Chapter 15 Red Blood Cell Membrane Disorders
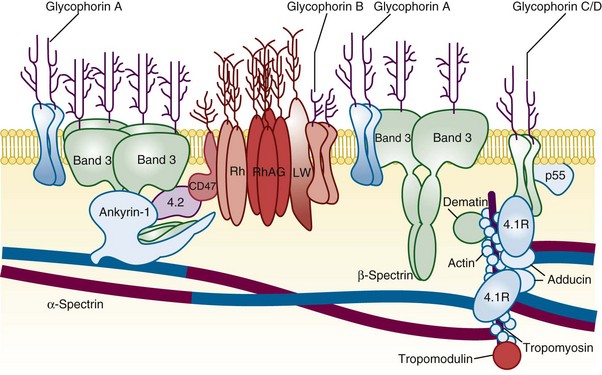
Figure 15-1 A SIMPLIFIED CROSS-SECTION OF THE ERYTHROCYTE MEMBRANE.
(From Perrotta S, Gallagher PG, Mohandas N: Hereditary spherocytosis. Lancet 372:1411, 2008.)
Table 15-1 Erythrocyte Membrane Abnormalities in Hereditary Spherocytosis, Hereditary Elliptocytosis, and Related Disorders
| Gene | Disorder | Comment |
|---|---|---|
| α-Spectrin | HS, HE, HPP, NIHF | Location of mutation determines clinical phenotype. α-Spectrin mutations are most common cause of typical HE. |
| Ankyrin | HS | Most common cause of typical dominant HS. |
| Band 3 | HS, SAO, NIHF | In HS “pincer-like” spherocytes on smear presplenectomy. SAO erythrocytes have transverse ridge or longitudinal slit. |
| β-Spectrin | HS, HE, HPP, NIHF | Location of mutation determines clinical phenotype. In HS, acanthrocytic spherocytes on smear presplenectomy. |
| Protein 4.2 | HS | Common in Japanese HS. |
| Protein 4.1 | HE | |
| Glycophorin C | HE | Concomitant protein 4.1 deficiency is basis of HE in glycophorin C defects. |
HE, Hereditary elliptocytosis; HPP, hereditary pyropoikilocytosis; HS, hereditary spherocytosis; NIHF, nonimmune hydrops fetalis; SAO, Southeast Asian ovalocytosis.
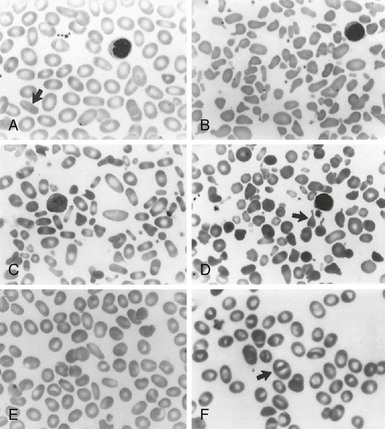
Table 15-2 Peripheral Blood Film Evaluation in a Patient With Red Cell Membrane Disorder
| Shape | Pathobiology | Diagnosis |
|---|---|---|
| Microspherocytes | Loss of membrane lipids leading to a reduction of surface area resulting from deficiencies of spectrin, ankyrin, or band 3 and protein 4.2 Removal of membrane material from antibody-coated red cells by macrophages Removal of membrane-associated Heinz bodies, with the adjacent membrane lipids, by the spleen |
HS Immunohemolytic anemias Heinz body hemolytic anemias |
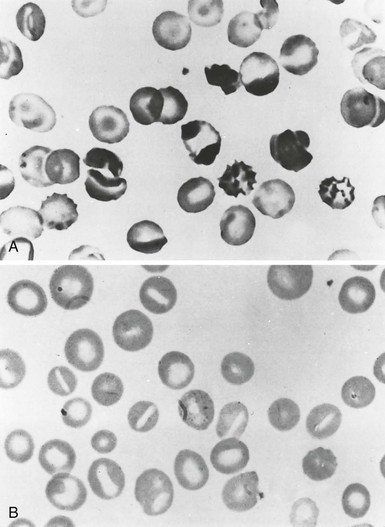
| Elliptocytes | Permanent red cell deformation resulting from a weakening of skeletal protein interactions (such as the spectrin dimer-dimer contact). This facilitates disruption of existing protein contacts during shear stress–induced elliptical deformation. Subsequently, new protein contacts are formed that stabilize elliptical shape Unknown |
Mild common HE Iron deficiency, megaloblastic anemias, myelofibrosis, myelophthisic anemias, myelodysplastic syndrome, thalassemias |
| Poikilocytes/Fragments | Weakening of skeletal protein contacts resulting from skeletal protein mutations Unknown |
Hemolytic HE/HPP Iron deficiency, megaloblastic anemias, myelofibrosis, myelophthisic anemias, myelodysplastic syndrome, thalassemias |
| Schistocytes, fragmented red cells | Red cells “torn” by mechanical trauma (fibrin strands, turbulent flow) | “Microangiopathic” hemolytic anemia associated with disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, vasculitis, heart valve prostheses |
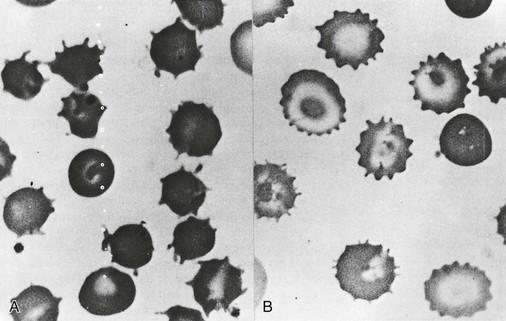
| Acanthocytes | Uptake of cholesterol and its preferential accumulation in the outer leaflet of the lipid bilayer Selective accumulation of sphingomyelin in the outer lipid leaflet Unknown |
Spur cell hemolytic anemia in severe liver disease Abetalipoproteinemia Chorea-acanthocytosis syndrome, malnutrition, hypothyroidism McLeod phenotype |
| Echinocytes | Expansion of the surface area of the outer hemileaflet of lipid bilayer relative to the inner hemileaflet Unknown |
Hemolytic anemia associated with hypomagnesemia and hypophosphatemia in malnourished patients, pyruvate kinase deficiency; in vitro artifact of low blood storage (ATP depletion), contact with glass or elevated pH Hemolysis in long-distance runners, renal failure |
| Stomatocytes | Expansion of the surface area of the inner hemileaflet of the bilayer relative to the outer leaflet Unknown |
Exposure of red cells to cationic anesthetics in vitro; in vivo the drug concentrations may not be sufficient to produce similar effect Alcoholism, inherited disorders of membrane permeability (hereditary stomatocytosis) |
| Target cells | Absolute excess of membrane lipids (both cholesterol and phospholipids: “symmetric” lipid gain), followed by an increase of cell surface area Relative excess of surface area because of a decrease in cell volume |
Obstructive jaundice, liver disease with intrahepatic cholestasis Thalassemias and some hemoglobinopathies (C, D, E) |
ATP, Adenosine triphosphate; HE, hereditary elliptocytosis; HPP, hereditary pyropoikilocytosis; HS, hereditary spherocytosis.
Splenectomy for Hereditary Spherocytosis
Indications for Splenectomy
Severe, symptomatic spherocytosis
Growth failure, skeletal changes, leg ulcers, and extramedullary hematopoietic tumors (signs of severe anemia)
Older patients with vascular compromise of vital organs
Moderate HS with compensated, asymptomatic anemia: controversial; decision should be individualized