Chapter 134 Remote Effects of Cancer on the Retina
Introduction
Neurologic manifestations from metastatic carcinoma have been recognized for over 100 years. However, paraneoplastic neurologic disorders have been recognized only for the past 50 years, with the initial descriptions of subacute cortical cerebellar degeneration associated with visceral carcinomas. Neurologic paraneoplastic syndromes have been estimated to occur in up to 15% of cancer patients, half of whom have primary carcinoma of the lung.1,2
The prevalence of ophthalmic paraneoplastic syndromes is unknown. Although uncommon, the remote effects of cancer on the eye are important to recognize because they may be the presenting signs of previously undiagnosed malignancy or may suggest recurrence of previously treated disease.3–5 Furthermore, the recognition of these syndromes in patients with unexplained visual loss may aid the ophthalmologist in palliative management, since the disabling ocular symptoms may be amenable to medical therapy.4,6
Paraneoplastic neuro-ophthalmic syndromes are part of a spectrum of autoimmune disorders that also encompass autoimmune retinopathy (Chapter 77, Autoimmune retinopathies) and autoimmune optic neuropathy.6,7
Cancer-associated retinopathy syndrome
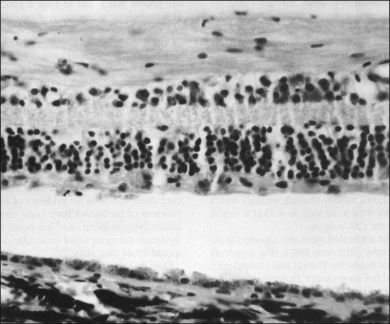
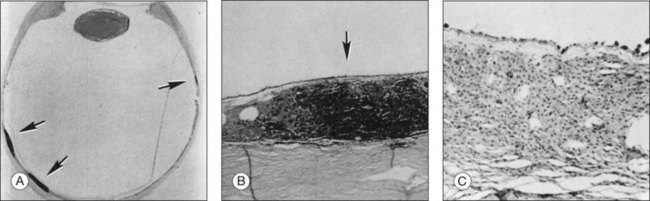
In 1976, Sawyer and coworkers3 described three patients with sudden onset of vision loss, positive visual phenomena, ring-like scotomata, and nyctalopia who developed light perception vision and were later diagnosed as having bronchogenic carcinoma. Fundus examination demonstrated only nonspecific pigment mottling in the posterior pole, normal optic nerves, and a slight degree of vascular attenuation with variable sheathing. Histopathologic examination in each case demonstrated widespread severe degeneration of the outer nuclear retinal layer and disintegration of photoreceptors, as well as the presence of macrophages containing phagocytosed granules from the retinal pigment epithelium (RPE) (Fig. 134.1). As opposed to eyes with retinitis pigmentosa, the RPE and choriocapillaris were remarkably preserved in all eyes examined. The authors believed that these features excluded a vascular basis for the observed findings and concluded that these represented a remote effect of bronchogenic carcinoma.3
Subsequently, Keltner and colleagues4 described a patient with undifferentiated cervical cancer presenting with progressive blindness, progressive retinal arteriolar narrowing, a few vitreous cells, and a flat electroretinogram (ERG). Antibodies were found in the patient’s serum against normal photoreceptors from fresh normal human retinal tissue. A trial of steroids was initiated, which improved vision. Histopathologic examination later disclosed a loss of retinal photoreceptors and the outer nuclear layer. The authors proposed a possible autoimmune mechanism for these findings.
Klingele and co-workers8 described a similar picture in a patient with mottled hyperfluorescence on fluorescein angiography, who was later diagnosed with breast carcinoma, and they termed this “paraneoplastic retinopathy.”
The term “cancer-associated retinopathy” (CAR) syndrome has subsequently come to be used to describe the clinical picture of subacute visual loss resulting from circulating antibodies against retinal proteins in the presence of systemic cancerous tumor growth and over 50 cases have been published to date.2,5,9,10
There is no gender predisposition in CAR syndrome, and the symptoms of decreased vision, a halo of missing peripheral vision, positive visual phenomena (“sparkles”), photosensitivity, impaired color vision and night blindness usually antedate the clinical signs and symptoms of systemic malignancy by a mean of 5 months in up to 50% of cases.11 The longest duration from the onset of CAR syndrome to diagnosis of the carcinoma was 11 years in a patient with a pulmonary carcinoma.12 Patients with CAR syndrome generally progress from initial stages of visual loss to blindness in 6–18 months.4,5,10
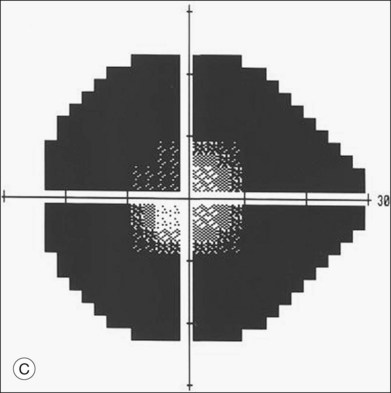
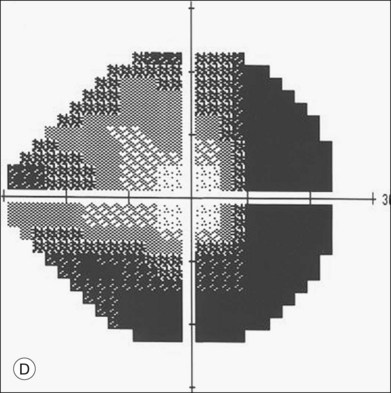
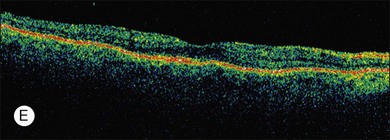
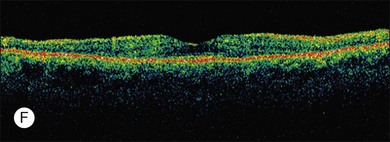
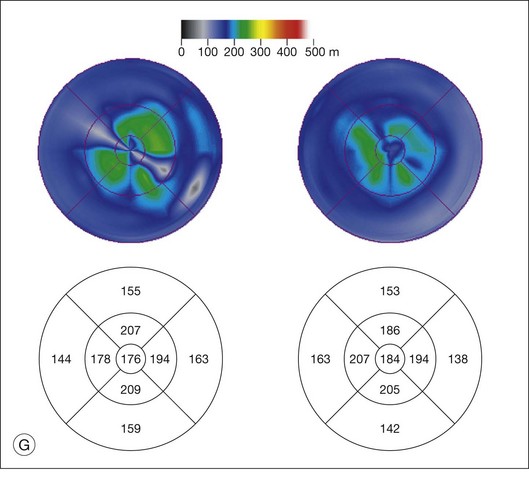
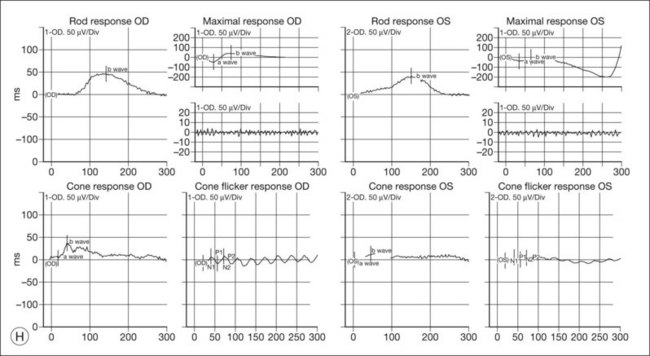
ERG findings in patients with CAR syndrome have been reported to show extinguished a and b waves or rod responses larger than cone responses (Fig. 134.2). Occult malignancy should be suspected in patients with acquired night blindness, minimal ocular findings to account for the visual difficulty, and abnormal ERG results.13 The presenting symptoms may be asymmetric, and some patients complain of glare and photosensitivity.14–17 Cells in the vitreous or anterior chamber are infrequently reported in CAR syndrome.4,8 Tonic pupils have been reported to be a potential ophthalmic finding in paraneoplastic syndromes.18 Reduced macular thickness on OCT examination may be observed prior to severe visual loss, and may be useful in demonstrating retinal damage in these patients.19
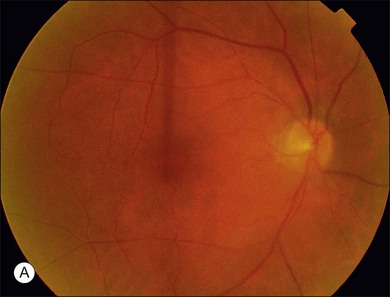
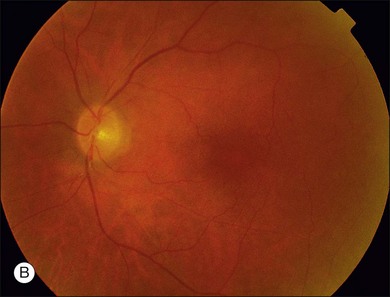
The most common malignancies related to CAR are carcinomas, and over half of all these patients have a pulmonary malignancy with the most common being small cell carcinoma of the lung.1,3,5,10,14–17,20–31 A chest X-ray should usually be obtained as part of the evaluation of suspect individuals. Other malignancies associated with CAR include those of the colon,32 endometrium/uterus,33–37 cervix,4,27,38 breast,8,39,40 prostate/bladder,39–41 thorax (rhabdomyosarcoma),42 abdominal liposarcoma,44 and systemic lymphoma.43 In rare instances, patients with documented antibodies to the retina and classic symptoms of CAR may not have an associated underlying malignancy. These patients constitute a minority of patients referred to as autoimmune retinopathy not associated with underlying malignancy and are discussed in greater detail in Chapter 77, Autoimmune retinopathies (Fig. 134.3).2,15,41,45–47
Based on an ultrastructural study showing immature melanin granules within melanolysosomes, Buchanan and co-workers48 suggested that a hormone-like substance produced by the tumor increased melanin synthesis in the pigment epithelium and that the increased melanin content in these cells compromised their ability to phagocytose and maintain normal turnover of photoreceptor outer segments. Since that report, a growing body of evidence has supported an autoimmune basis for CAR syndrome.49–51 In 1982, Kornguth et al.23 reported on the presence of 23 kDa (kilo Dalton) serum antiretinal ganglion cell antibodies in patients with visual dysfunction and small cell carcinoma of the lung. Subsequently, Grunwald et al.14 described the reaction of such antibodies with specific retinal antigens in the ganglion cells and inner nuclear layers and immunologically related antigens in tumor cells (as well as their absence in patients with tumors but with no visual defects). They proposed that the small cell tumor produces antigens normally associated with retinal tissue. These antigens are recognized as foreign, since the retina is an immunologically privileged site without antiself suppressor mechanisms.14,51 CAR syndrome can also be associated with uveitis (vitritis and vascular sheathing)4,35,52,53; as the concentrations of autoantibodies reach a critical threshold, they cross the abnormally permeable blood–retina barrier. It is postulated that a cross-reaction with retinal tissue occurs, and subsequently a nonspecific immune-mediated common pathway of retinal degeneration with apoptosis ensues.
The 23 kDa antigen to which antibodies have been produced in the majority of individuals with CAR syndrome has been shown to be recoverin, a calcium channel photoreceptor protein.17,21,27,29,46,54–57 No clear role for recoverin in the normal functioning of photoreceptor cells has been demonstrated, but binding to this protein results in cellular death by some unknown mechanism. Antibodies to the CAR antigen (recoverin) were not previously demonstrated in any other form of retinopathy, nor were these antibodies believed to be expressed by tumors without associated retinopathy.5,17 Recently, however, a clinically distinctive condition has been described resembling CAR with a cellular immune response against recoverin (recoverin-associated retinopathy).58
Now available commercially, the Western blot analysis for the CAR antigen-antibody may be negative even in the presence of CAR syndrome.52 Moreover, the titers of antibody versus recoverin do not necessarily correlate with the severity of the clinical retinopathy. Hence, clinical criteria and a high index of suspicion remain the mainstay of diagnosis.
Thirkill and co-workers10 reported on isolation of antibodies reacting strongly with the 23 kDa retinal protein in addition to yet another 65 kDa lung cancer antigen. Other antiretinal antibodies have been found in patients with retinopathy (abnormal ERGs, ring scotomata or depressed visual fields), but these patients did not have demonstrable cancer. However, these patients differ from patients with CAR syndrome on the basis of lower antiretinal antibody titers, as well as on the basis of their reaction to a different 40 kDa retinal protein.17,59
Adamus and colleagues56 reported several cases of CAR syndrome in association with antibodies to a 46 kDa antigen identified as human alpha-enolase, a glycolytic enzyme. They postulate that antigens aberrantly expressed in tumor cells of affected individuals trigger an immune response that cross-reacts with similar antigens in the retina causing cell damage. Further work by Adamus has led to the hypothesis that antibodies against recoverin and other retinal proteins such as alpha-enolase, are cytotoxic by triggering apoptosis via a caspase 3-apoptotic pathway.60–62 Therefore multiple antigens may be capable of inducing retinal damage and a clinical autoimmune retinopathy.6,58,63,64 There is a high degree of correlation between the presence of serum autoantibodies, the development of visual symptoms, and the presence of retinal degeneration.63
Cutaneous melanoma-associated retinopathy (MAR) syndrome
A paraneoplastic syndrome of acquired night blindness associated with rapidly developing vitiligo of the skin and uveal tract was reported in two patients with metastatic cutaneous melanoma.65,66 This process was described originally in 1984 by Gass as “an acute Vogt–Koyanagi–Harada-like syndrome.” These patients, like those with CAR syndrome, had ERG a and b waves that were markedly reduced in amplitude, indicating widespread photoreceptor cell dysfunction.
“Paraneoplastic acquired night blindness” has since been described in over 60 patients with cutaneous malignant melanoma and is being reported in a greater frequency than that of CAR syndrome.2,13,15,38,66–76 Melanoma-associated retinopathy (MAR) syndrome is thought to be a clinically distinctive subset of cancer-associated retinopathy. Specifically, this form of cancer-associated retinopathy is clinically differentiated from CAR syndrome by being nonprogressive, causing central visual loss (versus ring scotomas), a sensation of shimmering or pulsating light and being associated with vitiligo in up to 20%.66 In contrast to patients with CAR syndrome and the “acute Vogt–Koyanagi–Harada-like syndrome,” patients with MAR syndrome demonstrate a substantial elevation of rod absolute thresholds and selective reductions in the rod and cone ERG b waves, resembling those of patients with congenital stationary night blindness. The photoreceptor function is intact but signal transmission between the photoreceptors and second-order interneurons appears to be defective. MAR syndrome, unlike CAR syndrome, is more likely to be visually symptomatic in more advanced stages of disease and, in general, does not manifest before the clinical diagnosis of cutaneous malignant melanoma.2,75,77,78 Unilateral presentation of MAR has been reported over 18 months of observation but this may represent asymmetric ocular involvement.79 However, recent studies demonstrate that subclinical evidence for MAR may be present in earlier stages of cutaneous melanoma and this may portend a worse overall prognosis for the patient.78 Antibodies to retinal bipolar cells in MAR syndrome have been reported, but not against the 23 kDa CAR antigen.72,75,76 The nature of the bipolar cell antigen is not yet known but studies have verified that the bipolar cells are the primary site of pathology in this condition.80–82 Finally, antibodies against transducin, aldolase A and aldolase C have also been documented in patients with MAR.83,84
Management of paraneoplastic retinopathy
An ERG should be considered in any adult patient who has symptoms of central or paracentral positive visual phenomena (“shimmering” or “dancing” lights), photopsias, and minimal retinal findings. If the ERG is abnormal or extinguished, then a chest X-ray is a common “next step,” since over half of patients with CAR syndrome have a bronchogenic tumor. A history of previous cutaneous malignant melanoma should be elicited in patients with nyctalopia. Central and midperipheral visual complaints also may be documented with visual field testing. Antibodies to the CAR-antigen may be obtained, but a negative test is not inconsistent with a diagnosis of CAR syndrome. Although some have suggested indirect immunohistologic investigations for retinal inner nuclear layer bipolar cell staining as being more reliable than antiretinal antibody testing, there have been no studies comparing these techniques.84 Moreover, given the lack of a typical ERG change in CAR or MAR, testing for antiretinal antibodies in the patients’ sera seems to be crucial for early detection of the disease.64
Difficulties remain with a lack of standardization, reproducibility, and validation of testing for circulating antiretinal antibodies as well as with our understanding of which antibodies are pathogenic.85,86
The Ocular Immunology Laboratory at Oregon Health and Science University offers a comprehensive panel of autoantibody tests for CAR, MAR, as well as for autoimmune retinopathy and optic neuropathy (http://www.ohsu.edu/xd/health/services/casey-eye/clinical-services/diagnostic-services/immunology-lab.cfm, accessed August 11, 2011).
Paraneoplastic retinopathy is also a diagnosis of exclusion, and retrobulbar optic neuropathy as a result of tumor spread, ischemia, or toxic effects of chemotherapy must be ruled out.2 If cancer is not detected initially then continued surveillance at regular intervals is necessary.87
An immune basis for CAR would suggest a possible role for immunosuppression therapy. Indeed, Keltner, and others4,8,15,26,70,88 have reported visual improvement or stabilization following treatment with oral corticosteroids. The optimal dose of prednisone for CAR patients has not been determined.9 We are unaware of cases managed with local depot steroids. No standardized protocols have been established for management of the immunosuppression therapies for CAR and MAR.7,11,84 Immunosuppression has been reported to be effective in improving the visual outcomes in up to 100% of patients with CAR but long-term data concerning the benefits and sustainability of treatment are not available.11,85,86
There have been no reports of visual improvement with treatment directed only at the site of primary tumor, and according to one investigator, vision usually worsens with such management alone.9 The optimal approach to immunosuppressive therapy for this condition is uncertain. Plasmapheresis may have no effect on the cancer antigens and may paradoxically lead to the production of high concentrations of high-affinity antibodies.10,54 Prednisone has successfully stabilized the visual function of CAR patients but may exert a negative effect on the patient’s own tumor immunosurveillance. All systemic therapies have the potential to alter survival, for better or worse, in addition to the potential beneficial effect on vision.
Bilateral diffuse uveal melanocytic proliferation
Machemer, in 1966, reported a case of bilateral and simultaneously appearing uveal lesions in a 57-year-old man with aqueous cells and flare, iris nodules, cataracts, and inferior retinal detachments in both eyes. At autopsy the patient was found to have a pancreatic carcinoma. Histopathologic examination of the enucleated eyes demonstrated predominantly benign cytologic characteristics of uveal melanocytic proliferation.89
Isolated case reports of similar cases by Curtin,90 Font,91 and Ryll92 were followed by a series of four cases in 1982 by Barr,93 who defined a syndrome consisting of: (1) simultaneous occurrence of bilateral diffuse melanocytic involvement of the uveal tracts; (2) predominant cytologic benignity; (3) absence of metastases from the melanocytic proliferation, and (4) associated histopathologically proven systemic malignant neoplasm. The cellular benignity has been questioned because of the presence of foci of malignant-appearing epithelioid cells in addition to evidence of scleral invasion.94 However, there has been no evidence of mitotic activity, and no cases of metastases have been reported from these melanocytic proliferations.95 It is possible that these patients died as a result of the more lethal nonocular disease before such spread could take place.94,96 To date, approximately 30 cases have been reported.97
Other clinical signs that may be present include iridocyclitis, glaucoma,65,93,98 dilated episcleral vessels,24,65,93,95 shallow anterior chambers, iris cysts, ciliary body cysts,98 and rapidly developing cataracts.
1. Development of multiple, slightly elevated (usually only up to 2 mm), pigmented, and nonpigmented uveal melanocytic tumors, as well as evidence of diffuse thickening of the uveal tract
2. Multiple, round or oval, subtle, red patches at the level of the RPE in the posterior fundus
3. A striking pattern of multifocal areas of early hyperfluorescence on fluorescein angiography corresponding with these patches
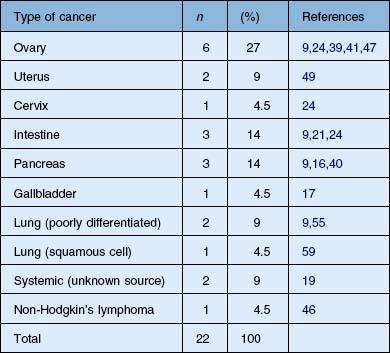
The findings of BDUMP usually antedate the findings of systemic malignancy by 3–12 months, and death usually ensues from the effects of the systemic malignancy within 8–24 months.65,99,100 The focal and diffuse thickening of the uveal tract can antedate the clinical findings of exudative retinal detachment and red patches by several months.99 Table 134.1 summarizes the cancers that have been reported to be associated with the clinical findings of BDUMP.
The pathogenesis of BDUMP remains obscure, but possibilities include: (1) a common oncogenic stimulus initiating both the ocular and nonocular tumors93; (2) a hormonal stimulus for ocular melanocytic proliferation by the nonocular tumor93; (3) an individual predisposed to simultaneous development of ocular melanocytic and nonocular visceral carcinomas;96 and (4) a proliferation of clinically unapparent hypopigmented diffuse bilateral choroidal nevi in response to a hormonal stimulus from the visceral carcinoma.65,98 An autoimmune basis for this condition is not clear and autoantibody testing is not indicated at this time.
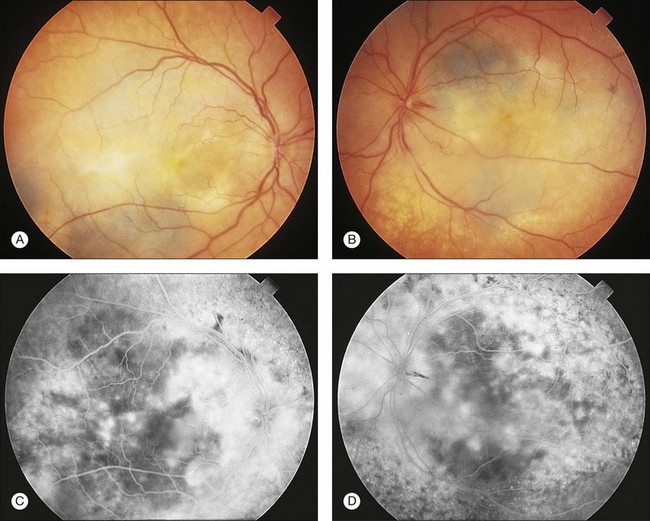
The characteristic multifocal red patches (or dark gray patches in patients with brunette fundi) that are angiographically seen to hyperfluoresce are of importance in the early diagnosis of this syndrome. Gass et al. and Margo et al. demonstrated by clinicopathologic correlation that these patches represent areas of depigmented RPE overlying hypopigmented, diffuse melanocytic proliferation (Fig. 134.5).65,94 These patches of melanocytic proliferation with overlying RPE changes are thought to enlarge progressively and become confluent, producing a reticular pattern that angiographically appears hypofluorescent against a background of normal choroidal hyperfluorescence.65,94 Gass proposes that the RPE changes are attributable to a toxic or immune-mediated process as a result of the melanocytic proliferation itself or to an interaction of the systemic malignancy and the RPE.65
No treatment method has successfully stabilized or improved the course of visual loss in patients with BDUMP syndrome. Prompt recognition of this paraneoplastic syndrome may lead to earlier treatment of the visceral carcinoma and conceivably provide the best hope for survival, although slow growth of the uveal melanocytic proliferation may continue unabated, with a poor prognosis for vision.65 Saito et al. suggested that oral steroid therapy in those individuals with BDUMP and who also manifest with antiretinal antibodies may be beneficial.97 Duong et al. reported a patient with BDUMP associated with ovarian carcinoma who survived the ovarian cancer but later developed metastatic amelanotic malignant melanoma, suggesting that the uveal manifestations of BDUMP may possibly have metastatic potential.101,102 This suggests the importance of long-term follow-up of treated individuals.
Conclusion
Because paraneoplastic syndromes involving the eye may be the presenting manifestation of systemic malignancy, it is important for ophthalmologists to be aware of this association so that they can initiate an early and aggressive search and treatment of visceral cancers. Evidence in support of an autoimmune basis for the CAR syndrome, as well as for MAR syndrome, suggests an early means of detection of nonocular malignancy, as well as a possible role for immunosuppression in the palliation of the visually disabling symptoms experienced by these unfortunate patients.85,86 Although no therapy has been shown to be successful in stabilizing or arresting the ocular manifestations of BDUMP syndrome, early identification of the associated malignancy could theoretically enhance the prognosis for life.
1 Spence AM, Sumi SM, Ruff R. Paraneoplastic syndromes that involve the nervous system. Curr Probl Cancer. 1983;8:4–43.
2 Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48:12–38.
3 Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;1976:606–613.
4 Keltner JL, Roth AM, Chang RS. Photoreceptor degeneration: possible autoimmune disorder. Arch Ophthalmol. 1983;101:48–53.
5 Thirkill C, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105:1987.
6 Keltner JL, Thirkill C, Roth AM. Autoimmune related retinopathy and optic neuropathy (ARRON syndrome). Invest Ophthalmol Vis Sci. 1988;29:178.
7 Powell SF, Dudek AZ. Treatment of melanoma-associated retinopathy. Curr Treat Options Neurol. 2010;12:54–63.
8 Klingele TG, Burde RM, Rappazzo JA, et al. Paraneoplastic retinopathy. J Clin Neuro-Ophthalmol. 1984;4:239–245.
9 Keltner JL, Thirkill CE, Tyler NK, et al. Management and monitoring of cancer-associated retinopathy. Arch Ophthalmol. 1992;110:48–53.
10 Thirkill CE, FitzGerald P, Sergott RC, et al. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic nerve, and cancer cells. N Engl J Med. 1989;321:1589–1594.
11 Damek DM. Paraneoplastic retinopathy/Optic neuropathy. Curr Treat Options Neurol. 2005;7:57–67.
12 Saito W, Kase S, Ohguro H, et al. Slowly progressive cancer-associated retinopathy. Arch Ophthalmol. 2007;125:1431–1433.
13 Berson EL, Lessell S. Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol. 1988;106:307–311.
14 Grunwald GB, Klein R, Simmonds MA, et al. Autoimmune basis for visual paraneoplastic syndrome in patients with small-cell lung carcinoma. Lancet. 1985;1:658–661.
15 Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28:162–167.
16 Stanford MR, Edelsten CE, Hughes JD, et al. Paraneoplastic retinopathy in association with large cell neuroendocrine bronchial carcinoma. Br J Ophthalmol. 1995:617–618. 1995
17 Thirkill CE, Keltner JL, Tyler NK, et al. Antibody reactions with retina and cancer-associated antigens in 10 patients with cancer-associated retinopathy. Arch Ophthalmol. 1993:931–937. 1993
18 Wabbels BK, Elflein H, Lorenz B, et al. Bilateral tonic pupils with evidence of anti-hu antibodies as a paraneoplastic manifestation of small cell lung cancer. Ophthalmologica. 2004;218:141–143.
19 Mohamed Q, Harper AC. Acute optical coherence tomographic findings in cancer-associated retinopathy. Arch Ophthalmol. 2007;125:1132–1133.
20 Gehrs KM, Tiedemann J. Hemeralopia in an older adult. Surv Ophthalmol. 1992;37:185–189.
21 Adamus G, Guy J, Schmied JL, et al. Role of antirecoverin autoantibodies in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:2626–2633.
22 Anderson NE, Rosenblum MK, Graus F, et al. Autoantibodies in paraneoplastic syndromes associated with small-cell lung cancer. Neurology. 1988;38:1391–1398.
23 Kornguth SE, Klein R, Appen R, et al. Occurrence of antiretinal ganglion cell antibodies in patients with small cell carcinoma of the lung. Cancer. 1982;50:1289–1293.
24 Murphy MA, Hart WM, Olk RJ. Bilateral diffuse uveal melanocytic proliferation simulating an arteriovenous fistula. J Neuro-Ophthalmol. 1997;17:77–83.
25 Murphy MA, Thirkill CE, Hart WM. Paraneoplastic retinopathy: a novel autoantibody reaction associated with small-cell carcinoma. J Neuro-Ophthalmol. 1997;17:166–169.
26 Ohnishi Y, Ohara S, Sakamoto T, et al. Cancer-associated retinopathy with retinal phlebitis. Br J Ophthalmol. 1993;77:795–798.
27 Thirkill CE, Tait RC, Tyler NK, et al. Intraperitoneal cultivation of small-cell carcinoma induces expression of the retinal cancer-associated retinopathy antigen. Arch Ophthalmol. 1993;111:974–978.
28 Thirkill CE, Roth AM, Keltner JL. Paraneoplastic retinopathy syndrome. Ophthalmology. 1993;100:147.
29 Thirkill CE, Tait RC, Tyler NK, et al. The cancer-associated retinopathy antigen is a recoverin-like protein. Invest Ophthalmol Vis Sci. 1992;33:2768–2772.
30 Van der Pol BAE, Planten JTH. A nonmetastatic remote effect of lung carcinoma. Doc Ophthalmol. 1987;67:89–94.
31 Vargas Núñez JA, Bonilla Velasco F, Casas Fernández-Tejerina J, et al. [Paraneoplastic retinal degeneration]. Med Clin (Barc). 1987;88:431.
32 Jacobson DM, Adamus G. Retinal anti-bipolar cell antibodies in a patient with paraneoplastic retinopathy and colon carcinoma. Am J Ophthalmol. 2001;131:806–808.
33 Campo E, Brunier MN, Merino MJ. Small cell carcinoma of the endometrium with associated ocular paraneoplastic syndrome. Cancer. 1992;69:2283.
34 Cogan DG, Kuwabara T, Currie J, et al. [Paraneoplastic retinopathy simulating cone dystrophy with achromatopsia]. Klin Monatsbl Augenheilkd. 1990;197:156.
35 Crofts JW, Bachynski BN, Odel JG. Visual paraneoplastic syndrome associated with undifferentiated endometrial carcinoma. Can J Ophthalmol. 1988;23:128.
36 Eltabbakh GH, Hoogerland DL, Kay MC. Paraneoplastic retinopathy associated with uterine sarcoma. Gynecol Oncol. 1995;58:120–123.
37 Ohkawa T, Kawashima H, Makino S, et al. Cancer-associated retinopathy in a patient with endometrial cancer. Am J Ophthalmol. 1996;122:740–742.
38 Kellner U, Bornfeld N, Foerster MH. Severe course of cutaneous melanoma-associated paraneoplastic retinopathy. Br J Ophthalmol. 1995;79:746–752.
39 Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117:471–477.
40 Pepkowitz S, Reader A, Jacobs A, et al. Paraneoplastic retinopathy: resolution with plasmapheresis. Transfusion. 1993;33:71.
41 Matsui Y, Mehta MC, Katsumi O, et al. Electrophysiological findings in paraneoplastic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1992;230:324.
42 Hammerstein W, Jurgens H, Gobel U. Retinadegeneration und embryonales rhabdomyosarkom des thorax. Fortschr Ophthalmol. 1991;88:463.
43 To KW, Thirkill CE, Jakobiec FA, et al. Lymphoma-associated retinopathy. Ophthalmology. 2002;109:2149–2153.
44 Kondo M, Mokuno K, Uemura A, et al. Paraneoplastic retinopathy associated with retroperitoneal liposarcoma. Clin Ophthalmol. 2010;4:243–245.
45 Mizener JB, Kimura AE, Adamus G, et al. Autoimmune retinopathy in the absence of cancer. Am J Ophthalmol. 1997;123:607–618.
46 Adamus G, Chan CC. Experimental autoimmune uveitides: multiple antigens, diverse diseases. Int Rev Immunol. 2002;21:209–229.
47 Adamus G. Antirecoverin antibodies and autoimmune retinopathy. Arch Ophthalmol. 2000;118:1577–1578.
48 Buchanan TAS, Gardiner TA, Archer DB. An ultrastructural study of retinal photoreceptor degeneration associated with bronchial carcinoma. Am J Ophthalmol. 1984;97:277–287.
49 Antel JP, Moumdjian R. Paraneoplastic syndromes: a role for the immune system. J Neurol. 1989;236:1–3.
50 Fine RM. Autoimmune basis for visual paraneoplastic syndrome. Int J Dermatol. 1985;24:509.
51 Kornguth SE, Kalinke T, Grunwald GB, et al. Antineurofilament antibodies in the sera of patients with small cell carcinoma of the lung and with visual paraneoplastic syndrome. Cancer Res. 1986;46:2588–2595.
52 Rizzo JF, Gittinger JW, Jr. Selective immunohistochemical staining in the paraneoplastic retinopathy syndrome. Ophthalmology. 1992;99:1286–1295.
53 Suzuki T, Obara Y, Sato Y, et al. Cancer-associated retinopathy with presumed vasculitis. Am J Ophthalmol. 1996;122:125–127.
54 Polans AS, Witkowska D, Haley TL, et al. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci U S A. 1995;92:9176–9180.
55 Matsubara S, Yamaji Y, Fujita T, et al. Cancer-associated retinopathy syndrome: a case of small cell lung cancer expressing recoverin immunoreactivity. Lung Cancer. 1996;14:265–271.
56 Adamus G, Aptsiauri N, Guy J, et al. The occurrence of serum antibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996;78:120–129.
57 Adamus G, Machnicki M, Siegel GM. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1997;38:283–291.
58 Whitcup SM, Vistica BP, Milam AH, et al. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998;126:230–237.
59 Peek R, Dijkstra BG, Meek B, et al. Autoantibodies to photoreceptor membrane proteins and outer plexiform layer in patients with cancer-associated retinopathy. Clin Exp Immunol. 2002;128:498–503.
60 Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2:63–68.
61 Adamus G, Sugden B, Shiraga S, et al. Anti-apoptotic effects of CNTF gene transfer on photoreceptor degeneration in experimental antibody-induced retinopathy. J Autoimmun. 2003;21:121–129.
62 Shiraga S, Adamus G. Mechanism of CAR syndrome: anti-recoverin antibodies are the inducers of retinal cell apoptotic death via the caspase 9- and caspase 3-dependent pathway. J Neuroimmunol. 2002;132:72–82.
63 Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. 2009;8:410–414.
64 Link B, Schlotzer-Schrehardt U, Junemann A. Carcinoma-associated retinopathy. An electrophysiological and immunohistochemical correlation. Retina. 2009;29:69–72.
65 Gass JD, Gieser RG, Wilkinson CP, et al. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol. 1990;108:527–533.
66 Gass JD. Stereoscopic atlas of macular diseases: diagnosis and treatment. St Louis: Mosby; 1997.
67 Alexander KR, Fishman GA, Peachey NS, et al. “On” response defect in paraneoplastic night blindness with cutaneous malignant melanoma. Invest Ophthalmol Vis Sci. 1992;33:477–483.
68 Andreasson S, Ponjavic V, Ehinger B. Full-field electroretinogram in a patient with cutaneous melanoma-associated retinopathy. Acta Ophthalmol Scand. 1993;71:487–490.
69 Gass JDM. Acute Vogt-Koyanagi-Harada-like syndrome occurring in a patient with metastatic cutaneous melanoma. In: Uveitis update: Proceedings of the International Symposium on Uveitis. Amsterdam: Elsevier; 1984.
70 Jacobson DM, Thirkill CE. Paraneoplastic cone dysfunction: an unusual visual remote effect of cancer. Arch Ophthalmol. 1995;113:1580–1582.
71 Kim RY, Retsas S, Fitzke FW, et al. Cutaneous melanoma-associated retinopathy. Ophthalmology. 1994;101:1837–1843.
72 Milam AH, Saari JC, Jacobson SG, et al. Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:91–100.
73 Remulla JF, Pineda R, Gaudio AR, et al. Cutaneous melanoma-associated retinopathy with retinal periphlebitis. Arch Ophthalmol. 1995;113:854–855.
74 Rush JA. Paraneoplastic retinopathy in malignant melanoma. Am J Ophthalmol. 1993;115:390–391.
75 Singh AD, Milam AH, Shields CL, et al. Melanoma-associated retinopathy. Am J Ophthalmol. 1995;119:369–370.
76 Weinstein JM, Kelman SE, Bresnick GH, et al. Paraneoplastic retinopathy associated with antiretinal bipolar cell antibodies in cutaneous malignant melanoma. Ophthalmology. 1994;101:1236–1243.
77 Myers DA, Bird BR, Ryan SM, et al. Unusual aspects of melanoma. Case 3. Melanoma-associated retinopathy presenting with night blindness. J Clin Oncol. 2004;22:746–748.
78 Pföhler C, Haus A, Palmowski A, et al. Melanoma-associated retinopathy: high frequency of subclinical findings in patients with melanoma. Br J Dermatol. 2003;149:74–78.
79 Janaky M, Palffy A, Kolozsvari L, et al. Unilateral manifestations of melanoma-associated retinopathy. Arch Ophthalmol. 2002;120:866–867.
80 Alexander KR, Barnes CS, Fishman GA, et al. Contrast-processing deficits in melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 2004;45:305–310.
81 Lei B, Bush RA, Milam AH, et al. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41:262–266.
82 Bazhin AV, Dalke C, Willner N, et al. Cancer-retina antigens as potential paraneoplastic antigens in melanoma-associated retinopathy. Int J Cancer. 2009;124:140–149.
83 Potter MJ, Adamus G, Szabo SM, et al. Autoantibodies to transducin in a patient with melanoma-associated retinopathy. Am J Ophthalmol. 2002;134:128–130.
84 Lu Y, Jia L, He S, et al. Melanoma-associated retinopathy. A paraneoplastic autoimmune complication. Arch Ophthalmol. 2009;127:1572–1580.
85 Ferreyra HA, Jayasundera T, Khan NW, et al. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127:390–397.
86 Jampol LM, Fishman GA. Immunosuppression for autoimmune retinopathy. Arch Ophthalmol. 2009;127:573–574.
87 Toothaker TB, Rubin M. Paraneoplastic neurological syndromes. Neurologist. 2009;15:21–33.
88 Oohira A, Tamaki Y, Nagahara K. A case of paraneoplastic retinopathy. Jpn J Ophthalmol. 1993;37:28–31.
89 Machemer R. Zur Pathogenese des flachenhaften malignen Melanoms. Klin Monatsbl Augenheilkd. 1966;149:641–652.
90 Curtin VT. Diffuse malignant melanoma of the uvea, mixed cell type. Eastern Ophthalmic Pathology Society Meeting, Philadelphia; 1974.
91 Font RL. Bilateral diffuse melanocytic tumor of uvea of questionable malignancy: adenocarcinoma of colon with widespread metastasis. Eastern Ophthalmic Pathology Society Meeting, Baltimore; 1978.
92 Ryll DL, Campbell RJ, Robertson DM, et al. Pseudo-metastatic lesions of the choroid. Ophthalmology. 1980;100:249–255.
93 Barr CC, Zimmerman LE, Curtin VT, et al. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms: a recently recognized syndrome. Arch Ophthalmol. 1982;100:249–255.
94 Margo CE, Pavan PR, Gendelman D, et al. Bilateral melanocytic uveal tumors associated with systemic nonocular malignancy: malignant melanomas or benign paraneoplastic syndrome? Retina. 1987;7:137–141.
95 Borruat FX, Othenin-Girard P, Uffer S, et al. Natural history of diffuse uveal melanocytic proliferation. Ophthalmology. 1992;99:1698–1704.
96 Mullaney J, Mooney D, O’Connor M, et al. Bilateral ovarian carcinoma with bilateral uveal melanoma. Br J Ophthalmol. 1984;68:261–267.
97 Saito W, Kase S, Yoshida K, et al. Bilateral diffuse uveal melanocytic proliferation in a patient with cancer-associated retinopathy. Am J Ophthalmol. 2005;140:942–945.
98 Prause JU, Jensen OA, Eisgart F, et al. Bilateral diffuse malignant melanoma of the uvea associated with large cell carcinoma, giant cell type, of the lung: case report of a newly described syndrome. Ophthalmologica. 1984;189:221–228.
99 Leys AM, Dierick HG, Sciot RM. Early lesions of bilateral diffuse melanocytic proliferation. Arch Ophthalmol. 1991;109:1590–1594.
100 Mooy CM, de Jong PTV, Strous C. Proliferative activity in bilateral paraneoplastic melanocytic proliferation and bilateral uveal melanoma. Br J Ophthalmol. 1994;78:483–484.
101 Duong HV, McLean IW, Beahm DE. Bilateral diffuse melanocytic proliferation associated with ovarian carcinoma and metastatic malignant amelanotic melanoma. Am J Ophthalmol. 2006;142:693–695.
102 Ko MW, Dalmau J, Galetta SL. Neuro-ophthalmic manifestations of paraneoplastic syndromes. J Neuro-Ophthalmol. 2008;28:58–68.