CHAPTER 31 Refractive lens exchange
History
Early in the year 1890, Fukala reported on refractive lens extraction in eyes with high myopia1. This procedure was subsequently abandoned due to the 10-fold increase of post-operative retinal detachment in comparison to unoperated myopic eyes. Since 1890, ophthalmic surgery has undergone steady changes by means of innovations in intraocular lens design, application of ophthalmic viscoelastics substances, and development of phacoemulsification and microincisional surgical techniques. These innovations improved the safety and efficacy of refractive lens exchange (RLE), making it an integral part of refractive surgery today.
Indications and patient selection
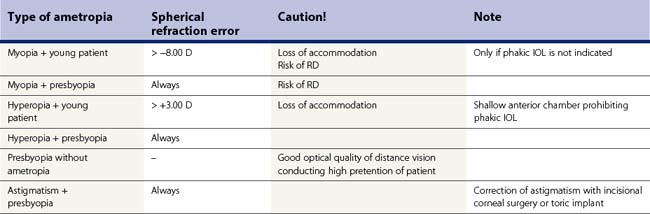
Indications for RLE are high myopia or hyperopia with coexistent presbyopia. The reason for the inclusion of presbyopia is the fact that RLE always leads to a complete loss of accommodation. Correction of underlying regular astigmatism in scope of RLE, which cannot be managed by corneal incisional techniques, is best accomplished by implantation of toric lens implants. Border indications for RLE are presbyopia without ametropia implying implantation of a multifocal lens implant, presbyopia with underlying astigmatism, and prepresbyopic patients with high hyperopia of from +5 to +10 D not amenable for keratorefractive surgery or phakic IOL due to shallow anterior chamber situation. Table 31.1 displays an overview over indications for RLE.
Preoperative calculation of IOL power
Exact calculation of IOL power is essential for a good postoperative outcome. It mainly depends on measurement of axial length, corneal power, and the IOL power calculation formula. In fourth-generation formulas, patients’ age, preoperative refraction, preoperative anterior chamber depth, lens thickness, and white to white measurement are also taken into consideration. In our own recent study, we assessed the predictive ability of third- (Hoffer Q and SRK/T) and fourth-generation (Holladay and Haigis) formulas in eyes with high ametropia2. All formulas tended to produce hyperopic surprises with the manufacturers’ lens constants in eyes having myopic RLE. With optimized lens constants, the Haigis formula performed best. In eyes with hyperopic RLE, all four formulas were almost equivalent2.
Complications in refractive lens exchange
Intraoperative complications
Myopic RLE may be difficult due to instability of the capsular bag. This bears an increased risk of tearing the capsule. Insertion of a capsular tension ring might alleviate the intraoperative situation, especially in cases of weak zonules or lysis of the zonules. In eyes with high axial length, the risk of an intraoperative subchoroidal hemorrhage is markedly increased as compared with that in normal eyes3. In eyes with an axial length of more than 25 mm rare capsular block syndrome appears more frequently4.
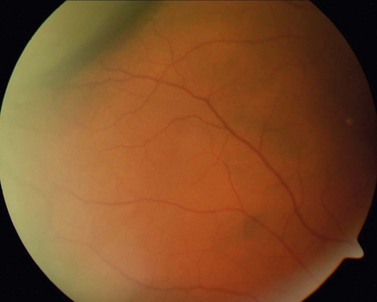
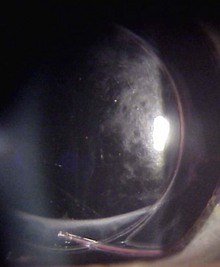
Hyperopic RLE surgery in eyes with an axial length below 21 mm is hampered by the narrow spatial situation and shallow anterior chamber. Compared with eyes with normal axial length, the risk of choroidal effusion syndrome (Fig. 31.1) is markedly increased5.
Postoperative complications
Retinal detachment
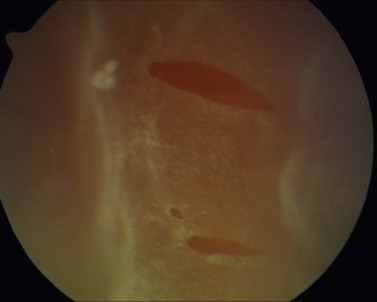
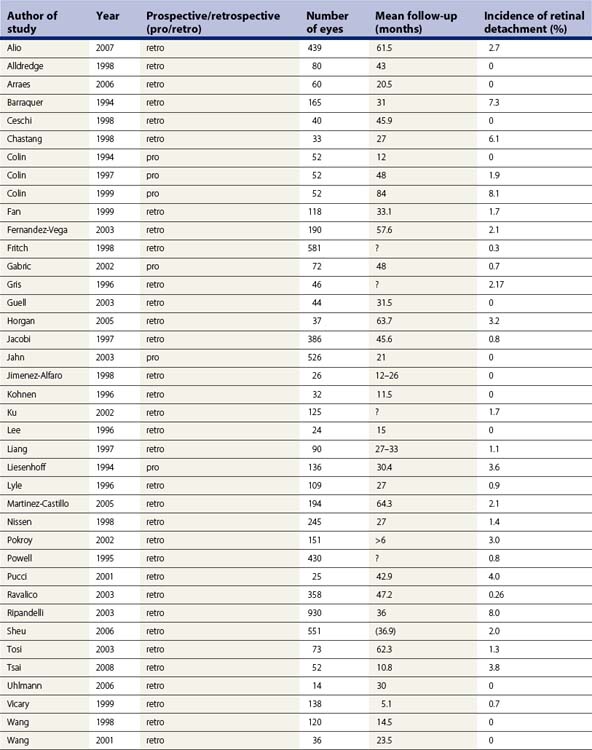
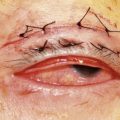
Incidence of rhegmatogenous retinal detachment (RD) (Fig. 31.2) in the general population is 0.02%6. The risk for development of RD increases with myopia due to higher degree of retinal and vitreous degenerations, posterior vitreous detachment, and retinal hole formation7. Phacoemulsification also increases the risk of RD and, as shown in Table 31.2, the combination of both myopia and phacoemulsification markedly enhances this risk. Intraoperative complications such as rupture of the capsular bag or vitreous loss elevate the risk up to 8.0%, even in the general population. Published data on the incidence of RD after phacoemulsification in myopes vary greatly depending on the particular study; from 0 to 8.1% in one single study as shown in Table 31.3, which includes a meta-analysis of publications from 1994 to 2008 dealing with this issue8. The majority of these publications are studies with a retrospective design. Considering the given incidences in Table 31.3, one must note the wide variation concerning the different particular operative techniques (e.g. ICCE, ECCE), the degree of myopia, and the length of follow-up between the studies. In addition, microincisional surgery and IOL with sharp optic edge design were not available until recently. The incidence of PCO was consequently higher in the young patient collectives and Nd:YAG-capsulotomy had to be performed more often. There is an overall tendency that all studies with a RD rate of 0 % embrace a follow-up of less than 4 years and studies with a longer follow-up indicate higher rates of RD. In order to estimate the real cumulative incidence of RD after RLE, an adequate long-term follow-up is mandatory as it has been shown that the cumulative risk of RD after lens surgery increases in a linear fashion over time9. Therefore, one should consider the cumulative risk of RD for young myopes that undergo RLE to be somewhat higher with respect to their significantly longer life expectancy.
| General population | 0.02% |
| Myope >10 D | 0.7% |
| General population after phacoemulsification | 1.2% (10 years) |
| Myope after phacoemulsification | 0–8.1% |
| Young myope (<50 years) after phacoemulsification | 5.2% |
| General population after complicated phacoemulsification | 8.0% |
| Myope after complicated phacoemulsification | >8.0% |
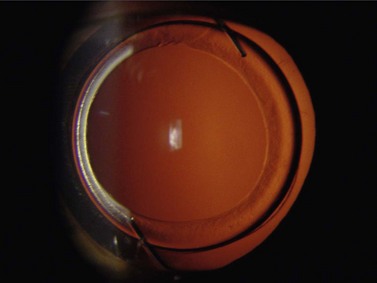
PCO after RLE
Independent of the type of implanted IOL, RLE causes an increased rate of posterior capsule opacification (PCO) (Fig. 31.3) due to the younger age of the patients. Thus, only IOL with sharp edge design should be used in RLE. IOL should be centered in the capsular bag and anterior capsulorrhexis should overlap the IOL (Fig. 31.4). Avoiding PCO is important in order to reduce complications associated with Nd:YAG capsulotomy such as elevation of IOP, iritis, vitreous prolapse, posterior vitreous detachment, cystoid macular edema, or RD. Published data concerning the incidence of RD after lens surgery and subsequent laser capsulotomy vary between 0 and 4%10,13. It is noteworthy that some authors postulate the incidence of RD after laser capsulotomy is not due to the application of the energy of the laser, but rather to the opening of the posterior capsule itself11. A direct correlation of mode, configuration, point of time after lens surgery, or level of applied energy of laser capsulotomy has not yet been substantiated in any study. However, the risk of RD after laser capsulotomy increases significantly with the degree in high myopia. Therefore after RLE, one should carefully weigh the risks of laser capsulotomy in eyes with beginning PCO and an axial length of more than 24 mm against the benefit of some gain in visual acuity12. In some cases it may be advisable to perform surgical polishing of the posterior capsule instead of using the laser in order to avoid opening of the posterior capsule.
1 Fukala V. Behandlung der höchstgradigen Myopie durch Aphakie. Graefes Arch Ophthalmol. 1890;36:230-244.
2 Terzi E, Wang L, Kohnen T. Accuracy of modern intraocular lens power calculation formulas in refractive lens exchange for high myopia and high hyperopia. J Cataract Refract Surg. 2009;35(7):1181-1189.
3 Speaker MG, Guerriero PN, Met JA, et al. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98(2):202-209. discussion 10
4 Kim HK, Shin JP. Capsular block syndrome after cataract surgery: clinical analysis and classification. J Cataract Refract Surg. 2008;34(3):357-363.
5 O’Grady RB. Nanophthalmos. Am J Ophthalmol. 1971;71(6):1251-1253.
6 Polkinghorne PJ, Craig JP. Northern New Zealand Rhegmatogenous Retinal Detachment Study: epidemiology and risk factors. Clin Exp Ophthalmol. 2004;32(2):159-163.
7 Perkins ES. Morbidity from myopia. Sight Sav Rev. 1979;49(1):11-19.
8 Colin J, Robinet A, Cochener B. Retinal detachment after clear lens extraction for high myopia: seven-year follow-up. Ophthalmology. 1999;106(12):2281-2284. discussion 5
9 Javitt JC. Clear-lens extraction for high myopia. Is this an idea whose time has come? Arch Ophthalmol. 1994;112(3):321-323.
10 Dardenne MU, Gerten GJ, Kokkas K, et al. Retrospective study of retinal detachment following neodymium:YAG laser posterior capsulotomy. J Cataract Refract Surg. 1989;15(6):676-680.
11 Winslow RL, Taylor BC. Retinal complications following YAG laser capsulotomy. Ophthalmology. 1985;92(6):785-789.
12 Koch DD, Liu JF, Gill EP, et al. Axial myopia increases the risk of retinal complications after neodymium-YAG laser posterior capsulotomy. Arch Ophthalmol. 1989;107(7):986-990.
13 Kohnen T, Fabian E, Gerl R, et al. Optic edge design as long-term factor for posterior capsular opacification rates. Ophthalmology. 2008;115:1308-1314.