Chapter 41 Recurrent Pregnancy Loss
INTRODUCTION
Recurrent pregnancy loss is a profound personal tragedy to couples seeking parenthood and a formidable clinical challenge to their physicians. Spontaneous abortion occurs in approximately 15% of clinically diagnosed pregnancies in reproductive-age women, but recurrent pregnancy loss occurs in about 1% to 2% of this same population.1 Great strides have been made in characterizing the incidence and diversity of this heterogeneous disorder, and a definite cause of pregnancy loss can be established in approximately two thirds of couples after a thorough evaluation.2 A complete evaluation will include investigations into genetic, endocrinologic, anatomic, immunologic, microbiologic, thrombophilic, and iatrogenic causes. Recurrent pregnancy losses may induce significant emotional distress; in some cases intensive supportive care may be necessary. Successful outcomes will occur in more than two thirds of all couples.3
DEFINITION OF PREGNANCY LOSS
Several studies have recently indicated that the risk of recurrent miscarriage after two successive losses is similar to the risk of miscarriage in women after three successive losses; thus, it is reasonable to start an evaluation after two or more consecutive spontaneous miscarriages to determine the etiology of their pregnancy loss, especially when the woman is older than age 35 or when the couple has had difficulty conceiving.3,4 Any loss before 20 gestational weeks is considered a miscarriage. Some authors further divide these into embryonic losses, which occur before the ninth gestational week, and fetal losses, which occur at any time from the 9th gestational week to 20 weeks’ gestation, although there is no developmental phase to justify this distinction. Those couples with primary recurrent loss have never had a previous viable infant; those with secondary recurrent loss have previously delivered a pregnancy beyond 20 weeks’ gestation and then suffered subsequent losses. Other investigators advocate the designation of tertiary recurrent loss to identify those women who have multiple miscarriages interspersed with normal pregnancies.
RECURRENCE RISK
The main concerns of couples who have had a spontaneous abortion are the cause and the risk of recurrence. In a first pregnancy, the overall risk of loss of a clinically recognized pregnancy is 15%.5,6 The true rate of early pregnancy loss, however, is estimated to be around 50% because of the high rate of losses that occur before the first missed menstrual period. Studies that evaluated the frequency of pregnancy loss, based on highly sensitive tests for quantitative hCG, indicated that the total clinical and preclinical losses in women age 20 to 30 is approximately 25%, whereas the loss rate in women age 40 or more is at least double that figure.7,8 For women who have had one miscarriage, the risk of another loss rises slightly, to 14% to 21%. After two or three miscarriages the rate rises to 24% to 29% and 31% to 33%, respectively.9 The ability to predict the risk of recurrence is influenced by several factors.
Maternal Age
Advancing maternal age is associated with a higher rate of pregnancy loss of both normal and abnormal conceptuses.10,11 This probably reflects poor oocyte quality and reduced endocrine function in this group. In one study of more than 1 million pregnancies, the overall rate of spontaneous abortion was 11% and the approximate rates of clinically recognized miscarriage according to maternal age were 9% to 17% (age 20 to 30), 20% (age 35), 40% (age 40), and 80% (age 45).10
Pregnancy Outcome
Although the risk of miscarriage increases with each successive pregnancy loss, a pregnancy ending in a live birth reduces the risk of miscarriage in the subsequent gestation.12,13
Parity
Increasing parity is associated with an increased rate of spontaneous abortion.14 This may be partially explained by the correlation between maternal age and parity.
ETIOLOGIES OF RECURRENT PREGNANCY LOSS
Genetic Factors
Chromosomal Disorders
Parental chromosome anomalies occur in 3% to 5% of couples with recurrent pregnancy loss as opposed to 0.2% in the general population. These include translocations, inversions, and the relatively rare ring chromosomes. Balanced translocations are the most common chromosomal abnormalities contributing to recurrent pregnancy loss.16 In recurrent pregnancy loss, this abnormality is found more frequently in the female partner, at a female-to-male ratio of from 2:1 up to 3:1.
Translocations
Robertsonian translocations involve two acrocentric chromosomes (numbers 13, 14, 15, 21, or 22) that fuse near the centromere region with loss of the short arms. Although a carrier of a Robertsonian translocation is phenotypically normal, there is a risk of unbalanced gametes and therefore unbalanced offspring. The risk of subsequent abortions varies with the type of translocation and the sex of the carrier. Robertsonian translocations are a more homogeneous group, and so the information on them is the most consistent. Studies indicate that when the Robertsonian translocation is maternal, there is a greater risk that the fetus will exhibit an unbalanced phenotype.17
Neri and coworkers found that carriers of these translocations had a 20% to 25% risk of spontaneous abortion. The only exceptions are translocations involving homologous chromosomes. If, for example, both copies of chromosome 22 fuse, the carrier should produce only trisomy 22 or monosomy 22, all of which would abort. Carriers of this type of translocation should therefore have a 100% rate of pregnancy loss, but two unexplained normal offspring from such carriers have been reported.18,19
Inversions
Chromosomal inversions occur less frequently than translocations. An inversion occurs when a single chromosome undergoes two breaks and is reconstituted, with the segment between the breaks inverted. Inversions are of two types: paracentric, in which both breaks occur in one arm, and pericentric, in which there is a break in each arm. An inversion does not usually cause an abnormal phenotype in carriers, because it is a balanced rearrangement. Its medical significance is for the progeny; a carrier of either type of inversion is at risk of producing abnormal gametes that may lead to pregnancy loss. A female inversion carrier is at an approximately 8% risk of producing anomalous offspring; the risk is reduced to 4% in male carriers.20
Ring Chromosomes
Ring chromosomes are formed when a chromosome undergoes two breaks and the broken ends of the chromosome reunite in a ring structure. Ring chromosomes are quite rare; because of mitotic instability, it is not uncommon for ring chromosomes to be found in only a proportion of cells. The medical literature is limited to isolated case reports, and the specifics of the effects of ring chromosomes on recurrent pregnancy loss are not known.21
Recurrent Aneuploidy
The first chromosomally abnormal abortus was documented in 1961, and since then a large body of data on the chromosomal status of spontaneous abortuses has accumulated. The overall frequency of chromosome abnormalities in spontaneous abortions is at least 50%. Of these abnormalities, most are numerical: 52% are trisomies, 29% are monosomy 45,X, 16% are triploidies, 6% are tetraploidies, and 4% are structural rearrangements.22 The earlier the abortion, the higher the chance of chromosomal abnormality. In Boúe’s study,22 66% of abortions at 2 to 7 weeks’ gestation were abnormal, whereas the percentage fell to 23% for abortions at 8 to 12 weeks’ gestation.
Evidence suggests that some couples are at risk for conceptions complicated by recurrent aneuploidy. Empirically, the birth of a trisomic infant places a woman at an approximate 1% increased risk for a subsequent trisomic conceptus.23 Germline mosaicism has been reported in recurrent cases of Down syndrome and may also be responsible for recurrent aneuploidy in some couples.24
Single-Gene Mutations
The role of single-gene mutations at the molecular level in recurrent pregnancy loss remains speculative. It is known from studies in transgenic mice that some selected mutations and inactivation of some specific transcription factors can cause pregnancy failure.25 It is speculated that similar mutations in genes required for embryonic, placental, or cardiac development in humans are likely to cause pregnancy loss.
X-Inactivation
In somatic cells of female mammals, only one X chromosome is active. Inactivation of the second X occurs early in embryonic life to achieve dosage compensation with males. The inactive X may be paternal or maternal; it is entirely a matter of chance which of the pair becomes inactivated in a cell. Occasionally, preferential inactivation of one allele occurs. Extremely skewed X-inactivation, defined as more than 90% inactivation of one allele, is observed in 2% of newborns and 4.5% of reproductive-age women.26
Several authors have found the skewed X-inactivation pattern in phenotypically normal women with recurrent pregnancy loss.27,28 Others have also found a statistically significant increase in the spontaneous abortion rate among family members who carried the skewed X trait.29 Presumably these women have an X-linked mutation or germline mosaicism.
Endocrinologic Factors
Luteal Phase Defect
Maintenance of early pregnancy depends on the production of progesterone by the corpus luteum. Between 7 and 9 weeks’ gestation the developing placenta takes over progesterone production. Luteal phase defect is defined as an inability of the corpus luteum to secrete progesterone in high enough amounts or for too short a duration. Abnormalities of the luteal phase have been reported to occur in up to 35% of women with recurrent abortion.30
Several potential mechanisms are thought to give rise to a luteal phase defect: decreased production of progesterone by the corpus luteum, decreased follicle-stimulating hormone (FSH) levels in the follicular phase, abnormal patterns of luteinizing hormone (LH) secretion, decreased response by the endometrium to already-secreted progesterone, and increased prolactin levels. The preponderance of evidence suggests that luteal phase defect is a preovulatory event most likely linked to an alteration in preovulatory estrogen stimulation, which may indicate poor oocyte quality and a poorly functioning corpus luteum.31,32
Some authors have advocated the measurement of serum progesterone levels in the luteal phase for the diagnosis of luteal phase defect, with levels below 10 ng/mL considered abnormal.33 However, progesterone levels are subject to large fluctuations because of pulsatile release of LH. Moreover, there is a lack of correlation between serum levels of progesterone and endometrial histology.34 Nonetheless, serum progesterone levels of 10 ng/mL or greater in the midluteal phase of the menstrual cycle are generally utilized to exclude luteal phase defect.
Untreated Hypothyroidism
Untreated hypothyroidism may increase the risk of miscarriage. A recent study of more than 700 patients with recurrent pregnancy loss identified 7.6% with hypothyroidism.35 Hypothyroidism is easily diagnosed with a sensitive thyrotropin test, and patients should be treated to become euthyroid before attempting a next pregnancy. It has also been suggested that thyroid antibodies are elevated in women with recurrent pregnancy loss. A retrospective study of 700 patients with recurrent pregnancy loss demonstrated that 158 women had antithyroid antibodies but only 23 of those women had clinical hypothyroidism on the basis of an abnormal thyrotropin value.36 The presence of antithyroid antibodies may imply abnormal T-cell function; therefore, an immune dysfunction rather than an endocrine disorder may be responsible for the pregnancy losses.
Insulin Resistance
Patients with poorly controlled diabetes are known to have an increased risk of spontaneous miscarriage, which is reduced to normal spontaneous loss rates when women are euglycemic preconceptually.37 Hyperglycemia and vascular complications resulting in compromised blood flow to the uterus may be responsible for the pregnancy loss. It is known that women with PCOS have an increased risk of miscarriage. The mechanism is unknown, but may be related to elevated serum LH levels and high testosterone and androstenedione concentrations, which may adversely affect the endometrium.38 It also may be related to the high prevalence of insulin resistance in these women.39 Testing for fasting insulin and glucose is simple, and treatment with insulin-sensitizing agents may reduce the risk of recurrent miscarriage.40
Elevated Day 3 FSH
Elevated day 3 FSH levels have been associated with decreased pregnancy rates in women undergoing in vitro fertilization (IVF). Although the frequency of elevated day 3 FSH levels in women with recurrent miscarriage is similar to the frequency in the infertile population, the prognosis for recurrent miscarriage is increased with increased day 3 FSH levels.41 Thus, it is speculated that women with recurrent pregnancy loss and elevated day 3 FSH levels may have poor quality oocytes with inherent chromosomal abnormalities. Although no treatment is available, testing should be performed in women over age 35 with recurrent pregnancy loss, and appropriate counseling should follow.
Hyperprolactinemia
Normal circulating levels of prolactin may play an important role in maintaining early pregnancy. Data from animal studies suggest that elevated prolactin levels may adversely affect corpus luteal function; however, this concept has not been proven in humans.42 A recent study of 64 hyperprolactinemic women showed that bromocriptine therapy was associated with a higher rate of successful pregnancy and that prolactin levels were significantly higher in women who miscarried.43
Anatomic Factors
Anatomic uterine defects are present in 15% to 20% of women evaluated for three or more consecutive spontaneous abortions.2 These anatomic abnormalities can be classified as congenital or acquired. Uterine malformations appear to predispose women to reproductive difficulties, including first- and second-trimester fetal losses, preterm labor, and abnormal fetal presentation.
Congenital Uterine Anomalies
Congenital malformations of the reproductive tract result from failure to complete bilateral duct elongation, fusion, canalization, or septal resorption of the müllerian ducts. Müllerian anomalies were found in 8% to 10% of women with three or more consecutive spontaneous abortions who underwent hysterosalpingography or hysteroscopic examination of their uteri.2,44 Inadequate vascularity, compromising the developing placenta, and reduced intraluminal volume have been theorized as possible mechanisms leading to pregnancy loss.
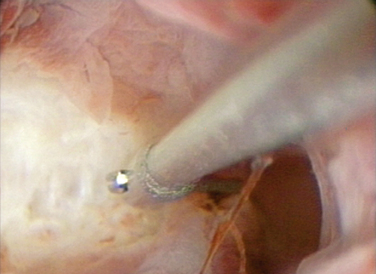
The most common abnormality associated with pregnancy loss is the septate uterus. The spontaneous abortion rate is high, averaging about 65% of pregnancies in some studies.45 A septum is primarily composed of fibromuscular tissue that is poorly vascularized (Fig. 41-1). This lack of vascularization may compromise decidual and placental growth. Alternatively, a uterine septum may impair fetal growth as a result of reduced endometrial capacity or a distorted endometrial cavity.45 Uncontrolled studies suggest that women who undergo resection of the uterine septum have higher delivery rates than women without treatment. Other congenital abnormalities, such as uterus didelphys, bicornuate uterus, and unicornuate uterus, are more frequently associated with later trimester losses or preterm delivery.
Diethylstilbestrol Exposure
Diethylstilbestrol (DES) is an orally active synthetic estrogen that was introduced in the 1940s for the treatment of recurrent pregnancy loss, premature delivery, and other complications of pregnancy. The use of DES in pregnancy was banned in 1971.46
Uterine abnormalities occur in 69% of women exposed to DES in utero.46 The most common abnormality is a T-shaped uterine cavity (70%). Other abnormalities include a small uterus, constriction rings, and intrauterine filling defects. In addition, 44% of DES-exposed women have structural changes in the cervix, including an anterior cervical ridge, cervical collar, hypoplastic cervix, and pseudopolyp. Women with a history of in utero exposure to DES appear to have a greater risk of adverse pregnancy outcome, including a twofold increased risk of spontaneous abortion and a ninefold increase in ectopic pregnancy rates.47
Acquired Uterine Abnormalities
Intrauterine Masses
Intrauterine cavity abnormalities, such as leiomyomas and polyps, can contribute to pregnancy loss. There are several hypotheses concerning how leiomyomas may be associated with recurrent pregnancy loss. Depending on the leiomyoma size and location, it may partially obliterate or alter the contour of the intrauterine cavity, providing a poorly vascularized endometrium for implantation or otherwise compromising placental development. Uterine leiomyomas and polyps may also act like an intrauterine device (IUD), causing subacute endometritis. Until recently, it was felt that only submucous leiomyomas should be surgically removed before subsequent attempts at pregnancy. However, several recent studies investigating the implantation rate in women undergoing IVF have clearly demonstrated decreased implantation with intramural leiomyomas in the range of 30 mm.48 When smaller leiomyomas are identified, it is unclear if myomectomy is beneficial.49
Incompetent Cervix
Cervical incompetence can be considered an acquired uterine anomaly that is associated with recurrent pregnancy loss. The diagnosis of cervical incompetence is based on the presence of painless cervical dilation resulting in the inability of the uterine cervix to retain a pregnancy. Cervical incompetence commonly causes pregnancy loss in the second trimester. It may be associated with congenital uterine abnormalities, such as septate or bicornuate uterus. Rarely, it may be congenital following in utero exposure to DES.47 It has been reported that trauma to the cervix from conization, loop electrosurgical excision procedures, dilation of the cervix during pregnancy termination, or short cervical lengths on ultrasound increase the risk of cervical incompetence.50
IMMUNOLOGIC FACTORS
The primary function of the immune system is to recognize and eliminate potentially pathogenic organisms from the body. To understand how immunologic factors can affect pregnancy, a basic understanding of the normal immune response is required. The immune response has two components: the innate, or nonspecific, immune response and the acquired, or adaptive, immune response.
Innate and Acquired Immune Responses
Acquired or adaptive immune responses are antigen-specific and lead to memory, but require 4 to 7 days to be effectively generated. T cells and B cells and a small proportion of NK cells largely mediate these responses. B cells differentiate in bone marrow. T and B cells occur in tissues in approximately equal numbers. T cells are responsible for cell-mediated immunity and are essential for B-cell function.51
T-helper Lymphocytes
T cells produce a range of cytokines following an immune challenge. The type of cytokine determines their differentiation into T helper 1 (Th1) and T helper 2 (Th2) lymphocytes. For example, Th1 lymphocytes secrete interleukin-2 (IL-2) and IFN-γ, creating a pro-inflammatory environment. Conversely, Th2 lymphocytes secrete cytokines, such as IL-4 and IL-10, that are predominantly involved in antibody production following an antigenic challenge. The actions of the two types of lymphocytes are intertwined, acting in concert and responding to counterregulatory effects of their cytokines. Hormones have a powerful effect on this action. During their reproductive years, women are more likely to develop a Th1 response after challenge with an infectious agent or antigen, except during pregnancy, when a Th2 environment prevails.52
Autoimmune Factors: Maternal Response to Self
In some instances, there is a failure in normal control mechanisms that prevents an immune reaction against self, resulting in an autoimmune response.53 Autoantibodies to phospholipids, thyroid antigens, nuclear antigens, and others have been investigated as possible causes for pregnancy loss.35 Antiphospholipid antibodies include both the lupus anticoagulant and anticardiolipin antibodies. There is still controversy concerning testing for other phospholipids, but an increasing number of studies suggest that antibodies to phosphatidyl serine are also associated with pregnancy loss.54
Systemic Lupus Erythematosus
Most clinical studies find that pregnancy loss is more common among women with systemic lupus erythematosus (SLE) than among normal women.55 The rate of spontaneous abortions among patients with SLE in one large study was estimated at 21%.56 Nearly three quarters of pregnancy losses appear to occur in the second and third trimesters.57 Most fetal deaths in women with SLE are associated with the presence of antiphospholipid antibodies.55,58 In a study of women with SLE and recurrent pregnancy loss, women with antiphospholipid antibodies had a tenfold increased risk of miscarriage compared to women with SLE but without antiphospholipid antibodies.55 Other factors associated with pregnancy loss in this population include (1) disease activity before conception, (2) the onset of SLE during pregnancy, and (3) underlying renal disease. Women with active SLE should be advised to delay conception until remission is established. Women with moderate renal insufficiency should be advised against pregnancy. Women should also be counseled about the higher rates of preeclampsia and premature delivery with SLE.57
Antiphospholipid Antibody Syndrome
Antiphospholipid antibody syndrome is an autoimmune condition characterized by the production of moderate to high levels of antiphospolipid antibodies and certain clinical features. The presence of antiphospholipid antibodies (anticardioplipin and lupus anticoagulant) during pregnancy is a major risk factor for adverse pregnancy outcome.59 In a large meta-analysis of studies of couples with recurrent abortion, the incidence of antiphospholipid antibody syndrome was between 15% and 20%, compared to about 5% in nonpregnant women without a history of obstetric complications.60 It is not yet understood how antiphospholipid antibodies arise in patients with the syndrome. Genetic factors and infection may play a role. The antibodies generated in patients with antiphospholipid antibody syndrome appear to recognize epitopes on phospholipid-binding proteins, unlike the antibodies that arise following infections such as syphilis and Lyme disease, which recognize phospholipids directly.61
Several mechanisms have been proposed by which antiphospholipid antibodies might mediate pregnancy loss. It appears that different coagulation proteins may be involved in binding to phospholipids, which may explain the predisposition to thrombosis. Antibodies against phospholipids could increase thromboxane and decrease prostacyclin synthesis within placental vessels. The resultant prothrombotic environment could promote vascular constriction, platelet adhesion, and placental infarction.60 Antiphospholipid antibodies appear to interfere with various components of the protein C antithrombotic pathway, including inhibiting the formation of thrombin, decreasing protein C activation by the thrombomodulin–thrombin complex, inhibiting assembly of protein C complex, inhibiting activated protein C activity, and binding to factors Va and VIIIa in ways that protect them from proteolysis by activated protein C.61 Another proposed mechanism is the disruption of the placental antithrombotic molecule, annexin V. The levels of annexin V are reduced in placental villi of women with recurrent pregnancy loss who are antiphospholipid antibody-positive.62 Antiphospholipid antibodies can also recognize heparin and heparinoid molecules and thereby inhibit antithrombin III activity.63 Antiphospholipid antibodies can interact with cultured human vascular endothelial cells with resultant injury or activation.61 A more direct action on the trophoblastic cells has been demonstrated and explains how antiphospholipid antibodies can cause early fetal loss. Antiphospholipid antibodies have been demonstrated to inhibit secretion of human placental chorionic gonadotropin and to inhibit the expression of trophoblast cell adhesion molecules (α1 and α5 integrins, E- and VE-cadherins).64
Antithyroid Antibodies
An increased frequency of antithyroid antibodies (antithyroid peroxidase, antithyroglobulin) have been reported in women with recurrent pregnancy loss. However, if the patient is euthyroid, the presence of antithyroid antibodies does not affect pregnancy outcome.65 Women who have positive antithyroid antibodies and are euthyroid are at an increased risk for hypothyroidism during and after pregnancy. These women should have their thyrotropin level tested during each trimester and postpartum for thyroiditis.66
Antinuclear Antibodies
Approximately 10% to 15% of all women will have detectable antinuclear antibodies regardless of their history of pregnancy loss. Their chance of successful pregnancy outcome is not dependent on the presence or absence of antinuclear antibodies. Treatments such as steroids have been shown to increase the maternal and fetal complications without benefiting live births67; thus, routine testing and treatment for antinuclear antibodies is not indicated.
Alloimmune Factors: Maternal Immune Response to Trophoblast
Implantation is the process by which the embryo is intimately connected with the maternal uterine and blood-borne cells. The implanting embryo possesses paternal antigens and therefore may be rejected as an allograft. However, in most cases, fetal rejection by the maternal immune system does not occur and seems to be prevented by mechanisms not yet defined. Alloimmune factors have been suggested to be associated with recurrent pregnancy loss. HLA sharing was thought to be associated with recurrent pregnancy loss based on a decreased maternal immune response and, thus, decreased production of blocking antibodies. Recent large studies, however, reveal no association between HLA (and HLA-DQα) homozygosity, and recurrent pregnancy loss.68 Other investigators have implicated certain embryotoxic factors, such as tumor necrosis factor (TNF-α) and IFN-δ, identified in the supernatants of peripheral blood lymphocytes from women with pregnancy loss; however, this has not been confirmed by independent studies. Immunophenotypes of endometrial cells from women with recurrent pregnancy loss demonstrate altered NK cell (CD56+) populations. Some have suggested that increased NK cells are associated with pregnancy loss, whereas others have indicated that decreased NK cells are associated with pregnancy loss. None of these findings have been clearly associated with pregnancy loss; thus, there are no definite recommended tests at this time.69 Several theories have been proposed to account for the immune-privileged state of the decidua.
Systemic Immunosuppression
Some studies have suggested that pregnancy-associated plasma protein A, human placental lactogen, human placental protein 14, and progesterone may have immune-depressant activity in lymphocytes.52
Lack of Expression of Human Leukocyte Antigens
The apparent lack of MHC gene expression suggests that the embryo may be protected from direct immunologic attack by the MHC-restricted T cells. However, because the NK cells of the innate immune system recognize and kill cells that do not express MHC, the trophoblast and the embryo could be vulnerable to those NK cells that are pervasive at sites of implantation. The expression of HLA-C, HLA-E, and HLA-G by trophoblast cells may protect against NK cell-mediated killing as well as modulate cytokine expression profiles at the maternal–fetal interface.70
Microbiologic Factors
Certain infectious agents have been identified more frequently in cultures from women who have had spontaneous pregnancy losses.72 These include Ureaplasma urealyticum, Mycoplasma hominis, and Chlamydia. Other less frequent pathogens include Toxoplasma gondii, rubella, herpes simplex virus, measles, cytomegalovirus, Coxsackievirus, and Listeria monocytogenes. It is important to be aware that none of these pathogens have been causally linked to recurrent pregnancy loss. Because of the clear association with sporadic pregnancy losses and the ease of diagnosis, women with recurrent pregnancy loss should be cultured for the three most common organisms and both partners should be treated if positive.
Thrombophilic Factors
The hemostatic system appears to play an important role in both the establishment and maintenance of pregnancy. Thrombophilia is defined as a tendency to thrombosis. Several authors have found an association between thrombotic predispositions and recurrent pregnancy loss. The proposed mechanisms for fetal loss include inhibition of the thrombolytic system, placental thrombosis, placental infarction, abnormal prostacyclin metabolism, and direct cytotoxic effects.73 Because maternal intervillous blood flow does not develop significantly before 8 weeks’ gestation, the theoretical role of thrombophilic defects in first-trimester abortion has been questioned. However, two recent meta-analyses of thrombophilias and early recurrent pregnancy loss established a significant association.74,75 Other obstetric complications associated with thrombophilia are midtrimester abortions, stillbirth, severe preeclampsia, intrauterine growth restriction, and placental abruption.74–76
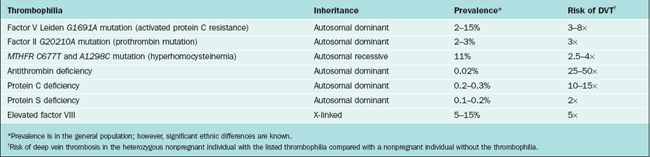
The most common inherited thrombophilias are heterozygosity for factor V Leiden (G1691A), factor II-prothrombin mutation (G20210A), and hyperhomocysteinemia (thermolabile MTHFR C677T and A1298C). Other possible abnormalities leading to hypercoagulable states that may be associated with recurrent miscarriage include antithrombin deficiency, protein C deficiency, protein S deficiency, and elevated factor VIII (Table 41-1).
Factor V Leiden Mutation
In 1994, the factor V Leiden mutation was discovered to be a very common single-gene predisposition to thrombosis.77 Roughly 3% to 6% of individuals of European ancestry carry this mutation. The Leiden mutation is a single nucleotide substitution in the factor V gene at nucleotide 1691 (G to A) that causes an amino acid substitution (glutamine for arginine) at position 506 in the factor V molecule. In normal clotting, activated protein C (APC) inactivates factor Va and VIIIa by cleavage at specific sites. In the presence of the mutation of factor V, the cleavage of this factor is inhibited, leading to enhanced thrombin generation and increased clot formation. The inheritance of the mutation is autosomal dominant. Carriers of factor V Leiden mutation are at greater risk of fetal loss and stillbirth. Heterozygotes for the mutation have a twofold higher risk of experiencing fetal loss or miscarriage.78
Prothrombin G20210A
In 1996, an additional factor involved in the etiology of thrombophilia was discovered: a point mutation, G to A at nucleotide position 20210 in the 3′-untranslated region of the prothrombin gene.79 This mutation is associated with higher plasma prothrombin concentrations, augmented thrombin generation, and increased risk of venous and arterial thrombotic disease. Heterozygosity for the mutation is present in 2% to 3% of the white population.
Hyperhomocysteinemia
Homocysteine is a sulfhydryl nonessential amino acid formed during metabolism of methionine. The levels of homocysteine are increased in a number of inherited and acquired conditions. Hyperhomocysteinemia is associated with the development of venous and arterial thrombosis.80 Homocysteine levels fall during normal pregnancy, and this fall is independent of folate intake.81 High homocysteine levels have been associated with a number of pregnancy-related complications, including neural tube defects, placental infarcts, intrauterine growth restriction, and placental abruption.
Folate deficiency is the most common acquired cause of hyperhomocysteinemia. Among the genetic causes a common one is a polymorphism that results from a C to T substitution at nucleotide position 677 in the MTHFR gene that converts alanine to a valine residue.82 Individuals homozygous for the mutation (6% to 12% of whites) have significantly elevated plasma homocysteine levels and are prone to the early development of arteriosclerosis. The reduced MTHFR activity and subsequent hyperhomocysteinemia may only be evident in the presence of folate deficiency or exacerbated vitamin B6 or B12 deficiency.83 Adequate folate supplementation may prevent phenotypic expression of the mutation.
It has been suggested that maternal hyperhomocysteinemia interferes with embryonic development through defective chorionic villus vascularization.84 A meta-analysis of the association between MTHFR and pregnancy loss before 16 weeks’ gestation suggests a weak association, with an odds ratio (OR) of 1.4 (95% CI, 1.0–2.0); however, the association between elevated fasting plasma homocysteine and early pregnancy loss was significant, with an OR of 2.7 (95% CI, 1.4–5.2).78,85
Antithrombin Deficiency
Antithrombin (previously called antithrombin III) is a serine protease inhibitor produced by the liver with naturally occurring anticoagulant properties. It inhibits thrombin as well as factors Xa, IXa, XIa, and XII and also accelerates the dissociation of the membrane bound tissue factor–VIIa complex. The antithrombin deficiency is inherited in an autosomal dominant fashion. The prevalence of heterozygous carriers is estimated at 1/2000 to 1/5000.86 It is present in 1% of patients with venous thromboembolism and is the most thrombogenic of the inherited thrombophilias, with a 50% lifetime risk of thrombosis.87 Antithrombin deficiency has been associated with an increased risk for stillbirth (OR, 5.25; 95% CI, 1.5–18.1) and fetal loss (OR, 1.7; 95% CI, 1.0–2.8).88
Protein C and Protein S Deficiency
Protein C and protein S are vitamin K-dependent serine protease inhibitors synthesized in the liver. Protein S is also made in endothelial cells and megakaryocytes. Protein S and protein C both exert their antithrombotic effects at those steps in the coagulation cascade that involve conversion of factor X to Xa and conversion of prothrombin to thrombin. The prevalence of protein C deficiency in the general population is 0.15% to 0.8%; prevalence is 2.7% to 4.6% in patients with a history of venous thromboembolism.86 The prevalence of protein S is less than 0.1% in the general population but is 2.2% in patients with a history of venous thromboembolism. Protein S and protein C deficiency do not appear to increase the risk of first-trimester miscarriages but are associated with an increased risk of second-trimester miscarriage and stillbirth.88,89
Combined Thrombophilia
The combination of thrombophilic states appears to increase the risk of recurrent pregnancy loss.88 In one study, although factor V Leiden was more common in women with pregnancy loss, factor II G20210A and homozygosity for MTHFR C677T contributed to pregnancy loss only when associated with other thrombophilic defects.90
Male Factor
Male factors potentially contributing to recurrent abortion are largely unexplored. It is known that chromosomal abnormalities limited to spermatogenic cells and not detected in somatic chromosomes are found in approximately 6% of patients in whom meiotic studies are performed.91 Preliminary data from assisted reproductive technology techniques appear to suggest that a paternal component may be implicated in pregnancy loss. Various studies looking the outcome of IVF and intracytoplasmic sperm injection (ICSI) treatment cycles have shown that there is a correlation between sperm DNA integrity and outcomes of these cycles.92,93 There also appears to be an increased risk of spontaneous abortion in women whose partners have poor sperm morphology. The ultrastructural studies of spermatozoa in these studies showed defects of chromatin condensation and irregular nuclei with vacuoles.94 These reports suggest that abnormal sperm chromosomes may be associated with recurrent pregnancy loss.
LIFESTYLE ISSUES AND ENVIRONMENTAL TOXINS
Cigarette Smoking
Cigarette smoking reduces fertility and increases the rate of spontaneous abortion. The data evaluating smoking and miscarriage are extensive and involve approximately 100,000 subjects. Although the studies are clinically heterogeneous, they nevertheless suggest a clinically significant detrimental effect of cigarette smoking that is dose-dependent, with a relative risk for miscarriage among moderate smokers (10 to 20 cigarettes a day) being 1.1 to 1.3.95
Alcohol Consumption
Alcohol consumption of more than two drinks per day is associated with a risk of spontaneous abortion twice that in nonalcohol-consuming controls.96 The minimum threshold dose for increasing the risk of spontaneous abortion appears to be 2 ounces of alcohol per week.97 When both personal habits, cigarette smoking and alcohol, are utilized in the same individual, the risk of pregnancy loss may increase fourfold. Couples should be counseled concerning these habits and strongly encouraged to discontinue these before attempting subsequent conception.98
Caffeine Intake
There is no evidence that modest caffeine intake (up to 150 mg/day or 1.5 cups of coffee per day) is associated with an increased risk for first-trimester spontaneous abortion or fetal malformations. Several studies have shown, however, that caffeine in excess of 300 mg/day (3 cups of coffee per day) is associated with a modest increase in spontaneous abortion, but it is not clear if this relationship is causal.99
Ionizing Radiation
The studies of atomic bomb survivors in Japan showed that in utero exposure to high-dose radiation increased the risk of spontaneous abortions, premature deliveries, and stillbirths.100,101 Diagnostic X-rays in the first trimester delivering less than 5 rads are not teratogenic.102 Large doses (360 to 500 rads) used in therapeutic radiation, however, induce abortion in offspring exposed in utero in the majority of cases. Adverse effects of chronic low-dose radiation on reproduction have not been identified in humans.95
Organic Solvents
Organic solvent is a broad term that applies to many classes of chemicals. Perchloroethylene, toluene, xylene, and styrene have all been linked to adverse reproductive outcomes in laboratory animals and in humans. Solvent intoxication is an established occupational hazard in painters and workers in the rubber, semiconductor, and dry-cleaning industries.95 In a meta-analysis of published studies from 1966 to 1994, an overall OR of 1.25 indicated that maternal occupational exposure to organic solvents by inhalation was associated with a small increased risk for spontaneous abortion.103 Overall, organic solvents as a class and toluene in particular are teratogenic at high exposure levels. There is no conclusive evidence that low-level exposure increases the risk of congenital malformations or other adverse reproductive outcomes.95
Heavy Metals
Exposure to organic mercury compounds such as methyl mercury or ethyl mercury may occur through consumption of contaminated fish or through the release from batteries, thermometers, and dental amalgams containing mercury. Methyl mercury crosses the placenta and accumulates in embryonic and fetal tissues, particularly in the brain, at concentrations exceeding those in the mother and can cause abnormal neurologic development. In nonhuman primates chronically treated with low-dose methyl mercury, reproductive failure was more likely to occur than in nontreated controls.104 There appears to be no similar association with human environmental exposure.105
Although federal guidelines are eliminating the use of lead, exposure can still occur from residual soil contamination from a variety of sources, including lead solders, pipes, storage batteries, lead-based paints, dyes, and wood preservatives. Exposures to high levels of lead are known to cause embryotoxicity, growth and mental retardation, and increased perinatal mortality. Women exposed to high concentrations of lead were known to have a higher risk of miscarriages in the 19th century. In fact, lead pills were sold as an abortifacient in the late 1800s and early 1900s.95 Lead concentrations in maternal blood should not exceed 25μg/dL. Federal standards mandate that women should not work in areas where air lead concentrations reach 50μg/cm3 because this may result in blood concentrations above 25 to 30μg/dL.106
Other Exposures
Adequately controlled prospective studies demonstrated that exposure to electromagnetic fields and video display terminals was not associated with increased pregnancy loss.107 Air travel, microwave radiation, and ultrasound exposure do not appear to increase the risk of spontaneous abortion. There is also no evidence for an increased incidence of spontaneous abortion with consumption of chemical sweeteners (aspartame, saccharin) or chocolate.95 Associations between maternal exposure to pesticides and adverse reproductive outcomes are speculative and remain inconclusive.108
EVALUATION FOR PREGNANCY LOSS
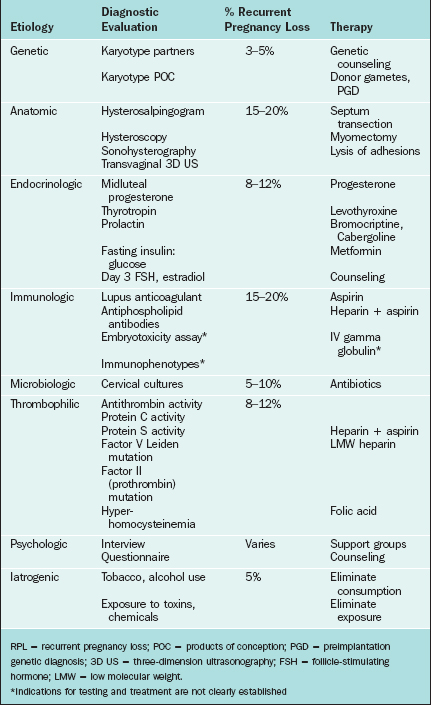
The evaluation of healthy women after a single loss is usually not recommended because this is a relatively common, sporadic event. The risk of another pregnancy loss after two miscarriages is only slightly lower (24% to 29%) than that of women with three or more spontaneous abortions.4,109 Therefore, evaluation and treatment can reasonably be started after two consecutive miscarriages. When the clinician makes the decision to initiate an evaluation for recurrent pregnancy loss, it is recommended that complete diagnostic testing be performed. This obviously includes a complete history, including documentation of prior pregnancies, any pathologic tests that were performed on prior miscarriages, any evidence of chronic or acute infections or diseases, any recent physical or emotional trauma, history of cramping or bleeding with a previous miscarriage, any family history of pregnancy loss, and any previous gynecologic surgery or complicating factor. A summary of the diagnosis and management of recurrent pregnancy loss includes an investigation of genetic, endocrinologic, anatomic, immunologic, microbiologic, iatrogenic, and thrombophilic causes (Table 41-2).
Genetic
Karyotyping of couples is part of the evaluation of recurrent pregnancy loss. Chromosomal abnormality in one of the parents can be found in up to 3% to 5% of couples who experience multiple spontaneous abortions. Cytogenetic analysis of aborted fetal material is also very important because this may have important prognostic implications and may direct future interventions. An abnormal karyotype is usually a sufficient explanation for a nonviable pregnancy and also may suggest karyotypic anomalies in the parents. The cytogenetic analysis of the aborted fetus depends on conventional tissue culturing and karyotyping. This technique is laborious and is subject to problems such as external contamination, culture failure, and selective growth of maternal cells. The efforts to develop methods to avoid such difficulties include the application of comparative genomic hybridization technology to recurrent pregnancy loss.110 This technique bypasses tissue culturing because it depends on DNA isolation from samples and provides a whole genome screen for chomosomal abnormalities.111
Endocrinologic
The diagnostic testing should include TSH and prolactin levels. Endometrial biopsy 10 days after LH surge or after day 24 of an idealized 28-day cycle is indicated for the diagnosis of luteal phase defect. The out-of-phase histology should be demonstrable in two consecutive cycles for the diagnosis of luteal phase defect. Recent data have challenged the entire concept of an endometrial biopsy for the diagnosis of a luteal phase disorder (see Chapter 8). In practice, many clinicians will use a midluteal progesterone level of al least 10 ng/mL to rule out a luteal phase defect. The fasting glucose-to-insulin ratio is recommended in patients with oligomenorrhea or with other signs and symptoms of PCOS. In obese PCOS patients and in patients with a glucose/insulin ratio less than 4.5, a 2-hour glucose tolerance test should be obtained. Evaluation of ovarian reserve is performed using day 3 FSH and estradiol levels. FSH over 15 mIU/mL and serum estradiol concentrations over 80 pg/mL on day 3 are associated with reduced oocyte numbers. Alternatively, clomiphene citrate challenge test (clomiphene citrate 100 mg daily administered on cycle days 5 to 9) may be used for evaluation of ovarian reserve. FSH levels are measured on day 3 and 10 and levels less than 15 mU/mL are considered normal.
Anatomic
In sexually active women, HSG is performed early in the follicular phase of the menstrual cycle after cessation of menses. Taking a nonsteroidal anti-inflammatory agent (ibuprofen 600 to 800 mg) 1 hour before the procedure has been shown to significantly decrease discomfort (see Chapter 29).
Modern imaging techniques can be used before hysteroscopy to determine the uterine contour.112 MRI and ultrasound have been used to measure uterine dimensions and are less invasive than laparoscopy. These techniques have the added potential advantage of being able to quantify the morphologic defect and estimate the likely success of surgical intervention. Three-dimensional ultrasound has been compared to HSG and laparoscopy in several studies and has shown a high level of agreement in the diagnosis of congenital uterine anomalies.113,114
Immunologic
An international consensus on classification criteria for definite antiphospholipid antibody syndrome was published in 2006.115 According to this criteria, one or more clinical and laboratory features must be present for the diagnosis of antiphospholipid antibody syndrome (Table 41-3). Clinical features must include one or more confirmed episodes of vascular thrombosis (venous, arterial, small vessel; or pregnancy complications (three or more consecutive spontaneous pregnancy losses at less than 10 weeks’ gestation, or one or more fetal deaths at greater than 10 weeks’ gestation, or one or more preterm births at less than 34 weeks’ gestation secondary to severe preeclampsia or placental insufficiency). Laboratory features should include positive plasma levels of anticardiolipin antibodies (IgG or IgM at greater than 40 phospholipid units) or positive plasma levels of lupus anticoagulant. The laboratory abnormalities should be present on two or more occasions at least 12 weeks apart.
Table 41-3 Clinical and Laboratory Characteristics of Antiphospholipid Antibody Syndrome
| Clinical | Laboratory |
|---|---|
Patients should have at least one clinical and one laboratory feature at some time in the course of their disease. Laboratory tests should be positive on at least two occasions more than 12 weeks apart.
GPL=IgG phospholipid units; MPL=IgM phospholipid units, PIH=pregnancy-induced hypertension.
Modified from Miyakis S, Lockshin MD, Atsumi T, et al: International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome. J Thromb Haemost 4:295–306, 2006.
Thrombophilia
TREATMENT OF RECURRENT PREGNANCY LOSS
The treatment of recurrent pregnancy loss should be directed at the cause. Given the good outcome for most couples with unexplained recurrent abortion in the absence of treatment, it is difficult to recommend unproven therapies, especially if they are invasive and expensive.44 Explanation and the appropriate emotional support are possibly the two most important aspects of therapy. In fact, in one study, antenatal counseling and psychological support for couples with recurrent abortion and no abnormal findings resulted in a pregnancy success rate of 86% compared with a success rate of 33% for women who were given no specific antenatal care.44
Genetic Causes
It is important to include genetic counseling as part of any treatment plan. The rate of recurrence of miscarriage often depends on the actual genetic abnormality discovered. Some couples with a known translocation or inversion can have a good pregnancy outcome. Others may need to turn to sperm or oocyte donation to avoid lethal abnormalities in their offspring.116 A recent development is preimplantation genetic diagnosis (PGD). This is a technique where biopsy of one or two cells from an in vitro embryo at the six- to eight-cell stage is performed for prenatal diagnosis of cytogenetic and mendelian disorders. Women who plan to utilize PGD must undergo IVF to conceive, even if infertility is not an issue for the couple. In some studies, PGD screening for aneuploidy has been shown to increase the implantation rate, lower the spontaneous loss rate, and likely improve pregnancy outcome in couples with recurrent pregnancy loss or IVF failure.117,118 Further investigation is required to confirm these preliminary findings.
Endocrine Abnormalities
The treatment of luteal phase defect is targeted at improving the endometrial responsiveness by enhancing the priming of the endometrium in the follicular phase. Various treatments have been described for luteal phase defect, including ovulation induction with clomiphene citrate or gonadotropins, hCG injection at the time of expected ovulation, and progesterone supplementation both during the luteal phase and first trimester of pregnancy.119,120 If the patient has a persistent luteal phase defect accompanied by hyperprolactinemia, bromocriptine, or cabergoline is recommended as a treatment option.121 It is unclear, however, if any of these treatments increase pregnancy rates in patients with recurrent miscarriage. A meta-analysis of randomized trials of pregnancies treated with progestational agents failed to find any evidence for a positive effect on the maintenance of pregnancy.122 Nevertheless, some physicians choose to administer progesterone either as intravaginal suppositories (50 to 100 mg twice daily starting the third day after LH surge and continuing for 8 to 10 weeks), as intramuscular injections (50 mg IM daily), or as orally administered micronized progesterone (100 mg orally two or three tablets daily). It should be mentioned that as many as 60% to 70% of women diagnosed with a luteal phase defect will carry a viable infant with the next pregnancy.
Immunologic Disorders
Antiphospholipid antibody syndrome treatments used for improving pregnancy outcome were originally directed toward suppressing the immune system but more recently have been directed to anticoagulation therapy. A combination of low-dose heparin (5000 to 10,000 units S.C. every 12 hours) appears to be effective and may reduce pregnancy loss by 54% in women with antiphospholipid syndrome.123 Aspirin alone does not appear to reduce miscarriage rates.124 A randomized, controlled trial comparing low-dose heparin (10,000 units S.C. every 12 hours) to prednisone (20 mg every 12 hours) found them to be equally effective in preventing pregnancy loss; however, treatment was not initiated until after the documentation of fetal heart. Moreover, because patients treated with prednisone experienced more complications (premature rupture of membranes and preeclampsia), it is not the preferred therapy.123 Initiating heparin before conception is potentially dangerous because of the risk of hemorrhage at the time of conception. Aspirin should be started preconceptually and heparin should be started after the first positive pregnancy test.125 Treatment should normally be continued until the time of delivery because these women are at an increased risk for thrombosis. Postpartum thromboprophylaxis is reasonable for a short interval to prevent thrombosis when the risk is high. The adverse reactions associated with heparin include bleeding, thrombocytopenia, and osteoporosis with fracture. Calcium (600 mg twice daily) with added vitamin D supplementation (400 IU twice daily) and weight-bearing exercise are encouraged to decrease the risk of osteoporosis. In any pregnant woman starting on heparin, the platelet count should be monitored weekly for the first 2 weeks and every 4 to 6 weeks thereafter.
Low molecular weight heparin (LMWH) appears to be a safe alternative to unfractionated heparin as an anticoagulant during pregnancy. It has a longer half-life, which results in a more predictable anticoagulant response and requires only one daily injection. The frequent monitoring with partial thromboplastin time is not needed, and there is a lower risk of osteoporosis and thrombocytopenia. LMWH does not cross the placenta and appears to be safe to the fetus.126 However, the optimal dose in the clinical setting of antiphospholipid antibody syndrome has not been determined.
Observational studies in patients with recurrent pregnancy loss have suggested that intravenous immunoglobulin (IVIG) may be effective.127 However, randomized trials have not confirmed this benefit; therefore, immunotherapy is not recommended for prevention of pregnancy loss at this time.123,128,129
Immunotherapy
Three primary types of immunotherapy have been described. Paternal leukocyte immunization was the first immunotherapy utilized to boost maternal immune recognition of the developing conceptus. The value of this treatment has never been proven, and its use is not recommended at this time. Trophoblast immune infusion is another form of active immunization that likewise has not been proven to confer any benefits in women with recurrent pregnancy loss. IVIG infusion is a passive form of immunotherapy that is used in an attempt to produce immunomodulatory effects, including neutralization of circulating antibodies, the inhibition of complement-mediated cytotoxicity, and the modulation of release of cytokines from lymphocytes.130 Although some randomized, double-blind studies have shown an increase in successful pregnancy outcomes, other have not confirmed these results.131–133 There is not yet firm evidence to suggest benefit from this form of treatment.134
A Cochrane review of 19 trials of various forms of immunotherapy did not show significant differences between treatment and control groups.135 Corticosteroids are known to suppress NK cell activity and also have anti-inflammatory effects. Oral corticosteroids for treatment of recurrent pregnancy loss have been used in the past but are not recommended because of uncertain efficacy and increase in pregnancy complications.67
Thrombophilia
Although the association between heritable thrombophilia and recurrent pregnancy loss is still disputed, several studies show that anticoagulation may improve both fetal and maternal outcome. In one study, 50 women with recurrent pregnancy loss and a variety of thrombophilic defects were treated with 40 to 120 mg of enoxaparin daily; in this group the chance of successful pregnancy outcome was 75%, which was significantly better than the 20% historical outcome in these women before the diagnosis of thrombophilia was made.136 In women without a history of prior thrombosis, prophylactic anticoagulation can be obtained with unfractionated heparin or LMWH.
Patients found to have elevated homocysteine levels should have appropriate supplementation with vitamin B6, vitamin B12, and folic acid prior to pregnancy, and conception can be attempted once homocysteine levels are normalized.137 Treatment should be continued throughout pregnancy and potentially lifelong to prevent thrombophilic events. There are no randomized, controlled trials to support this approach; however, the toxicity of this therapy is negligible and folic acid has proven value in reducing neural tube defects. Prophylactic heparin should be considered in patients with a history of thromboembolism, in those who have a homozygous MTHFR mutation, or patients with adverse pregnancy outcomes who are unresponsive to vitamin supplementation.
OUTCOME
In approximately 70% of all cases of recurrent pregnancy loss, a complete evaluation will reveal a possible etiology.2,138 Abnormal findings during the evaluation should be corrected before attempting any subsequent pregnancy. If no cause can be found, the majority of couples will eventually have a successful pregnancy outcome with supportive therapy alone.44,139 Once a pregnancy occurs, the patient should be monitored closely with evaluation of quantitative hCG levels at least twice and documentation of adequate progesterone levels. Early sonography should be scheduled and any encouraging results should be communicated to the couple. In women with a history of recurrent pregnancy loss, the presence of a normal embryonic heart rate between 6 and 8 gestational weeks is associated with a live birth rate of 82%.
Women who suffer recurrent pregnancy loss have already begun to prepare for their baby, both emotionally and physically, as compared to couples with infertility who have never conceived. When a miscarriage occurs, a couple may have great difficulty informing friends or family about the loss. Feelings of hopelessness may continue long after the loss. Patients may continue to grieve and have episodes of depression on the expected due date or the date of the pregnancy loss. Participation in support groups or referral for grief counseling may be beneficial in many cases (SHARE, Pregnancy and Infant Loss Support, Inc., www.national%20shareoffice.com).
1 Kutteh WH. Recurrent pregnancy loss. In: Rebar RW, editor. An update in Obstetrics and Gynecology. Washington, D.C.: American College of Obstetrics and Gynecology; 2002:151-161.
2 Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24-29.
3 Kutteh WH. Recurrent pregnancy loss. Semin Reprod Med. 2006;24:3-4.
4 Carson SA, Branch DW. Management of early recurrent pregnancy loss. ACOG Educ Bull. 2001;24:1-12.
5 Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673-675.
6 Scott JR. Recurrent miscarriage: Overview and recommendations. Clin Obstet Gynecol. 1994;37:768-773.
7 Clifford K, Rai R, Regan L. Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Hum Reprod. 1997;12:387-389.
8 Mills JL, Simpson JL, Driscoll SG. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. NEJM. 1998;319:1617-1623.
9 Stirrat GM. Recurrent miscarriage I: Definition and epidemiology. Lancet. 1990;336:673-675.
10 Nybo Andersen AM, Wohlfahrt J, Christens P. Maternal age and fetal loss: Population-based register linkage study. BMJ. 2000;304:1708-1712.
11 Scott JR. Recurrent miscarriage: Overview and recommendations. Clin Obstet Gynecol. 1994;37:768-773.
12 Harger JH, Archer DF, Marchese SG, et al. Etiology of recurrent pregnancy losses and outcome of subsequent pregnancies. Obstet Gynecol. 1983;62:574-581.
13 Quenby SM, Farquharson RG. Predicting recurring miscarriage: What is important? Obstet Gynecol. 1993;82:132-138.
14 Roman E. Fetal loss rates and their relationship to pregnancy order. J Epidemiol Community Health. 1984;38:29.
15 Hatasaka HH. Recurrent miscarriage: Epidemiologic factors, definitions, and incidence. Clin Obstet Gynecol. 1994;37:625-634.
16 Sierra S, Stephenson M. Genetics of recurrent pregnancy loss. Semin Reprod Med. 2006;24:17-24.
17 Boué A, Gallano P. A collaborative study of the segregation of inherited chromosome structural arrangements in 1356 prenatal diagnoses. Prenatal Diagn. 1984;1984:45-67.
18 Kirkels VG, Hustinx TW, Scheres JM. Habitual abortion and translocation (22q;22q): Unexpected transmission from a mother to her phenotypically normal daughter. Clin Genet. 1980;18:456-461.
19 Palmer CG, Schwartz S, Hodes ME. Transmission of a balanced homologous t(22q;22q) translocation from mother to normal daughter. Clin Genet. 1980;17:418-422.
20 Byrne JL, Ward K. Genetic factors in recurrent abortion. Clin Obstet Gynecol. 1994;37:693-704.
21 Lacassie Y, Arriaza MI, Vargas A, La Motta I. Ring 2 chromosome: Ten-year follow-up report. Am J Med Genet. 1999;85:122-177.
22 Boué A, Boué J, Gropp A. Cytogenetics of pregnancy wastage. Annu Rev Genet. 1985;14:1-57.
23 Stene J, Stene E, Mikkelsen M. Risk for chromosome abnormality at amniocentesis following a child with a non-inherited chromosome aberration. Prenatal Diagn. 1984;4:81-495.
24 Sachs ES, Jahoda MG, Los FJ, et al. Trisomy 21 mosaicism in gonads with unexpectedly high recurrence risks. Am J Med Genet. 1990;7:186-188.
25 Copp AJ. Death before birth: Clues from gene knockouts and mutations. Trends Gene. 1995;11:87-93.
26 Busque L, Mio R, Mattioli J, et al. Nonrandom X-inactivation patterns in normal females: Lyonization ratios vary with age. Blood. 1996;88:59-65.
27 Sangha KK, Stephenson MD, Brown CJ, Robinson WP. Extremely skewed X-chromosome inactivation is increased in women with recurrent spontaneous abortion. Am J Hum Genet. 1999;65:913-917.
28 Lanasa MC, Hogge WA, Kubik C, et al. Highly skewed X-chromosome inactivation is associated with idiopathic recurrent spontaneous abortion. Am J Hum Genet. 1999;65:252-254.
29 Pegoraro E, Whitaker J, Mowery-Rushton P, et al. Familial skewed X inactivation: A molecular trait associated with high spontaneous-abortion rate maps to Xq28. Am J Hum Genet. 1997;61:160-170.
30 Insler V. Corpus luteum defects. Curr Opin Obstet Gynecol. 1992;4:203-211.
31 Tuckerman E, Laird SM, Stewart R, et al. Markers of endometrial function in women with unexplained recurrent pregnancy loss. Hum Reprod. 2004;19:196-205.
32 Jacobs MH, Balasch J, Gonzalez-Merlo JM. Endometrial cytosolic and nuclear progesterone receptors in the luteal phase defect. J Clin Endocrinol Metab. 1987;64:472-478.
33 Cumming DC, Honore LH, Scott JZ, Williams KP. The late luteal phase in infertile women: Comparison of simultaneous endometrial biopsy and progesterone levels. Fertil Steril. 1985;43:715-719.
34 Shepard MK, Senturia YD. Comparison of serum progesterone and endometrial biopsy for confirmation of ovulation and evaluation of luteal function. Fertil Steril. 1977;28:541-548.
35 Ghazeeri GS, Clark DA, Kutteh WH. Immunologic factors in implantation. Infertil Reprod Med Clin North Am. 2001;12:315-337.
36 Kutteh WH, Yetman DL, Carr AC, et al. Increased prevalence of antithyroid antibodies identified in women with recurrent pregnancy loss but not in women undergoing assisted reproduction. Fertil Steril. 1999;71:843-848.
37 Mills JL, Simpson JL, Driscoll SG, et al. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. NEJM. 1998;319:1617-1623.
38 Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril. 1998;69:682-690.
39 Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. 2002;78:487-490.
40 Sills ES, Perloe M, Palermo GD. Correction of hyperinsulinemia in oligo-ovulatory women with clomiphene-resistant polycystic ovary syndrome: A review of therapeutic rationale and reproductive outcomes. Eur J Obstet Gynecol Reprod Biol. 2000;91:135-141.
41 Hofmann GE, Khoury J, Thie J. Recurrent pregnancy loss and diminished ovarian reserve. Fertil Steril. 2000;74:1192-1195.
42 Dlugi AM. Hyperprolactinemic recurrent spontaneous pregnancy loss: A true clinical entity or a spurious finding? Fertil Steril. 1998;70:253-255.
43 Hirahara F, Andoh N, Sawai K, et al. Hyperprolactinemic recurrent miscarriage and results of randomized bromocriptine treatment trials. Fertil Steril. 1998;70:246-252.
44 Stray-Pedersen B, Stray-Pedersen S. Etiologic factors and subsequent reproductive performance in 195 couples with a prior history of habitual abortion. Am J Obstet Gynecol. 1984;148:140-146.
45 Propst AM, Hill JA3rd. Anatomic factors associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:341-350.
46 Kaufman RH, Adam E, Binder GL, Gerthoffer E. Upper genital tract changes and pregnancy outcome in offspring exposed in utero to diethylstilbestrol. Am J Obstet Gynecol. 1980;137:299-308.
47 Goldberg GM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril. 1999;72:1-7.
48 Stovall DW, Parrish SB, Van Voorhis BJ, et al. Uterine leiomyomas reduce the efficacy of assisted reproduction. Hum Reprod. 1998;13:192-197.
49 Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization–embryo transfer cycle outcome. Fertil Steril. 2001;75:405-419.
50 Devi Wold AS, Pham N, Arici A. Anatomic factors in recurrent pregnancy loss. Semin Reprod Med. 2006;24:25-32.
51 Vince GS, Christmas SE, Johnson PM. Understanding cellular and humoral immunity. Infertil Reprod Med Clin North Am. 2002;13:1-17.
52 Mor G. Immunology of implantation. Infertil Reprod Med Clin North Am. 2002;13:113-128.
53 Kutteh WH. Immunology of multiple endocrinopathies associated with premature ovarian failure. Endocrinologist. 1996;6:462-466.
54 Franklin RD, Kutteh WH. Antiphospholipid antibodies (APA) and recurrent pregnancy loss: Treating a unique APA positive population. Hum Reprod. 2002;17:2981-2985.
55 Kutteh WH, Lyda EC, Abraham SM, Wacholtz MC. Association of anticardiolipin antibodies and pregnancy loss in women with systemic lupus erythematosus. Fertil Steril. 1993;60:449-455.
56 Petri M, Allbriton J. Fetal outcome of lupus pregnancy: A retrospective case-control study of the Hopkins Lupus Cohort. J Rheumatol. 1993;20:650-656.
57 Fausett MB, Branch DW. Autoimmunity and pregnancy loss. Semin Reprod Med. 2000;18:379-392.
58 Ogasawara M, Aoki K, Hayashi Y. A prospective study on pregnancy risk of antiphospholipid antibodies in association with systemic lupus erythematosus. J Reprod Immunol. 1995;28:159-164.
59 Out HJ, Bruinse HW, Christians CML, et al. A prospective, controlled multicenter study of the obstetric risks of pregnant women with antiphospholipid antibodies. BJOG. 1992;167:26-32.
60 Wu S, Stephenson MD. Obstetrical antiphospholipid syndrome. Semin Reprod Med. 2006;24:40-53.
61 Rand JH. The antiphospholipid syndrome. Annu Rev Med. 2003;54:409-424.
62 Rand JH, Wu XX, Guller S, et al. Reduction of annexin-V (placental anticoagulant protein-I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171:1566-1572.
63 Shibata S, Harpel PC, Gharavi A, et al. Autoantibodies to heparin from patients with antiphospholipid antibody syndrome inhibit formation of antithrombin III–thrombin complexes. Blood. 1994;83:2532-2540.
64 Di Simone N, Ferrazani S, Castellani R, et al. Heparin and low-dose aspirin restore placental human chorionic gonadotropin secretion abolished by antiphospholipid antibody containing sera. Hum Reprod. 1997;12:2061-2065.
65 Rushworth FH, Backos M, Rai R, et al. Prospective pregnancy outcome in untreated recurrent miscarriers with thyroid autoantibodies. Hum Reprod. 2000;15:1637-1639.
66 Esplin MS, Branch DW, Silver R, Stagnaro-Green A. Thyroid autoantibodies are not associated with recurrent pregnancy loss. Am J Obstet Gynecol. 1998;179:1583-1586.
67 Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. NEJM. 1997;337:148-153.
68 Ober C, Karrison T, Odem RR, et al. Mononuclear-cell immunisation in prevention of recurrent miscarriages: A randomised trial. Lancet. 1999;354:365-369.
69 Laird SM, Tuckerman EM, Cork BA, et al. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003;9:163-174.
70 King A, Hiby SE, Gardner L, et al. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors—a review. Placenta. 2000;21(SupplA14):S81-S85.
71 Tafuri A, Alferink J, Moller P, et al. T cell awareness of apternal alloantigens during pregnancy. Science. 1995;270:630-633.
72 Penta M, Lukic A, Conte MP, et al. Infectious agents in tissues from spontaneous abortions in the first trimester of pregnancy. New Microbiol. 2003;26:329-337.
73 Kutteh WH, Triplett DA. Thrombophilias and recurrent pregnancy loss. Semin Reprod Med. 2006;24:54-65.
74 Kovalevsky G, Gracia CR, Berlin JA, et al. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss: A meta-analysis. Arch Intern Med. 2004;164:558-563.
75 Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: A meta-analysis. Lancet. 2003;361:901-908.
76 Regan L, Rai R. Thrombophilia and pregnancy loss. J Reprod Immunol. 2002;55:163-180.
77 Bertina RM, Loelman BP, Koster T, Rosendaal FR. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64-67.
78 Krabbendam I, Francks A, Bots ML, Fijnheer R, Bruinse HW. Thrombophilias and recurrent pregnancy loss: A critical appraisal of the literature. Eur J Obstet Gynecol Reprod Biol. 2005;118:143-153.
79 Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene and the risk for arterial thrombotic disease. Blood. 1996;88:3698-3703.
80 Welch G, Loscalzo J. Homocysteine and artherothrombosis. NEJM. 1998;338:1042-1051.
81 Bonnette RE, Caudill MA, Boddie AM, et al. Plasma homocysteine concentrations in pregnant and non-pregnant women with controlled folate intake. Obstet Gynecol. 1999;92:167-170.
82 Frosst P, Blom HG, et al. MR: A candidate genetic risk factor for vascular disease: A common mutation in the methylene tetrahydrofolate reductase. Nature Genet. 1995;10:111-113.
83 Jacques P, Bostom A, Williams R. Relation between folate status and a common mutation in the methylenetetrahydofolate reductase and plasma homocysteine concentrations. Circulation. 1996;93:7-9.
84 Nelen WL, Bulten J, Steegers EA, et al. Maternal homocysteine and chorionic vascularization in recurrent early pregnancy loss. Hum Reprod. 2000;15:954-960.
85 Nelen WL, Blom HJ, Steegers EA, et al. Hyperhomocysteinemia and recurrent early pregnancy loss: A meta-analysis. Fertil Steril. 2000;74:1196-1199.
86 Tait R, Perry D, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87:106-112.
87 Adelberg AM, Kuller JA. Thrombophilias and recurrent miscarriage. Obstet Gynecol Survey. 2002;57:703-709.
88 Preston FE, Rosendaal FR, Walker ID, et al. Increased fetal loss in women with heritable thrombophilia. Lancet. 1996;348:913-916.
89 Coumans AB, Huijgens PC, Jakobs C, et al. Haemostatic and metabolic abnormalities in women with unexplained recurrent abortion. Hum Reprod. 1999;14:211-214.
90 Sarig G, Younis JS, Hoffman R, et al. Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage. Fertil Steril. 2002;77:342-347.
91 De Braekeleer, Dao TN. Cytogenetic studies in male infertility: A review. Hum Genet. 1991;6:245-250.
92 Lopes S, Sun JG, Juriscova A, et al. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with fertilisation in ICSI. Fertil Steril. 1998;69:528-532.
93 Sakkas D, Turner F, Bizzaro D, et al. Sperm nuclear DNA damage and altered chromatin structure: Effect on fertilisation and embryo development. Hum Reprod. 1998;13(Suppl4):11-19.
94 Gopalkrishnan K, Padwal V, Meherji PK, et al. Poor quality of sperm as it affects repeated early pregnancy loss. Arch Androl. 2000;45:111-117.
95 Gardella JR, Hill JA3rd. Environmental toxins associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:407-424.
96 Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2:173-178.
97 Kline J, Shroat P, Stein ZA, et al. Drinking during pregnancy and spontaneous abortion. Lancet. 1980;2:176-180.
98 Ness RB, Grisso JA, Hrischinger N, et al. Cocaine and tobacoo use and the risk of spontaneous abortion. NEJM. 1999;340:333-339.
99 Dlugosz L, Bracken MB. Reproductive effects of caffeine: A review and theoretical analysis. Epidemiol Rev. 1992;4:83-100.
100 Miller RW. Effects of ionizing radiation from the atomic bomb on Japanese children. Pediatrics. 1968;41:257.
101 Yamazaki JN, Schull WH. Perinatal loss and neurological abnormalities among children of the atomic bomb: Nagasaki and Hiroshima revisited, 1949 to 1989. JAMA. 1990;264:605-609.
102 Brent RL. The effects of embryonic and fetal exposure to x-ray, microwaves, and ultrasound. Clin Perinatol. 1986;13:615-648.
103 McMartin KI, Chu M, Kopecky E, et al. Pregnancy oucome following maternal organic solvent exposure: A meta-analysis of epidemiologic studies. Am J Indust Med. 1998;34:288-292.
104 Burbacher TM, Monett C, Grant KS, Mottet NK. Methylmercury exposure and reproductive dysfunction in the nonhuman primate. Toxicol Applied Pharmacol. 1984;75:18-24.
105 Brodsky JB, Cohen EN, Whitcher C, et al. Occupational exposure to mercury in dentistry and pregnancy outcome. J Am Dental Assoc. 1985;111:779.
106 CDC. Preventing lead poisoning in young children in the United States. MMWR. 1985;34:66.
107 Schnorr TM, Grajewski BA, Hornung RW, et al. Video display terminals and the risk of spontaneous abortion. NEJM. 1991;325:811-813.
108 Nurminen T. Maternal pesticide exposure and preganancy outcome. J Occupation Environ Med. 1995;37:935-940.
109 Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673-675.
110 Daniely M, Aviram-Goldring A, Barkai G, Goldman B. Detection of chromosomal aberration in fetuses arising from recurrent spontaneous abortion by comparative genomic hybridization. Hum Reprod. 1998;13:805-809.
111 Bryndorf T, Kirchhoff M, Maahr J, et al. Comparative genomic hybridization in clinical cytogenetics. Am J Hum Genet. 1995;57:1211-1220.
112 Salim R, Jurkovic D. Assessing congenital uterine anomalies: The role of three-dimensional ultrasonography. Best Pract Res Clin Obstet Gynaecol. 2004;18:29-36.
113 Jurkovic D, Geipel A, Gruboeck K, et al. Three-dimensional ultrasound for the assessment of uterine anatomy and detection of congenital anomalies: A comparison with hysterosalpingography and two-dimensional sonography. Ultrasound Obstet Gynecol. 1995;5:233-237.
114 Raga F, Bonilla-Musoles F, Blanes J, Osborne NG. Congenital Müllerian anomalies: Diagnostic accuracy of three dimensional ultrasound. Fertil Steril. 1996;65:523-528.
115 Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
116 Munne S, Morrison L, Fung J, et al. Spontaneous abortions are reduced after preconception diagnosis of translocations. J Assist Reprod Genet. 1998;15:290-296.
117 Gianaroli L, Magli MC, Ferraretti AP, et al. The role of preimplantation diagnosis for aneuploidies. Reprod Biomed Online. 2002;4(Suppl 3):31-36.
118 Rubio C, Simon C, Vidal F. Chromosone abnormalities and embryo development in recurrent miscarriage couples. Hum Reprod. 2003;18:182-188.
119 Karamardian LM, Grimes S. Luteal phase deficiency: Effect of treament on pregnancy rates. Am J Obstet Gynecol. 1992;167:1391-1398.
120 Kutteh WH. Recurrent Pregnancy Loss. Stamford, Conn.: Appleton and Lange, 1998.
121 Roberts CP, Murphy AA. Endocrinopathies associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:357-362.
122 Goldstein P, Berrier J, Rosen S, et al. A meta-analysis of randomized control trials of progestational agents in pregnancy. BJOG. 1989;96:265-274.
123 Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: A systematic review of therapeutic trials. Obstet Gynecol. 2002;99:135-144.
124 Pattison NS, Chamley LS, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183:1008-1012.
125 Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: Treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol. 1996;174:1584-1589.
126 Silver RM, Branch DW. Immunologic Disorders. Philadelphia: WB Saunders Co, 1999.
127 Vaquero E, Lazzarin N, Valensise H, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: A comparative study of intravenous immunoglobulin versus prednisone plus low-dose aspirin. Am J Reprod Immunol. 2001;45:174-179.
128 Branch DW, Porter TF, Paidas MJ, et al. Obstetric uses of intravenous immunoglobulin: Successes, failures, and promises. J Allergy Clin Immunol. 2001;108(4 Suppl):S133-S138.
129 Triolo G, Ferrante A, Ciccia F, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis Rheum. 2003;48:728-731.
130 Li TC, Makris M, Tomsu M, et al. Recurrent miscarriage: Aetiology, management and prognosis. Hum Reprod Update. 2002;8:463-481.
131 Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. Am J Reprod Immunol. 1995;34:333-337.
132 Jablonowska B, Selbing A, Palfi M, et al. Prevention of recurrent spontaneous abortion by intravenous immunoglobulin: A double-blind placebo-controlled study. Hum Reprod. 1999;14:838-841.
133 Stephenson MD, Dreher K, Houlihan E, Wu V. Prevention of unexplained recurrent spontaneous abortion using intravenous immunoglobulin: A prospective, randomized, double-blinded, placebo- controlled trial. Am J Reprod Immunol. 1998;39:82-88.
134 Daya S, Gunby J, Porter F, et al. Critical analysis of intravenous immunoglobulin therapy for recurrent miscarriage. Hum Reprod Update. 1999;5:475-482.
135 Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2003. CD000112.
136 Brenner B, Hoffman R, Blumenfeld Z, et al. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated by enoxaparin. Thromb Haemost. 2000;83:693-697.
137 Quere I, Mercier E, Bellet H, et al. Vitamin supplementation and pregnancy outcome in women with recurrent early pregnancy loss and hyperhomocysteinemia. Fertil Steril. 2001;75:823-825.
138 Jaslow CR, Carney J, Norman L, Fong S, Ke RW, Kutteh WH. Etiology of recurrent pregnancy loss in 1018 women. Fertil Steril. 2006;86:S472.
139 Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod. 1999;14:2868-2871.