CHAPTER 68 Radiosurgical Treatment of Epilepsy
Radiosurgical Therapy for Temporal Lobe Epilepsy
Radiosurgery is the precise application of focused radiation under stereotactic guidance to a targeted volume area within the brain identified on magnetic resonance imaging (MRI).1 Conceptualized by Lars Leksell for use in functional neurosurgery, radiosurgical treatment of neurological disorders has progressively widened its utility2–34 and is now also a treatment modality option for several neoplastic35–84 and vascular indications.62,85–118 Unlike standard-dose fractionated radiotherapy, radiosurgery allows the neurosurgeon to deliver effective, precise, and accurate doses of radiation to a smaller volume without affecting large portions of normal parenchyma, thereby allowing a powerful radiobiologic effect on the chosen targeted volume.1,119–121
Epilepsy is one of the most common serious neurological diseases and has a prevalence of 0.5% to 1.0% in the U.S. population.122,123 Approximately, 20% of patients with epilepsy have seizures that are medically refractory (i.e., failing to respond to medications). Despite modern advances in new antiepileptic medications, the percentage of patients with medically refractory epilepsy has not significantly improved. Patients with medically refractory seizures may be referred for possible surgical management, and approximately half of them are found to be suitable candidates for open surgical resection of a seizure focus.124 Focal partial epilepsies such as temporal lobe epilepsy are typically responsive to open surgical resection and are increasingly being treated with “structural” treatment modalities.117,125
The most common type of open surgery performed for temporal lobe epilepsy is anterior temporal lobectomy, which is resection of a portion of the temporal lobe.123,125–127 With modern advances in surgical and anesthetic techniques, microsurgical resection of mesial temporal lobe structures can be performed with low morbidity and even lower mortality.121 Open invasive surgical procedures, however, have inherent risks, including damage to the brain (either directly or indirectly by injury to important blood vessels), bleeding (which can require reoperation), blood loss (which can require transfusion), infection, and general anesthetic risks.128–131 In addition, significant postoperative pain can result from surgical incisions and scars. Several clinical studies evaluating the morbidity and mortality associated with open microsurgery for temporal lobe epilepsy have reported that approximately 5% to 23% of patients undergoing open microsurgery experience a symptomatic neurological deficit postoperatively.126,128,129,132,133 Furthermore, open surgical procedures require several days of care in the hospital, typically including one night in the intensive care unit, which contributes to the economic costs of open microsurgical treatment.117 There is also a significant population of patients with medically intractable epilepsy who are unsuitable for conventional open microsurgery.117 These patients may have their epileptic focus in regions that are difficult to access or in eloquent functional regions of the brain where surgical resection could result in irreversible language, motor, or visual impairment.117,118
Radiosurgery is now being evaluated as an alternative treatment modality to open resective microsurgery for intractable temporal lobe epilepsy. High-dose radiation is toxic to all living cells, but the highly focused nature of radiosurgery allows stereotactic guidance and sparing of adjacent tissues from the damaging effects of radiation. Although performed in a hospital setting, radiosurgery is relatively noninvasive, with frame-based radiosurgery using just frame pins that penetrate only the skin to firmly fix the stereotactic frame to the skull. Typically, patients can return to full activity within 1 to 2 days after treatment. Currently, radiosurgery is under investigations as a treatment modality for epilepsy associated with vascular malformations, hypothalamic hamartomas, and medial temporal lobe epilepsy (MTLE) associated with mesial temporal sclerosis (MTS).*
Preclinical Evidence
Preclinical studies investigating focused high-dose radiosurgery in animal models of epilepsy have demonstrated the potential utility of radiosurgical treatment applied to nonhuman epilepsy models. Early animal experiments indicated the efficacy of focused irradiation in a feline model of epilepsy in reducing seizure activity.117,134,135 At doses between 10 and 20 Gy (1 Gy is equivalent to 1 J of energy per kilogram of tissue), cats with epileptic foci treated with an implanted cobalt radiation source had reduced seizure activity. Histologic analysis of these radiosurgically treated animal specimens revealed “neuronal reafferentation” as a proposed potential mechanism for amelioration of seizures with focused irradiation.134,135
Recently, Sun and colleagues reported that radiosurgery successfully reduced seizure activity and raised seizure thresholds in a nonhuman epilepsy model.118 In this preclinical investigation, a linear accelerator (LINAC) was used to deliver radiosurgery doses of 10 or 40 Gy at the 90% isodose line. These investigators reported that seizure thresholds in these radiosurgically treated rats were significantly increased and that the length of afterdischarges was significantly decreased in the group treated with 40 Gy. These antiepileptic effects were observed 1 week after radiosurgical treatment, and the antiseizure effects persisted at the 3-month follow-up period.118
In animal studies from the University of Virginia, the effects of radiosurgery on a chronic spontaneous epilepsy model in rodents were reported.153 In this preclinical investigation, hippocampal electrodes were implanted to produce a rodent temporal lobe epilepsy model. Ten weeks later, Gamma Knife radiosurgery (GKRS) with radiation doses between 10 and 40 Gy was applied as the therapeutic modality. Although the group receiving the lowest dose (10 Gy) showed no improvement in seizure activity, the 20-Gy group did exhibit a gradual and progressive reduction in seizures 2 to 6 months after radiosurgery. Also reported in this animal study, the 40-Gy group displayed a more dramatic and earlier reduction in seizures by the second month of follow-up. On histologic analysis of temporal lobe regions treated by radiosurgery in this rodent study, no necrosis in the tissue specimens was reported. Synaptically driven neuronal firing was reported to be intact in these radiosurgically treated rodent brain slices, thus suggesting that functional neuronal death was not responsible for the identified reduction in seizures.153
Recent preclinical experiments from the University of Pittsburgh were designed to estimate the radiation dose in radiosurgery that was needed to reduce seizure activity in a rat kainic acid–induced epilepsy model.144 In this animal investigation, rats underwent stereotactic injection of kainic acid into the hippocampus to induce seizures. Ten days after the injections, the focal epileptic injection site was treated by GKRS at a dose range of 20 to 100 Gy. Even animals treated with the lowest dose of 20 Gy in this study were reported to demonstrate a progressive reduction in the number of daily seizures during each week of observation after radiosurgery. Furthermore, 3 weeks after radiosurgery, all treated rats in this study at each radiosurgery dose—20, 40, 60, and 100 Gy—showed a statistically significant reduction in seizure activity. The authors reported that histologic evaluation revealed radiation-induced necrosis only at the highest 100-Gy radiosurgery dose. However, injection of kainic acid induces a loss of CA3 neurons in all animals, and for this reason, interpretation of the histologic findings, especially for radiation-induced necrosis, is extremely difficult and problematic in this kainic acid–based epilepsy model. Small areas of kainic acid–induced necrosis were, however, reported in 2 of 20 control animals and in 14 of 37 radiosurgically treated animals, but only in the animals treated with 100 Gy of radiosurgery did the observed histologic necrosis match the collimator size.144
A second preclinical study using the same kainic acid–induced epilepsy model was undertaken to further evaluate the histopathologic and behavioral effects of “subnecrotic” radiosurgery doses.154 Stereotactic hippocampal kainic acid injections were subsequently followed by GKRS at doses of either 30 or 60 Gy. A statistically significant reduction in seizures was reported in all radiosurgically treated animals, and this antiepileptic effect was observed earlier in the animals treated with the higher radiosurgery dose (weeks 5 to 9 versus weeks 7 to 9). Furthermore, in this preclinical investigation, no animals treated with radiosurgery were reported to demonstrate a deficit in new memory attainment tasks on water maze testing in comparison to control animals only injected with kainic acid, but both groups showed “cognitive” impairment when compared with rats that did not receive any kainic acid injection or radiosurgical treatment. For the histopathologic analysis in this study, two blinded observers evaluated the specimens from all animals at 13 weeks after radiosurgery. Typical changes with kainic acid injections were seen in all injected animals. Furthermore, in 25 of 46 injected animals, unilateral hippocampal atrophy was also observed. Again as noted earlier, histopathologic assessment is difficult given the use of kainic acid, but radiation-induced necrosis matching the target volume of radiation was not reported in any of the animals treated with radiosurgery.154 These preclinical animal findings suggest that reduction of seizure activity after radiosurgery does not require necrosis or concomitant functional loss of treated neurons.154
With the suggestion that necrosis is not necessary for reduction of seizures, the radiosurgery group in Prague reported on their preclinical characterization of a “subnecrotic” dose of radiosurgery in a rat model.155,156 This preclinical investigation evaluated radiosurgery doses of 25, 50, 75, or 100 Gy delivered bilaterally to the rat hippocampus and then assessed the rats with cognitive tests, MRI, and histopathologic examinations at 1, 3, 6, and 12 months after radiosurgery. A progressive time- and dose-dependent response curve was observed in cognitive memory function, edema on MRI, and necrotic histopathology. All animals radiosurgically treated with the 100-Gy dose died by 6 months after radiation therapy, and all histopathology specimens from these rats had radiation-induced necrotic lesions. All animals treated with 75 Gy displayed cognitive memory functional impairments, edema on MRI, and radiation-induced necrotic lesions, whereas only one of the animals treated with the 50-Gy radiosurgery dose had observable edema and necrosis. Animals treated with radiosurgery doses of 25 and 50 Gy were not reported to demonstrate any functional or structural impairments after radiosurgery.155 This observation of a potential subnecrotic radiosurgery dose that could improve seizure activity prompted a second follow-up preclinical study in which a 35-Gy radiosurgery dose was used with a long-term follow-up period of 16 months.156 In this study, 6 months after radiosurgical treatment, edema was observed on MRI, and this edema was most pronounced at 9 months after radiosurgery. After 16 months, two of six treated animals were reported to have radiation-induced necrotic cavities after treatment with a 35-Gy dose of radiosurgery. The four treated animals without frankly necrotic cavities had other notable histopathologic findings such as severe atrophy of the corpus callosum, loss of thickness of the somatosensory cortex, and damage to the stratum oriens hippocampi.156 These preclinical animal studies suggest that the full radiobiologic and histopathologic effect of radiosurgery may be manifested only several months after radiosurgery.
These preclinical studies reported amelioration of seizures, as well as histologic neuronal changes associated with radiosurgical treatment in different animal epilepsy models. These animal studies suggested that the antiepileptic efficacy of radiosurgery is dose dependent.121,144,153,154 Most of these studies suggest that a radiosurgery dose of approximately 25 Gy is required to induce a therapeutic antiepileptic effect and that the full histologic and other toxicity may require several months to fully develop.117,118,144,153–156 Animal models, however, may be poor predictors of radiation effects for translation to human biologic responses.
Clinical Evidence
The first application of radiosurgery for epilepsy surgery is attributed to Talairach in the 1950s, who implanted radioactive yttrium in patients with temporal lobe epilepsy without a lesion.117,118,121 Further clinical experiences with GKRS and LINAC-based radiosurgery for the treatment of arteriovenous malformations and low-grade tumors also reported the incidental antiseizure effects of radiosurgery.* Although it is not clear whether lesion resolution itself may contribute to the reduction in seizure activity, these clinical reports of improvement in seizures with radiosurgery provided the impetus for investigating radiosurgery as a potentially effective treatment of medically intractable epilepsy.
Medial Temporal Lobe Epilepsy
MTLE associated with MTS is perhaps the most well defined epilepsy syndrome responsive to structural intervention. MTS is an idiopathic process associated with extensive loss of neurons and an increase in astrocytes in the mesial temporal structures, which include the amygdala and hippocampus in the temporal lobe. When temporal lobe epilepsy is due to underlying MTS, improvements in seizures with open microsurgical structural resections can be expected in between 65% and 90% of patients.117,123,125,127,157–162 This form of temporal lobe epilepsy is particularly amenable to structural interventions such as radiosurgery because 80% to 90% of these patients show detectable changes on MRI.120,159
Radiosurgery has also been explored as an alternative to open microsurgery for MTS-associated MTLE. In a small series of patients with MTLE treated with GKRS, Régis and coauthors reported clinical and effective amelioration of seizures with minimal morbidity.145,146 A recent, prospective, multicenter European study evaluating GKRS for MTS showed comparable efficacy rates (65%) for reduction of seizures by conventional microsurgery and radiosurgery after 2 years of follow-up.121 Using a marginal dose of 24 Gy, Régis and colleagues reported that radiosurgery can be used as an alternative to conventional open microsurgery to effectively treat MTLE associated with MTS and improve quality of life with comparable rates of morbidity and mortality.121 In the United States, a multicenter pilot trial is currently being conducted, and the initial results show that 85% of patients treated with 24 Gy (to the 50% isodose line) delivered to the medial temporal lobe, including the amygdala, anterior hippocampus, and nearby cortex, are seizure free at 2 years of follow-up with minimal morbidity (Barbaro and coworkers, unpublished). This study group is also planning a follow-up, phase III multicenter trial to compare open microsurgery with radiosurgery for patients with clinically and radiographically defined MTS-associated MTLE.
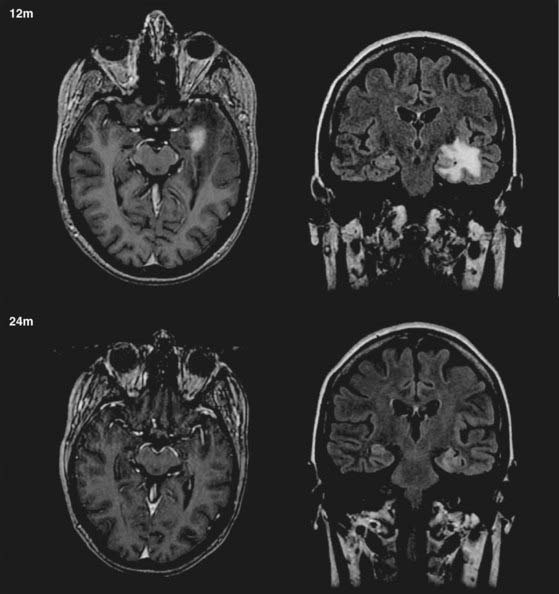
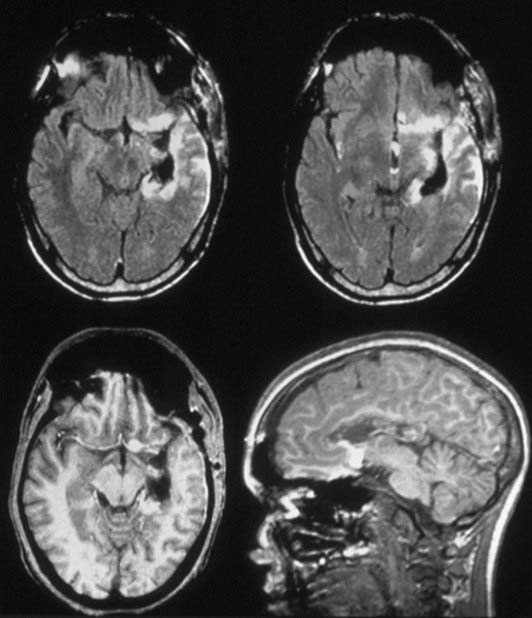
Although radiosurgery has proved effective and safe in improving MTLE-associated seizures, the beneficial effects of radiosurgery are not demonstrated immediately. Typically, patients with MTLE treated by radiosurgery can achieve improvement in seizures between 9 and 12 months and dramatic improvement in seizures between 18 and 24 months after treatment. A transient increase in partial seizures (auras) has been reported at approximately the same time that the complex seizures decrease.121 Most patients require a temporary course of corticosteroids to treat the delayed radiation-induced edema associated with the initial radiosurgical effect, commonly 10 to 15 months after treatment (Fig. 68-1) (Barbaro, personal observation).121
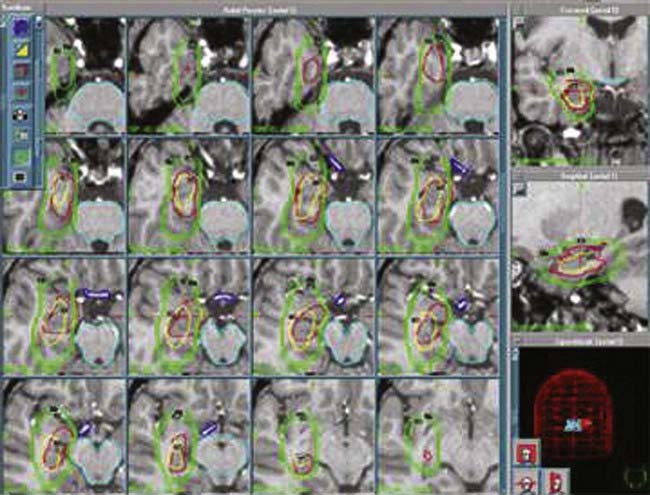
One of the difficulties in applying radiosurgery broadly as an application for intractable MTLE is the definition of the radiosurgical target (Fig. 68-2). Because the MTS associated with MTLE is not clearly defined anatomically, the precise boundaries and structures for radiosurgical treatment are yet not well characterized. Hence, standardization among different academic and community treatment centers has not been implemented and is difficult to achieve thus far, and consensus on treatment targets remains elusive. Successful radiosurgical treatment, however, is correlated with the targets treated with radiosurgery. For example, in recent reports, Régis and colleagues radiosurgically targeted the mesial temporal lobe structures in their series, whereas Kawai and associates restricted their treatment to the amygdala or hippocampus structures, and each series reported varying rates of successful amelioration of MTLE.121,140,145,146 Although target definition may vary among different neurosurgeons, radiosurgery for MTS-associated MTLE remains an attractive therapeutic option because of its effectiveness, low morbidity and mortality, and the consistent manifestations of this disease with identifiable imaging characteristics on MRI. Moreover, conventional open microsurgical temporal lobectomy is still possible if the initial radiosurgical treatment is ineffective after sufficient time has elapsed for the delayed radiosurgical antiepileptic effect.121
Furthermore, recent dose studies have also suggested that a lower dose of 20 Gy at the margins may be less effective than higher marginal doses in reducing seizure activity. Cmelak and coauthors reported unsuccessful reduction of seizures with a 15-Gy marginal radiosurgery dose.163 Similarly, Kawai and coworkers reported two cases of radiosurgery with an unsuccessful antiepileptic effect at a marginal radiosurgery dose of 18 Gy.140 Finally, Srikijvilaikul and colleagues from the Cleveland Clinic also reported their series of ineffective radiosurgical treatment for seizure control with a 20-Gy marginal dose.164
Histologic Evaluation after Radiosurgical Treatment of Medial Temporal Lobe Epilepsy
Histologic examination of radiosurgically treated human mesial temporal tissue for MTLE has been limited because of the efficacy of radiosurgery for MTS-associated MTLE. However, histologic analysis of radiation-treated tissues has been reported in patients who underwent resection as a result of ineffective seizure control after radiosurgery.140,163,164 Using a subtherapeutic dose, Cmelak and coauthors reported no radiation-induced histopathologic changes in tissues treated with radiosurgery at 15 Gy.163 In another series in which two patients were treated with 18 Gy, one patient was noted to have a necrotic focus with some prominent vascular changes consisting of vessel wall thickening and fibrinoid and hyaline degeneration, whereas the other patient treated with this subtherapeutic dose showed no necrosis or vascular histopathologic changes.140 When treated with a higher, yet subtherapeutic dose of 20 Gy, all five patients from a series reported from the Cleveland Clinic demonstrated histopathologic necrosis, perivascular sclerosis, and macrophage infiltration on resection and evaluation.164 These reports suggest that in the clinical use of radiosurgery, significant identifiable histologic changes may be observed only with radiosurgical doses of 20 Gy or greater. These radiobiologic and histologic markers such as necrosis and vascular changes may be required for an effective antiseizure effect to become manifested. Thus, a dose that produces some tissue necrosis and histopathologic effects without inducing an excessive biologic response (e.g., edema), such as 24 Gy, may be the optimal effective dose for the radiosurgical treatment of MTLE.121,145,146
Currently, the radiobiology of radiosurgery in the setting of MTS-associated MTLE is not yet completely understood. Although some preclinical studies have suggested an antiepileptic effect of radiation with subnecrotic doses,154 human clinical studies have suggested that a certain amount of tissue necrosis and histopathologic changes may be required to produce significant amelioration of MTS-associated seizures. The importance of this issue on biologic effect is that radiosurgical treatment of eloquent brain regions would be possible if an effective subnecrotic dose could be found.
The Antiepileptic Radiosurgery Mechanism
Although radiosurgery has been shown to reduce seizures in various forms of medically intractable epilepsy, the mechanism by which this abatement occurs is not well understood. It has been suggested that radiation itself has a direct antiepileptic effect that may operate through several mechanisms. Because glial cells are more radiosensitive than neurons, Barcia-Salorio proposed that low-dose radiosurgery may reduce glial scar formation, allowing increased dendritic sprouting and improved cortical reorganization that results in fewer seizures.137 Elomaa theorized that the antiepileptic effect of radiation is further mediated through the effects of somatostatin.165 Although the clinical results of the most recent human studies suggest that the therapeutic efficacy of radiosurgery is linked to histopathologic changes and identifiable necrosis of mesial temporal structures, proof of this theory would need to come from direct observation and histologic evaluation of tissue samples from patients in whom radiosurgery has effectively controlled the seizures. This is unlikely to occur because only patients with persistent seizures after radiosurgery are likely to undergo further open resective microsurgery.
Surrogate markers of radiation effect and radiobiology such as changes on MRI have thus far shown variable results. Radiation-induced edema typically becomes evident in most patients 9 to 15 months after radiosurgery (see Fig. 68-1). These imaging findings, however, are usually time-limited and are often followed by focal atrophic changes. Thus, changes on MRI may not be diagnostic or indicative of true radiation necrosis. Furthermore, our pilot clinical trials have shown that MRI changes and peak MRI effects are poorly correlated with post-treatment symptoms. The actual biomechanism by which high-dose radiation and radiosurgery reduce neuronal hyperexcitability to ameliorate seizures will probably not be found or elucidated from human studies.
Although preclinical evidence and the results from early clinical human trials suggest that control of seizures might be possible with doses of radiosurgery that are lower than those typically applied to tumors,136,139 recent case reports also demonstrate the failure of low-dose radiosurgery to control seizures.140,163,164 Although failure of seizure control is easy to identify, it is a much more difficult task to determine that lack of seizure control is caused by an insufficient radiation dose. The time dependence of radiosurgical effects is also a confounding factor that has not been fully elucidated, and a consensus among different treating radiosurgical centers of when radiosurgical treatment has “failed” has not yet been reached.149 Furthermore, radiosurgery patients reported to have inadequate reduction of seizures commonly received radiation doses of 20 Gy or less, and these patients showed little evidence of radiation-induced necrosis or histopathologic changes in their tissue specimens.140,163,164 Thus, the best evidence to date from human and animal preclinical experiments suggests that there is a steep dose-response curve for seizure reduction and that some neuronal necrosis is required to produce abatement of seizures. This suggests that the radiosurgery dose required to reduce seizures is very close to the absolute tolerability threshold of human brain tissue.
Radiosurgical Treatment of Epilepsy Associated with Hypothalamic Hamartomas and Cavernomas
Radiosurgery is, by definition, a neurosurgical procedure that uses stereotactically focused, converging narrow ionizing beams to induce a desired biologic effect in a predetermined target with minimal radiation delivered to surrounding tissues and without opening the skull. Lars Leksell, a Swedish neurosurgeon, was the first to introduce the concept of radiosurgery in 1951.166 His goal in performing stereotactic procedures was to avoid the risk associated with craniectomy, notably bleeding and infection. The increased worldwide use of GKRS to treat various pathologies has shown the side-effect profile of radiosurgery to be rare, generally transient, and quite easily predictable.57 Once it is established that resection of a small, deeply seated lesion is associated with a significant risk for surgical complications or functional worsening (or both), GKRS must be discussed as an alternative. For these indications, GKRS frequently compares favorably with microsurgical removal in terms of safety, efficacy, and cost-effectiveness.
The first radiosurgical treatments in epilepsy surgery were performed by Talairach in the 1950s.167 Unlike Leksell, he already had expertise in epilepsy surgery and led one of the first large comprehensive programs for epilepsy surgery. As early as 1974, Talairach reported on the use of radioactive yttrium implants in patients with MTLE without space-occupying lesions and showed a high rate of seizure control in patients with epilepsies confined to the mesial structures of the temporal lobe.167 In 1980, Elomaa165 promoted the idea of the use of focal irradiation for the treatment of temporal lobe epilepsy based on the preliminary reports of Tracy, Von Wieser, and Baudouin.168,169 Furthermore, clinical experience with the use of GKRS and LINAC-based radiosurgery for arteriovenous malformations and cortical-subcortical tumors (mostly metastases and low-grade glial tumors) revealed an anticonvulsive effect of radiosurgery in the absence of tissue necrosis.138,170,171 A series of experimental studies in small animals confirmed this effect134,172 and emphasized a relationship to the dose delivered.153,154 Barcia Salorio and colleagues and later Lindquist and coauthors reported on a small and heterogeneous group of patients treated by radiosurgery for the purpose of alleviating seizures. However, their data were poor173–175 and unfortunately were never published in peer-reviewed papers, so precise data are unavailable.
The Department of Functional Surgery in Marseille is a major referral center for epilepsy surgery and radiosurgery and has reported the first comprehensively evaluated series of MTLE successfully treated by GKRS. The first use of GKRS for MTLE took place in 1993 and was reported in 1995 by this group.145 Several prospective trials by this group have demonstrated (1) the safety and efficacy of this approach,121,146 (2) a very specific timetable for the clinical and radiologic events,146,176 (3) the importance of the anterior parahippocampal cortex for seizure cessation,177,178 (4) the importance of the marginal dose (24 Gy) for efficacy,178 (5) sparing of verbal memory in dominant-side epilepsy,121 and (6) the nonlesional mechanism of action of radiosurgery.179 Recently, all these findings have been confirmed by a prospective trial in the United States.180 Since 1993, among a total of 8590 GKRS procedures, the Marseille group performed GKRS for epilepsy in 155 patients. The majority of these patients had MTLE (56 patients) or hypothalamic hamartoma (HH, 55 patients). The rest of the patients suffered from severe epilepsy associated with small benign lesions such as cavernous malformations (CMs), for which the epileptic zone was considered to be confined to the surrounding cortex.181
Cessation of seizures may be generated by a specific neuromodulatory effect of radiosurgery, without induction of a significant amount of histologic necrosis.145,176,179,182,183 Selection of the appropriate technical parameters (e.g., dose, volume target) that allow us to accurately achieve the desired functional effect without histologic damage remains an important challenge. A review of these cases, as well as other clinical and experimental data, suggests that the use of radiosurgery is beneficial only in patients in whom a strict preoperative definition of the extent of the epileptogenic network has been achieved184 and in whom strict rules of dose planning have been followed.178 The strategy is to identify patients in whom the safety-to-efficacy ratio makes radiosurgery advantageous or at least comparable to craniotomy and cortectomy.
In patients with HH, GKRS offers very low morbidity with efficacy similar to that of microsurgical alternatives.148,185 This has led us to systematically consider radiosurgery as the first-line treatment in patients with limited type I, II, and III and possibly type IV HH.185
Patients with CMs and a longer duration of epilepsy are thought to have a more widespread epileptogenic zone involving distant cortical structures.186–188 In patients with seizures arising from eloquent cortex surrounding the lesion, GKRS appears to be a suitable alternative. It is essential that electroclinical correlation be established for this strategy to work. Microsurgical excision remains the preferred approach for cortical-subcortical epileptogenic CMs that are not located in functional cortex.
Hypothalamic Hamartomas
HHs are rare congenital heterotopic lesions that are intrinsically epileptogenic when closely connected to the mammillary bodies.185,189 Patients classically experience gelastic seizures during the first years of life.190 In more severe forms of the disease, an epileptic encephalopathy characterized by drug resistance, various types of seizures with generalization (including drop attacks),190 cognitive decline,191–193 and severe psychiatric comorbidity develops in affected patients during the following years.194 Usually, the seizures begin early in life and are often particularly drug resistant from the onset. Commonly, the seizure semiology suggests the involvement of temporal or frontal lobe regions and a phenomenon of secondary epileptogenesis. HHs may also be asymptomatic or be associated with precocious puberty, neurological disorders (including epilepsy, behavior disturbances, and cognitive impairment), or both.
In 1969, Paillas and coworkers first showed that the epilepsy associated with HH can be alleviated by surgical resection of the HH lesion itself.189 Direct proof of the role of HH in generating seizure activity was provided by Munari and associates,195 who relied on data obtained from stereo encephalographic recordings from implanted depth electrodes.196,197 This evidence was reinforced by ictal single-photon emission computed tomographic studies198–200 and by other studies using depth electrodes.201,202
The natural history is unfavorable in the majority of patients because of behavioral symptoms (particularly aggressive behavior) and mental decline, which occur as a direct effect of the seizures.203 Interestingly, in our experience, reversal of these behavioral symptoms after radiosurgery seems to begin even before complete cessation of the seizures and appears to be correlated to the improvement in background electroencephalographic (EEG) activity. It is the authors’ speculation that these continuous discharges lead to the disorganization of several systems, including the limbic system, and that their disappearance accounts for the improvement seen in attention, memory, cognitive performance, and impulsive behavior. In these cases, radiosurgery’s role in reversal of the behavioral symptoms may be as or more important than its effect on decreasing seizure occurrence. Consequently, we consider it essential to operate on these young patients as early as possible, whatever the surgical approach being considered (resection or radiosurgery).
Surgical Approach
Even though the first successful and safe removal of an HH was reported by Paillas and coauthors in 1969,189 interest in surgical cure of this specific group of patients developed only in the 1990s. According to Valdueza and colleagues, epilepsy-related HH is observed only with medium/large sessile HHs broadly attached to tuber cinereum or mammillary body.204 Microsurgical resection in this critical area is associated with a significant risk for oculomotor palsy, hemiparesis, and visual field deficits.205,206 The first clinical series evaluating microsurgical resection via pterional and midline frontal approaches did not emphasize complications.204,207–210 However, in 2002, Palmini and coworkers observed severe complications after microsurgical resection in 7 of 13 patients by analyzing patients treated in several of the best centers for epilepsy surgery around the world.211 These complications included thalamocapsular infarcts with contralateral hemiplegia in 4 patients (with subtotal recovery), a transient third nerve paresis in 4 patients, central diabetes insipidus, and nonreversible hyperphagia.211 Nonetheless, they confirmed the efficacy of surgery for this kind of pathologic condition. More specifically, 3 patients showed complete seizure cessation and the remaining 10 patients had a greater than 90% reduction in the frequency of their seizures.
The rationale for surgical disconnection treatment of HH is that the lesion is not a neoplasm and removal of it is therefore not mandatory. A further factor favoring a disconnection technique is the possibility of avoiding the complications that may occur during dissection in the cisterns, a maneuver necessary for microsurgical resection. Delalande and associates actually stressed this point as favoring the simple disconnection of an HH versus complete excision of it because of the occurrence of severe complications in their first patient.205 When the clinical result is not satisfactory and the upper part of the lesion is mainly in the third ventricle, Delalande and colleagues proposed a second step via an endoscopic approach to the third ventricle. In 2003, Delalande and Fohlen published a series of 17 patients with a follow-up of between 1 month and 5.4 years.205a A second intervention (usually endoscopic) was necessary in 8 patients. In this excellent series, 47% of the patients (8 of 17) were seizure free, including 3 patients operated on twice. The authors reported some permanent severe complications, namely, 1 patient with hemiplegia, 1 with hemiparesis, 2 with hyperphagia, 1 with panhypopituitarism, 1 with hypothyroidism, and 1 with growth hormone deficiency. Transient morbidity included one case of meningitis and two cases of diabetes insipidus. In addition, the authors reported a postoperative frontal lobe ischemic complication that was apparently asymptomatic. In conclusion, only 6 patients (35%) were seizure free with no permanent toxicity. In contrast to reports of the use of a transcallosal approach, Delalande and Fohlen did not observe any memory deficit. Finally, the authors found a correlation between the completeness of exclusion and control of the seizures.
Transcallosal Interforniceal Approach
The transcallosal interforniceal route was initially proposed by the Melbourne team to approach HH via the third ventricle.212,213 In January 2001 this team published a series of 5 patients operated on with this approach. Two of the patients were seizure free, 2 others were very much improved, and 1 failed to improve. No permanent complications were reported except an increased appetite in 2 subjects (obesity developing in 1 of them). Some months later, they reported a series of 25 patients operated on by the same route, including 21 with sufficient follow-up for evaluation (follow-up of 3 to 52 months). The results were not as favorable with regard to complications in this larger group but more favorable in terms of seizure cessation rate (67% instead of 40%). Resection was nearly complete (95% to 100%) in 13 of the 21 (62%) patients. Some transient side effects were reported: hypernatremia (>150 mEq/L) in 12 patients (57%), somnolence in 7 (33%), body temperature instability in 5 (24%), and third nerve palsy in 1 patient. Permanent complications were also reported: thalamic infarct in 2 patients (capsulothalamic in 1), appetite stimulation in 10 patients (48%) with permanence in 5 (24%); low thyroxin level in 6 (29%), permanent in 4 (19%); anxiety and depression in 4 (19%), permanent in 3 (14%); and short-term memory deficit in 8 patients (38%), permanent in 3 (14%). In this group of 21 patients, a total of 38 transient complications and 18 permanent complications occurred. The more important concern, according to the authors, was the short-term memory deficits. Thus, transcallosal interforniceal resection is a very attractive approach for stage I, II, and III HH.185 Both the Barrow214 and Melbourne215 series report very good results, with 54% and 52% of patients, respectively, seizure free postoperatively. The major problem with these approaches is the frequent injury to the fornices. In fact, a clear short-term memory deficit was observed in 58% and 48% of the children, respectively. The long-term neuropsychological outcomes were not known precisely because the majority of the children lived in places distant from the hospital and could not be monitored adequately with appropriate neuropsychological testing, especially in the Kerrigan team report.216 With this limitation, it is noteworthy that a persistent major short-term memory deficit was described in 8% and 14% of the children. In the Barrow series, major weight gain was reported in 19% of the patients on short-term postoperative observation, but no reduction in the long-term observation. Diabetes insipidus was reported in 15% of the children in short-term and in 4% in long-term follow-up. Permanent hemianopia was observed in 4%. According to family perception, behavioral function was better in 88.5% of the patients operated on and worse in 3.8%. Kerrigan and coworkers found age, duration of the epilepsy, lesion size, and extent of resection to be predictors of complete seizure cessation after transcallosal resection of HH.216 The mean percent resection in seizure-free patients was 90% versus 76% in non–seizure-free patients. The interhemispheric transchoroidal approach may be an interesting alternative to the transcallosal interforniceal one in reducing the risk for postoperative permanent memory deficit.
Stereotactic Radiofrequency Thermocoagulation
Stereotactic radiofrequency thermocoagulation was proposed by some authors for lesioning of HH instead of the direct microsurgical approach.199,217,218 Because of the irregular conformation and close proximity to the normal hypothalamus, mammillary bodies, and visual pathways, direct lesioning with a stereotactic probe carries a certain risk. The interface between HH and the normal hypothalamus may also be unclear, so damage to perforating vessels “en passage” (e.g., thalamoperforating vessels) may be possible. Guthrie (personal communication, Montreal, 2001) reported on 12 patients who underwent thermocoagulation (mainly for small lesions). Of these 12 patients, only 3 were seizure free (25%) after thermocoagulation, thus demonstrating that this procedure is less successful than microsurgery or radiosurgery. Additionally, the author reported some transient complications in 2 patients (1 with transient third nerve palsy and 1 with a transient amnesic deficit) and a very severe complication in 1 (brainstem infarction). Finally, according to the author, the main disadvantages of this technique are blind passage of the probe, difficulty accessing the hamartoma, absence of control over the extent of the physical effect, and the theoretical requirement for multiple passes of the probe (implying greater increments of risk) because of the complexity of shape and relationship to critical structures in the majority of cases. In addition, we know from the literature that a 1% to 2% risk for hemorrhage is associated with all insertions of a stereotactic probe into the brain.
Stimulation of the Hypothalamic Hamartoma
Other authors, such as the Grenoble team, proposed stimulation of the HH. The condition of the patient reported by this team was significantly improved after stimulation, but the severity of the side effects (weight gain) led them to renounce this therapy.219 Sadikot and Dubeau reported a patient treated by implantation in both the anterior thalamus and the HH, with an unsatisfactory clinical result (Dubeau, personal communication, Montreal, 2001).
Vagus Nerve Stimulation
Finally, left vagus nerve stimulation was reported by Murphy and colleagues in six children with HH and epilepsy.220 In this small group, none of the patients were seizure free after stimulation; three were improved, but no significant improvement was seen in three. Brandberg and coauthors reported the Swedish experience, including five patients treated with this technique. In the five patients with implants, none demonstrated any kind of improvement.221 This experience clearly indicates that vagus nerve stimulation is a palliative and poorly effective therapy in comparison to radiosurgery or microsurgery, in which almost all patients have their conditions improved and more than 50% become seizure free. This limitation in efficacy, cost, the requirement for changing the battery every 4 to 5 years, and the 3% risk for infection related to pacemaker implantation, in our opinion, must lead one to consider vagus nerve stimulation as only a second or third choice when radiosurgery, microsurgery, or both have failed or are contraindicated. However, in such circumstances there are several arguments in favor of vagus nerve stimulation instead of callosotomy. The efficacy of callosotomy is actually very limited,222 surgical risk is high, and the effect on behavior and psychiatric symptoms is very poor. Conversely, in Murphy and coworkers’ report of four patients with severe autistic behavior, all four were dramatically improved by intermittent stimulation.220
Interstitial Radiosurgery
Interstitial radiosurgery has recently been proposed as an additional possible treatment of HH.223 The Freiburg neurosurgical team has a large experience with the use of brachytherapy for a variety of neurosurgical conditions. This team has recently reported a series of seven patients with a relatively short follow-up. The efficacy of the treatment was quite poor, with a seizure cessation rate of just 28.6% (two of seven).223 The authors have not reported any “major” permanent deficits up to the present in this series. The principle of interstitial radiosurgery is insertion of a seed of radioactive material (in this case 125I) into the lesion. The advantage of this technique is the very reliable falloff of the radioisotopic dose. Its major limitation is the absence of any possibility of shaping of the field of irradiation. Because of the close vicinity of the mammillary body and fornix, this limitation forces the operator to undercover the part of the lesion surrounding these structures. This technical failure perhaps explains the very low rate of cessation of seizures and makes the method poorly adapted to the treatment of HH. On the contrary, GKRS allows the shaping of complex, very conformal irradiation fields and specific dose planning, thus making it more adapted to HH.
Radiosurgery
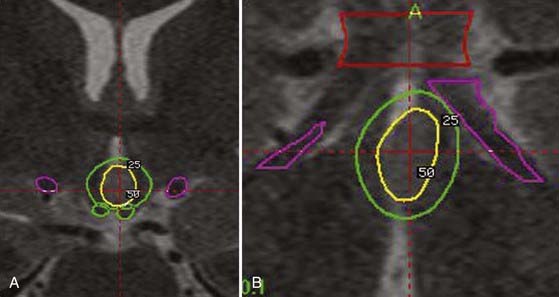
We retrospectively analyzed the results of radiosurgery in a series of 10 patients from centers around the world.148 The excellent safety-to-efficacy ratio (all improved, 50% cured, and no adverse effects except 1 patient with poikilothermia) led us to organize a prospective multicenter trial. Our series of 55 prospectively evaluated patients is unique in number and has been published in a preliminary report.185 Satisfactory follow-up was available for 27 patients. The preoperative cognitive deficits, behavioral disturbances, and relationship of seizure severity and anatomic type to cognitive abilities were characterized.191 The goal of the preoperative work-up was to adequately select candidates for inclusion and evaluate the patients’ baseline neurological and endocrinologic function. All radiosurgical procedures were carried out with the Leksell 201-source Cobalt 60 Gamma Knife (Elekta Instrument, Stockholm). We consistently used multi-isocentric complex dose planning of high conformity and selectivity (Fig. 68-3). We used low peripheral doses to take into account the close relationship with the optic pathways and the hypothalamus (median, 17 Gy; range, 13 to 26 Gy). The lesions treated were generally small (median, 9.5 mm; range, 5 to 26 mm). We paid special attention to the dose delivered to the mamillary body and to the fornix, and we always tried to tailor the dose plan for each patient based on the use of a single run of shots with the 4-mm collimator. Patients were evaluated with respect to seizures, cognition, behavior, and endocrine status 6, 12, 18, 24, and 36 months after radiosurgery and then every year thereafter. Among these patients, 10 are seizure free (37%) and 6 are very much improved (22.2%) with a huge reduction in seizures (usually only rare residual gelastic seizures) associated with a dramatic improvement in behavior and cognition. Overall, an excellent result was obtained in 60% of the patients. According to our policy, the patient and family are offered a second radiosurgery in the event of a partial benefit when the lesion is anatomically small and well defined. Five patients (18.5%) with small hamartomas were only modestly improved and are being considered for a second session of radiosurgery. Two have reported no significant improvement up to the present. A microsurgical approach was performed in 4 patients (14.8%) with quite large HHs and poor efficacy of radiosurgery. Of these patients, 2 have been cured and 2 failed to respond. The radiosurgical treatment was carried out twice in 9 patients.
Topologic Hypothalamic Hamartoma Classification and Treatment Strategy
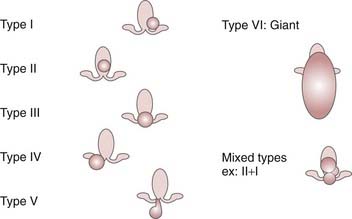
Topologic classification of the lesion based on a good high-resolution MRI scan is a key feature in the decision-making process (Fig. 68-4).185 Previous classifications have been based on anatomic51,224,225 or surgical51 considerations. These classifications do not describe the large diversity of these lesions and their therapeutic consequences. As underlined by Palmini and coworkers, the exact location of the lesion in relation to the interpeduncular fossa and the walls of the third ventricle correlates with the extent of excision, seizure control, and complication rate.211 Accordingly, we classify HHs according to their topology based on our original classification.226 In our experience, this classification correlates with clinical semiology and severity and is especially critical for selection of the surgical strategy.
In type II lesions (when the lesion is small and mainly in the third ventricle), radiosurgery is certainly a safer alternative. Even though the endoscopic and transcallosal interforniceal approaches have been well described, the risks of short-term deterioration in memory, endocrinologic disturbance (hyperphagia with obesity, low thyroxin, disturbance in sodium metabolism), and thalamic or thalamocapsular infarcts have been reported even in the hands of highly skilled and experience neurosurgeons. In exceptional cases of very severe recurrent status epilepticus, we recommend open surgery though either a transcallosal interforniceal approach or an endoscopic approach (depending on the width of the third ventricle). If the lesion is small and the third ventricle large, an endoscopic approach is chosen (see Fig. 68-3).
In type IV lesions (the lesion is sessile in the cistern), disconnection can be discussed (pterional approach with or without orbitozygomatic osteotomy). However, if the lesion is small, GKRS can be recommended because of its safety and ability to simultaneously treat the small associated part of the lesion in the hypothalamus itself, which is frequently visible on high-resolution MRI. In Delalande and Fohlen’s experience, just 2 of 14 patients were seizure free after a single disconnection through a pterional approach.51 Consequently, we recommend this approach only for lesions too large for GKRS as a first step in a staged approach. In most circumstances, the patient is improved but not seizure free after the first surgical step, and GKRS is initiated at 3 months as a second step in the treatment.
Limits and Strengths of Radiosurgery for Hypothalamic Hamartoma
Two major questions remain. First, we know that complete treatment or resection of the lesion is not always necessary for control,213,227,228 but we do not know how to predict in an individual patient the amount (and mapping) of the HH that must be treated to obtain a complete antiepileptic effect. Second, we know that these patients frequently have an electroclinical semiology that suggests involvement of the temporal or frontal lobe and that it can mimic a secondary epileptogenesis phenomenon.195,229 In our experience, some of these patients can be cured completely by isolated treatment of the HH, whereas in others, a partial result is obtained, with residual seizures despite significant overall psychiatric and cognitive improvement. In this second group, it is tempting to propose that a secondary epileptogenic area is accounting for the partial failure.
Cavernous Malformations
CMs are congenital vascular malformations of the brain. They may cause hemorrhage and repetitive neurological deficits, but the most frequent initial symptom is epilepsy.230 The epileptogenicity of these lesions results from the ongoing deposition of iron and blood products at the margin of the lesion.231 The incidence of drug-resistant epilepsy is not well known. However, in one large series, 40% of patients with a supratentorial CM had drug-resistant epilepsy.232
GKRS for CMs is controversial, and the few available studies have reported conflicting results concerning its protection from hemorrhage.93,141,233,234 In view of the previously observed relatively high rate of radiation-induced side effects93 and the safety and efficacy of microsurgical excision,231 the latter technique is usually recommended for this indication. Nevertheless, a low risk of clinical bleeding235 associated with CMs located in cortical/subcortical sites, unlike the expectation with deep-seated lesions, has clearly been demonstrated. These findings explain why seizure control is often the main indication for surgery in patients with this condition. Microsurgery is considered the best treatment option in patients with intractable epilepsy. However, in some series, a beneficial effect on the course of epilepsy was reported for supratentorial CMs treated by GKRS.233 Because GKRS is generally associated with a minimal risk of injury to the surrounding nontarget neural tissues, this technique could be a good alternative for this indication, particularly when the CM is located in a brain region associated with high surgical risk.
The effectiveness of GKRS in the management of drug-resistant seizures associated with CMs has been evaluated in a retrospective multicenter study (Marseille, France; Graz, Austria; Komaki, Japan; Sheffield, England; and Prague, Czech Republic).147 Forty-nine patients with cortical/subcortical CMs and severe long-term drug-resistant epilepsy were included and had been observed for longer than 1 year after GKRS. The mean duration of epilepsy before these GKRS procedures was 7.5 (±9.3) years. The mean frequency of seizures was 6.9 per month (±14). The mean marginal radiation dose was 19.17 ± 4.4 Gy. Among the 49 patients, 35% had a CM located in or involving a highly functional area. At the last follow-up examination, 53% were seizure free (Engel’s class I), including 49% in class IA and 4% with occasional auras. A highly significant decrease in the number of seizures was achieved in 20% (class IIB). The remaining 27% of patients showed little or no improvement. The mediotemporal site was associated with a higher risk for failure. One patient bled during the observation period, and another experienced radiation-induced edema with transient aphasia. Excision was performed after radiosurgery in 5 patients, and a second radiosurgical treatment was carried out in 1 patient.
Management Strategy
The management strategy for CM is complex, but the general consensus is that microsurgical resection of the lesion is the best treatment in patients with intractable epilepsy. Two surgical strategies can be applied, depending on the date of onset and features of the seizures.196 For patients with seizures of recent onset in whom correlation between symptoms and location of the CM is good, resection of the lesion is the best method (lesionectomy). Epilepsy surgery strategies should be preferred for patients with a long history of chronic epilepsy in whom correlation of clinical and EEG findings is unclear. In these cases, a multimodal approach that sometimes includes invasive techniques (depth electrodes if necessary) should be considered. In this context, surgery should aim to control the epilepsy rather than remove the lesion. The appropriate use or nonuse of these criteria probably explains why the success rate for surgical excision of CM varies from 20% to 80% in the literature.186,188,196,231,232,235–240 The effect of GKRS on epilepsy is probably different from that of interventions in which prevention of bleeding is the objective. Of the 11 patients with intractable epilepsy before GKRS in the series of Kida and colleagues, 7 (64%) achieved good seizure control after GKRS even though there was no apparent change in the size of the CM.233 The apparent size of a CM seems to fluctuate and depend on the production and absorption of blood pigments.
Although a reliable prospective study with long-term follow-up is still needed, this retrospective series indicates that GKRS can be effective for this indication and that morbidity can be low. It should be emphasized that the GKRS procedure treats the lesion and the immediate surrounding tissue. The results observed in this study were comparable to those reported in the recent literature for lesionectomy.186,188,196,231,232,235–240 The most valuable prognostic factor for outcome after GKRS was lesion location. A probable explanation for this finding is that the relationship between the lesion and the epileptogenic zone is particularly direct in certain regions, especially simple motor partial seizures associated with CMs in the rolandic region. In contrast, when a CM is located in the mediotemporal region, a more complex organization probably accounts for the extended epileptogenic zone, and epilepsy surgery strategies should be preferred. This probably explains why patients with complex partial seizures had a less favorable outcome than did patients with simple partial seizures in our study.
Bleeding Risk
We lack convincing data concerning the efficacy of GKRS in prevention of the bleeding associated with CMs. Early authors reported no effect.93,233,234 Recently, some authors have reported a decrease in bleeding 2 years after GKRS,141,241 but the majority reported no effect. Thus, the role of radiosurgery in the management of these lesions remains controversial.141 Moreover, in some studies, the incidence of radiation-induced complications was particularly high after radiosurgical treatment of the CM.93,242 This is perhaps explained by poor radiologic localization and the dose-planning software available at that time. This problem was countered by the use of a lower radiation dose than usually applied for other vascular targets.
Technical Questions
Target Definition
Some articles reporting microsurgical resection of occult malformations suggest that resection of the hemosiderin-stained tissue surrounding the lesion is critical for eliminating seizures.243 Obviously, the reason for cessation of seizures is not the dose received by the margin of the target (even if defined generously) but the ionizing radiation received by the epileptogenic zone located close to the CM.
Dose Selection
Various doses have been tested in experimental studies on animals183,244,245 and in clinical experience with GKRS on epileptic lesions.148,246,247 There is clearly a relationship between dose and effectiveness in abating seizures. Depending on the dose, animals undergo cessation of seizures, reduction in seizure frequency, no change, or worsening of epilepsy.245 Dose selection is obviously crucial for clinical control of seizures. We believe that at present satisfactory evidence is lacking for recommending systematic use of GKRS for this indication (cortical-subcortical CM associated with drug-resistant epilepsy). Although this series is very encouraging, the aim of this publication is not to promote widespread use of GKRS. Our main interest is to gain insight into the relationship between dose selection, target volume, and effectiveness in treating epilepsy in this unique population. All patients treated worldwide have received comparable doses (around a 20-Gy peripheral dose) and volumes, which may explain the absence of a statistical correlation between these two parameters and outcome (Fig. 68-5). Similarly, all patients have had a long history of epilepsy (mean duration of epilepsy before GKRS, 7.5 ± 9.3 years), which prevented us from correlating the duration of the epilepsy with the probability of seizure cessation.
Barcia-Salorio JL, Barcia JA, Hernandez G, et al. Radiosurgery of epilepsy. Long-term results. Acta Neurochir Suppl. 1994;62:111-113.
Barcia Salorio JL, Roldan P, Hernandez G, et al. Radiosurgery treatment of epilepsy. Appl Neurophysiol. 1985;48:400-403.
Bien CG, Kurthen M, Baron K, et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42:1416-1421.
Cascino GD, Andermann F, Berkovic SF, et al. Gelastic seizures and hypothalamic hamartomas: evaluation of patients undergoing chronic intracranial EEG monitoring and outcome of surgical treatment. Neurology. 1993;43:747-750.
Engel JJr. Surgery for seizures. N Engl J Med. 1996;334:647-652.
Gaffey C, Monotoya V, Lyman J, et al. Restriction of the spread of epileptic discharges in cats by mean of Bragg Peak intracranial irradiation. Int J Appl Radiat Isotope. 1981;32:779-787.
Kitchen N. Experimental and clinical studies on the putative therapeutic efficacy of cerebral irradiation (radiotherapy) in epilepsy. Epilepsy Res. 1995;20:1-10.
Maesawa S, Kondziolka D, Dixon C, et al. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93:1033-1040.
Munari C, Kahane P, Francione S, et al. Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study). Electroencephalogr Clin Neurophysiol. 1995;95:154-160.
Ng YT, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia. 2006;47:1192-1202.
Nguyen DK, Spencer SS. Recent advances in the treatment of epilepsy. Arch Neurol. 2003;60:929-935.
Porter P, Willinsky R, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
Regis J, Bartolomei F, de Toffol B, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery. 2000;47:1343-1351.
Régis J, Bartolomei F, Kida Y, et al. Radiosurgery of epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. 2000;47:1091-1097.
Régis J, Bartolomei F, Rey M, et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40:1551-1556.
Regis J, Hayashi M, Eupierre LP, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Acta Neurochir Suppl. 2004;91:33-50.
Régis J, Peragut JC, Rey M, et al. First selective amygdalohippocampic radiosurgery for mesial temporal lobe epilepsy. Stereotact Funct Neurosurg. 1994;64(suppl 1):191-201.
Regis J, Rey M, Bartolomei F, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504-515.
Spencer SS, Berg AT, Vickrey BG, et al. Initial outcomes in the Multicenter Study of Epilepsy Surgery. Neurology. 2003;61:1680-1685.
Talairach J, Bancaud J, Szikla G, et al. Approche nouvelle de la neurochirurgie de l’epilepsie. Méthodologie stéréotaxique et résultats thérapeutiques. Neurochirurgie. 1974;20:92-98.
Whang CJ, Kwon Y. Long-term follow-up of stereotactic Gamma Knife radiosurgery in epilepsy. Stereotact Funct Neurosurg. 1996;66(suppl 1):349-356.
Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
Yang I, Barbaro NM. Advances in the radiosurgical treatment of epilepsy. Epilepsy Curr. 2007;7:31-35.
1 Nguyen DK, Spencer SS. Recent advances in the treatment of epilepsy. Arch Neurol. 2003;60:929-935.
2 Kreil W, Luggin J, Fuchs I, et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
3 Lavine S, Petrovich Z, Cohen Gadol A, et al. Gamma knife radiosurgery for metastatic melanoma: an analysis of survival, outcome, and complications. Neurosurgery. 1999;44:59-64.
4 Lee JY, Niranjan A, McInerney J, et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65-72.
5 Linskey ME, Lunsford LD, Flickinger JC. Tumor control after stereotactic radiosurgery in neurofibromatosis patients with bilateral acoustic tumors. Neurosurgery. 1992;31:829-838.
6 Linskey ME, Lunsford LD, Flickinger JC, et al. Stereotactic radiosurgery for acoustic tumors. Neurosurg Clin N Am. 1992;3:191-205.
7 Lunsford LD, Kamerer DB, Flickinger JC. Stereotactic radiosurgery for acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1990;116:907-909.
8 Lunsford LD, Kondziolka D, Flickinger JC. Radiosurgery as an alternative to microsurgery of acoustic tumors. Clin Neurosurg. 1992;38:619-634.
9 Lunsford LD, Kondziolka DS, Flickinger JC. Radiosurgery of tumors of the cerebellopontine angle. Clin Neurosurg. 1994;41:168-184.
10 Nicolato A, Ferraresi P, Foroni R, et al. Gamma Knife radiosurgery in skull base meningiomas. Preliminary experience with 50 cases. Stereotact Funct Neurosurg. 1996;66(suppl 1):112-120.
11 Nicolato A, Ria A, Foroni R, et al. Gamma knife radiosurgery in brain metastases from testicular tumors. Med Oncol. 2005;22:45-56.
12 Noren G. Gamma knife radiosurgery of acoustic neurinomas. A historic perspective. Neurochirurgie. 2004;50:253-256.
13 Norén G, Greitz D, Hirsch A. Gamma knife surgery in acoustic tumours. Acta Neurochir Suppl. 1993;58:104-107.
14 Nwokedi EC, DiBiase SJ, Jabbour S, et al. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50:41-46.
15 Ojemann SG, Sneed PK, Larson DA, et al. Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg. 2000;93(suppl 3):62-67.
16 Okunieff P, Schell MC, Ruo R, et al. Long-term management of patients with multiple brain metastases after shaped beam radiosurgery. Case report and review of the literature. J Neurosurg. 2004;101(suppl 3):406-412.
17 Payne BR, Prasad D, Szeifert G, et al. Gamma surgery for intracranial metastases from renal cell carcinoma. J Neurosurg. 2000;92:760-765.
18 Pollock BE. Stereotactic radiosurgery for intracranial meningiomas: indications and results. Neurosurg Focus. 2003;14(5):e4.
19 Pollock BE, Link MJ, Foote RL, et al. Radiosurgery as primary management for meningiomas extending into the internal auditory canal. Stereotact Funct Neurosurg. 2004;82:98-103.
20 Pollock BE, Lunsford LD, Kondziolka D, et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36:215-224.
21 Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745-759.
22 Samii M, Matthies C. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:892-894.
23 Selch MT, Pedroso A, Lee SP, et al. Stereotactic radiotherapy for the treatment of acoustic neuromas. J Neurosurg. 2004;101(suppl 3):362-372.
24 Seung SK, Sneed PK, McDermott MW, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998;4:103-109.
25 Shaw E, Scott C, Souhami L, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996;34:647-654.
26 Somaza S, Kondziolka D, Lunsford LD, et al. Stereotactic radiosurgery for cerebral metastatic melanoma. J Neurosurg. 1993;79:661-666.
27 Spiegelmann R, Gofman J, Alezra D, et al. Radiosurgery for acoustic neurinomas (vestibular schwannomas). Isr Med Assoc J. 1999;1:8-13.
28 Subach BR, Lunsford LD, Kondziolka D, et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443.
29 Torres RC, Frighetto L, De Salles AA, et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003;14(5):e5.
30 Unger F, Schröttner O, Haselsberger K, et al. Gamma knife radiosurgery for hypothalamic hamartomas in patients with medically intractable epilepsy and precocious puberty. Report of two cases. J Neurosurg. 2000;92:726-731.
31 Unger F, Walch C, Haselsberger K, et al. Radiosurgery of vestibular schwannomas: a minimally invasive alternative to microsurgery. Acta Neurochir (Wien). 1999;141:1281-1285.
32 Unger F, Walch C, Papaefthymiou G, et al. Radiosurgery of residual and recurrent vestibular schwannomas. Acta Neurochir (Wien). 2002;144:671-676.
33 Warnick RE, Darakchiev BJ, Breneman JC. Stereotactic radiosurgery for patients with solid brain metastases: current status. J Neurooncol. 2004;69:125-137.
34 Weil MD. Advances in stereotactic radiosurgery for brain neoplasms. Curr Neurol Neurosci Rep. 2001;1:233-237.
35 Adler JR, Cox RS, Kaplan I, et al. Stereotactic radiosurgical treatment of brain metastases. J Neurosurg. 1992;76:444-449.
36 Alexander E3rd, Loeffler JS. Radiosurgery for primary malignant brain tumors. Semin Surg Oncol. 1998;14:43-52.
37 Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265-1278.
38 Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
39 Bakay RA. Stereotactic radiosurgery in the treatment of brain tumors. Clin Neurosurg. 1992;39:292-313.
40 Battista RA, Wiet RJ. Stereotactic radiosurgery for acoustic neuromas: a survey of the American Neurotology Society. Am J Otol. 2000;21:371-381.
41 Becker G, Duffner F, Kortmann R, et al. Radiosurgery for the treatment of brain metastases in renal cell carcinoma. Anticancer Res. 1999;19(2C):1611-1617.
42 Black PM. Solitary brain metastases. Radiation, resection, or radiosurgery? Chest. 1993;103(4 suppl):367S-369S.
43 Boyd TS, Mehta MP. Stereotactic radiosurgery for brain metastases. Oncology (Williston Park). 1999;13:1397-1409.
44 Boyd TS, Mehta MP. Radiosurgery for brain metastases. Neurosurg Clin N Am. 1999;10:337-350.
45 Brada M. Radiosurgery for brain tumours. Eur J Cancer. 1991;27:1545-1548.
46 Brada M, Laing R. Radiosurgery/stereotactic external beam radiotherapy for malignant brain tumours: the Royal Marsden Hospital experience. Recent Results Cancer Res. 1994;135:91-104.
47 Brophy BP. Acoustic neuroma—surgery or radiosurgery? Stereotact Funct Neurosurg. 2000;74:121-128.
48 Buatti JM, Friedman WA, Bova FJ, et al. Treatment selection factors for stereotactic radiosurgery of intracranial metastases. Int J Radiat Oncol Biol Phys. 1995;32:1161-1166.
49 Coffey RJ, Lunsford LD, Flickinger JC. The role of radiosurgery in the treatment of malignant brain tumors. Neurosurg Clin N Am. 1992;3:231-244.
50 Combs SE, Thilmann C, Debus J, et al. Long-term outcome of stereotactic radiosurgery (SRS) in patients with acoustic neuromas. Int J Radiat Oncol Biol Phys. 2006;64:1341-1347.
51 Delalande O, Fohlen M. Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo). 2003;43:61-68.
52 DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
53 Engenhart R, Kimmig BN, Höver KH, et al. Long-term follow-up for brain metastases treated by percutaneous stereotactic single high-dose irradiation. Cancer. 1993;71:1353-1361.
54 Ewend MG, Carey LA, Brem H. Treatment of melanoma metastases in the brain. Semin Surg Oncol. 1996;12:429-435.
55 Fedorcsák I, Sipos L, Horváth A, et al. Multiple intracranial melanoma metastases treated with surgery and radiosurgery with long term control. A case report. J Neurooncol. 1993;16:173-176.
56 Flickinger JC, Kondziolka D, Lunsford LD. Dose and diameter relationships for facial, trigeminal, and acoustic neuropathies following acoustic neuroma radiosurgery. Radiother Oncol. 1996;41:215-219.
57 Flickinger JC, Kondziolka D, Lunsford LD. Clinical applications of stereotactic radiosurgery. Cancer Treat Res. 1998;93:283-297.
58 Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797-802.
59 Fohlen M, Lellouch A, Delalande O. Hypothalamic hamartoma with refractory epilepsy: surgical procedures and results in 18 patients. Epileptic Disord. 2003;5:267-273.
60 Foote KD, Friedman WA, Buatti JM, et al. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440-449.
61 Forster DM, Kemeny AA, Pathak A, et al. Radiosurgery: a minimally interventional alternative to microsurgery in the management of acoustic neuroma. Br J Neurosurg. 1996;10:169-174.
62 Friedman WA, Bova FJ, Bollampally S, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-307.
63 Friedman WA, Murad GJ, Bradshaw P, et al. Linear accelerator surgery for meningiomas. J Neurosurg. 2005;103:206-209.
64 Gerosa M, Nicolato A, Foroni R. The role of gamma knife radiosurgery in the treatment of primary and metastatic brain tumors. Curr Opin Oncol. 2003;15:188-196.
65 Gerosa M, Nicolato A, Foroni R, et al. Gamma knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002;97(5 suppl):515-524.
66 Gerosa M, Nicolato A, Severi F, et al. Gamma Knife radiosurgery for intracranial metastases: from local tumor control to increased survival. Stereotact Funct Neurosurg. 1996;66(suppl 1):184-192.
67 Gerosa MA, Nicolato A, Berlucchi S, et al. Gamma Knife radiosurgery of primary and metastatic malignant brain tumors, a preliminary report. Stereotact Funct Neurosurg. 1995;64(suppl 1):56-66.
68 Gieger M, Wu JK, Ling MN, et al. Response of intracranial melanoma metastases to stereotactic radiosurgery. Radiat Oncol Invest. 1997;5:72-80.
69 Goyal S, Prasad D, Harrell FJr, et al. Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg. 2005;103:218-223.
70 Hakim R, Alexander E3rd, Loeffler JS, et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-453.
71 Harris AE, Lee JY, Omalu B, et al. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60:298-305.
72 Hawighorst H, Debus J, Schreiber W, et al. Contrast-enhanced magnetization transfer imaging: improvement of brain tumor conspicuity and delineation for radiosurgical target volume definition. Radiother Oncol. 1997;43:261-267.
73 Hayhurst C, Dhir J, Dias PS. Stereotactic radiosurgery and vestibular schwannoma: hydrocephalus associated with the development of a secondary arachnoid cyst: a report of two cases and review of the literature. Br J Neurosurg. 2005;19:178-181.
74 Huang CF, Tu HT, Lo HK, et al. Radiosurgery for vestibular schwannomas. J Chin Med Assoc. 2005;68:315-320.
75 Hudgins WR. Patients’ attitude about outcomes and the role of gamma knife radiosurgery in the treatment of vestibular schwannomas. Neurosurgery. 1994;34:459-463.
76 Hudgins WR, Barker JL, Schwartz DE, et al. Gamma Knife treatment of 100 consecutive meningiomas. Stereotact Funct Neurosurg. 1996;66(suppl 1):121-128.
77 Iwai Y, Yamanaka K. Gamma Knife radiosurgery for skull base metastasis and invasion. Stereotact Funct Neurosurg. 1999;72(suppl 1):81-87.
78 Iwai Y, Yamanaka K, Ishiguro T. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas. Neurosurgery. 2003;52:517-524.
79 Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59:283-289.
80 Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64:207-212.
81 Kamath R, Ryken TC, Meeks SL, et al. Initial clinical experience with frameless radiosurgery for patients with intracranial metastases. Int J Radiat Oncol Biol Phys. 2005;61:1467-1472.
82 Kobayashi T, Kida Y, Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
83 Koc M, McGregor J, Grecula J, et al. Gamma Knife radiosurgery for intracranial metastatic melanoma: an analysis of survival and prognostic factors. J Neurooncol. 2005;71:307-313.
84 Kondziolka D, Lunsford LD, Coffey RJ, et al. Stereotactic radiosurgery of meningiomas. J Neurosurg. 1991;74:552-559.
85 Barker FG2nd, Butler WE, Lyons S, et al. Dose-volume prediction of radiation-related complications after proton beam radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 2003;99:254-263.
86 Betti OO, Munari C, Rosler R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311-321.
87 Bollet MA, Anxionnat R, Buchheit I, et al. Efficacy and morbidity of arc-therapy radiosurgery for cerebral arteriovenous malformations: a comparison with the natural history. Int J Radiat Oncol Biol Phys. 2004;58:1353-1363.
88 Chang TC, Shirato H, Aoyama H, et al. Stereotactic irradiation for intracranial arteriovenous malformation using stereotactic radiosurgery or hypofractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:861-870.
89 de Tribolet N. Radiosurgery of deep arteriovenous malformations. J Neurosurg. 2004;100:205-206.
90 Duffner F, Freudenstein D, Becker G, et al. Combined treatment effects after embolization and radiosurgery in high-grade arteriovenous malformations. Case report and review of the literature. Stereotact Funct Neurosurg. 2000;75:27-34.
91 Eisenschenk S, Gilmore RL, Friedman WA, et al. The effect of LINAC stereotactic radiosurgery on epilepsy associated with arteriovenous malformations. Stereotact Funct Neurosurg. 1998;71:51-61.
92 Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional analysis of complication outcomes after arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 1999;44:67-74.
93 Karlsson B, Kihlström L, Lindquist C, et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
94 Karlsson B, Lax I, Söderman M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49:1045-1051.
95 Karlsson B, Lax I, Söderman M, et al. Prediction of results following Gamma Knife surgery for brain stem and other centrally located arteriovenous malformations: relation to natural course. Stereotact Funct Neurosurg. 1996;66(Suppl 1):260-268.
96 Kida Y, Kobayashi T, Tanaka T, et al. Seizure control after radiosurgery on cerebral arteriovenous malformations. J Clin Neurosci. 2000;7(suppl 1):6-9.
97 Kurita H, Ueki K, Shin M, et al. Headaches in patients with radiosurgically treated occipital arteriovenous malformations. J Neurosurg. 2000;93:224-228.
98 Kwon Y, Jeon SR, Kim JH, et al. Analysis of the causes of treatment failure in gamma knife radiosurgery for intracranial arteriovenous malformations. J Neurosurg. 2000;93(suppl 3):104-106.
99 Levegrün S, Hof H, Essig M, et al. Radiation-induced changes of brain tissue after radiosurgery in patients with arteriovenous malformations: correlation with dose distribution parameters. Int J Radiat Oncol Biol Phys. 2004;59:796-808.
100 Levy EI, Niranjan A, Thompson TP, et al. Radiosurgery for childhood intracranial arteriovenous malformations. Neurosurgery. 2000;47:834-841.
101 Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75:512-524.
102 Maesawa S, Flickinger JC, Kondziolka D, et al. Repeated radiosurgery for incompletely obliterated arteriovenous malformations. J Neurosurg. 2000;92:961-970.
103 Maruyama K, Kawahara N, Shin M, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352:146-153.
104 Miyawaki L, Dowd C, Wara W, et al. Five year results of LINAC radiosurgery for arteriovenous malformations: outcome for large AVMS. Int J Radiat Oncol Biol Phys. 1999;44:1089-1106.
105 Nataf F, Ghossoub M, Schlienger M, et al. Bleeding after radiosurgery for cerebral arteriovenous malformations. Neurosurgery. 2004;55:298-305.
106 Nicolato A, Lupidi F, Sandri MF, et al. Gamma Knife radiosurgery for cerebral arteriovenous malformations in children/adolescents and adults. Part I: differences in epidemiologic, morphologic, and clinical characteristics, permanent complications, and bleeding in the latency period. Int J Radiat Oncol Biol Phys. 2006;64:904-913.
107 Pan DH, Guo WY, Chung WY, et al. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000;93(suppl 3):113-119.
108 Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239-1244.
109 Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery. 2003;52:1291-1296.
110 Schlienger M, Atlan D, Lefkopoulos D, et al. LINAC radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
111 Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.
112 Smyth MD, Sneed PK, Ciricillo SF, et al. Stereotactic radiosurgery for pediatric intracranial arteriovenous malformations: the University of California at San Francisco experience. J Neurosurg. 2002;97:48-55.
113 Tanaka T, Kobayashi T, Kida Y, et al. Comparison between adult and pediatric arteriovenous malformations treated by Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 1996;66(suppl 1):288-295.
114 Vernimmen FJ, Slabbert JP, Wilson JA, et al. Stereotactic proton beam therapy for intracranial arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2005;62:44-52.
115 Zabel-du Bois A, Milker-Zabel S, Huber P, et al. Stereotactic LINAC-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys. 2006;64:1044-1048.
116 Zipfel GJ, Bradshaw P, Bova FJ, et al. Do the morphological characteristics of arteriovenous malformations affect the results of radiosurgery? J Neurosurg. 2004;101:393-401.
117 Kitchen N. Experimental and clinical studies on the putative therapeutic efficacy of cerebral irradiation (radiotherapy) in epilepsy. Epilepsy Res. 1995;20:1-10.
118 Sun B, DeSalles AA, Medin PM, et al. Reduction of hippocampal-kindled seizure activity in rats by stereotactic radiosurgery. Exp Neurol. 1998;154:691-695.
119 Kondziolka D, Lunsford LD, Witt TC, et al. The future of radiosurgery: radiobiology, technology, and applications. Surg Neurol. 2000;54:406-414.
120 Dillon WP, Barbaro N. Noninvasive surgery for epilepsy: the era of image guidance. AJNR Am J Neuroradiol. 1999;20:185.
121 Régis J, Rey M, Bartolomei F, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504-515.
122 Cascino GD. Epilepsy: contemporary perspectives on evaluation and treatment. Mayo Clin Proc. 1994;69:1199-1211.
123 Engel JJr. Surgery for seizures. N Engl J Med. 1996;334:647-652.
124 Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513-524.
125 Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
126 Spencer SS. Long-term outcome after epilepsy surgery. Epilepsia. 1996;37:807-813.
127 Spencer SS, Berg AT, Vickrey BG, et al. Initial outcomes in the Multicenter Study of Epilepsy Surgery. Neurology. 2003;61:1680-1685.
128 Behrens E, Schramm J, Zentner J, et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41:1-9.
129 Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49:51-56.
130 Siegel AM, Cascino GD, Meyer FB, et al. Surgical outcome and predictive factors in adult patients with intractable epilepsy and focal cortical dysplasia. Acta Neurol Scand. 2006;113:65-71.
131 Sperling MR, Feldman H, Kinman J, et al. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46:45-50.
132 Guldvog B, Løyning Y, Hauglie-Hanssen E, et al. Surgical versus medical treatment for epilepsy. I. Outcome related to survival, seizures, and neurologic deficit. Epilepsia. 1991;32:375-388.
133 Guldvog B, Løyning Y, Hauglie-Hanssen E, et al. Surgical versus medical treatment for epilepsy. II. Outcome related to social areas. Epilepsia. 1991;32:477-486.
134 Barcia Salorio JL, Roldan P, Hernandez G, et al. Radiosurgical treatment of epilepsy. Appl Neurophysiol. 1985;48:400-403.
135 Barcia-Salorio JL, Vanaclocha V, Cerdá M, et al. Response of experimental epileptic focus to focal ionizing radiation. Appl Neurophysiol. 1987;50:359-364.
136 Barcia-Salorio JL, Barcia JA, Hernández G, et al. Radiosurgery of epilepsy. Long-term results. Acta Neurochir Suppl. 1994;62:111-113.
137 Barcia-Salorio JL. Radiosurgery in epilepsy and neuronal plasticity. Adv Neurol. 1999;81:299-305.
138 Heikkinen ER, Konnov B, Melnikov L, et al. Relief of epilepsy by radiosurgery of cerebral arteriovenous malformations. Stereotact Funct Neurosurg. 1989;53:157-166.
139 Heikkinen ER, Heikkinen MI, Sotaniemi K. Stereotactic radiotherapy instead of conventional epilepsy surgery. A case report. Acta Neurochir (Wien). 1992;119:159-160.
140 Kawai K, Suzuki I, Kurita H, et al. Failure of low-dose radiosurgery to control temporal lobe epilepsy. J Neurosurg. 2001;95:883-887.
141 Kondziolka D, Lunsford LD, Flickinger JC, et al. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
142 Kurita H, Kawamoto S, Suzuki I, et al. Control of epilepsy associated with cerebral arteriovenous malformations after radiosurgery. J Neurol Neurosurg Psychiatry. 1998;65:648-655.
143 Kurita H, Suzuki I, Shin M, et al. Successful radiosurgical treatment of lesional epilepsy of mesial temporal origin. Minim Invasive Neurosurg. 2001;44:43-46.
144 Mori Y, Kondziolka D, Balzer J, et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery. 2000;46:157-165.
145 Régis J, Peragui JC, Rey M, et al. First selective amygdalohippocampal radiosurgery for ‘mesial temporal lobe epilepsy.’. Stereotact Funct Neurosurg. 1995;64(suppl 1):193-201.
146 Régis J, Bartolomei F, Rey M, et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40:1551-1556.
147 Régis J, Bartolomei F, Kida Y, et al. Radiosurgery for epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. 2000;47:1091-1097.
148 Régis J, Bartolomei F, de Toffol B, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery. 2000;47:1343-1351.
149 Régis J, Bartolomei F. Comment on: Failure of Gamma Knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 2004;54:1404.
150 Régis J, Delsanti C, Roche PH, et al. [Functional outcomes of radiosurgical treatment of vestibular schwannomas: 1000 successive cases and review of the literature.]. Neurochirurgie. 2004;50:301-311.
151 Sutcliffe JC, Forster DM, Walton L, et al. Untoward clinical effects after stereotactic radiosurgery for intracranial arteriovenous malformations. Br J Neurosurg. 1992;6:177-185.
152 Whang CJ, Kwon Y. Long-term follow-up of stereotactic Gamma Knife radiosurgery in epilepsy. Stereotact Funct Neurosurg. 1996;66(suppl 1):349-356.
153 Chen ZF, Kamiryo T, Henson SL, et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg. 2001;94:270-280.
154 Maesawa S, Kondziolka D, Dixon CE, et al. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93:1033-1040.
155 Liscák R, Vladyka V, Novotný JJr, et al. Leksell gamma knife lesioning of the rat hippocampus: the relationship between radiation dose and functional and structural damage. J Neurosurg. 2002;97(5 suppl):666-673.
156 Herynek V, Burian M, Jirák D, et al. Metabolite and diffusion changes in the rat brain after Leksell Gamma Knife irradiation. Magn Reson Med. 2004;52:397-402.
157 Bien CG, Kurthen M, Baron K, et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42:1416-1421.
158 Engel JJr. Finally, a randomized, controlled trial of epilepsy surgery. N Engl J Med. 2001;345:365-367.
159 Engel JJr. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992). Neurology. 1993;43:1612-1617.
160 Cascino GD. Structural neuroimaging in partial epilepsy. Magnetic resonance imaging. Neurosurg Clin N Am. 1995;6:455-464.
161 Cascino GD. Clinical correlations with hippocampal atrophy. Magn Reson Imaging. 1995;13:1133-1136.
162 Garcia PA, Laxer KD, Barbaro NM, et al. Prognostic value of qualitative magnetic resonance imaging hippocampal abnormalities in patients undergoing temporal lobectomy for medically refractory seizures. Epilepsia. 1994;35:520-524.
163 Cmelak AJ, Abou-Khalil B, Konrad PE, et al. Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure. 2001;10:442-446.
164 Srikijvilaikul T, Najm I, Foldvary-Schaefer N, et al. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 2004;54:1395-1402.
165 Elomaa E. Focal irradiation of the brain: an alternative to temporal lobe resection in intractable focal epilepsy? Med Hypotheses. 1980;6:501-503.
166 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chirurg Scand. 1951;102:316-319.
167 Talairach J, Bancaud J, Szikla G, et al. Approche nouvelle de la neurochirurgie de l’epilepsie. Méthodologie stéréotaxique et résultats thérapeutiques. Neurochirurgie. 1974;20:92-98.
168 Baudouin M, Stuhl L, Perrard A. Un cas d’épilepsie focale traité par la radiothérapie. Rev Neurol. 1951;84:60-63.
169 Von Wieser W. Die Roentgentherapie der traumatischen Epilepsie. Monatsschr Psychiat Neurol. 1939;101:422-424.
170 Rogers L, Morris H, Lupica K. Effect of cranial irradiation on seizure frequency in adults with low-grade astrocytoma and medically intractable epilepsy. Neurology. 1993;43:1599-1601.
171 Rossi G, Scerrati M, Roselli R. Epileptogenic cerebral low grade tumors: effect of interstital stereotactic irradiation on seizures. Appl Neurophysiol. 1985;48:127-132.
172 Gaffey C, Monotoya V, Lyman J, et al. Restriction of the spread of epileptic discharges in cats by mean of Bragg Peak intracranial irradiation. Int J Appl Radiat Isotope. 1981;32:779-787.
173 Barcia Salorio JL, Garcia JA, Hernandez G, et al. Radiosurgery of epilepsy: long-term results. Paper presented at the European Society for Stereotactic and Functional Neurosurgery, 1993.
174 Lindquist C, Hellstrand E, Kilström L, et al. Stereotactic localisation of epileptic foci by magnetoencephalography and MRI followed by gamma surgery. Paper presented at the International Stereotactic Radiosurgery Symposium, 1991.
175 Lindquist C, Kihlström L, Hellstrand E, et al. Stereotactic radiosurgery instead of conventional epilepsy surgery. Paper presented at the European Society for. Stereotactic and Functional Neurosurgery. 1993.
176 Régis J, Semah F, Bryan R, et al. Early and delayed MR and PET changes after selective temporomesial radiosurgery in mesial temporal lobe epilepsy. AJNR Am J Neuroradiol. 1999;20:213-216.
177 Hayashi M, Régis J, Hori T. [Current treatment strategy with gamma knife surgery for mesial temporal lobe epilepsy.]. No Shinkei Geka. 2003;31:141-155.
178 Régis J, Bartolomei F, Hayashi M. Radiosurgery for intractable epilepsy. Tech Neurosurg. 2003;9:191-203.
179 Régis J, Bartolomei F, Hayashi M, et al. Gamma Knife surgery, a neuromodulation therapy in epilepsy surgery!. Acta Neurochir Suppl. 2002;84:37-47.
180 Yang I, Barbaro NM. Advances in the radiosurgical treatment of epilepsy. Epilepsy Curr. 2007;7(2):31-35.
181 Régis J, Bartolomei F, Hayashi M, et al. The role of gamma knife surgery in the treatment of severe epilepsies. Epileptic Disord. 2000;2:113-122.
182 Régis J, Roberts D. Gamma Knife radiosurgery relative to microsurgery: epilepsy. Stereotact Funct Neurosurg. 1999;72(suppl 1):11-21.
183 Régis J, Kerkerian-Legoff L, Rey M, et al. First biochemical evidence of differential functional effects following Gamma Knife surgery. Stereotact Funct Neurosurg. 1996;66(suppl 1):29-38.
184 Rheims S, Ryvlin P, Scherer C, et al. Analysis of clinical patterns and underlying epileptogenic zones of hypermotor seizures. Epilepsia.. 2008;49:2030-2040.
185 Régis J, Hayashi M, Eupierre LP, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Acta Neurochir Suppl. 2004;91:33-50.
186 Cohen D, Zubay G, Goodman R. Seizure outcome after lesionectomy for cavernous malformations. J. Neurosurg. 1995;83:237-242.
187 Kahane P, Tassi L, Hoffmann D, et al. Crises dacrystiques et hamartome hypothalamique: à propos d’une observation vidéo-stéréo-EEG. Epilepsies. 1994;6:259-279.
188 Zevgaridis D, Van Velthoven V, Ebelling U, et al. Seizure control following surgery in supratentorial cavernous malformations : a retrospective study in 77 patients. Acta Neurochir (Wien). 1996;138:672-677.
189 Paillas JE, Roger G, Toga M, et al. Hamarthome de l’hypothalamus. Rev Neurol. 1969;120:177-194.
190 Tassinari CA, Riguzzi P, Rizzi R, et al. Gelastic Seizures. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Current Problems in Epilepsy, Vol 1. London: John Libbey; 1997:429-446.
191 Frattali CM, Liow K, Craig GH, et al. Cognitive deficits in children with gelastic seizures and hypothalamic hamartoma. Neurology. 2001;57:43-46.
192 Nguyen D, Singh S, Zaatreh M, et al. Hypothalamic hamartomas: seven cases and review of the literature. Epilepsy Behav. 2003;4:246-258.
193 Quiske A, Frings L, Wagner K, et al. Cognitive functions in juvenile and adult patients with gelastic epilepsy due to hypothalamic hamartoma. Epilepsia. 2006;47:153-158.
194 Weissenberger AA, Dell ML, Liow K, et al. Aggression and psychiatric comorbidity in children with hypothalamic hamartomas and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2001;40:696-703.
195 Munari C, Kahane P, Francione S, et al. Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study). Electroencephalogr Clin Neurophysiol. 1995;95:154-160.
196 Kahane P, Munari C, Hoffmann D, et al. Approche chirurgicale multimodale des angiomes caverneux épileptogènes. Epilepsies. 1994;6:113-130.
197 Tassi L, Quarato P, Francione S. Chronological relationships between ictal laughing and smiling and intralesional discharges: stereo-EEG study of a patient with gelastic epilepsy and hypothalamic hamartoma. Epilepsia. 1995;36:239-240.
198 Donley D, Kuzniecky R, Mountz J, et al. Ictal SPECT findings in hypothalamic hamartoma and epilepsy [abstract]. Epilepsia. 1994;35(suppl 8):146.
199 Kuzniecky R, Guthrie B, Mountz J, et al. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol. 1997;42:60-67.
200 Kuzniecky R, Guthrie B, Mountz J, et al. Hypothalamic hamartomas and gelastic seizures: evidence for subcortical seizure generation by ictal SPECT and cerebral stimulation. Epilepsia. 1995;36(suppl 3):s266.
201 Arroyo S, Santamaria J, Sanmarti F, et al. Ictal laughter associated with paroxysmal hypothalamopituitary dysfunction. Epilepsia. 1997;38:114-117.
202 Berkovic SF, Andermann F, Melanson D, et al. Hypothalamic hamartoma and associated ictal laughter. Evolution of a characteristic epileptic syndrome and diagnostic value of magnetic resonance imaging. Ann. Neurol. 1988;23:429-439.
203 Deonna T, Ziegler AL. Hypothalamic hamartoma, precocious puberty and gelastic seizures: a special model of “epileptic” developmental disorder. Epileptic Disord. 2000;2:33-37.
204 Valdueza JM, Cristante L, Dammann O, et al. Hypothalamic hamartomas: with special reference to gelastic epilepsy and surgery. Neurosurgery. 1994;34:949-958.
205 Delalande O, Fohlen M, Jalin C, et al. Surgical treatment of epilepsy due to hypothalamic hamartoma: technique and preliminary results in five cases. Epilepsia. 1998;39(suppl 6):90-91.
205a Delalande O, Fohlen M. Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo). 2003;43:61-68.
206 Stewart L, Steinbok P, Daaboul J. Role of surgical resection in the treatment of hypothalamic hamartomas causing precocious puberty. Report of six cases. J Neurosurg. 1998;88:340-345.
207 Machado HR, Hoffmann HJ, Hwang PA. Gelastic Seizures treated by resection of a hypothalamic hamartoma. Childs Nerv System. 1991;7:462-465.
208 Nishio S, Fujiwara S, Aiko Y, et al. Hypothalamic hamartoma. Report of two cases. J Neurosurg. 1989;70:640-645.
209 Nishio S, Morioka T, Fukui M, et al. Surgical treatment of intractable seizures due to hypothalamic hamartoma. Epilepsia. 1994;35:514-519.
210 Nishio S, Shigeto H, Fukui M. Hypothalamic hamartoma: the role of surgery. Neurosurg Rev. 1993;16:157-160.
211 Palmini A, Chandler C, Andermann F, et al. Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy. Neurology.. 2002;58:1338-1347.
212 Rosenfeld JV, Freeman JL, Harvey AS. Operative technique: the anterior transcallosal transseptal interforniceal approach to the third ventricle and resection of hypothalamic hamartomas. J Clin Neurosci. 2004;11:738-744.
213 Rosenfeld JV, Harvey AS, Wrennall J, et al. Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery. 2001;48:108-118.
214 Ng YT, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia. 2006;47:1192-1202.
215 Harvey AS, Freeman JL, Berkovic SF, et al. Transcallosal resection of hypothalamic hamartomas in patients with intractable epilepsy. Epileptic Disord. 2003;5:257-265.
216 Kerrigan JF, Ng YT, Chung S, et al. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol. 2005;12:119-131.
217 Fujimoto Y, Kato A, Saitoh Y, et al. Stereotactic radiofrequency ablation for sessile hypothalamic hamartoma with an image fusion technique. Acta Neurochir (Wien). 2003;145:697-700.
218 Parrent AG. Stereotactic radiofrequency ablation for the treatment of gelastic seizures associated with hypothalamic hamartoma. Case report. J Neurosurg. 1999;91:881-884.
219 Kahane P, Ryvlin P, Hoffmann D, et al. From hypothalamic hamartoma to cortex: what can be learnt from depth recordings and stimulation? Epileptic Disord. 2003;5:205-217.
220 Murphy JV, Wheless JW, Schmoll CM. Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Pediatr Neurol. 2000;23:167-168.
221 Brandberg G, Raininko R, Eeg-Olofsson O. Hypothalamic hamartoma with gelastic seizures in Swedish children and adolescents. Eur J Paediatr Neurol. 2004;8:35-44.
222 Pallini R, Bozzini V, Colicchio G, et al. Callosotomy for generalized seizures associated with hypothalamic hamartoma. Neurol Res. 1993;15:139-141.
223 Schulze-Bonhage A, Homberg V, Trippel M, et al. Interstitial radiosurgery in the treatment of gelastic epilepsy due to hypothalamic hamartomas. Neurology. 2004;62:644-647.
224 Feiz-Erfan I, Horn EM, Rekate HL, et al. Surgical strategies for approaching hypothalamic hamartomas causing gelastic seizures in the pediatric population: transventricular compared with skull base approaches. J Neurosurg. 2005;103(4 suppl):325-332.
225 Maixner W. Hypothalamic hamartomas—clinical, neuropathological and surgical aspects. Childs Nerv Syst. 2006;22:867-873.
226 McCord MW, Buatti JM, Fennell EM, et al. Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biology Phys. 1997;39:437-444.
227 Pascual-Castroviejo I, Moneo JH, Viano J, et al. [Hypothalamic hamartomas: control of seizures after partial removal in one case.]. Rev Neurol. 2000;31:119-122.
228 Watanabe T, Enomoto T, Uemura K, et al. [Gelastic seizures treated by partial resection of a hypothalamic hamartoma.]. No Shinkei Geka. 1998;26:923-928.
229 Cascino GD, Andermann F, Berkovic SF, et al. Gelastic seizures and hypothalamic hamartomas: evaluation of patients undergoing chronic intracranial EEG monitoring and outcome of surgical treatment. Neurology. 1993;43:747-750.
230 Maraire J, Abdulrauf S, Berger S, et al. De novo development of cavernous malformation of the spinal cord following spinal axis radiation. J Neurosurg Spine. 1999;90:234-238.
231 Maraire JN, Awad IA. Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery. 1995;37:591-605.
232 Casazza M, Broggi G, Franzini A, et al. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and post-operative outcome. Neurosurgery. 1996;39:26-34.
233 Kida Y, Kobayashi T, Tanaka T. Radiosurgery of symptomatic angiographically occult vascular malformations with Gamma Knife. In: Kondziolka D, editor. Radiosurgery. Basel: Karger; 1995:207-217.
234 Seo Y, Fukupka S, Takanashi M, et al. Gamma Knife surgery for angiographically occult vascular malformations. Stereotact Funct Neurosurg. 1997;64:98-109.
235 Porter P, Willinsky R, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
236 Engel J, VanNess P, Rasmussen T, et al. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:609-622.
237 Dodick D, Cascino G, Meyer F. Vascular malformations and intractable epilepsy: outcome after surgical treatment. Mayo Clin Proc. 1994;69:741-745.
238 Acciari N, Giulioni M, Padovani R, et al. Surgical management of cerebral cavernous angiomas causing epilepsy. J. Neurosurg. Sci. 1995;39:13-20.
239 Giulinoni M, Acciari N, Padovani R, et al. Results of surgery in children with cerebral cavernous angiomas causing epilepsy. Br J Neurosurg. 1995;9:135-141.
240 Schroeder H, Gaab M, Runge U. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and post-operative outcome. Neurosurgery. 1996;12:1271.
241 Kondziolka D, Lunsford D, Flickinger J. Stereotactic radiosurgery for cavernous malformations. In: Lunsford D, editor. Gamma Knife Brain Surgery, Vol 14. Basel: Karger; 1998:78-88.
242 Amin-Hanjani S, Ogilvy CS, Candia GJ, et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at Harvard cyclotron. Neurosurgery. 1998;42:12291238.
243 Kraemer D, Griebel M, Lee N, et al. Surgical outcome in patients with epilepsy with occult vascular malformations treated with lesionectomy. Epilepsia. 1998;39:600-607.
244 Barcia Salorio JL, Vanaclocha V, Cerda M, et al. Focus irradiation in epilepsy. Experimental study in the cat. Appl Neurophysiol. 1985;48:152.
245 Kondziolka D, Mori Y, Blazer J, et al. Stereotactic radiosurgery for the treatment of epilepsy evaluated in the rat kainic acid model. Paper presented at the 8th international Leksell Gamma Knife Society Meeting, 1997, Marseille, France.
246 Régis J, Bartolomei F, Metellus P, et al. Radiosurgery for trigeminal neuralgia and epilepsy. Neurosurg Clin N Am. 1999;10:359-377.
247 Régis J, Bartolomei F, Rey M, et al. Role of Gamma Knife surgery in the treatment of severe epilepsies, 3rd ed. Wyllie E, editor. The Treatment of Epilepsy: Principle and Practice, Vol 1. Baltimore: Williams & Wilkins. 2000.