Red blood cell and platelet transfusion
Red blood cells
Collection, storage, and administration
Red blood cell transfusion
The sole reason to transfuse RBCs is to increase the content of O2 in the blood, thereby increasing O2 delivery (![]() ), which is a product of hemoglobin concentration, arterial O2 saturation, and cardiac output (CO, which itself is a product of stroke volume and heart rate). A specific hematocrit value may sustain adequate
), which is a product of hemoglobin concentration, arterial O2 saturation, and cardiac output (CO, which itself is a product of stroke volume and heart rate). A specific hematocrit value may sustain adequate ![]() if CO is adequate but may be insufficient when cardiac output is limited or when arterial saturation is impaired by the presence of a transpulmonary shunt. Therefore, despite the widely accepted hemoglobin trigger of 7 g/dL, the decision to transfuse should take into consideration the current hemoglobin level, estimated blood loss, cardiac reserve, vital signs, the likelihood of ongoing hemorrhage, and the risk of tissue ischemia. The dynamic nature of surgical hemorrhage requires a more aggressive approach to blood replacement in the operating room, compared with sites elsewhere in the hospital. In patients with chronic anemia, increased 2,3-DPG levels make O2 transport (see Chapter 21) more efficient; in acute anemia, cardiovascular mechanisms of compensation (e.g., increased CO, heart rate, myocardial O2 consumption) are more important.
if CO is adequate but may be insufficient when cardiac output is limited or when arterial saturation is impaired by the presence of a transpulmonary shunt. Therefore, despite the widely accepted hemoglobin trigger of 7 g/dL, the decision to transfuse should take into consideration the current hemoglobin level, estimated blood loss, cardiac reserve, vital signs, the likelihood of ongoing hemorrhage, and the risk of tissue ischemia. The dynamic nature of surgical hemorrhage requires a more aggressive approach to blood replacement in the operating room, compared with sites elsewhere in the hospital. In patients with chronic anemia, increased 2,3-DPG levels make O2 transport (see Chapter 21) more efficient; in acute anemia, cardiovascular mechanisms of compensation (e.g., increased CO, heart rate, myocardial O2 consumption) are more important.
Indications for transfusion of red blood cells
The binding of O2 to hemoglobin is represented by a sinusoidal relationship known as the oxyhemoglobin dissociation curve, (see Chapter 21), which facilitates efficient O2 loading of hemoglobin in the lungs (where PO2 is high) and unloading of hemoglobin in the tissues (where PO2 is low). Because the vast majority of O2 carried in the blood is noncovalently bound to hemoglobin, ![]() is the product of CO and O2 content:
is the product of CO and O2 content:
< ?xml:namespace prefix = "mml" />
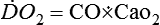
where O2 content is calculated as follows:
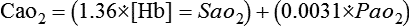
Platelet transfusions
Platelet preparation and storage
Indications for platelet transfusion
Box 102-1 summarizes the indications for platelet concentrate transfusion listed in the American Society of Anesthesiologists’ Guidelines for Perioperative Transfusion Therapy. Patients with abnormal platelet function or thrombocytopenia are likely to benefit from administration of platelet transfusions if the platelet disorder is thought to induce or exacerbate their bleeding. Platelet counts of less than 10 × 109/L often occur in patients receiving chemotherapeutic agents. Platelet transfusions are used to prevent spontaneous intracranial and gastrointestinal hemorrhages in these patients.
Risks of platelet transfusion
Platelet alloimmunization and platelet refractoriness
Other risks of platelet transfusion
The other major risks associated with platelet transfusion overlap with the risks associated with the transfusion of RBCs: febrile transfusion reactions, allergic reactions, and transmission of infectious disease. Although platelet concentrates are drawn from single donors, many units are usually given at a time, increasing the risk of complications. Platelet transfusions also contain more donor plasma and are more likely to cause lung injury. Bacteria can proliferate in platelet concentrates because they are stored at room temperature; they are often implicated in septic transfusion reactions (see Chapter 104).





