Radionuclide imaging in oncology and infection
Positron emission tomography imaging
The widespread acceptance of PET as a major advance is due to two major factors:
1. There is increased recognition in the literature of the role of the main PET tracer 18F-FDG, a glucose analogue that is taken up in tissue in proportion to cellular glucose metabolism. This is particularly useful for tumour imaging, since most tumours have increased glucose metabolism and will concentrate FDG. Malignant cells are characterized by increased glucose transporter molecules at the cell surface. FDG is phosphorylated by the enzyme hexokinase to a polar intermediate which does not cross cell membranes well and is, therefore, trapped in the cell. Hexokinase levels and activity are increased in malignant cells. The reverse reaction (glucose-6-phosphatase) is slow and the enzyme is commonly deficient in cancer cells.
2. Integrated PET CT scanners are now widely available and are the standard of care. These units have separate PET and CT scanners installed in the same gantry. The patient undergoes a conventional CT scan (usually performed with low exposure factors to reduce radiation dose) immediately followed by a PET scan without moving, on the same table-top. This allows fusion of the anatomical information from CT with the functional data from the PET scan, and hence accurate anatomical localization of metabolically active disease and recognition of normal anatomical and physiological uptake. The density data from the CT scan are also used to correct the PET data for differential attenuation of the emitted photons within the patient.
Normal physiological uptake is seen in organs that are hypermetabolic and big glucose users, especially the brain and the heart, or active or recently active skeletal muscle. Variable uptake is seen in the gut and there is normal excreted urinary activity in the urinary tract. One confounding factor for interpretation may be normal physiological uptake in brown fat – particularly in the neck and paraspinal regions. Differentiation of this normal activity from pathology is greatly aided by the image registration afforded by combined PET CT scanners.
2-[18F]fluoro-2-deoxy-d-glucose (18F-FDG) PET scanning
Indications (oncology)
General
1. Distinguishing benign from malignant disease, e.g. lung nodules, brain lesions, etc.
2. Establishing the grade of malignancy, e.g. brain tumours, soft-tissue masses
3. Establishing the stage of disease, e.g. lung cancer, lymphoma, etc.
4. Establishing whether there is recurrent or residual disease, e.g. lymphoma, teratoma, seminoma, etc.
5. Establishing the site of disease in the face of rising tumour markers, e.g. colorectal, germ cell tumours, etc.
6. Establishing the response to therapy – pre, during and post therapy imaging
7. Identifying occult malignancy, e.g. paraneoplastic syndrome or the unknown primary in the setting of proven metastatic disease.
Patient preparation
1. Fasting for 4–6 h, with plenty of non-sugary fluids. Even a small snack or sweet can alter the pattern of 18FDG uptake.
2. Measure blood glucose before injection to ensure it is not elevated. Elevated blood glucose levels result in diversion of glucose to muscle. Consider rescheduling patient if blood glucose elevated. Administration of insulin is counter-productive as this diverts glucose uptake to muscle.
3. A mild sedative such as diazepam may be given to reduce physiological muscle and brown fat uptake.
4. Oral contrast may be administered to aid interpretation of the CT. Previous concerns about attenuation correction artifact on the PET images secondary to the high-density contrast has been shown not to be clinically significant.
Technique
1. Up to the UK limit of 400 MBq 18FDG intravenously (i.v.) (10 mSv effective dose (ED)) is administered.
2. To reduce muscle uptake of FDG, patients should remain in a relaxed environment such as lying in a darkened room (without talking if head and neck area are being imaged) between injection and scan.
3. Image at 1 h post injection.
4. Imaging is preferred with the arms above the head to reduce beam hardening artifact on the CT.
(a) Low dose (example exposure factors 120 kv, 80 mAs)
(c) Positive or negative oral contrast agents according to local protocol may be used, but care should be taken with image interpretation as there may be bowel movement between the short initial CT scan and the 15–30 minutes taken for the PET acquisition.
(a) From the base of skull to upper thighs (occasionally extended if melanoma or limb or head and neck tumours)
7. In some instances a diagnostic standard dose CT with i.v. contrast may be acquired as well, but in routine practice a diagnostic scan will usually be already available. A scan performed with i.v. contrast may result in attenuation artefacts on the reconstructed PET images if used for attenuation correction.
Cook, GJ, Wegner, EA, Fogelman, I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 2004; 34(2):122–133.
Kapoor, V, McCook, BM, Torok, FS. An introduction to PET CT imaging. RadioGraphics. 2004; 24(2):523–543.
Rohren, EM, Turkington, TG, Coleman, RE. Clinical applications of PET in oncology. Radiology. 2004; 231(2):305–332.
The Royal College of Physicians and the Royal College of Radiologists. Evidence based guidelines for the use of PET CT in the United Kingdom 2012. London: The Royal College of Physicians and the Royal College of Radiologists; 2012.
von Schulthess, GK, Steinert, HC, Hany, TF. Integrated PET/CT: current applications and future directions. Radiology. 2006; 238(2):405–422.
Gallium radionuclide tumour imaging
This is rarely used, having almost entirely been superseded by cross-sectional techniques and PET scanning.1 The main disadvantages are the high radiation dose, the extended nature of the investigation, its non-specific nature, and difficulties in interpretation in the abdomen due to normal bowel activity.
Indications
1. Hodgkin’s and non-Hodgkin’s lymphoma: assessment of residual masses after therapy and early diagnosis of recurrence1
2. Gallium imaging has been used with variable success in a variety of other tumours, e.g. hepatoma, bronchial carcinoma, multiple myeloma and sarcoma
3. It has also been used in benign conditions such as sarcoidosis and for localization of infection or in suspected orthopaedic infection.
Images
1. 48 and 72 h. Whole-body, spot views and single photon emission computed tomography (SPECT) as appropriate. SPECT can increase the sensitivity and specificity of the investigation
2. Non-specific bowel activity can be discriminated by imaging on two separate occasions. Activity in bowel contents should move between scans and abnormal areas of accumulation will be stationary. If there is still any doubt at 72 h, later images at up to 7 days may prove helpful.
Bombardieri, E, Aktolun, C, Baum, RP, et al. 67Ga scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2003; 30(12):BP125–BP131.
Front, D, Bar-Shalom, R, Israel, O. The continuing clinical role of gallium 67 scintigraphy in the age of receptor imaging. Semin Nucl Med. 1997; 27(1):68–74.
Palestro, CJ, Love, C, Tronco, GG, et al. Role of radionuclide imaging in the diagnosis of postoperative infection. RadioGraphics. 2000; 20:1649–1660.
Radioiodine metaiodobenzylguanidine scan
Metaiodobenzylguanidine (MIBG) is a noradrenaline (norepinephrine) analogue. It is taken up actively across cell membranes of sympathetic and adrenal medullary tissue into intracellular storage vesicles. There is no further metabolism, and it remains sequestered and localized in the storage vesicles of catecholamine-secreting tumours and tumours of neuroendocrine origin.1
Radiopharmaceuticals
1. 123Iodine(I)-metaiodobenzylguanidine (MIBG), 250 MBq typical, 400 MBq max (6 mSv ED with thyroid blockade). The 13-h half-life of 123I allows imaging up to 48 h
2. 131I-labelled MIBG is also available, and is still in common use outside Europe where 123I-MIBG is not made commercially. However, the higher photon energy of the emitted gamma rays renders it an inferior imaging agent and results in a higher radiation dose to the patient. It should only be considered where 123I-MIBG cannot be obtained.
Patient preparation
1. Where possible, stop medications that interfere with MIBG uptake.2 These include tricyclic antidepressants, antihypertensives, cocaine, sympathomimetics, decongestants containing pseudoephedrine, phenylpropanolamine and phenylephrine (many available over the counter) and others.
2. Thyroid blockade, to reduce radiation dose to the thyroid continuing for 24 h after 123I-MIBG injection:
(a) Adults – either oral potassium perchlorate (400 mg, 1 h before MIBG injection, then 200 mg every 6 h) or oral potassium iodate (85 mg twice daily starting 24 h before MIBG injection)
(b) Children – Lugol’s iodine 0.1–0.2 ml diluted with water or milk three times a day starting 48 h before MIBG injection. Potassium iodate is more palatable – the tablets need splitting for paediatric dosage.
Additional techniques
1. If therapy with 131I-MIBG is being considered, quantitative assessment can be performed using geometric mean and attenuation correction to calculate percentage of administered dose residing in tumour at 24 h
2. 111In-octreotide, which binds to somatostatin receptors frequently expressed in neuroendocrine and other tumours, is an alternative imaging agent to MIBG. It appears to be more sensitive for carcinoids, and may be useful in cases where the MIBG scan is negative. It also has therapeutic analogues under development
3. PET imaging with novel radiopharmaceuticals such as 18-F fluorodopamine and 18-F fluorodopa is available in only a few centres but has been reported to offer additional value over MIBG.3,4 Conventional 18-F FDG PET imaging is also of value in assessing metastatic disease.
References
1. Ilias, I, Divgi, C, Pacak, K. Current role of metaiodobenzylguanidine in the diagnosis of pheochromocytoma and medullary thyroid cancer. Semin Nucl Med. 2011; 41:364–368.
2. Solanki, KK, Bomanji, J, Moyes, J, et al. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG). Nucl Med Commun. 1992; 13(7):513–521.
3. Chrisoulidou1, A, Kaltsas, G, Ilias, I, et al. The diagnosis and management of malignant phaeochromocytoma and paragangliomas. Endocrine-Related Cancer. 2007; 14:569–585.
4. Timmers, H, Chen, C, Jorge, A, et al. Comparison of 18f-fluoro-l-dopa, 18f-fluoro-deoxyglucose, and 18f-fluorodopamine pet and 123i-mibg scintigraphy in the localization of pheochromocytoma and paragangliomas. J Clin Endocrinol Metab. 2009; 94:4757–4767.
Somatostatin receptor imaging
Somatostatin is a physiological neuropeptide which has biological effects including inhibition of growth hormone release, and suppression of insulin and glucagon excretion. Octreotide (a long-acting analogue of the human hormone, somatostatin) can be used therapeutically to inhibit hormone production by carcinoids, gastrinomas and insulinoma, etc. A number of tumours, particularly those of neuroendocrine origin, express neuroendocrine receptors. Imaging after the administration of radionuclide-labelled somatostatin analogues such as octreotide, therefore, allows their localization.1,2
Indications
Localization and staging of the following tumours of neuroendocrine origin:
Although sometimes used for the assessment of insulinomas, these latter tumours are more variably visible with octreotide (approx. 50%) than carcinoids, gastrinomas and phaeochromocytomas, which are seen in 80–100% of cases.
Radiopharmaceuticals
111Indium (In) pentetreotide (a DTPA conjugate of octreotide) 220 MBq i.v. (ED 17 mSv).
Patient preparation
1. Some centres use bowel preparation
2. Oral hydration to help with renal clearance. There is usually high renal uptake.
3. In patients with possible insulinoma i.v. glucose should be available because of a small risk of inducing hypoglycaemia
4. Discontinue oral somatostatin to avoid competitive inhibition.
Technique
Image at 24 h and 48 h if necessary:
1. Anterior and posterior abdomen, 10–20 min per view
2. Whole-body imaging for comprehensive search for metastases
3. SPECT and now hybrid CT-SPECT scanners are increasingly available and will help anatomical localization, and improve specificity by differentiating tumour uptake from bowel uptake for example.
Additional techniques
MIBG may be positive in octreotide-negative tumours and vice versa.
Other novel agents for conventional isotope and PET imaging have been investigated.2
References
1. Krenning, EP, Kwekkeboom, DJ, Bakker, WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1] and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993; 20(8):716–731.
2. Rufini, V, Calcagni, M, Baum, R. Imaging of neuroendocrine tumors. Sem Nucl Med. 2006; 36:228–247.
Lymph node and lymphatic channel imaging
Radionuclide lymphoscintigraphy
Lymphoscintigraphy provides a less invasive alternative to conventional lymphography. High-resolution anatomical detail is not possible (see MR lymphangiography under further techniques below).
Indications
1. Localization of the ‘sentinel’ node in breast carcinoma2,3 and malignant melanoma4 using a hand-held probe. In recent years, this has become the major indication for lymphoscintigraphy. The technique in itself does not diagnose nodes affected by malignancy; rather it identifies the node most likely to be involved and, therefore, to allow histological sampling. Although still awaiting completion of long-term clinical trials, the early indications are that if the first or ‘sentinel’ node in the lymphatic drainage chain from the primary site is shown to have negative histology (approx. 60% of cases in breast cancer), then more extensive nodal clearance and associated morbidity can be avoided. SPECT CT can improve anatomical localization4
Radiopharmaceuticals
1. 99mTechnetium (Tc)-nanocolloidal albumin (particle size <80 nm), 40 MBq maximum (0.4 mSv ED) total for all injections. The colloid is injected intradermally and cleared from the interstitial space by lymphatic drainage.
2. A number of other colloids are used around the world for sentinel node imaging, and other radiopharmaceuticals have been used for lymphoscintigraphy.
Technique
(a) breast carcinoma: inject 99mTc-colloid in approximately 5 ml volume intradermally for palpable lesions and around the tumour under US guidance for non-palpable lesions
(b) melanoma: inject 99mTc-colloid intradermally in a ring of locations around the melanoma site, with a volume of about 0.1 ml for each injection.
2. Other anatomical sites of investigation or for investigation of lymphatic drainage/lymphoedema:
(a) 99mTc-colloid in 0.1–0.3 ml volume is injected intradermally at sites depending upon the area to be studied, e.g. for nodes below diaphragm and lower limb drainage – injections in each foot in the first and second web spaces for drainage or over the lateral dorsum of the foot for lymphatics or for axillary nodes and upper limb drainage injections in each hand in the second and third web spaces
(b) Static images are taken of the injection site(s) immediately, followed by injection site, drainage route and liver images at intervals, e.g. 15, 30, 60 and 180 min, continuing up to 24 h or until the liver is seen. Visualization of the liver indicates patency of at least one lymphatic channel (except early liver activity within 15 min, which implies some colloid entry into blood vessels).
References
1. Harisinghani, MG, Barentsz, J, Hahn, PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New Engl J Med. 2003; 348(25):2491–2499.
2. Krynyckyi, BR, Kim, CK, Goyenechea, MR, et al. Clinical breast lymphoscintigraphy: optimal techniques for performing studies, image atlas, and analysis of images. RadioGraphics. 2004; 24(1):121–145.
3. Husarik, DB, Steinert, HC. Single-photon emission computed tomography/computed tomography for sentinel node mapping in breast cancer. Semin Nucl Med. 2007; 37(1):29–33.
4. Intenzo, CM, Truluck, CA, Kushen, MC, et al. Lymphoscintigraphy in cutaneous melanoma: an updated total body atlas of sentinel node mapping. RadioGraphics. 2009; 29(4):1125–1135.
5. Witte, CL, Witte, MH, Unger, EC, et al. Advances in imaging of lymph flow disorders. RadioGraphics. 2000; 20(6):1697–1719.
6. Lohrmann, C, Foeldi, E, Speck, O, et al. High-resolution MR lymphangiography in patients with primary and secondary lymphedema. Am J Roentgenol. 2006; 187(2):556–561.
Radionuclide imaging of infection and inflammation
A number of radionuclide techniques exist for this, the most commonly used of which is radionuclide-labelled leucocyte imaging.1,2 The ready availability and sensitivity for collections and inflammation of anatomical imaging techniques such as US and CT has, however, reduced the demand for radionuclide procedures.
Radiopharmaceuticals
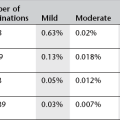
1. 111In-labelled leucocytes, 20 MBq maximum (9 mSv ED). 111In-oxine, tropolonate and acetylacetonate are highly lipophilic complexes that will label leucocytes, erythrocytes and platelets. The leucocytes have to be labelled in vitro and the labelled cell suspension reinjected. ABO/Rh-matched donor leucocytes can be used with neutropenic patients or to reduce infection hazard in HIV-positive patients.111In has a half-life of 67 h and principal gamma emissions at 171 and 245 keV. There is no confounding uptake in bowel and this technique may be more suitable for chronic or more low-grade infections because of the longer imaging window (4–48 h).
2. 99mTc-hexamethylpropyleneamineoxime (HMPAO)-labelled leucocytes, 200 MBq max (3 mSv ED). HMPAO is also a highly lipophilic complex which preferentially labels granulocytes. The cell-labelling technique is similar to that for 111In, but HMPAO has the advantage that kits can be stocked and used at short notice. There is more bowel uptake as a result of biliary excretion than with 111In-labelled leucocytes, so images must be taken earlier than 4 h post injection for diagnosis of abdominal infection. The 99mTc label delivers a lower radiation dose than 111In-labelled leucocytes and has better imaging resolution, which can, for example, help to identify inflammation in small bowel.
3. 67Ga-gallium citrate, 150 MBq max (17 mSv ED). This localizes in inflammatory sites. Formerly the most commonly used agent, it has now largely been replaced by labelled leucocyte imaging. There is significant bowel activity up to 72 h, so delayed imaging may be necessary for suspected abdominal infection, and accuracy in the abdomen is less than elsewhere. 67Ga, with a T1/2 of 78 h and principal γ-emissions at 93, 185 and 300 keV, delivers a significantly higher radiation dose than 99mTc-HMPAO- and 111In-labelled leucocytes, but it has the advantage of requiring no special preparation.
4. 99mTc- or 111In-human immunoglobulin (HIG). This is an agent for which a commercial kit is available for 99mTc labelling. It has the advantage of not requiring a complex preparation procedure, but its place relative to labelled leucocytes is still a matter of debate.
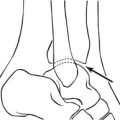
5. 99mTc-sulesomab (Leukoscan). This is another commercial agent comprising a labelled antigranulocyte monoclonal antibody fragment. It also has a simple preparation procedure, and is finding a role in the diagnosis of orthopaedic infections.
6. 18F-FDG PET has been used to evaluate obscure infection or suspected infection of orthopaedic hardware.
Technique
1. The radiopharmaceutical is administered intravenously
2. Image timing depends upon the radiopharmaceutical used and the suspected source of infection. Whole-body imaging may be employed for all of the radiopharmaceuticals:
(a) 111In-labelled white cells. Static images are acquired at 3 and 24 h post injection. Further imaging at 48 h may prove helpful
(b) 99mTc-HMPAO-labelled white cells. For suspected abdominal infections, image at 0.5 and 2 h, i.e. before significant normal bowel activity is seen. For other sites, image at 1, 2 and 4 h. Additional 24-h images may be useful
(c) 67Ga-citrate. Images are acquired at 48 and 72 h for regions where normal bowel, urinary and blood pool activity may obscure abnormal collection sites. Later images may prove helpful in non-urgent cases. Extremities and urgent cases may be imaged from as early as 6 h. If not contraindicated, laxatives given for 48 h post injection will help to clear bowel activity.