529.1 Acute Renal Failure
Acute renal failure (ARF), also termed acute renal insufficiency, is a clinical syndrome in which a sudden deterioration in renal function results in the inability of the kidneys to maintain fluid and electrolyte homeostasis. ARF occurs in 2-3% of children admitted to pediatric tertiary care centers and in as many as 8% of infants in neonatal intensive care units. A classification system has been proposed to standardize the definition of acute kidney injury in adults. These criteria of risk, injury, failure, loss, and end-stage renal disease were given the acronym of RIFLE. A modified RIFLE criteria (pRIFLE) has been developed to characterize the pattern of acute kidney injury in critically ill children (Table 529-1). Because RIFLE focuses on glomerular filtration rate (GFR), a modification (Acute Kidney Injury Network, AKIN) categorizes severity by rise in serum creatinine: stage 1 >150%, stage II >200%, stage III >300%.
| CRITERIA | ESTIMATED CCl | URINE OUTPUT |
|---|---|---|
| Risk | eCCl decrease by 25% | <0.5 mL/kg/hr for 8 hr |
| Injury | eCCl decrease by 50% | <0.5 mL/kg/hr for 16 hr |
| Failure | eCCl decrease by 75% or eCCl <35 ml/min/1.73 m2 | <0.3 mL/kg/hr for 24 hr or anuric for 12 hr |
| Loss | Persistent failure >4 wk | |
| End-stage | End-stage renal disease (persistent failure >3 mo) |
eCCl, estimated creatinine clearance; pRIFLE, pediatric risk, injury, failure, loss and end-stage renal disease.
Pathogenesis
ARF has been conventionally classified into 3 categories: prerenal, intrinsic renal, and postrenal (Table 529-2).
Table 529-2 COMMON CAUSES OF ACUTE RENAL FAILURE
PRERENAL
INTRINSIC RENAL
POSTRENAL
Intrinsic renal ARF includes a variety of disorders characterized by renal parenchymal damage, including sustained hypoperfusion and ischemia. Many forms of glomerulonephritis, including postinfectious glomerulonephritis, lupus nephritis, Henoch-Schönlein purpura nephritis, membranoproliferative glomerulonephritis, and anti-glomerular basement membrane nephritis, can cause ARF. Hemolytic-uremic syndrome (HUS) has been described as the most common cause of intrinsic ARF in the USA (Chapter 512).
Tumor lysis syndrome is a specific form of ARF related to spontaneous or chemotherapy-induced cell lysis in patients with lymphoproliferative malignancies. This disorder is primarily caused by obstruction of the tubules by uric acid crystals (Chapters 489 and 490). Acute interstitial nephritis is an increasingly common cause of ARF and is usually a result of a hypersensitivity reaction to a therapeutic agent or various infectious agents (Chapter 526).
Clinical Manifestations and Diagnosis
The physical examination must be thorough, with careful attention to volume status. Tachycardia, dry mucous membranes, and poor peripheral perfusion suggest inadequate circulating volume and the possibility of prerenal ARF (Chapter 54). Peripheral edema, rales, and a cardiac gallop suggest volume overload and the possibility of intrinsic ARF from glomerulonephritis or ATN. The presence of a rash and arthritis might suggest systemic lupus erythematosus (SLE) or Henoch-Schönlein purpura nephritis. Palpable flank masses might suggest renal vein thrombosis, tumors, cystic disease, or urinary tract obstruction.
Laboratory Findings
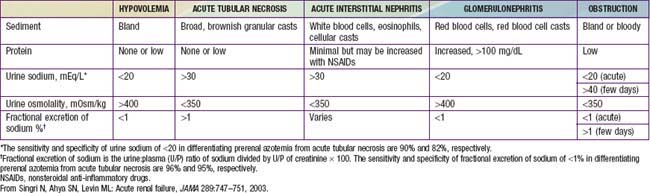
Urinary indices may be useful in differentiating prerenal ARF from intrinsic ARF (Table 529-3). Patients whose urine shows an elevated specific gravity (>1.020), elevated urine osmolality (UOsm > 500 mOsm/kg), low urine sodium (UNa < 20 mEq/L), and fractional excretion of sodium (FENa) <1% (<2.5% in neonates) most likely have prerenal ARF. Those with a specific gravity of <1.010, low urine osmolality (UOsm < 350 mOsm/kg), high urine sodium (UNa > 40 mEq/L), and FENa > 2% (>10% in neonates) most likely have intrinsic ARF.
Treatment
Medical Management
Determination of the volume status is of critical importance when initially evaluating a patient with ARF. If there is no evidence of volume overload or cardiac failure, intravascular volume should be expanded by intravenous administration of isotonic saline, 20 mL/kg over 30 min. In the absence of blood loss or hypoproteinemia, colloid-containing solutions are not required for volume expansion. Severe hypovolemia may require additional fluid boluses (Chapters 53, 54, and 64). Determination of the central venous pressure may be helpful if adequacy of the blood volume is difficult to determine. After volume resuscitation, hypovolemic patients generally void within 2 hr; failure to do so points to intrinsic or postrenal ARF. Hypotension due to sepsis requires vigorous fluid resuscitation followed by a continuous infusion of norepinephrine.
In ARF, rapid development of hyperkalemia (serum potassium level >6 mEq/L) can lead to cardiac arrhythmia, cardiac arrest, and death. The earliest electrocardiographic change seen in patients with developing hyperkalemia is the appearance of peaked T waves. This may be followed by widening of the QRS intervals, ST segment depression, ventricular arrhythmias, and cardiac arrest (Chapter 52.4). Procedures to deplete body potassium stores should be initiated when the serum potassium value rises to >6.0 mEq/L. Exogenous sources of potassium (dietary, intravenous fluids, total parenteral nutrition) should be eliminated. Sodium polystyrene sulfonate resin (Kayexalate), 1 g/kg, should be given orally or by retention enema. This resin exchanges sodium for potassium and can take several hours to take effect. A single dose of 1 g/kg can be expected to lower the serum potassium level by about 1 mEq/L. Resin therapy may be repeated every 2 hr, the frequency being limited primarily by the risk of sodium overload.
Mild metabolic acidosis is common in ARF because of retention of hydrogen ions, phosphate, and sulfate, but it rarely requires treatment. If acidosis is severe (arterial pH < 7.15; serum bicarbonate < 8 mEq/L) or contributes to hyperkalemia, treatment is required. The acidosis should be corrected partially by the intravenous route, generally giving enough bicarbonate to raise the arterial pH to 7.20 (which approximates a serum bicarbonate level of 12 mEq/L). The remainder of the correction may be accomplished by oral administration of sodium bicarbonate after normalization of the serum calcium and phosphorus levels. Correction of metabolic acidosis with intravenous bicarbonate can precipitate tetany in patients with renal failure as rapid correction of acidosis reduces the ionized calcium concentration (Chapter 52).
Hypertension can result from hyperreninemia associated with the primary disease process and/or expansion of the extracellular fluid volume and is most common in ARF patients with acute glomerulonephritis or HUS. Salt and water restriction is critical, and diuretic administration may be useful (Chapter 439). Isradipine (0.05-0.15 mg/kg/dose, maximum dose 5 mg qid) may be administered for relatively rapid reduction in blood pressure. Longer-acting agents such as calcium channel blockers (amlodipine, 0.1-0.6 mg/kg/24 hr qd or divided bid) or β-blockers (propranolol, 0.5-8 mg/kg/24 hr divided bid or tid; labetalol, 4-40 mg/kg/24 hr divided bid or tid) may be helpful in maintaining control of blood pressure. Children with severe symptomatic hypertension (hypertensive urgency or emergency) should be treated with continuous infusions of sodium nitroprusside (0.5-10 µg/kg/min), labetalol (0.25-3.0 mg/kg/hr), or esmolol (150-300 µg/kg/min) and converted to intermittently dosed antihypertensives when more stable.
Dialysis
Indications for dialysis in ARF include the following:
An additional indication for dialysis is the inability to provide adequate nutritional intake because of the need for severe fluid restriction. In patients with ARF, dialysis support may be necessary for days or for up to 12 wk. Many patients with ARF require dialysis support for 1-3 wk. The advantages and disadvantages of the 3 types of dialysis are shown in Table 529-4.
Table 529-4 COMPARISON OF PERITONEAL DIALYSIS, INTERMITTENT HEMODIALYSIS, AND CONTINUAL RENAL REPLACEMENT THERAPY
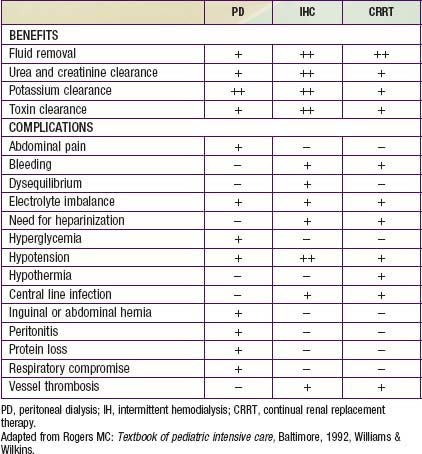
Table 529-4 compares the relative risks and benefits of the various renal replacement therapies.
Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028-1035.
Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24:253-263.
Borthwick E, Ferguson A. Perioperative acute kidney injury: risk factors, recognition, management, and outcomes. BMJ. 2010;341:85-90.
Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
Lameire N, Van Biesen W, Vanholder R. Acute kidney injury. Lancet. 2008;372:1863-1865.
Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118(5):2119-2125.
Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316-1325.
Nigwekar SU, Navaneethan SD, Parikh CR, et al: Atrial natriuretic peptide for preventing and treating acute kidney injury, Cochrane Database Syst Rev (4):CD006028, 2009.
Pannu N, Klarenbach S, Wiebe N, et al. Renal replacement therapy in patients with acute renal failure. JAMA. 2008;299:793-805.
Pickering JW, Endre ZH. GFR shot by RIFLE: errors in staging acute kidney injury. Lancet. 2009;373:1318-1320.
RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627-1638.
Sterns RH, Rojas M, Bernstein P, et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol. 2009;24:37-48.
529.2 Chronic Kidney Disease
Pathogenesis
CKD may be viewed as a continuum of disease, with increasing biochemical and clinical manifestations as renal function deteriorates. The pathophysiologic manifestations of CKD are outlined in Table 529-5. The terminology to describe the stages of chronic kidney disease is standardized (Table 529-6). End-stage renal disease (ESRD) is an administrative term in the USA, defining all patients treated with dialysis or kidney transplantation. Patients with ESRD are a subset of the patients with stage 5 CKD.
| MANIFESTATION | MECHANISMS |
|---|---|
| Accumulation of nitrogenous waste products | Decrease in glomerular filtration rate |
| Acidosis |
Table 529-6 STANDARDIZED TERMINOLOGY FOR STAGES OF CHRONIC KIDNEY DISEASE
| STAGE | DESCRIPTION | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | >90 |
| 2 | Kidney damage with mild decrease in GFR | 60-89 |
| 3 | Moderate decrease in GFR | 30-59 |
| 4 | Severe decrease in GFR | 5-29 |
| 5 | Kidney failure | <15 or on dialysis |
GFR, glomerular filtration rate.
Clinical Manifestations
The physical examination in patients with CKD can reveal pallor and a sallow appearance. Patients with long-standing untreated CKD can have short stature and the bony abnormalities of renal osteodystrophy (Chapter 523.4). Children with CKD due to chronic glomerulonephritis (or children with advanced renal failure from any cause) can have edema, hypertension, and other signs of extracellular fluid volume overload.
Laboratory Findings
where k is 0.33 for low-birthweight infants <1 yr old, 0.45 for term infants <1 yr old whose weight is appropriate for gestational age, 0.55 for children and adolescent girls, and 0.70 for adolescent boys.
Treatment
Eknoyan G. Artificial kidneys: progress and promise. Lancet. 2009;370:1977-1978.
ESCAPE Trial Group. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med. 2010;363(19):1833-1844.
Ingelfinger JR. Blood-pressure control and delay in progression of kidney disease in children. N Engl J Med. 2009;361:1701-1702.
Köttgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712-717.
Kretzler M, Allred L. Notch inhibition reverses kidney failure. Nat Med. 2008;14:246-247.
Lilien MR, Groothoff JW. Cardiovascular disease in children with CKD or ESRD. Nat Rev Nephrol. 2009;5:229-235.
National Kidney Foundation. KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Am J Kidney Dis. 2009;53(3 Suppl 2):S1-S124.
National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):s1-s266.
National Kidney Foundation. KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50(3):471-530.
Palmer SC, Navaneethan SD, Craig JC, et al. Systematic review: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23-33.
Sanchez CP. Mineral metabolism and bone abnormalities in children with chronic renal failure. Rev Endocrin Metab Disord. 2008;9:131-137.
Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839-1848.
Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease. JAMA. 2009;301:629-634.
VanDeVoorde RG, Warady BA. Management of chronic kidney disease. In: Avner ED, Harmon WE, Niaudet P, et al, editors. Pediatric nephrology. ed 6. Heidelberg, Germany: Springer-Verlag; 2009:1693-1712.
Wühl E, Schaefer F. Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol. 2008;23:705-716.
529.3 End-Stage Renal Disease
ESRD represents the state in which a patient’s renal dysfunction has progressed to the point at which homeostasis and survival can no longer be sustained with native kidney function and maximal medical management. At this point, renal replacement therapy (dialysis or renal transplantation) becomes necessary. The ultimate goal for children with ESRD is successful kidney transplantation (Chapter 530) because it provides the most normal lifestyle and possibility for rehabilitation for the child and family.
Peritoneal dialysis may be provided either as continuous ambulatory peritoneal dialysis or as any of several forms of automated therapies using a cycler (continuous cyclic peritoneal dialysis, intermittent peritoneal dialysis, or nocturnal intermittent peritoneal dialysis). The majority of U.S. children treated with peritoneal dialysis use cycler-driven therapy, which allows the child and family to be free of dialysis demands during the waking hours. The exchanges are performed automatically during sleep by machine. This permits an uninterrupted day of activities, a reduction in the number of dialysis catheter connections and disconnections (which should decrease the risk of peritonitis), and a reduction in the time required by patients and parents to perform dialysis, reducing the risk of fatigue and burnout. Because peritoneal dialysis is not as efficient as hemodialysis, it must be performed daily rather than 3 times weekly as in hemodialysis. The merits of peritoneal dialysis are outlined in Table 529-7.
Table 529-7 MERITS OF PERITONEAL DIALYSIS IN PEDIATRIC PATIENTS WITH END-STAGE RENAL DISEASE
ADVANTAGES
DISADVANTAGES
Giessing M, Muller D, Winkelmann B, et al. Kidney transplantation in children and adolescents. Transplant Proc. 2007;39:2197-2201.
Goldstein SL. Advances in renal replacement therapy as a bridge to renal transplantation. Pediatr Transplant. 2007;11:463-470.
Mahan JD, Patel HP. Recent advances in pediatric dialysis: a review of selected articles. Pediatr Nephrol. 2008;23:1737-1747.
Shroff R, Ledermann S. Long-term outcome of chronic dialysis in children. Pediatr Nephrol. 2009;24:463-474.

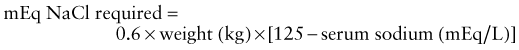
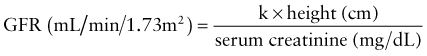
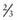 of children with ESRD are treated with peritoneal dialysis, whereas
of children with ESRD are treated with peritoneal dialysis, whereas 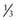 are treated with hemodialysis. Age is a defining factor in dialysis modality selection: 88% of infants and children from birth to 5 yr of age are treated with peritoneal dialysis, and 54% of children >12 yr of age are treated with hemodialysis.
are treated with hemodialysis. Age is a defining factor in dialysis modality selection: 88% of infants and children from birth to 5 yr of age are treated with peritoneal dialysis, and 54% of children >12 yr of age are treated with hemodialysis.