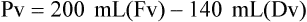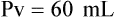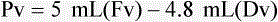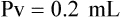Reconstitution of Powders or Crystals into Liquid Medications
• Read labels of powders or crystals (lyophilized) medications to determine correct diluent and correct volume necessary to reconstitute powders
• Check labels for expiration dates and storage conditions before and after reconstitution of solid medication to a liquid form
• Understand the importance of labeling reconstituted medications with date, time, and initials of person performing the medication reconstitution
• Determine the appropriate amount of diluent necessary when using a single-dose container of a powder or crystals
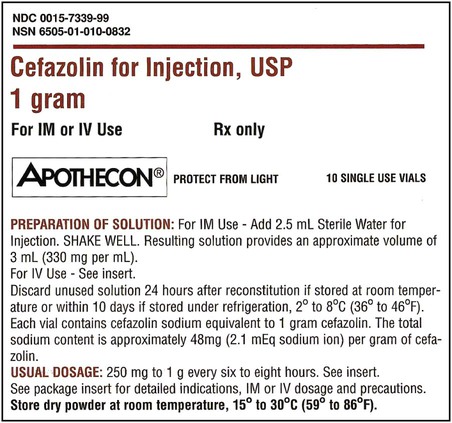
• Determine the appropriate amount of diluent necessary when preparing a multidose container of a powder or crystals
• Determine the appropriate dilution concentration when more than one dosage strength in the multidose container is possible, and then determine amount of diluent necessary to meet desired concentration
• Calculate the amount of medication of a reconstituted medication to be dispensed to meet the physician’s order
Reconstitution of Powders into Liquid Medications
Most oral preparations are found in bottles that are larger than the medication volume present. This allows space for shaking medications before administration. Because the usual vehicle for reconstitution of oral medications is distilled water, graduates are used to measure the quantity of liquid to be added (Figure 9-1). In some pharmacies, a computerized dispenser for distilled water, such as a Fillmaster electronic pharmaceutical water dispenser, is available. When indicating the correct amount of water to be dispensed, this machine provides one half of the necessary diluent, stops and allows time for mixing the powder medication with the diluent, and then dispenses the remainder of the water on demand.
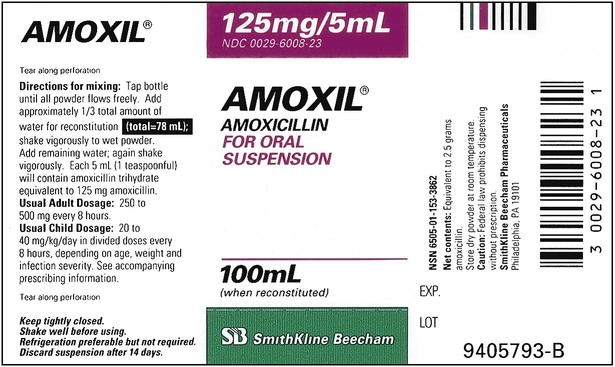
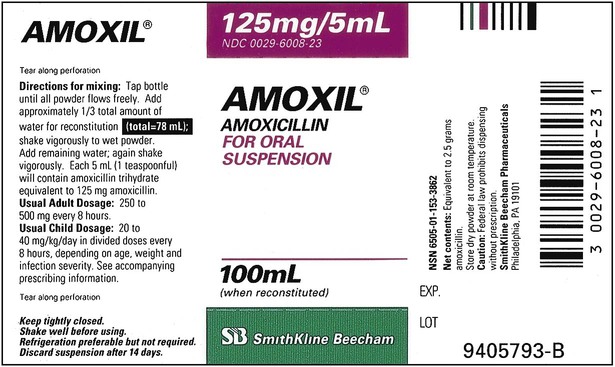
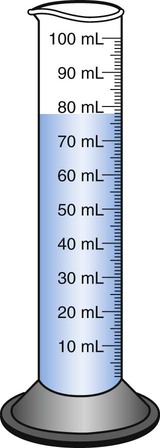
When powders are dissolved in the liquid, the weight or strength of the medication will always remain the same as the amount given on the label. For example, if a label for amoxicillin states that the container holds a total of 2.5 g as a powder, the total weight or strength of the medication will be 2.5 g after reconstitution has occurred. When medications are supplied in dry powder or crystalline form for stability, the space occupied by the powder is known as powder volume or powder displacement. The total liquid volume of the medication will be that of the amount of medication plus the amount of liquid. In the label shown in Figure 9-2, the final volume of the bottle is 100 mL when reconstituted with 78 mL of water (Figure 9-3). So with this reconstitution the powder volume or displacement is 22 mL (100 mL total volume − 78 mL diluent = 22 mL powder volume). Powder volume (Pv) is the difference between the final volume (Fv) and the diluent volume (Dv). This can be shown with the formula Pv = Fv − Dv.
Basic Principles of Reconstitution
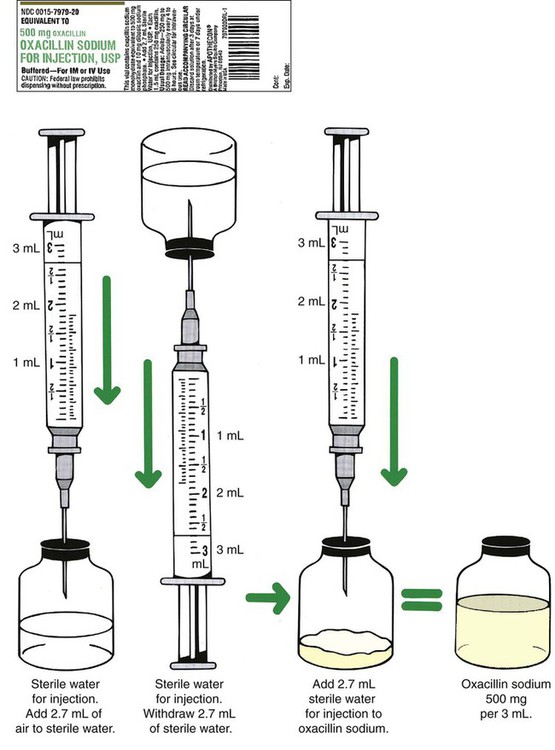
The usual diluent for mixing a powder for an oral medication is either distilled water or, if different, as indicated by the manufacturer. The diluent with injectable medications may vary with each drug. Some injectable powders come with the diluent in two separate chambers, one for the powder and another for the diluent as with Solu-Medrol (methylprednisolone sodium succinate) or hydrocortisone sodium succinate (Figure 9-4). This system is called the Act-O-Vial system. In this case when the plunger is dislodged by pushing, the diluent drops from the upper chamber into the lower chamber which contains the powder. In other cases when a special diluent is necessary, this diluent may be packaged separately and supplied with the medication such as with some immunization agents. In these cases, finding the designated diluent is not necessary, because the manufacturer has included it.

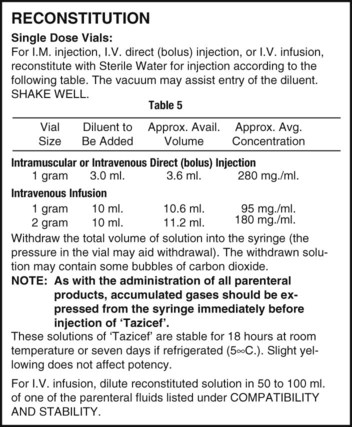
Once the label has been read and the diluent of choice is known, the amount of diluent for the desired dosage must be verified. In single-dose vials, the amount of diluent will be one volume because a single dose will be given. In multidose vials, multiple instructions for different strengths following reconstitution may be possible and will be indicated on the label, each showing the amounts necessary for reconstituting to a particular strength (Figure 9-5). Read the possible concentrations or strengths following reconstitution, because typically the desired strength per volume should be the closest to the amount of the order and should also match the intended route of administration. Decide on the amount of diluent that will be added and prepare this for use. Remember that when adding diluent, the less fluid added to a specific container of medication, the more concentrated the medication—or “less is more” (Figure 9-6).
• The expiration date and time for injectable medications or length of time before expiration with oral medications
• The dose strength or final concentration after it has been mixed (e.g., 250 mg/5 mL) is important when preparing the medication dose for administration
Remember that the final volume of medication in the container will always be greater than the amount of diluent added because of powder volume. Thus the total volume prepared should always be checked to ensure there is an adequate amount for dispensing of the prescription or order; if not, be sure the correct medication strength has been chosen and reconstituted as required to meet the order. Usually the medication label also gives the total volume of medication after mixing according to indicated directions (e.g., see Figure 9-2, in which the label for Amoxil indicates the total amount of reconstituted medication is 100 mL).
Review
Several steps are necessary in preparing reconstituted medications:
1. Read all of the directions on the medication label before starting the procedure.
2. Tap the bottle to loosen the powder in the bottle or vial.
3. Use the diluent that is designated by the manufacturer in the amount that is appropriate for the concentration or strength of medication necessary for the physician’s order. If this information is not on the vial or bottle, use a drug reference for the appropriate diluent and amount.
4. After reconstitution of injectable label the medication with the initials of the person who reconstituted the medication, the date and time of reconstitution, the strength of the medication, and the expiration date and time for oral medications, be sure the time of expiration is excluded on the container label.
5. Remember that the final volume of the reconstituted powder will always be greater than the amount of diluent added because of the powder volume (Pv = Fv − Dv).
Review of Rules
Reconstitution of Powdered Medications
• Before reconstituting any powdered medication for dispensing, carefully read all directions on the medication.
• Use the exact diluent designated by the manufacturer in the amount that is appropriate for the desired concentration or strength of medication. If this information is not available on the label, use a drug reference for the appropriate diluent and amount. Be sure that the diluent does not have an expiration date that falls before its use date.
• After reconstitution, label the medication with the initials of the person who did the reconstitution, the strength of the medication as reconstituted, and the expiration date and time for parenteral medications. For reconstituted oral medications, the length of time before expiration or the date of expiration should be noted.
• Remember that the volume of medication after reconstitution will always be greater than the diluent added because of powder volume.
• Powder volume may be calculated by subtracting diluent volume from the total or final volume (Pv = Fv − Dv).