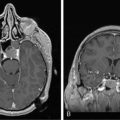
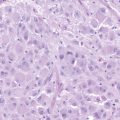
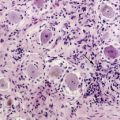
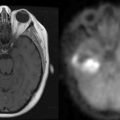
2 Recent Advances in Epidemiology of Brain Tumors
Refinements in Histological Categorization of Brain Tumors and Associated Developments
The International Classification of Diseases for Oncology, Third Edition (ICD-O-3) is the standard classification system for the registration of cancers in the United States (and most areas of the world) and contains widely-accepted histologic categories of brain tumors. Brain tumors are classified into the following major histologic groupings: tumors of neuroepithelial tissue (hereafter referred to as glioma, including astrocytoma [grade II], anaplastic astrocytoma [grade III], glioblastoma [grade IV], oligodendroglioma, and ependymoma), tumors of meninges (including meningioma and hemangioblastoma), germ cell tumors, and tumors of the sellar region (including pituitary tumors and craniopharyngioma). Glioma is the most common histological category, followed by meningioma, the category that includes the highest proportion of benign brain tumors (approximately 96% of meningiomas are considered benign).1 Criteria for classifying brain tumors have varied substantially across time and geographic region. Prior to the past decade, most epidemiologic studies presented results for crude categories such as “all central nervous system (CNS) tumors” or “brain tumors.” It is now well established that both the descriptive and analytic epidemiology of brain tumors varies considerably according to histologic grouping. A meaningful contribution of the Central Brain Tumor Registry of the United States (CBTRUS), which collects information on brain tumors occurring among residents of 19 U.S. states, has been the presentation of descriptive statistics according to categories detailed in the ICD-O-3.1
Progress in Understanding the Descriptive Epidemiology of Brain Tumors
Glioma incidence rates increased during the 1970s and 1980s (probably reflecting the use of new diagnostic imaging technologies2) and have remained relatively stable since the 1980s. Incidence rates for high grade gliomas among older age groups have increased over time from the late 1970s to the early 1990s, as have those for oligodendroglioma and mixed tumor histologies at the expense of less specific histologies.2 A similar description of American meningioma incidence rates over time is not possible, because benign brain tumors were only recently required to be reported to American central cancer registries. However, Klaeboe et al.3 report an increase in European meningioma incidence rates, which is also probably explained by new imaging technology introduced in the 1970s.
For all brain and CNS tumors combined, benign and malignant (brain tumors accounting for approximately 88%), the age-adjusted average annual (2000 to 2004) incidence rate for females (17.2 per 100,000 females) is greater than that for males (15.8 per 100,000 males).1 Table 2-1 shows age-adjusted average annual (2000 to 2004) incidence rates and median ages at diagnosis for the major histologic groupings and their selected common histologic subtypes of brain tumors.1 As shown in Table 2-1, men have higher incidence rates of gliomas, germ cell tumors and cysts, while women have a higher incidence rate of meningiomas. As described in a following section, results from analytic studies concerning reproductive and hormonal factors have suggested compelling possible explanations for the sex difference in glioma and meningioma risks.
TABLE 2-1 Number of Cases, Median Ages at Diagnosis and Age-Adjusted Average Annual (2000-2004) Incidence Ratesa of Primary Brain Tumors (Major Histologic Groupings and Selected Histologic Subtypes), According to Sex.
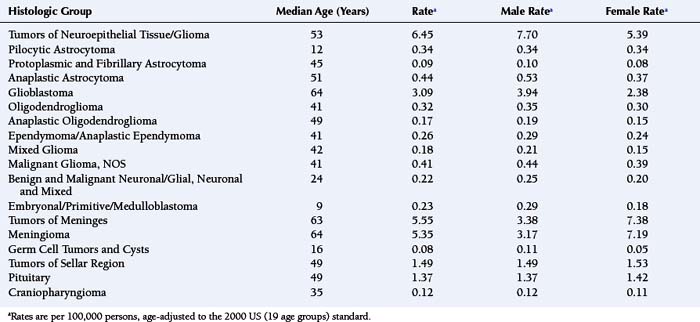
Newly diagnosed glioma is approximately two times more common among whites than among blacks, as are germ cell tumors. In contrast, meningioma incidence rates are similar between whites and blacks.1 Although there is no well-accepted mechanism for the race differences in glioma risk, differences in the distribution of human leukocyte antigens (HLA) among races, described later in the chapter, may explain it. In the United States, for diagnoses made between 2000 and 2004, the median age at diagnosis of a primary brain tumor was 57 years.1 For all major histologic groups except germ cell tumors and cysts, incidence rates increase with advancing age.1 Rates of cancers of nearly every anatomic site increase with advancing age; still, the reason for this increase is not known.
Brain tumor incidence rates vary moderately by geographic region in the United States and throughout the world.1,4 However, differences in diagnostic practices, completeness of reporting, access to and quality of health care, make geographic and especially international comparisons difficult to interpret.5,6
Brain tumor survival time varies greatly by histologic type and age at diagnosis. In every adult age group, the lowest relative 2-year survival is found for patients with glioblastoma multiforme (GBM), ranging from 30.4% among those aged 20 to 44 years to 1.3% among those aged 75 years and older.1 In general, within histologic types, survival time decreases with advancing age. The mechanisms for the strong and consistent inverse association between age and survival are poorly understood and deserve further exploration. Among adult (ages 20 years and older) patients diagnosed with primary malignant brain tumors between 2000 and 2005, only 32.5% and 23.7% survived 2 and 5 years, respectively, from the time of diagnosis.4 Although there has been little change in the poor survival rates for patients diagnosed with GBM, for adult (ages 20 years and older) patients diagnosed with all malignant brain tumors combined, 2-year survival has increased from 21.7% in 1975 to 31.9% in 2004.4 Among patients diagnosed with glioma, the largest improvements in survival occurred among patients with earlier stage glioma and those who were younger than 65 years of age. Population-based data from Norway and Finland suggest that survival for patients diagnosed with meningioma also improved between the 1950s and 1990s.7,8 McCarthy et al.9 estimate 5-year survival of 69% for meningioma, with those younger at diagnosis and those with benign meningioma having a more favorable prognosis.9 It is likely that improvements in imaging technology, which allow the earlier identification of tumors, explain the progress for both glioma and meningioma. GBM probably has only a brief preclinical period; therefore, there may be little opportunity for technology-associated improvement in GBM survival. In the past decade, there has been very little improvement in 2-year survival among adults diagnosed with a primary malignant brain tumor (1.4% difference between the 2-year survival among patients diagnosed in 1994 and those diagnosed in 2004). The only relatively important difference in length of survival following GBM diagnosis, in the past decade, has resulted from concomitant addition of, and maintenance with, the chemotherapeutic agent, Temodar, which has improved median survival time for GBM patients by only 2.5 months.10
Advances in Identifying Prognostic Factors for Brain Tumors
Previous research has shown that GBM prognosis is associated with the following factors: age, marital status, tumor size, Karnofsky Performance Scale (KPS) score, patient condition before radiation therapy, degree of necrosis, enhancement on preoperative magnetic resonance imaging studies, therapeutic approach including extent of resection and capacity for complete resection, volume of residual disease, location of tumor, patient deterioration, presurgical serum albumin level, and persistent hyperglycemia 1 to 3 months following surgical resection.11–16
Most recent advances in the search for prognostic factors related to GBM and other high-grade gliomas have focused on molecular or serologic factors or on inherited genetic variation. Loss of heterozygosity (LOH) on chromosome 10q has been associated with shorter duration of survival from GBM,17,18 and the combined LOH on 1p and 19q may afford a more favorable prognosis to GBM patients.18 For oligodendroglioma, it is now well established that the combined tumor loss of 1p and 19q confers a more favorable prognosis.19 Results submitted by Yang et al. showed that two genotypes associated with the 19q deletion region, GLTSCR1-exon-1 and ARCC2-exon-22, are independent predictors of glioma survival.20 Results reported in the past decade provide evidence for the following as prognostic indicators for GBM and other glioma subtypes: p53 mutation and expression,18,21–29 overexpression or amplification of epidermal growth factor receptor (EGFR),22,24,25,27–29 CDKN2A alterations and deletions,22,24,27 and MDM2 amplifications.21,24,27,29,30 For example, EGFR expression is associated with nearly three-fold poorer survival among anaplastic astrocytoma patients.29 In addition, Wrensch et al.29 reported that glutathione-S-transferase (GST) theta (T)1 deletion afforded a less favorable prognosis for glioma patients, while higher survival is afforded to glioma patients with the ERCC1 (a DNA excision repair gene) C8092A polymorphism.
Recently, we have learned that p53 protein expression probably decreases with advancing age,22,31 and investigators have reported interactions between age and molecular prognostic factors. For example, Simmons et al.31 showed that there was a shorter survival time among younger patients whose tumors overexpressed EFGR but had normal p53 immunohistochemistry.31 Age-dependent associations between GBM survival and 1p and CDKN2A have also been identified.22
GBM survival time varies with change in the MNS16A human telomerase polymorphism. Median survival time is 24.7 months for the SS-genotype, compared to 14.0 months and 13.1 months for the SL- and LL-genotypes respectively.32 These results suggest that MNS16A may be used as a biomarker of treatment success.
In order to develop and progress, brain tumors must evade anti-tumor immunity. Recently, several immunological factors have been implicated in glioma prognosis. GBM patients with elevated immunoglobulin (Ig) E live an average of 9 months longer than do patients with lower or normal IgE levels.29 Furthermore, amplification of interleukin (IL)-6, a cytokine which stimulates immune response, is significantly associated with decreased GBM survival time.33
Prognostic factors for meningioma patients have not been as thoroughly evaluated as those for glioma, perhaps because meningioma patients have more favorable prognoses. The recent requirement of central cancer registries to report benign brain tumors may increase our knowledge of demographic and geographic factors related to meningioma prognosis. Generally, younger meningioma patients survive longer than do older patients. A large study revealed that age, tumor size, and surgical and radiation treatments were associated with benign meningioma survival; however, only age and surgical and radiation treatments were associated with malignant meningioma survival.9 Loss of tumor suppressor in lung cancer-1 (TSLC1) protein and abnormalities of chromosome 14 have been associated with meningioma prognosis.34,35 We know little about the molecular and genetic factors that determine meningioma prognosis, but studies of such characteristics are being conducted at the present time.
Established Environmental Causal Factors for Brain Tumors
There are consistent and strong results from prospective studies of people exposed to ionizing radiation, providing unquestionable evidence of a linear dose-response association between ionizing radiation exposure and glioma risk.36 Exposure to ionizing radiation comes from therapeutic and diagnostic medical procedures, occupation, atmospheric testing of nuclear weapons, natural sources, industrial accidents, and atomic bomb explosions. Therapeutic doses of ionizing radiation probably contribute to the development of only a small proportion of brain tumors, as exposure to therapeutic levels of ionizing radiation is rare.37 Atomic bomb survivors have higher incidence rates of glioma and meningioma, as well as of schwannoma and pituitary tumors,38 and meningioma risk increases with estimated radiation dose to the brain.39 Ionizing radiation used to treat conditions such as tinea capitis in infants and children is associated with elevated relative risks for both glioma and meningioma, along with nerve sheath tumors and pituitary adenoma.38,40,41 There are mixed results pertaining to diagnostic and therapeutic x-rays of the head and neck.42–45 Second primary brain tumors occur more frequently than expected among patients previously treated for brain tumors with radiation therapy46; however, these results are confounded by the fact that people with higher grade tumors are more likely both to receive radiation and to have a recurrence. Future studies should consider the potential for underreporting of ionizing radiation exposure and imprecise estimates of age at first exposure.36 There may be interaction between ionizing radiation and both age at exposure and genetic variation that may mediate exposure; future studies should consider these possible interactions.
In addition to rare mutations in penetrant genes and ionizing radiation, exogenous hormone use among women is now an established risk factor for meningioma. In part because females have a greater meningioma risk than do males, investigators have examined factors associated with estrogen (menopausal status, ages at menarche and menopause, parity, and uses of oral contraceptives and hormone replacement therapy [HRT]). The ratio of the female-to-male meningioma incidence rate has increased in European countries over time with the increased use of hormone replacement therapy by perimenopausal and postmenopausal women.3 Several results suggest that meningioma risk is greatest among women during their reproductive years7,47,48; however, SEER statistics reveal that ratios of female-to-male meningioma incidence rates are greatest (greater than 2.5) during the ages 30 to 54 years.4 There is no consistent or convincing evidence that parity and oral contraceptive use are associated with meningioma risk.47–50 However, results from a population-based case-control study conducted by Wigertz et al.48 revealed elevated meningioma risks among women who had used long-acting hormonal contraceptives (odds ratio [OR] for at least 10 years of use = 2.7; 95% CI: 0.9-7.5) and postmenopausal women who had ever used HRT (OR = 1.7; 95% CI: 1.0-2.8). While exogenous female hormones probably play a role in the development of some meningiomas, our understanding of mechanisms governing their role is limited, perhaps in part because the menstrual and reproductive factors that have been examined are insufficient to accurately characterize lifetime estrogen or other hormonal exposure.
Probable Causal Factors for Brain Tumors
FAMILY HISTORY
The progressive accumulation of genetic and/or epigenetic alterations, permitting cells to evade normal regulatory mechanisms and/or escape destruction by the immune system, is thought to govern the development of glioma and meningioma, although these mechanisms have not yet been fully defined.51–53 Evidence for the presence of genetic involvement in the causal pathways of glioma and meningioma is demonstrated most simply by studies which have shown an increased risk of brain tumors in close relatives of brain tumor patients, especially those with gliomas. Although brain tumors clearly aggregate in families, it can be difficult to distinguish shared environmental exposures from inherited characteristics. In fact, Grossman et al.54 showed that brain tumors occur commonly in families with no known predisposing hereditary disease, and that the pattern of occurrence in many families suggests environmental, rather than genetic, causes. However, important results presented by Malmer et al.55 suggest that first-degree relatives, but not spouses, have a significantly increased brain tumor risk.
ALLERGIC AND ASSOCIATED IMMUNOLOGICAL CONDITIONS AND GLIOMA
There is consistent evidence for an association between allergy-related immune responses and glioma risk. Over the past two decades, results from ten case-control56–65 and one of two cohort studies66 show that self-reported allergies are inversely related to glioma risk. Linos et al.67 conducted a formal meta-analysis of a subset of these studies and found that self-reported allergies are related to a 40% reduction in the risk of glioma (OR = 0.61; 95% CI: 0.55-0.67). Further evidence for this inverse association was contributed by Wiemels et al.68 who found that total IgE levels were lower in glioma cases than in controls. Although mechanisms governing potential protection have not been identified, they may arise from the anti-inflammatory effects of IL- 4 and IL-13 cytokines involved in allergic and autoimmune disease69 or from increased tumor immunosurveillance among those with allergies and autoimmune disease.70 It is also possible that the inverse association results from immune suppression by a preclinical tumor.71
In addition to their role in allergic conditions, IL-4 and IL-13 cytokines also inhibit growth of glioma cell lines. IL-4 is strongly expressed during brain injury,72 where invading T cells may be a source of this cytokine,73 and IL-4 increases the number of T-cell precursors in GBM patients.74 Barna et al.73 found that three normal astrocytic, two low-grade astrocytoma, and three out of four GBM cell lines they evaluated expressed IL-4R alpha receptors. However, IL-4 suppressed DNA synthesis and cell proliferation only in the normal astrocytic and low-grade astrocytoma cell lines, not in the GBM cell lines. IL-4 could play a role in the inhibition of GBMs that arise from astrocytomas, but it may not be involved in de novo GBMs.75 In view of a possible role for IL-4 and IL-13 in both allergic conditions and glioma, Schwartzbaum et al.63 identified polymorphisms of the IL-4R alpha and IL-13 genes that increase allergic condition risk. Although these germline genetic variants are not sensitive indicators of the presence of allergic conditions, they do provide a measure of risk of these conditions free of recall bias. The working hypothesis was that individuals with IL-4R alpha or IL-13 polymorphisms that increase allergic condition risk would have a decreased risk of GBM. Using data from a small case-control study (111 GBM cases, 422 controls), the authors found results consistent with their hypothesis; each of the two IL-4R alpha and IL-13 single nucleotide polymorphisms (SNPs) associated with increased allergic condition risk were also related to decreased GBM risk. Wiemels et al.76 confirmed their finding for one of the IL-13 SNPs in a larger case-control study of glioma (456 glioma cases, 541 controls). Furthermore, they reported that this IL-13 SNP was inversely associated with IgE levels among controls (p = 0.04). However, they did not find associations between the IL-4R alpha SNPs and glioma as had Schwartzbaum et al. They did note a borderline association between an IL-4R alpha haplotype (OR = 1.5; 95% CI: 1.0-2.3) and glioma. They also saw that a rare IL-4 haplotype was associated with decreased glioma risk (OR = 0.23; 95% CI: 0.07-0.83).
A larger study of the original four IL-4R alpha and IL-13 genetic variants by Schwartzbaum et al.77 did not provide strong support for their original observations. Nonetheless, they found an IL-4R alpha haplotype associated with GBM (OR = 2.26; 95% CI: 1.13-4.52) and inversely related to self-report of hay fever or asthma among controls (OR= 0.39; 95% CI: 0.16-0.98). Although Wiemels et al. also found suggestive evidence for an association of between an IL-4R alpha haplotype and glioma, when they restricted their haplotype to the same IL-4R alpha SNPs that Schwartzbaum et al. examined, they observed no evidence for an association with glioma (OR = 1.13; 95% CI: 0.83-1.53).
Immunosuppressive regulatory T-cells and their associated cytokines TGF-beta and IL-10 repress effective anti-glioma reactions and may provide a conceptual and mechanistic framework to explain an indirect relationship between allergies and anti-glioma immune reactions. The unique architecture of the brain does not exclude glioma from immune system interaction, although immune responses may be attenuated compared to those found in other organs. In addition, there is now evidence for infiltration of T and B cells into the brains of brain tumor patients; the enhancement of such responses is likely to form the basis of future effective glioma therapies. In recent in vitro studies of glioma, human glioma cell lines were found to secrete immunosuppressive cytokines that can selectively recruit regulatory T cells into the tumor microenvironment.78 In addition, Chahlavi et al.79 demonstrated that glioma cell lines mediate immunosuppression by promoting T cell death through tumor-associated antigens and gangliosides. Two of the major immunosuppressive cytokines that are present in both the glioma microenvironment and the peripheral blood of glioma patients, IL-10 and TGF-beta, induce immune tolerance, thereby inhibiting allergy and asthma.80 Elevated IgE concentrations may therefore indicate low levels of immunosuppression and the resulting ability to conduct anti-tumor immunosurveillance against incipient glioma. Alternatively, the relative absence of allergies in glioma patients may merely show that these tumor-induced cytokines have suppressed the immune system.
Results pertaining to HLA—cell surface molecules that mediate interactions of tumor cells with the host immune response, in part by presenting antigenic peptides to T-lymphocytes—also suggest the importance of immunological responses in glioma development. Facoetti et al.81 found that HLA class I antigen loss was significantly (P<0.025) correlated with tumor grade: HLA class I antigens were lost in approximately half of GBM tumors, but only in 20% of grade II astrocytoma tumors; selective HLA-A2 antigen loss was observed in approximately 80% of GBM lesions and half of the grade II astrocytoma tumors. GBM is positively associated with the HLA genotype B*13 and the HLA haplotype B*07-Cw*07 (P=0.01 and P<0.001, respectively), and is inversely associated with the genotype Cw*01.82 Interestingly, these results could partially explain the increased GBM incidence among whites, because B*07 and B*07-Cw*07 are more common among whites than among nonwhites.
VARICELLA-ZOSTER VIRUS INFECTION AND ASSOCIATED IMMUNOGLOBULIN G AND GLIOMA
A reported history of varicella-zoster virus (VZV) infection and positive IgG to VZV is also associated with a reduced risk of glioma.83–85 Results from case-control studies suggest that past clinical disease associated with VZV infection and anti-VZV IgG levels may be inversely associated with adult glioma risk.83–85 It may be the specific nature of immune system response to antigens, and not exposure to the antigen per se, that is responsible for this inverse association with glioma.68,86 Associations of glioma risk with VZV and IgG, along with other potentially important viral or bacterial infections, should be validated in studies in which serologic measurement of viral or bacterial exposure is ascertained before the development of brain tumors (to prevent the possibility of the tumor affecting the serologic assessment) and in which there is serologic or symptom-based confirmation of infection.
Possible Causal Factors for Brain Tumors Requiring Additional Study
CELLULAR TELEPHONE USE
In general, with the exception of analog cellular telephone use (which has largely been replaced by digital cell phone use), meningioma risk does not appear to increase as the result of cell phone use.87–89,90,91 Mixed results have been reported for associations between cell phone use and acoustic neuroma risk90–95; however, relative risks from studies of more than one histologic type of brain tumor have been greater for acoustic neuroma, as compared to meningioma and glioma, especially when cell phone use is on the same side of the head as the tumor (ipsilateral).93 Further study of cell phone use and acoustic neuroma risk is warranted. A relatively large number of epidemiologic studies of cell phone use and glioma risk have suggested that short-term cell phone use is probably not associated with glioma risk.87–89,91,96–104 There are limited data and inconsistent results pertaining to long-term use and glioma risk,87–89,91,97–99,102,104,105 with the most compelling results suggesting evidence of increased glioma risk as the result of ipsilateral cell phone use.89,91,97,99,102 However, these results have likely been affected by small sample sizes, and selection and recall biases.87,106 Some of the increased risk resulting from ipsilateral cell phone use may be attributable to recall bias because contralateral cell phone use appears to reduce risk; in the absence of recall bias, one would not expect cell phone use to decrease risk. The largest population-based case-control study reported to date (1522 glioma cases and 3301 controls) conducted in five Nordic countries and the United Kingdom102 found no consistent evidence overall for increased risk of glioma related to use of cell phones; nor did they find increased glioma risk among the most highly-exposed group. However, if the latency period is at least 5 years long, then earlier studies lacked sufficient numbers of long-term cell phone users to adequately evaluate the relationship. The association of glioma risk with long-term cell phone use has not yet been convincingly demonstrated but will continue to be examined in the context of more refined studies, which will have greater statistical power because of the increasing numbers of the population who are long-term cell phone users and the potential release of individual records by cellular telephone companies for better exposure assessments.
POLYMORPHIC VARIATION IN DETOXIFICATION, DNA STABILITY AND REPAIR, AND CELL CYCLE REGULATION AND GLIOMA
Results from studies of polymorphic variation in detoxification, DNA stability and repair, and cell cycle regulation genes have produced suggestive but not definitive evidence for an association of these genes with glioma risk. Cytochrome p450s (CYP) and GST are involved in metabolizing many electrophilic compounds, including carcinogens, mutagens, cytotoxic drugs, metabolites, and products of reactive oxidation. For brain tumors, studies of CPY and GST have produced mixed results. Results from a recent meta-analysis of eight studies suggest that, although the T1 null genotype is significantly associated with a nearly twofold risk of meningioma, there are no associations between any of the GSTP1 105 and GSTP1 114 SNPs and glioma risk. Wrensch et al.107 found little evidence for a general association of GST polymorphisms with glioma, but did show an association of GSTT1 deletion for glioma with p53 mutations. In a large Nordic and British population-based case-control study, Schwartzbaum et al.108 reported no associations between the GSTM3, GSTP1 NQ01, CYP1A1, GSTM1, or GSTT1 polymorphisms and adult brain tumor risk; however, they found a weak association between the G-C (Val-Ala) GSTP1 105/114 haplotype and glioma.
Because DNA repair is important in maintaining DNA integrity, inherited variation in components of DNA repair pathways has been examined as potentially being related to glioma risk. Associations with glioma have been reported for variants in ERCC1,109,110 ERCC2,20,109,111 the nearby gene glioma tumor suppressor candidate of unknown function (GLTSCR1),20 PRKDC (also known as XRCC7—a gene involved in nonhomologous end joining double-strand break repair),112 and O6–methylguanine-DNA methyltransferase (MGMT, which encodes a DNA repair protein that removes alkyl groups from an important DNA site—O6),113,114 but there are too few studies to assess consistency. Bethke et al.115 recently reported that 16 SNPs (among over 1500 haplotype-tagging and putative functional SNPs selected to capture most common variation in the known 136 DNA repair genes) were significantly associated with glioma risk. Because DNA repair is complex (involving at least 136 known genes), studies focusing on constellations of variants involved in DNA repair pathways, as well as their interactions, might elucidate the roles of variants in gliomagenesis and progression.
Dysregulation of the cell cycle (control of proliferation and apoptosis) is a hallmark feature of most gliomas.116 Results from large series of glioma cases and controls suggest that inherited variation in CASP8, a regulator of apoptosis, may be important in glioma development; risk for glioma was elevated among those with carrier status for the rare allele of D302H in CASP8 (OR = 1.7; 95% CI: 1.10-1.70).117 In addition, MDM2, a key molecule in maintaining the fidelity of proliferation and apoptosis, appears to negatively regulate TP53 expression.118,119 Findings from a recent study suggest that SNP309, in the promoter region of MDM2 and associated with MDM2 protein expression level, is not associated with histologic grade of glioma, age at onset, or p53 mutation rate or gliomagenesis.120 In a study of glioma patients with Li-Fraumeni syndrome,121 the G variant of SNP309 in the MDM2 promoter led to higher expression of MDM2 with concomitant reduced expression of TP53, and was significantly associated with earlier age of tumor development and multiple tumor sites. Associations between MDM2 and TP53 remain poorly understood and should be examined in studies with large sample sizes because of the potential need for smaller subgroup analyses.
HUMAN CYTOMEGALOVIRUS AND GLIOMA
Although several case-control studies found no evidence suggesting that human cytomegalovirus (HCMV) plays a role in the development of glioma,85,122 HCMV nucleic acids and proteins have been observed in glioblastoma tissue and HCMV DNA has been found in the peripheral blood of glioblastoma patients.123 Scheurer et al.124 found HCMV infection in 21 glioblastoma tumors that they evaluated. However, the presence of HCMV gene products in blood or tumor tissue may result from reactivation of infection caused by tumor-induced immunosuppression or from infected tumor cells shedding viral DNA123 rather than from any role of HCMV in glioma development.
GENETIC FACTORS (OTHER THAN FAMILY HISTORY AND RARE MUTATIONS) AND MENINGIOMA
Mutations in the NF2 gene (probably accounting for more than half of meningiomas125) and loss of chromosome 22q are the most common genetic alterations associated with the initiation of meningiomas.125,126 However, we do not understand the mechanisms governing the factors that allow meningioma to develop. Genetic results that may shape future studies of meningioma include the following findings: genetic changes of the APC gene play a role in meningioma formation;127 a candidate common LOH region on 1p36.11 may harbor tumor suppressor genes related to malignant progression of meningioma;128 the genotype combination of CC-CG-CC formed by three polymorphisms in p53 increases meningioma risk, but only among those with a family history of cancer;129 SNPs in the Ki-ras and ERCC2 genes may be involved in meningioma formation; and SNPs in cyclin D1 and p16 may mark genes that have an inverse effect on risk of developing both radiation-associated and sporadic meningioma. One of the most intriguing findings concerning genetic factors related to meningioma resulted from a recent analysis of 136 DNA repair genes.130 Bethke et al. found that the SNP rs4968451, which maps to the gene that encodes breast cancer susceptibility gene 1–interacting protein 1 (BRIP1), was consistently associated, across five studies, with an increased risk of developing meningioma.130 This is compelling because: (1) greater than one fourth of the European population are carriers of at-risk genotypes for rs4968451; therefore, the variant is likely to contribute to 16% of meningiomas; and (2) BRIP1 encodes a helicase which interacts with BRCA1 and has BRCA1-dependent DNA repair and checkpoint functions, and this provides a possible explanation for the fact that both breast cancer and meningioma most commonly occur among women ages 50 to 70 years and that women with breast cancer have approximately 50% greater risk of meningioma and vice versa.130
Summary
However, this year (2009) two published genome wide association studies for glioma, based on five sets of genome wide genotyping in glioma cases (University of California at san Francisco, Mayo Clinic, MD Anderson Cancer Center, UK and The Cancer Genome Atlas) and several control groups discovered and confirmed the following five regions for glioma risk: chromosome 5p15.33 (TERT), 9p21.3 (CDKN2B), 8q24, 11q23 (PHLDB1), and, 20q13.3 (RTEL1).131,132 These new discoveries open up tremendous opportunities for discovering mechanisms of glioma risk.
1. CBTRUS. Statistical Report: Primary Brain Tumors in the United States 2000–2004. 2008.
2. B.J. McCarthy. Descriptive Epidemiology of Glioma. Principles and Practices of Neuro-Oncology: A Multidisciplinary Approach. 2008. (In Press)
3. L. Klaeboe, L. Stefan, D. Scheie, et al. Incidence of intracranial meningioma in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer. 2005;117(6):996-1001.
4. SEER. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission. 2008.
5. P.D. Inskip, M.S. Linet, E.F. Heineman. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17(2):382-414.
6. M. Wrensch, Y. Minn, T. Chew, M. Bondy, M.S. Berger. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol. 2002;4(4):278-299.
7. A. Helseth, Incidence and survival of intracranial meningioma patients in Norway 1963–1992. Neuroepidemiology, 16;2. 1997:53-59.
8. R. Sankila, M. Kallio, J. Jaaskelainen, T. Hakulinen. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland. Comparison of the observed and expected survival rates in a population-based series. Cancer. 1992;70(6):1568-1576.
9. B.J. McCarthy, F.G. Davis, S. Freels, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88(5):831-839.
10. M.H. Cohen, J.R. Johnson, R. Pazdur. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11(19 Pt 1):6767-6771.
11. M. Lacroix, D. Abi-Said, D.R. Fourney, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198.
12. J. Lutterbach, W. Sauerbrei, R. Guttenberger. Multivariate analysis of prognostic factors in patients with glioblastoma. Strahlentherapie und Onkologie. 2003;179(1):8-15.
13. B. Jeremic, B. Milicic, D. Grujicic, A. Dagovic, J. Aleksandrovic. Multivariate analysis of clinical prognostic factors in patients with glioblastoma multiforme treated with a combined modality approach. J Cancer Research Clinical Oncol. 2003;129(8):477-484.
14. J. Schwartzbaum, P. Lal, W. Evanoff, et al. Presurgical serum albumin levels predict survival time from glioblastoma multiforme. J Neuro-oncol. 1999;43:35-41.
15. S. Chang, F.G. Barker2nd. Marital status, treatment, and survival in patients with glioblastoma multiforme. Cancer. 2008;104(9):1975-1984.
16. M.J. McGirt, K.L. Chaichana, M. Gathinji, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurg. 2008;63(2):286-291.
17. H. Ohgaki, P. Dessen, B. Jourde, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892-6899.
18. M.C. Schmidt, S. Antweiler, N. Urban, et al. Impact of genotype and morphology on the prognosis of glioblastoma. J Neuropathol Exp Neurol. 2002;61(4):321-328.
19. K. Aldape, P.C. Burger, A. Perry. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med. 2007;131(2):242-251.
20. P. Yang, T.M. Kollmeyer, K. Buckner, W. Bamlet, K.V. Ballman, R.B. Jenkins. Polymorphisms in GLTSCR1 and ERCC2 are associated with the development of oligodendrogliomas. Cancer. 2005;1(103):2363-2372.
21. Y. Ushio, K. Tada, S. Shiraishi, et al. Correlation of molecular genetic analysis of p53, MDM2, p16, PTEN, and EGFR and survival of patients with anaplastic astrocytoma and glioblastoma. Front Biosci. 2003;8:e281-e288.
22. T.T. Batchelor, R.A. Betensky, J.M. Esposito, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 Pt 1):228-233.
23. M. Stander, A. Peraud, B. Leroch, F.W. Kreth. Prognostic impact of TP53 mutation status for adult patients with supratentorial World Health Organization Grade II astrocytoma or oligoastrocytoma: a long-term analysis. Cancer. 2004;101(5):1028-1035.
24. L.M. Backlund, B.R. Nilsson, L. Liu, K. Ichimura, V.P. Collins. Mutations in Rb1 pathway-related genes are associated with poor prognosis in anaplastic astrocytomas. Brit J Cancer. 2005;93(1):124-130.
25. P. Deb, M.C. Sharma, A.K. Mahapatra, D. Agarwal, C. Sarkar. Glioblastoma multiforme with long term survival. Neurol India. 2005;53(3):329-332.
26. R.E. McLendon, J.E. Herndon2nd, B. West, et al. Survival analysis of presumptive prognostic markers among oligodendrogliomas. Cancer. 2005;104(8):1693-1699.
27. C. Houillier, J. Lejeune, A. Benouaich-Amiel, et al. Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer. 2006;106(10):2218-2223.
28. L.J. Layfield, C. Willmore, S. Tripp, C. Jones, R.L. Jensen. Epidermal growth factor receptor gene amplification and protein expression in glioblastoma multiforme: prognostic significance and relationship to other prognostic factors. Appl Immunohistochem Mol Morphol. 2006;14(1):91-96.
29. M. Wrensch, J.K. Wiencke, J. Wiemels, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66(8):4531-4541.
30. S.M. Ranuncolo, M. Varela, A. Morandi, et al. Prognostic value of Mdm2, p53 and p16 in patients with astrocytomas. J Neuro-oncol. 2004;68(2):113-121.
31. M.L. Simmons, K.R. Lamborn, M. Takahashi, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61(3):1122-1128.
32. L. Wang, L.E. Wang, R. El-Zein, et al. Human telomerase genetic variation predicts survival of patients with glioblastoma multiforme (Abstract number 2823). Proc Amer Assoc Cancer Res. 46, 2005.
33. A. Tchirkov, T. Khalil, E. Chautard, et al. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Brit J Cancer. 2007;96(3):474-476.
34. E.I. Surace, E. Lusis, Y. Murakami, B.W. Scheithauer, A. Perry, D.H. Gutmann. Loss of tumor suppressor in lung cancer-1 (TSLC1) expression in meningioma correlates with increased malignancy grade and reduced patient survival. J Neuropathol Exp Neurol. 2004;63(10):1015-1027.
35. A. Maillo, A. Orfao, J.M. Sayagues, et al. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J Clin Oncol. 2003;21(17):3285-3295.
36. Sadetzki S. Exposure to Ionizing Radiation and Glioma Risk. Principles and Practices of Neuro-Oncology: A Multidisciplinary Approach. (In Press).
37. M. Blettner, B. Schlehofer, F. Samkange-Zeeb, G. Berg, K. Schlaefer, J. Schuz. Medical exposure to ionising radiation and the risk of brain tumours: Interphone study group, Germany. Eur J Cancer. 2007;43(13):1990-1998.
38. S. Preston-Martin. Epidemiology of primary CNS neoplasms. Neurol Clin. 1996;14(2):273-290.
39. T. Shintani, N. Hayakawa, M. Hoshi, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40(1):49-57.
40. M. Wrensch, Y. Minn, T. Chew, M. Bondy, M.S. Berger. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol. 2002;4(4):278-299.
41. Y. Juven, S. Sadetzki. A possible association between ionizing radiation and pituitary adenoma: a descriptive study. Cancer. 2002;95(2):397-403.
42. M. Wrensch, R. Miike, M. Lee, J. Neuhaus. Are prior head injuries or diagnostic X-rays associated with glioma in adults? The effects of control selection bias. Neuroepidemiology. 2000;19(5):234-244.
43. L. Hardell, K.H. Mild, A. Pahlson, A. Hallquist. Ionizing radiation, cellular telephones and the risk for brain tumours. Eur J Cancer Prev. 2001;10(6):523-529.
44. W.T.J. Longstreth, L.E. Phillips, M. Drangsholt, et al. Dental X-rays and the risk of intracranial meningioma: a population-based case-control study. Cancer. 2004;100(5):1026-1034.
45. S. Preston-Martin, M.C. Yu, B.E. Henderson, C. Roberts. Risk factors for meningiomas in men in Los Angeles County. J Natl Cancer Inst. 1983;70(5):863-866.
46. E. Salminen, E. Pukkala, L. Teppo. Second cancers in patients with brain tumours impact of treatment. Eur J Cancer. 1999;35(1):102-105.
47. B. Schlehofer, M. Blettner, J. Wahrendorf. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84(17):1346-1349.
48. A. Wigertz, S. Lonn, T. Mathiesen, et al. Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol. 2006;164(7):629-636.
49. B.S. Jhawar, C.S. Fuchs, G.A. Colditz, M.J. Stampfer. Sex steroid hormone exposures and risk for meningioma. J Neurosurg. 2003;99(5):848-853.
50. E.E. Hatch, M.S. Linet, J. Zhang, et al. Reproductive and hormonal factors and risk of brain tumors in adult females. Intl J Cancer. 2005;114(5):797-805.
51. J.L. Fisher, J. Schwartzbaum, M. Wrensch, J.L. Wiemels. Epidemiology of Brain Tumors. Neurologic clinics. Brain Tumors in Adults. 2007;25(4):867-890.
52. J.A. Schwartzbaum, J.L. Fisher, K.D. Aldape, M. Wrensch. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494-503.
53. M. Wrensch, J.L. Fisher, J.A. Schwartzbaum, M. Bondy, M. Berger, K.D. Aldape. The molecular epidemiology of gliomas in adults. Neurosurg Focus. 2005;19(5):E5.
54. S.A. Grossman, M. Osman, R. Hruban, S. Piantadosi. Central nervous system cancers in first-degree relatives and spouses. Cancer Invest. 1999;17(5):299-308.
55. B. Malmer, R. Henriksson, H. Gronberg. Familial brain tumours-genetics or environment? A nationwide cohort study of cancer risk in spouses and first-degree relatives of brain tumour patients. Int J Cancer. 2003;106(2):260-263.
56. A.V. Brenner, M.S. Linet, H.A. Fine, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99(2):252-259.
57. F.M. Cicuttini, S.F. Hurley, A. Forbes. Association of adult glioma with medical conditions, family, and reproductive history. Int J Cancer. 71(203–7), 1997.
58. F. Hochberg, P. Toniolo, P. Cole, M. Salcman. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8(1):55-60.
59. P. Ryan, M.W. Lee, B. North, A.J. McMichael. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51(1):20-27.
60. B. Schlehofer, M. Blettner, N. Becker, C. Martinsohn, J. Wahrendorf. Medical risk factors and the development of brain tumors. Cancer. 1992;69:2541-2547.
61. B. Schlehofer, M. Blettner, S. Preston-Martin, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer. 1999;82(2):155-160.
62. M.J. Schoemaker, A.J. Swerdlow, S.J. Hepworth, P.A. McKinney, M. van Tongeren, K.R. Muir. History of allergies and risk of glioma in adults. Int J Cancer. 2006.
63. J. Schwartzbaum, A. Ahlbom, B. Malmer, et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 2005;65(14):6459-6465.
64. A. Wigertz, S. Lonn, J. Schwartzbaum, et al. Allergic Conditions and Brain Tumor Risk. Am J Epidemiol. 2007.
65. J.L. Wiemels, J.K. Wiencke, J.D. Sison, R. Miike, A. McMillan, M. Wrensch. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98(4):609-615.
66. J. Schwartzbaum, F. Jonsson, A. Ahlbom, et al. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003;106(3):423-428.
67. E. Linos, T. Raine, A. Alonso, D. Michaud. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544-1550.
68. J.L. Wiemels, J.K. Wiencke, J. Patoka, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64(22):8468-8473.
69. C.A. Dinarello. Setting the cytokine trap for autoimmunity. Nat Med. 2003;9(1):20-22.
70. G.P. Dunn, A.T. Bruce, H. Ikeda, L.J. Old, R.D. Schreiber. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991-998.
71. M.J. Schoemaker, A.J. Swerdlow, S.J. Hepworth, P.A. McKinney, M. van Tongeren, K.R. Muir. History of allergies and risk of glioma in adults. Int J Cancer. 2006;119(9):2165-2172.
72. H. Liu, R.A. Prayson, M.L. Estes, et al. In vivo expression of the interleukin 4 receptor alpha by astrocytes in epilepsy cerebral cortex. Cytokine. 2000;12(11):1656-1661.
73. B.P. Barna, M.L. Estes, J. Pettay, K. Iwasaki, P. Zhou, G.H. Barnett. Human astrocyte growth regulation: interleukin-4 sensitivity and receptor expression. J Neuroimmunol. 1995;60(1–2):75-81.
74. C. Faber, E. Terao, E. Morga, P. Heuschling. Interleukin-4 enhances the in vitro precursor cell recruitment for tumor-specific T lymphocytes in patients with glioblastoma. J Immunother. 2000;23(1):11-16.
75. D.N. Louis. A molecular genetic model of astrocytoma histopathology. Brain Pathol. 1997;7(2):755-764.
76. J.L. Wiemels, J.K. Wiencke, K.T. Kelsey, et al. Allergy-related polymorphisms influence glioma status and serum IgE levels. Cancer Epidemiol Biomarkers Prev. 2007.
77. J. Schwartzbaum, A. Ahlbom, S. Lönn, et al. An international case-control study of IL-4Ralpha, IL-13 and cyclooxygenase-2 polymorphisms and glioblastoma risk. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2448-2454.
78. J.T. Jordan, W. Sun, S.F. Hussain, G. DeAngulo, S.S. Prabhu, A.B. Heimberger. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57(1):123-131.
79. A. Chahlavi, P. Rayman, A.L. Richmond, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65(12):5428-5438.
80. D.T. Umetsu, R.H. DeKruyff. The regulation of allergy and asthma. Immunol Rev. 2006;212:238-255.
81. A. Facoetti, R. Nano, P. Zelini, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin. Cancer Res. 2005;11(23):8304-8311.
82. J. Tang, W. Shao, M.T. Dorak, et al. Positive and negative associations of human leukocyte antigen variants with the onset and prognosis of adult glioblastoma multiforme. Cancer Epidemiol Biomarkers Prev. 2005;14(8):2040-2044.
83. M. Wrensch, A. Weinberg, J. Wiencke, et al. Does prior infection with varicella-zoster virus influence risk of adult glioma? Am J Epidemiol. 1997;145(7):594-597.
84. M. Wrensch, A. Weinberg, J. Wiencke, R. Miike, G. Barger, K. Kelsey. Prevalence of antibodies to four herpesviruses among adults with glioma and controls. Am J Epidemiol. 2001;154(2):161-165.
85. M. Wrensch, A. Weinberg, J. Wiencke, et al. History of Chickenpox and Shingles and Prevalence of Antibodies to Varicella-Zoster Virus and Three Other Herpesviruses among Adults with Glioma and Controls. Am J Epidemiol. 2005;161(10):929-938.
86. J.L. Wiemels, J.K. Wiencke, J.D. Sison, R. Miike, A. McMillan, M. Wrensch. History of allergies among adults with glioma and controls. Intl J Cancer. 2002;98(4):609-615.
87. J. Schuz, E. Bohler, G. Berg, et al. Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany). Am J Epidemiol. 2006;163(6):512-520.
88. H.C. Christensen, J. Schuz, M. Kosteljanetz, et al. Cellular telephones and risk for brain tumors: a population-based, incident case-control study. Neurology. 2005;64(7):1189-1195.
89. S. Lonn, A. Ahlbom, P. Hall, M. Feychting, M. Feychting. Long-term mobile phone use and brain tumor risk. Am J Epidemiol. 2005;161(6):526-535.
90. L. Hardell, M. Carlberg, K. Hansson Mild. Pooled analysis of two case-control studies on the use of cellular and cordless telephones and the risk of benign brain tumours diagnosed during 1997–2003. Intl J Oncol. 2006;28(2):509-518.
91. L. Hardell, M. Carlberg, K. Hansson Mild. Case-control study on cellular and cordless telephones and the risk for acoustic neuroma or meningioma in patients diagnosed 2000–2003. Neuroepidemiology. 2005;25(3):120-128.
92. T. Takebayashi, S. Akiba, Y. Kikuchi, et al. Mobile phone use and acoustic neuroma risk in Japan. Occup Environ Med. 2006;63(12):802-807.
93. M.J. Schoemaker, A.J. Swerdlow, A. Ahlbom, et al. Mobile phone use and risk of acoustic neuroma: results of the Interphone case-control study in five North European countries. Brit J Cancer. 2005;93(7):842-848.
94. M. Kundi, K. Mild, L. Hardell, M-O Mattsson. Mobile telephones and cancer—a review of epidemiological evidence. J Toxicol Environ Health. 2004;7(5):351-384.
95. H.C. Christensen, J. Schuz, M. Kosteljanetz, H.S. Poulsen, J. Thomsen, C. Johansen. Cellular telephone use and risk of acoustic neuroma. Am J Epidemiol. 2004;159(3):277-283.
96. A. Auvinen, M. Hietanen, R. Luukkonen, R-S. Koskela. Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology. 2002;13(3):356-359.
97. L. Hardell, K.H. Mild, M. Carlberg. Case-control study on the use of cellular and cordless phones and the risk for malignant brain tumours. Intl J Rad Biol. 2002;78(10):931-936.
98. L. Hardell, A. Nasman, A. Pahlson, A. Hallquist, K. Hansson Mild. Use of cellular telephones and the risk for brain tumours: A case-control study. Intl J Oncol. 1999;15(1):113-116.
99. S.J. Hepworth, M.J. Schoemaker, K.R. Muir, A.J. Swerdlow, M.J.A. van Tongeren, P.A. McKinney. Mobile phone use and risk of glioma in adults: case-control study. BMJ. 2006;332(7546):883-887.
100. P.D. Inskip, R.E. Tarone, E.E. Hatch, et al. Cellular-telephone use and brain tumors. N Eng J Med. 2001;344(2):79-86.
101. C. Johansen, J.J. Boice, J. McLaughlin, J. Olsen. Cellular telephones and cancer—a nationwide cohort study in Denmark. J Natl Cancer Inst. 2001;93(3):203-207.
102. A. Lahkola, A. Auvinen, J. Raitanen, et al. Mobile phone use and risk of glioma in 5 North European countries. Intl J Cancer. 2007;120(8):1769-1775.
103. J.E. Muscat, M.G. Malkin, S. Thompson, et al. Handheld cellular telephone use and risk of brain cancer. JAMA. 2000;284(23):3001-3007.
104. Feychting M, Anders A. Radiofrequency fields and glioma. Principles and Practices of Neuro-Oncology: A Multidisciplinary Approach. (In Press).
105. A. Ahlbom, A. Green, L. Kheifets, D. Savitz, A. Swerdlow, A. Swerdlow. Epidemiology of health effects of radiofrequency exposure. Environ Health Perspect. 2004;112(17):1741-1754.
106. L. Hardell, M. Carlberg, K. Hansson Mild. Pooled analysis of two case-control studies on use of cellular and cordless telephones and the risk for malignant brain tumours diagnosed in 1997–2003. Intl Arch Occup Environ Health. 2006;79(8):630-639.
107. M. Wrensch, K.T. Kelsey, M. Liu, et al. Glutathione-S-transferase and adult glioma. Cancer Epidemiol Biomarkers Prev. 2004;13(3):461-467.
108. J.A. Schwartzbaum, A. Ahlbom, S. Lonn, et al. An international case-control study of glutathione transferase and functionally related polymorphisms and risk of primary adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2007;16(3):559-596.
109. M. Wrensch, K.T. Kelsey, M. Liu, et al. ERCC1 and ERCC2 polymorphisms and adult glioma. Neuro-oncol. 2005;7(4):495-507.
110. P. Chen, J. Wiencke, K. Aldape, et al. Association of an ERCC1 polymorphism with adult-onset glioma. Cancer Epidemiol Biomarkers Prev. 2000;9(8):843-847.
111. M. Caggana, J. Kilgallen, J.M. Conroy, et al. Associations between ERCC2 polymorphisms and gliomas. Cancer Epidemiol Biomarkers Prev. 2001;10(4):355-360.
112. L-E Wang, M.L. Bondy, H. Shen, et al. Polymorphisms of DNA repair genes and risk of glioma. Cancer Res. 2004;64(16):5560-5563.
113. J.K. Wiencke, K. Aldape, A. McMillan, et al. Molecular features of adult glioma associated with patient race/ethnicity, age, and a polymorphism in O6-methylguanine-DNA-methyltransferase. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1774-1783.
114. R. Inoue, M. Isono, M. Abe, T. Abe, H. Kobayashi. A genotype of the polymorphic DNA repair gene MGMT is associated with de novo glioblastoma. Neurol Res. 2003;25(8):875-879.
115. L. Bethke, E. Webb, A. Murray, et al. Comprehensive analysis of the role of DNA repair gene polymorphisms on risk of glioma. Hum Mol Genet. 2007;17(6):800-805.
116. K. Ichimura, H. Ohgaki, P. Kleihues, V.P. Collins. Molecular pathogenesis of astrocytic tumours. J Neuro-oncol. 2004;70(2):137-160.
117. L. Bethke, K. Sullivan, E. Webb, et al. The common D02H variant of CASP8 is associated with risk of glioma. Cancer Epidemiol Biomarkers Prev. 2008;17(4):987-989.
118. G.L. Bond, W. Hu, A.J. Levine. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5(1):3-8.
119. M.E. Halatsch, U. Schmidt, A. Unterberg, V.I. Vougioukas. Uniform MDM2 overexpression in a panel of glioblastoma multiforme cell lines with divergent EGFR and p53 expression status. Anticancer Res. 2006;26(6B):4191-4194.
120. H. Tsuiki, T. Nishi, H. Takeshima, et al. Single nucleotide polymorphism 309 affects murin-double-minute 2 protein expression but not glioma tumorigenesis. Neurol Med Chir (Tokyo). 2007;47(5):203-208. discussion 8–9
121. G.L. Bond, W. Hu, E.E. Bond, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119(5):591-602.
122. S. Poltermann, B. Schlehofer, K. Steindorf, P. Schnitzler, K. Geletneky, J.R. Schlehofer. Lack of association of herpesviruses with brain tumors. J Neurovirol. 2006;12:90-99.
123. D.A. Mitchell, W. Xie, R. Schmittling, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-oncol. 10(1), 2008.
124. M.E. Scheurer, M. Bondy, K. Aldape, T. Albrecht, R. El-Zein. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116:79-86.
125. M. Simon, J.P. Bostrom, C. Hartmann. Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery. 2007;60(5):787-798. discussion -98
126. M.J. Riemenschneider, A. Perry, G. Reifenberger. Histological classification and molecular genetics of meningiomas. Lancet neurol. 2006;5(12):1045-1054.
127. N. Pecina-Slaus, T. Nikuseva Martic, D. Tomas, V. Beros, M. Zeljko, H. Cupic. Meningiomas exhibit loss of heterozygosity of the APC gene. J Neuro-oncol. 2008;87(1):63-70.
128. Y. Guan, N. Hata, D. Kuga, et al. Narrowing of the regions of allelic losses of chromosome 1p36 in meningioma tissues by an improved SSCP analysis. Intl J Cancer. 2008;122(8):1820-1826.
129. B. Malmer, M. Feychting, S. Lonn, A. Ahlbom, R. Henriksson. p53 Genotypes and risk of glioma and meningioma. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2220-2223.
130. L. Bethke, A. Murray, E. Webb, et al. Comprehensive analysis of DNA repair gene variants and risk of meningioma. J Natl Cancer Inst. 2008;100(4):270-276.
131. S. Sanjay, F.J. Hosking, L.B. Robertson, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nature Genetics. 2009;41(8):899-904.
132. M. Wrensch, R.B. Jenkins, J.S. Chang, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature Genetics [published online ahead of print. July 5, 2009;41(8):905-908.