Chapter 10 Radiographic Evaluation of Spinal Canal Tumors
INTRODUCTION
Imaging of spinal canal tumors and tumor-like masses has been revolutionized by the advent of magnetic resonance imaging (MRI), which is a sensitive modality for the identification and characterization of spinal lesions. Radiologic evaluation of these lesions focuses on localizing lesions into extradural, intradural extramedullary, and intramedullary compartments. Although this approach is somewhat artificial, because many lesions transgress compartments or can be found in different compartments, it is very useful for generating an appropriate differential diagnosis for a spinal mass (Table 10-1). Clinical information (in particular patient age) is helpful to further narrow and order the differential diagnosis of spinal lesions. This chapter begins with a brief discussion of imaging techniques used in evaluating spinal masses. This is followed by a discussion of imaging characteristics of the most common lesions that occur in each of the aforementioned categories, including a description of tumors, tumor-like cysts, and non-neoplastic tumor mimics. A discussion of spinal vascular malformations is beyond the scope of this chapter.
Table 10-1 Classification of Spinal Canal Tumors and Tumor-Like Masses
| Extradural | Intradural Extramedullary | Intramedullary |
|---|---|---|
| NEOPLASTIC | ||
| Metastases lymphoma (and epidural extension of other vertebral body and paraspinal lesions) Epidural lipomatosis Angiolipoma |
Schwannoma Neurofibroma Meningioma Paraganglioma Metastases (Hemangioblastoma) |
Astrocytoma Ependymoma Ganglioglioma Hemangioblastoma Metastases (Schwannoma) |
| NON-NEOPLASTIC | ||
| Abscess Hematoma Degenerative changes Arachnoid cyst OPLL |
Arachnoid cyst |
Syrinx MS Transverse myelitis Cord infarct |
MS, multiple sclerosis; OPLL, ossification of the posterior longitudinal ligament
IMAGING MODALITIES
Radiographs, computed tomography (CT), CT myelography, MRI, and angiography are the modalities available for assessment of spinal canal lesions. Radiographs can be a useful screening test but are limited, demonstrating mainly the secondary effects of spinal lesions on the adjacent bony and paraspinal soft tissues. Expansion of the central canal, bony destruction and/or remodeling (including vertebral body scalloping and neural foraminal widening), thickening of the paraspinal soft tissues, and associated scoliosis are some of the findings that may be seen in the setting of an intraspinal lesion. A normal radiograph does not exclude the presence of an underlying spinal mass. CT demonstrates bony and soft tissue changes with a greater degree of conspicuity, but the intraspinal lesion itself may be subtle or impossible to detect. CT myelography, a technique whereby myelographic contrast is introduced directly into the thecal sac followed by CT imaging, increases sensitivity for detection of intraspinal disease over conventional CT. However, it is still less effective than MRI for assessing intramedullary and extradural disease, and, although equally sensitive to contrast-enhanced MRI for detection of intradural extramedullary spinal masses, it is more invasive.1–3 CT myelography, therefore, typically is reserved for patients who have contraindications for MRI and sometimes for those with spinal hardware that causes excessive artifact on MRI. CT myelography demonstrates expansion of the cord or mass effect on the cord by spinal lesions but is limited in its ability to characterize the underlying lesion and may be insensitive for intramedullary lesions that have only minimal mass effect. Occasionally, CT myelography is a useful adjunct to MRI for cystic lesions within the spinal canal, where it is used to assess for communication with the cerebrospinal fluid (CSF) space.4,5 In patients without contraindications, MRI is undoubtedly the modality of choice for evaluation of spinal canal lesions. It is the most sensitive and specific modality for both identifying and characterizing intraspinal lesions. Spinal angiography is reserved for vascular lesions, providing important information for preoperative planning and guiding endovascular treatment.
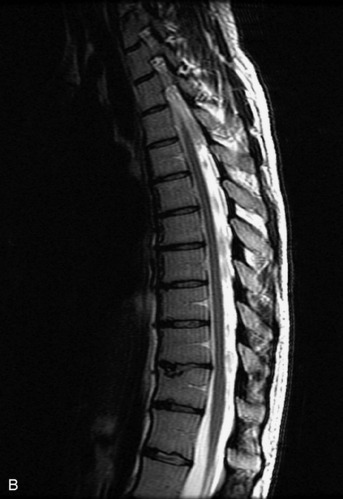
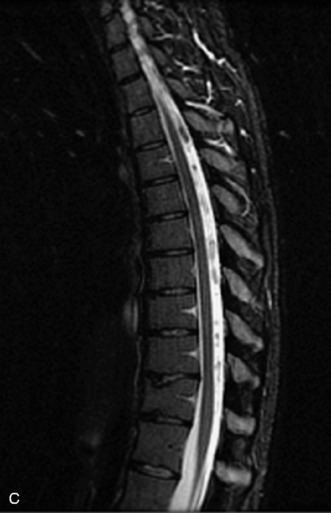
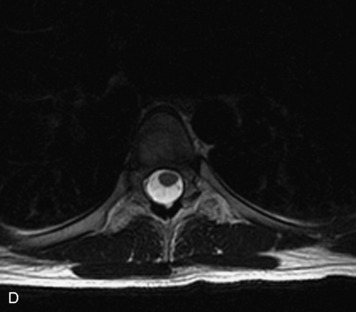
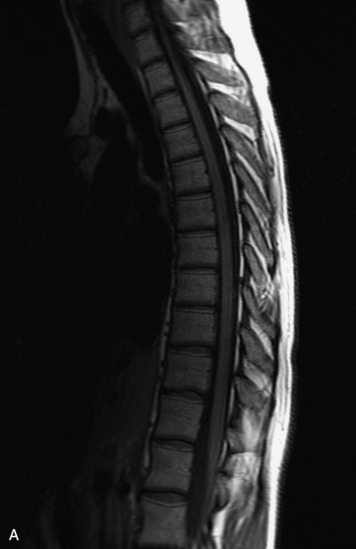
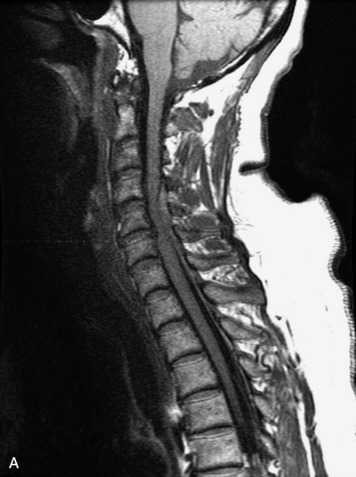
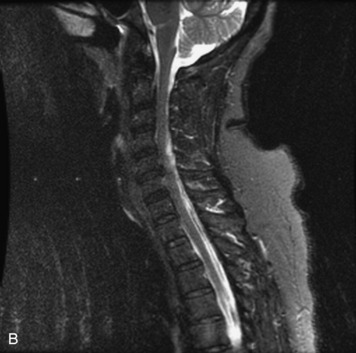
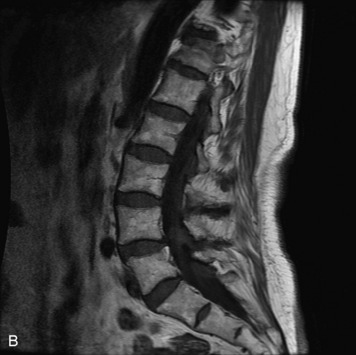
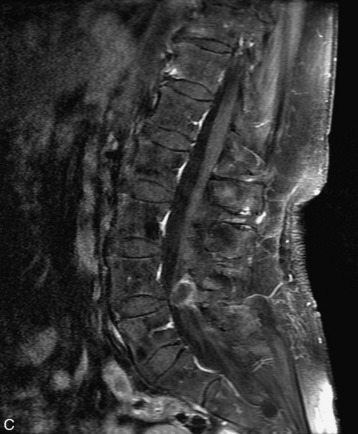
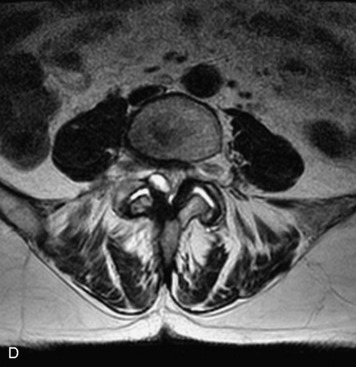
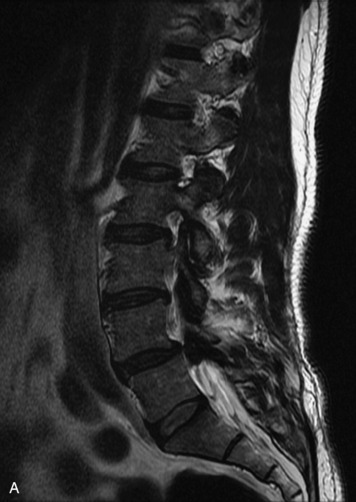
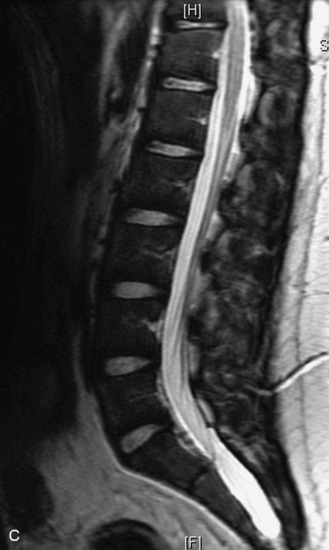
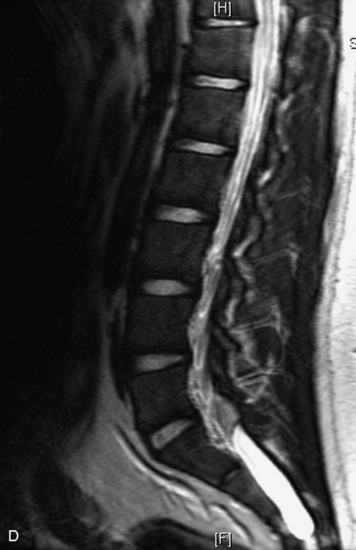
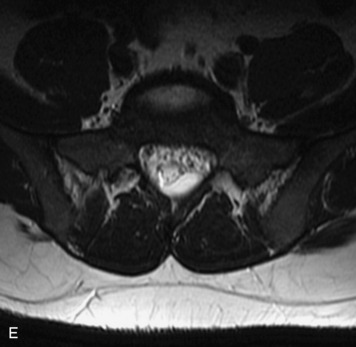
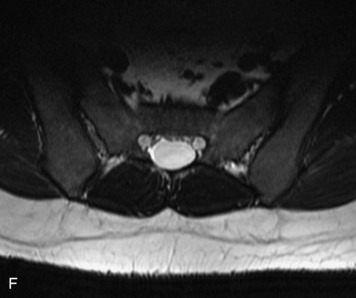
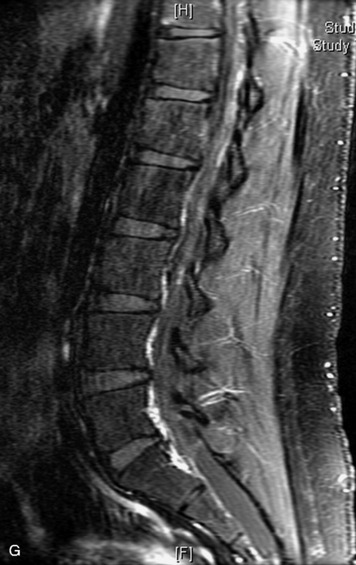
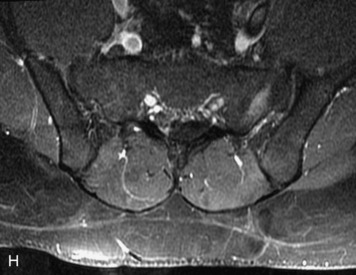
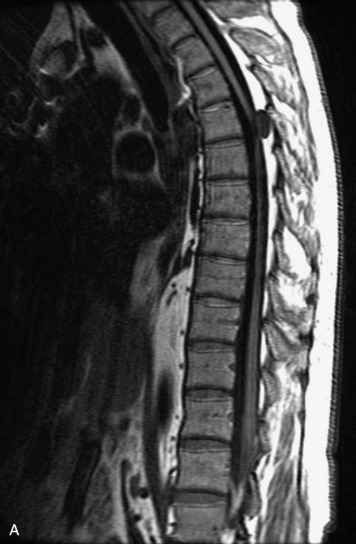
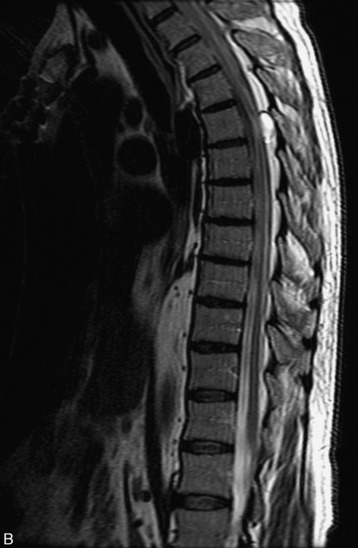
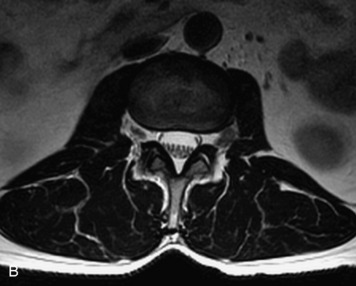
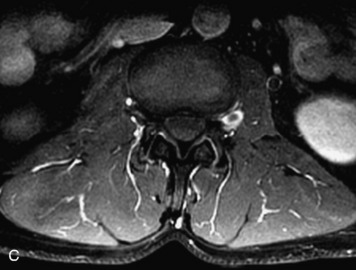
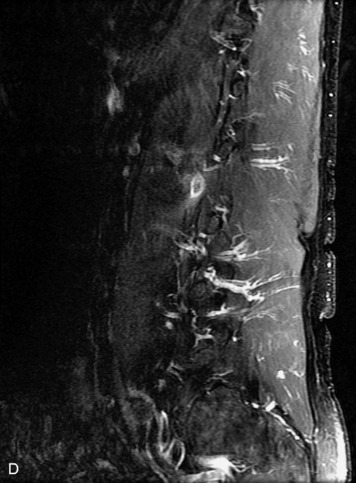
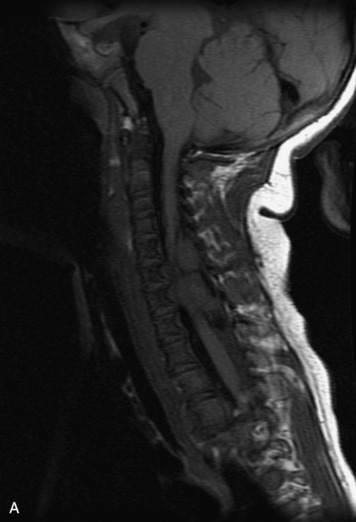
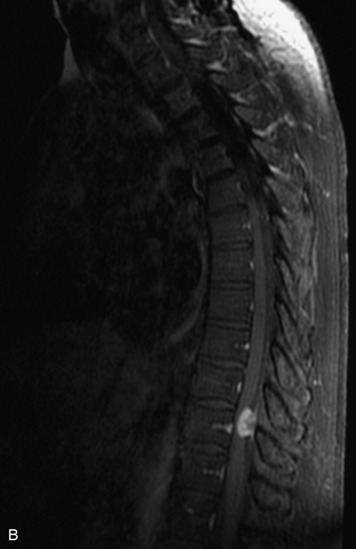
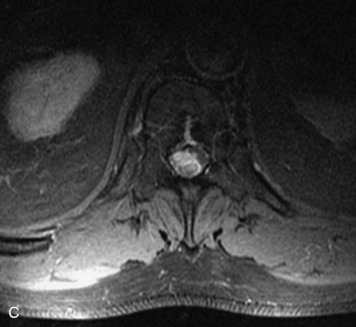
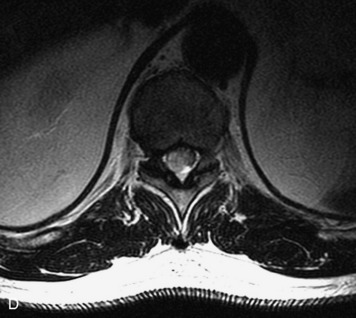
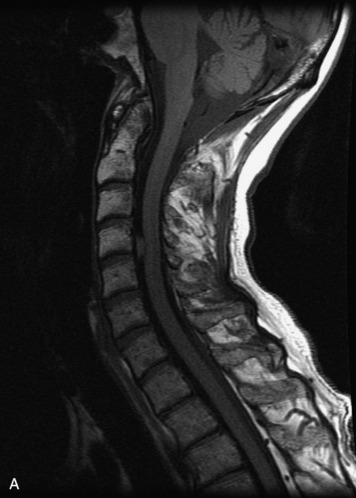
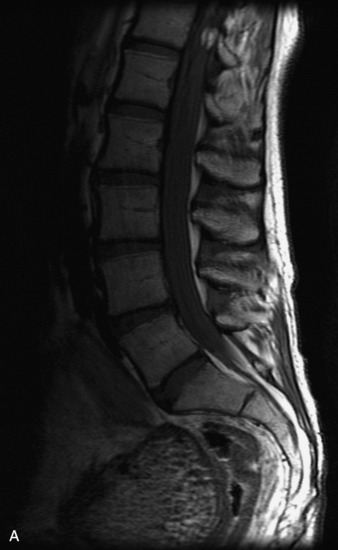
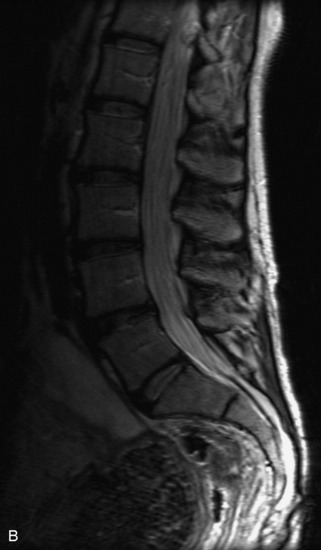
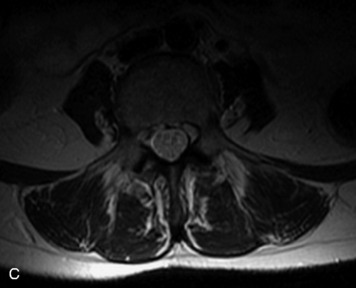
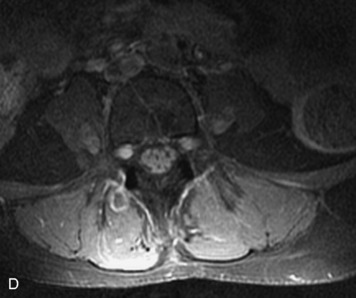
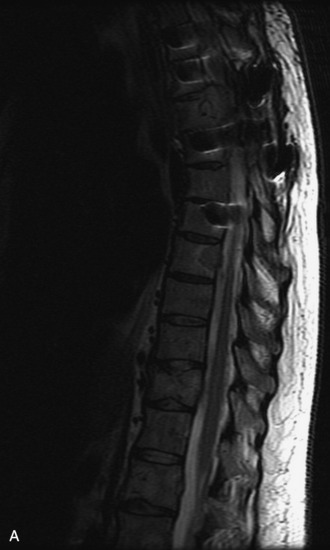
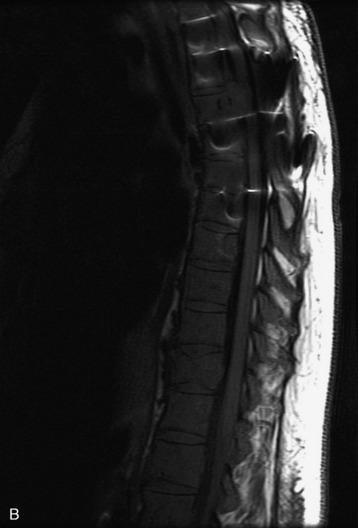
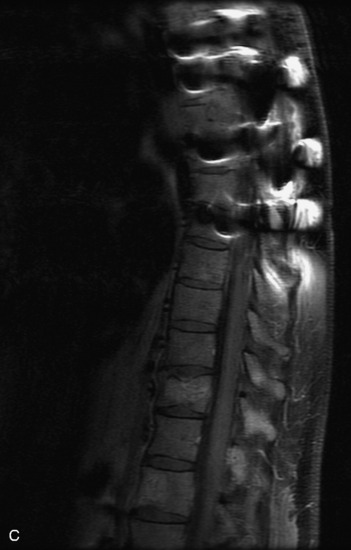
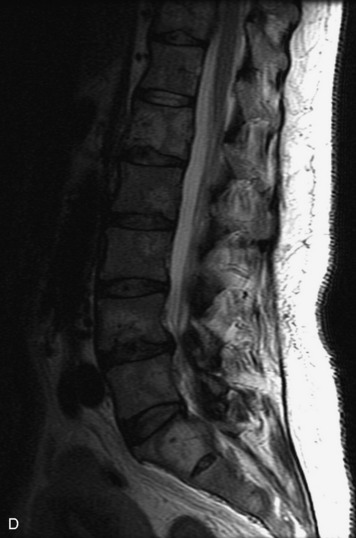
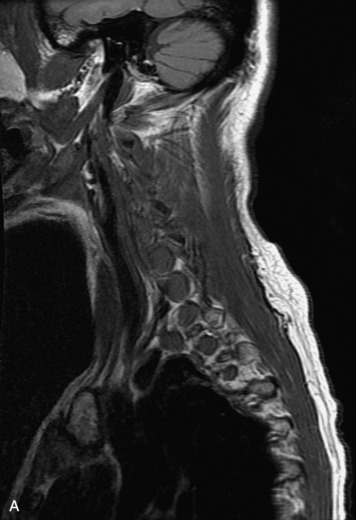
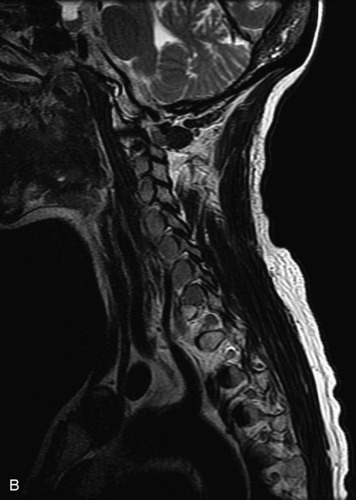
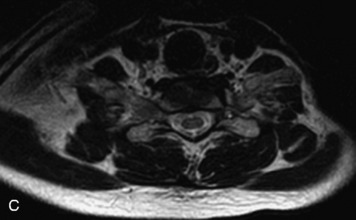
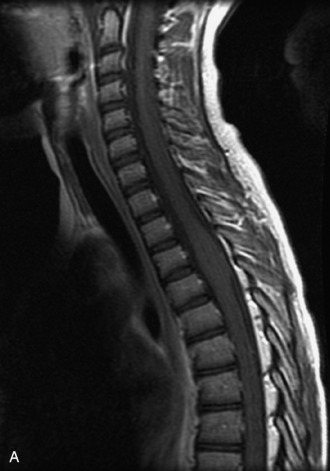
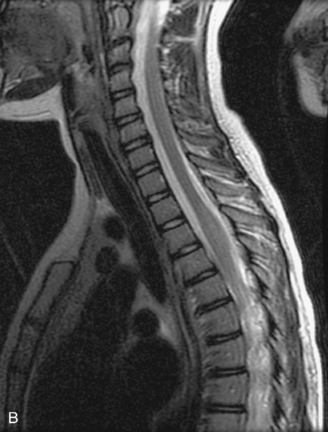
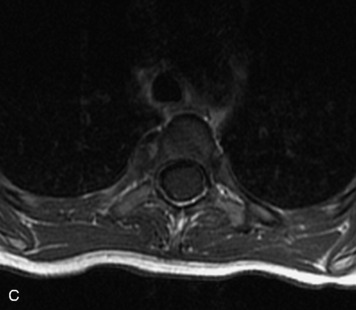
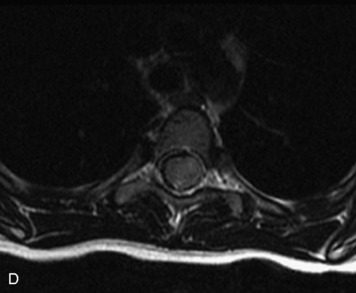
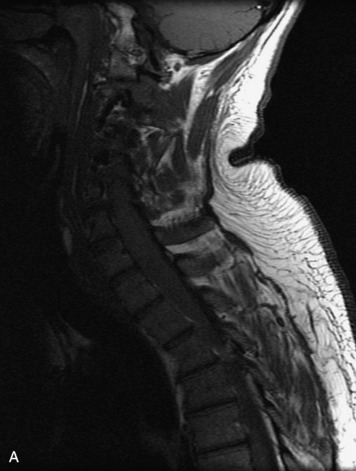
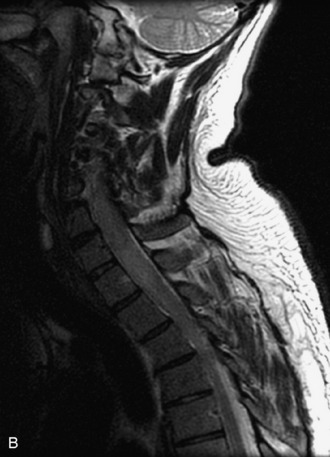
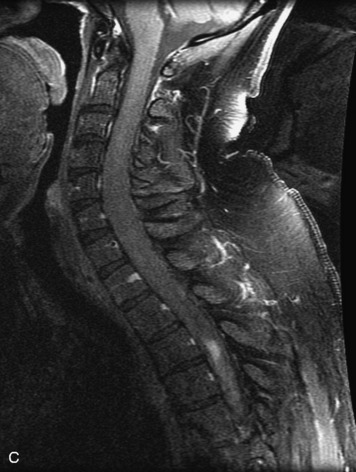
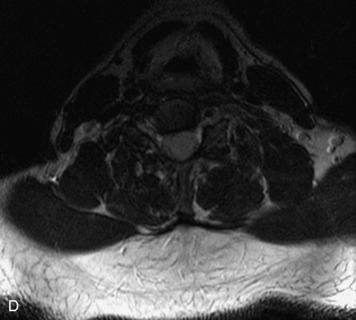
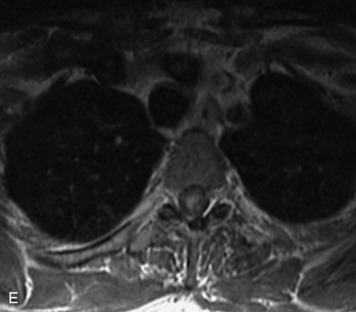
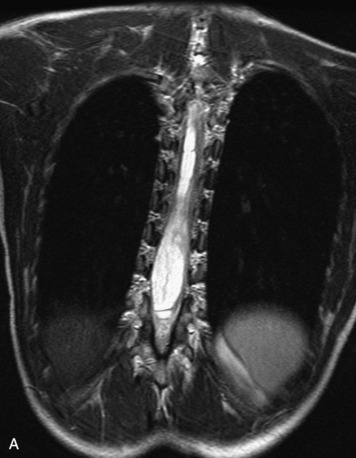
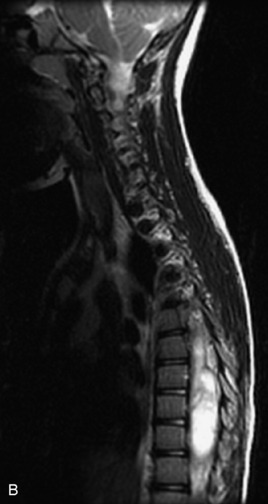
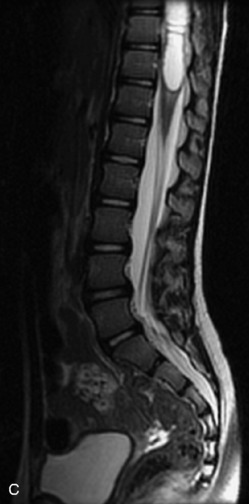
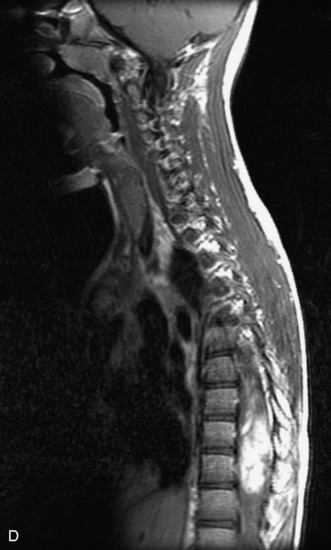
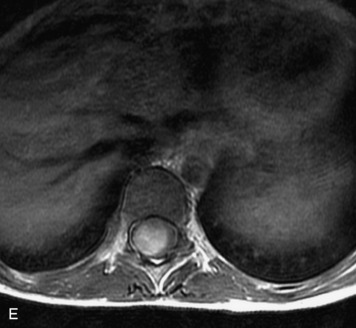
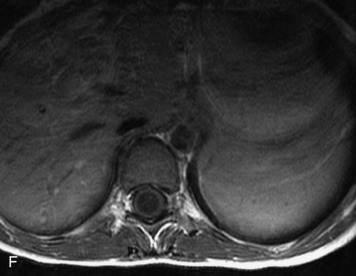
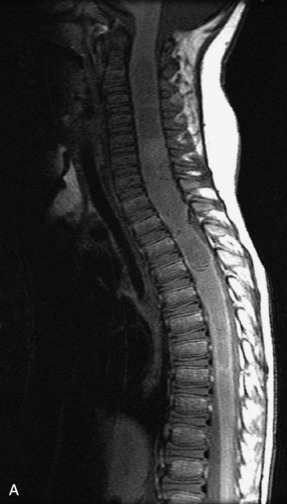
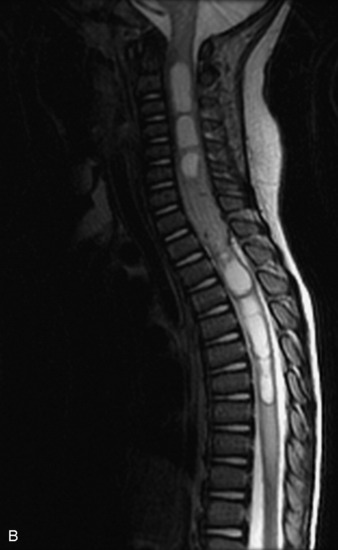
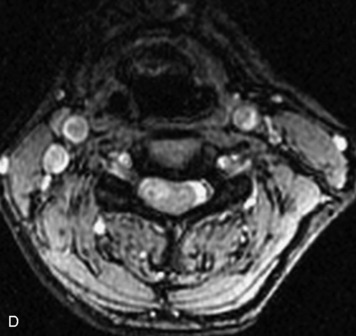
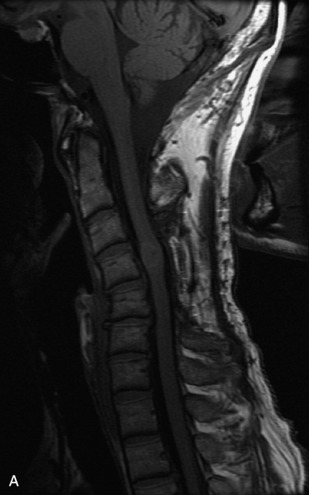
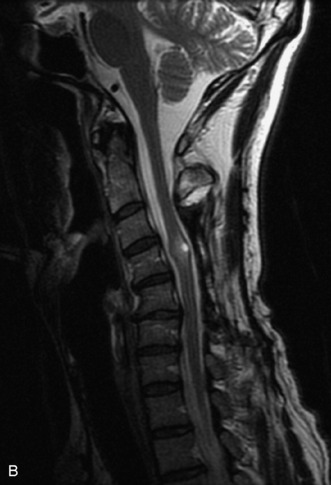
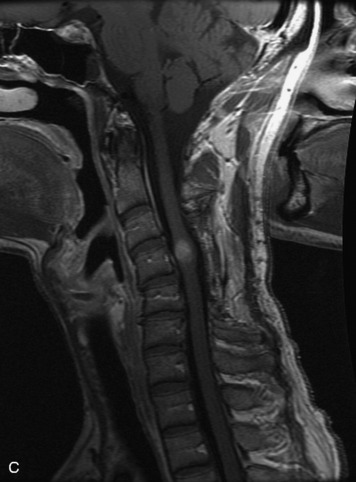
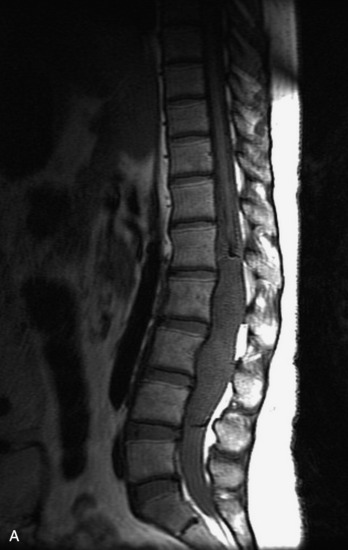
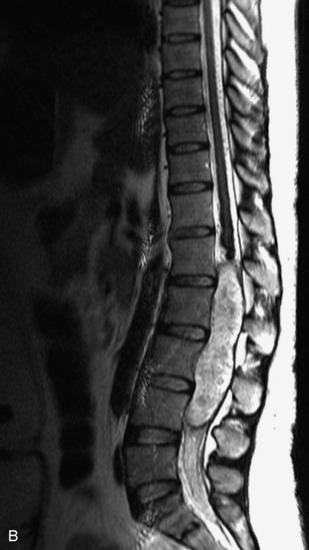
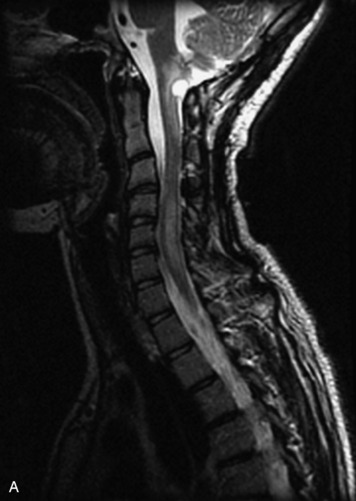
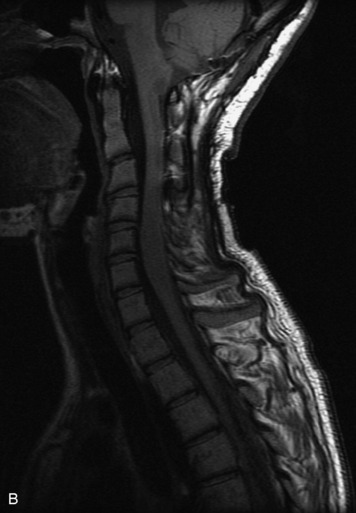
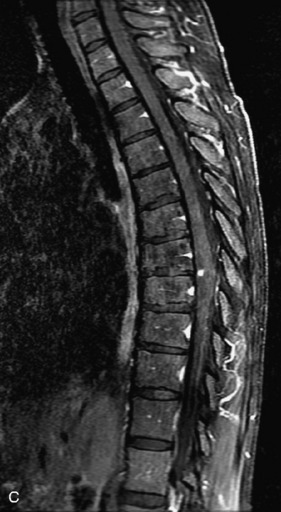
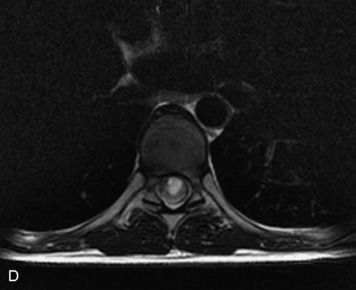
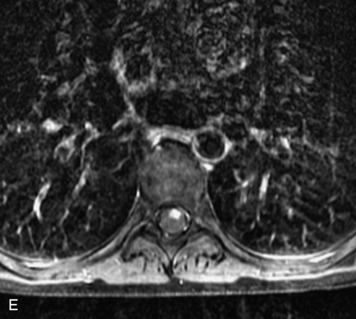
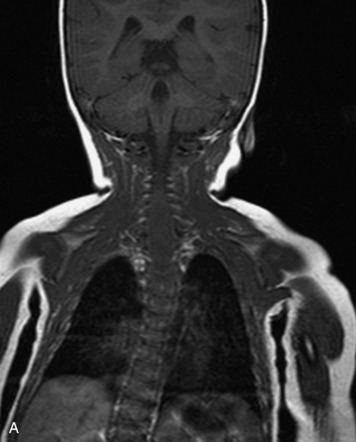
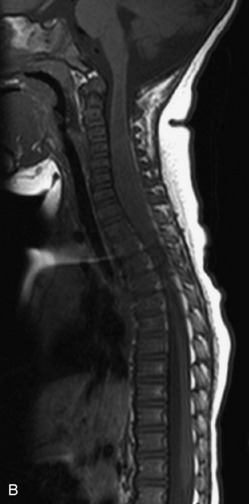
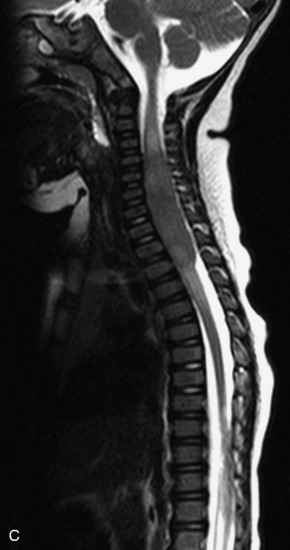
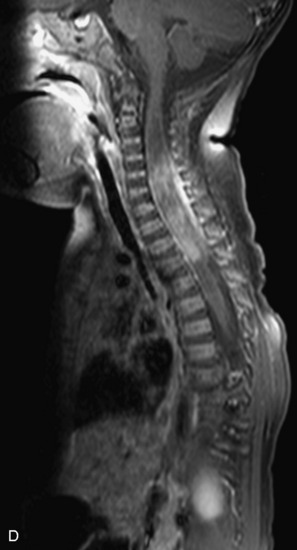
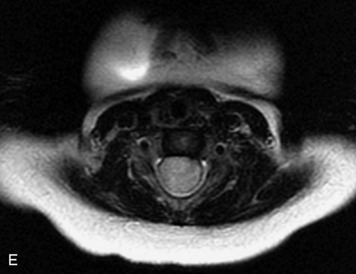
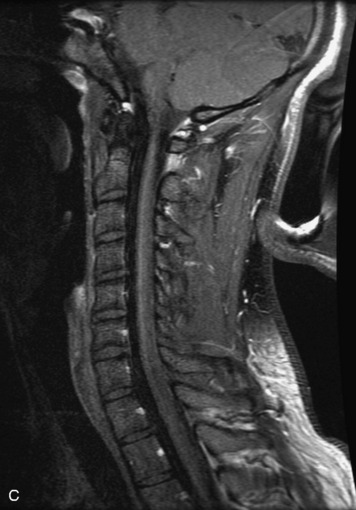
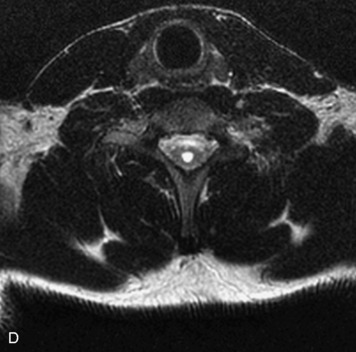
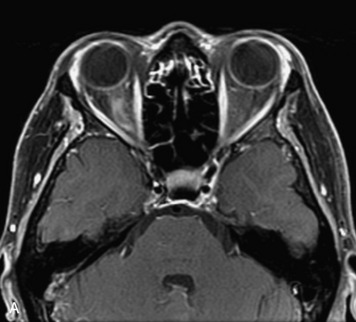
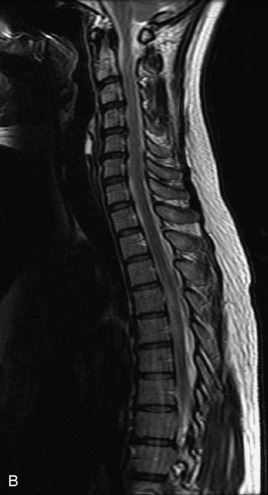
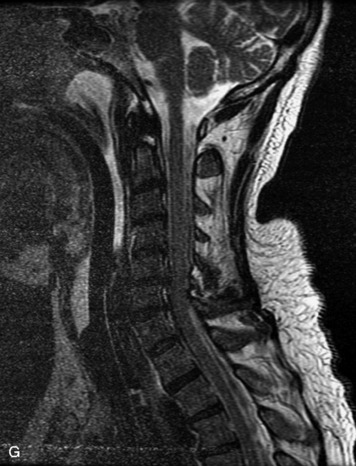
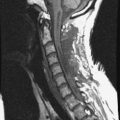
MRI of the spine involves greater technical challenges than imaging of the brain. The presence of bone housing the spinal canal creates large susceptibility gradients. The small size of the spinal canal and spinal cord limits spatial resolution. In addition, CSF pulsation introduces motion artifacts. MR techniques used in screening for spinal lesions include at least a T1- and T2-weighted sequence. Addition of a gradient echo (GRE) sequence is useful to evaluate for blood products. Intravenous gadolinium improves detection of intradural extramedullary disease and helps to characterize and delineate intramedullary lesions.3,6 Evaluation in both the sagittal and transverse planes is important to confirm true findings and exclude artifacts. The role of these standard sequences in evaluating spinal masses is described in Table 10-2. Examples of the normal appearance of the spine when imaged with these standard sequences are shown in Figs. 10-1 to 10-3.
Table 10-2 Standard MRI Sequences for the Evaluation of Spinal Tumors
| T1-weighted image (T1WI) Spin echo (SE) |
Excellent morphologic detail, especially cord size; dark CSF; important information about cysts, fat, and blood |
| T2-weighted image (T2WI) Fast spin echo (FSE) |
FSE has mostly replaced spin echo T2; sensitive for identification of intramedullary tumor and edema; hyperintense CSF; susceptible to CSF motion artifacts, especially in axial plane in cervical spine |
| Gradient echo (GRE) | Sensitive to magnetic susceptibility artifact from hemorrhage; often used in axial plane in cervical spine instead of FSE T2 to reduce CSF pulsation artifact |
| T1-weighted + gadolinium | Useful for characterization and accurate assessment of borders of a lesion; distinguishes tumor from adjacent edema, and differentiates solid tumor from tumor cysts and syrinx; fat suppression improves contrast between enhancing lesion and background, distinguishes fat from hemorrhage, and is particularly important in extramedullary lesions |
CSF, cerebrospinal fluid.
Some newer pulse sequences have been investigated for use in spinal imaging with variable results. These include fluid-attenuation inversion recovery (FLAIR), short tau inversion recovery (STIR), diffusion weighted imaging (DWI), diffusion tensor imaging (DTI), and MR spectroscopy (MRS) (Table 10-3). FLAIR is a heavily T2-weighted sequence that nulls signal from CSF and thus may increase conspicuity of hyperintense lesions in close proximity to CSF. Despite its utility in brain imaging, FLAIR has been less successful in the spine and has shown to be less sensitive than standard T2-weighted sequences in assessment of cord lesions.7–10 STIR (a fat suppressed T2-weighted sequence) is commonly used to assess for vertebral body involvement in the setting of trauma or infection but also may have useful application in detecting intramedullary disease. Several studies have demonstrated increased sensitivity of STIR for cord disease in multiple sclerosis (MS) relative to other T2-weighted sequences.10,11 However, STIR is limited by reduced signal to noise ratio and greater sensitivity to motion than standard T2-weighted sequences.8 DWI is an MR technique that is sensitive to the random motion of water molecules in tissues over microscopic distances (5–20 microns) and can generate a map of the average apparent diffusion coefficient (ADC). Reduced ADC is seen in acute cerebral stroke and aids early detection of infarcts, improving accuracy over conventional MRI.12 DWI also has become an invaluable technique for assessing other intracranial diseases, including applications in infection, tumors, and cysts. DWI in the spinal cord and spinal canal has been more difficult to implement than in the brain because of technical factors, in particular susceptibility from the vertebrae and limited spatial resolution. Although DWI is not currently routinely used for spinal cord imaging at most institutions, DWI techniques have been developed that are practical for use in the clinical setting.13,14 DTI, an application of DWI that measures diffusion anisotropy and thus can be used to trace white matter tracts within the brain and spinal cord using statistical methods, is of great interest in spinal cord tumor imaging. DTI demonstrates a high degree of anisotropy in the normal spinal cord, which is disrupted by spinal cord tumors, a feature that may be helpful to depict the full extent of spinal cord lesions more accurately than conventional imaging and perhaps eventually map out involvement of key spinal cord white matter tracts.15,16 MRS is a technique that can obtain biochemical information about tissues being studied by creating a spectrum of metabolites within a region of interest. Currently, a number of technical factors preclude use of MRS in the spine, but future advances may make this technique both possible and useful in spinal cord imaging.8
Table 10-3 Potential Sequences for Evaluation of Spinal Tumors
| FLAIR | CSF-suppressed T2-weighted sequence; intramedullary lesions may be less conspicuous on FLAIR than on FSE T2; CSF pulsation artifact accentuated |
| STIR | Fat-suppressed T2-weighted sequence; intramedullary lesions shown to be more conspicuous; particularly useful in assessment of trauma and infection; lower signal to noise than FSE T2; sensitive to motion. |
| DWI | Currently limited in spine because of motion, spatial resolution, and susceptibility; in vivo application has been performed in spinal cord infarcts and MS |
| DTI | Application of DWI that measures diffusion anisotropy and may be used to trace white matter tracts; potential use in imaging spinal cord tumors, but not yet feasible in the clinical setting |
| MRS | Demonstrates spectrum of metabolites in region of interest; currently limited by spatial resolution and susceptibility artifacts and cannot yet be performed adequately in spine |
MS, multiple sclerosis.
EXTRADURAL LESIONS
Extradural lesions, which by definition are outside of the dura, usually arise from the osseous spinal structures, intervertebral discs, and surrounding soft tissues (including vessels, nerves, fat, and paraspinal muscles). These lesions cause focal displacement of the thecal sac and its contents away from the mass. Radiographs may demonstrate secondary effects of the lesion on the bony structures, including bone destruction or scalloping, expansion of the central spinal canal, and paraspinal extension. CT myelography shows extrinsic compression of the thecal sac and decrease in the ipsilateral CSF space. MRI shows the dura draped over the mass and may show an epidural fat cap (Box 10-1).17 The most common epidural masses are degenerative, including disc protrusions, osteophytes, and synovial cysts. Infection and hematomas may present as epidural masses. Of neoplastic lesions, metastases to bone with extradural extension are the most common.17,18 Imaging of lesions of the vertebrae (with and without epidural extension) was discussed in the previous chapter. Here we discuss epidural lesions that were not previously mentioned.
EPIDURAL LIPOMATOSIS
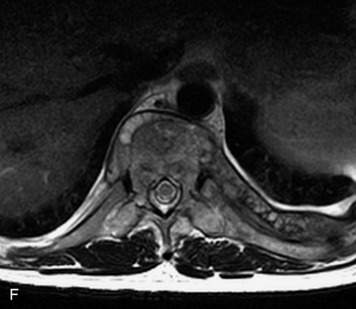
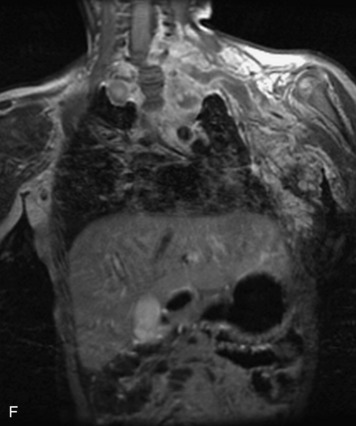
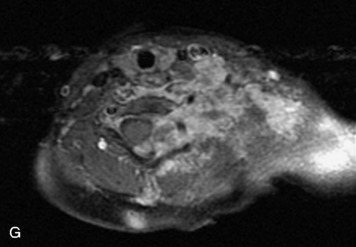
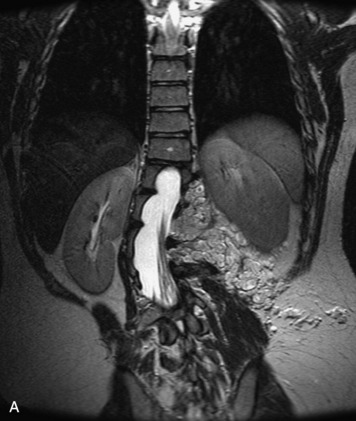
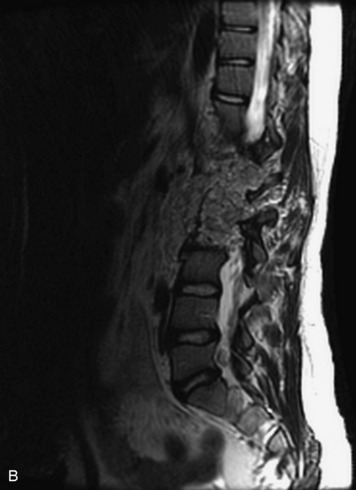
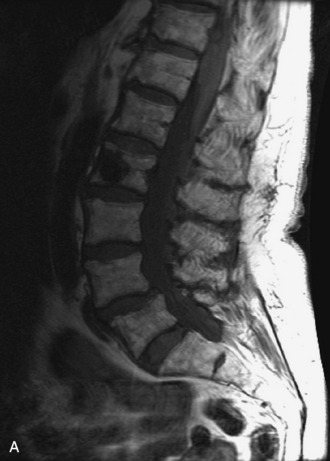
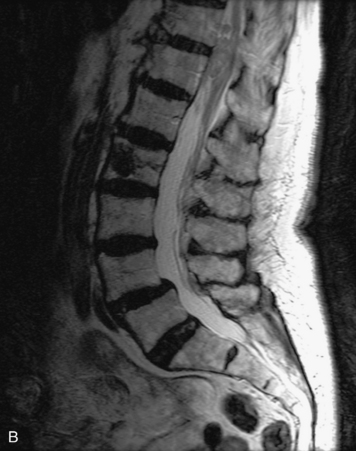
This is a rare condition manifested by excessive deposition of unencapsulated fat in the epidural space, resulting in cord and cauda equina compression with neurologic symptoms. Most cases are seen in association with long-term exogenous steroid use or conditions in which there is elevated endogenous production of steroid (such as in Cushing’s disease). Cases may occur as a result of morbid obesity or with no cause evident.19 In approximately 60% of cases, involvement of the mid to lower thoracic spine is seen with fat predominantly posterior to the cord. The remaining 40% of cases involve mainly the lower lumbar region with fat surrounding the circumference of the thecal sac (Box 10-2). A combination of thoracic and lumbar involvement is sometimes seen.20 CT and MR demonstrate increased epidural fat and diminished subarachnoid space, with tapering of the thecal sac and a Y-shaped configuration seen in the transverse plane (Fig. 10-4).21,22 Epidural lipomatosis does not enhance nor does it cause bony erosion.17,20 Fat suppression is helpful on MRI to confirm the presence of adipose tissue (Box 10-2).
ANGIOLIPOMA
Angiolipomas are benign tumors composed of adipose and vascular elements that are uncommon in the spine. They are almost always epidural, but intramedullary angiolipomas have been reported.23 Lesions usually extend over more than two vertebral body segments and may manifest as either a focal or infiltrating mass.24,25 On CT, angiolipomas usually demonstrate low attenuation relative to the cord with scattered soft tissue reticulation.26 On MRI, lesions are hyperintense on unenhanced T1-weighted images (T1WI) because of fat content and are hyperintense on T2-weighted images (T2WI) (Box 10-3). Mild, heterogeneous enhancement is seen on post-gadolinium fat-saturated images (Box 10-3).24
EPIDURAL HEMATOMA
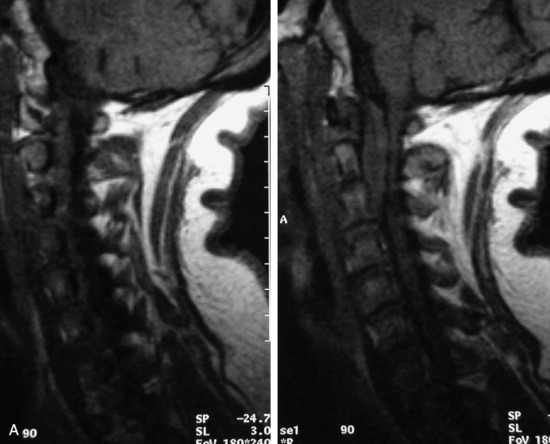
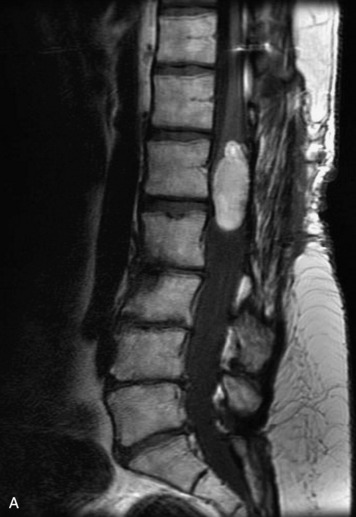
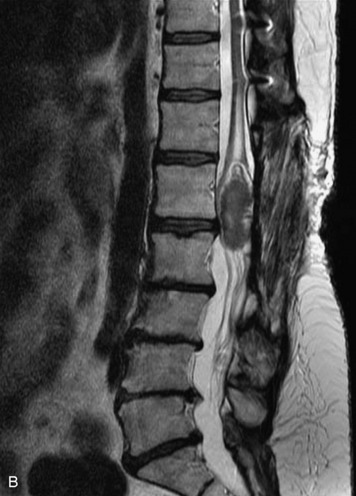
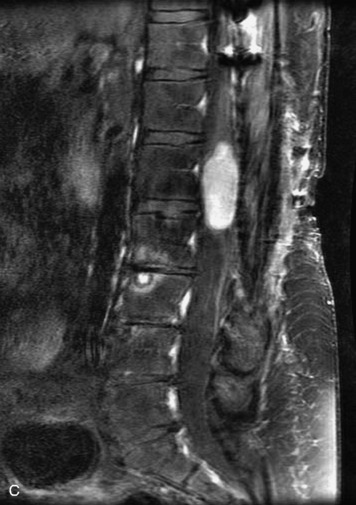
Extravasation of blood into the epidural compartment of the spine may be caused by trauma or instrumentation but also can occur spontaneously, especially in the setting of anticoagulation or coagulopathy (Box 10-4). An epidural hematoma is rarely the sequelae of hemorrhage from a spinal vascular malformation.27,28 MR and CT myelography demonstrate an elongated, often multisegment, extra-axial mass encasing or displacing the thecal sac.20 MRI signal characteristics depend on the age of blood products and are usually heterogeneous. Hyperintensity on T1WI, heterogeneity with foci of low signal on T2WI, and susceptibility demonstrated with GRE are helpful features in making the diagnosis (Fig. 10-5).29,30 Enhancement may be seen, especially along the margins of an epidural hematoma (Box 10-4).20,30
Box 10-4 Epidural Hematoma
EPIDURAL ABSCESS
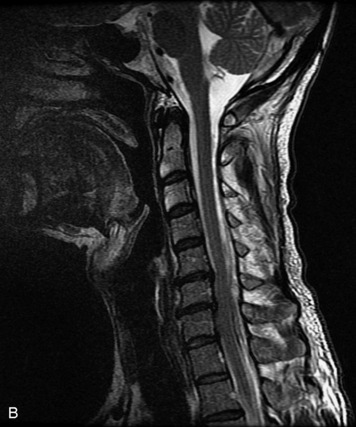
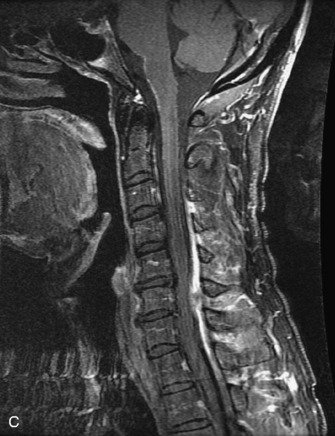
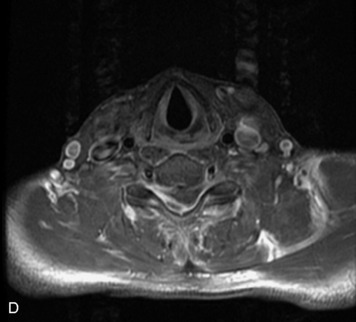
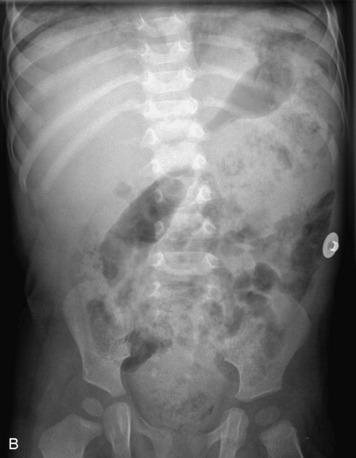
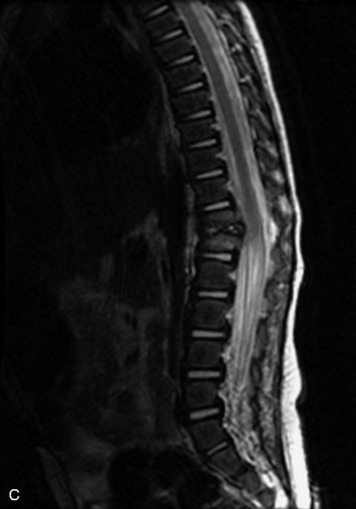
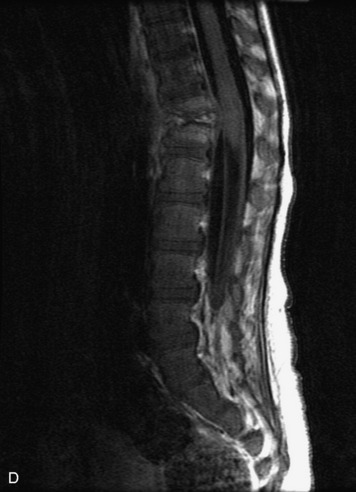
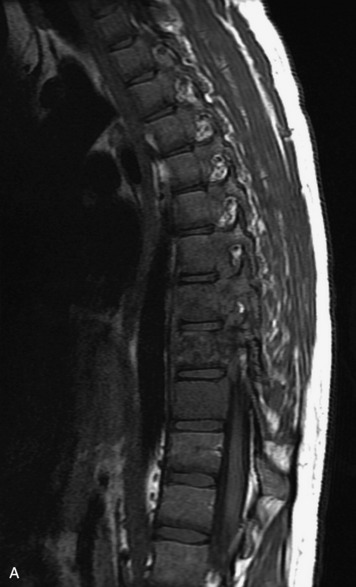
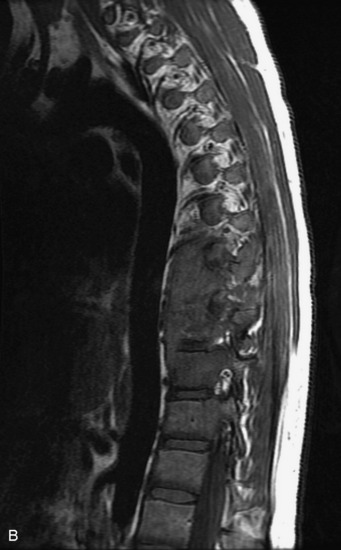
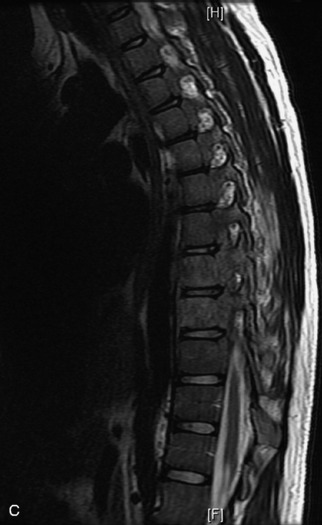
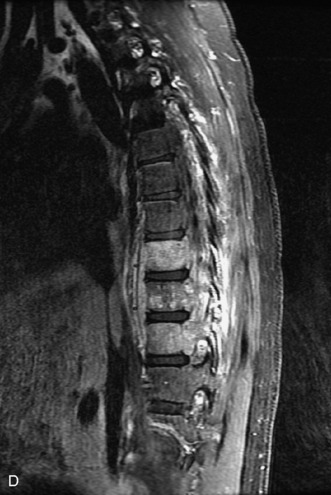
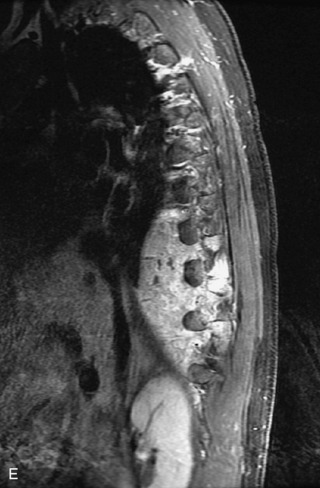
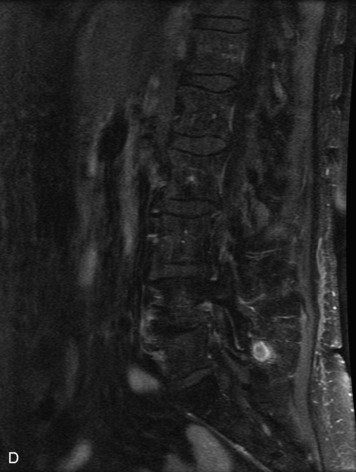
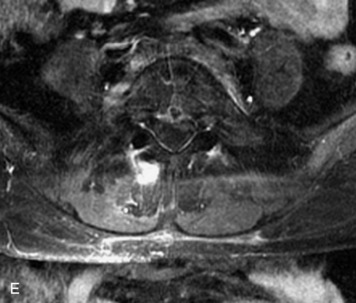
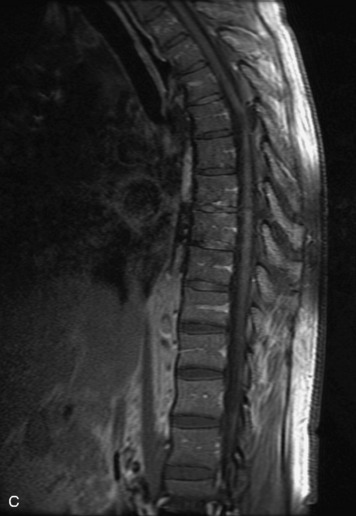
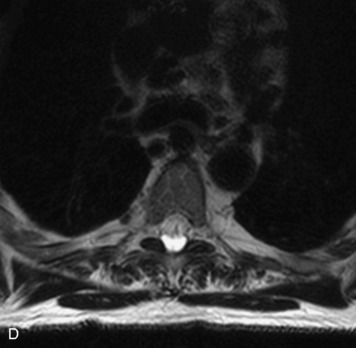
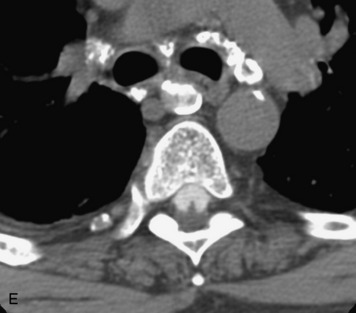
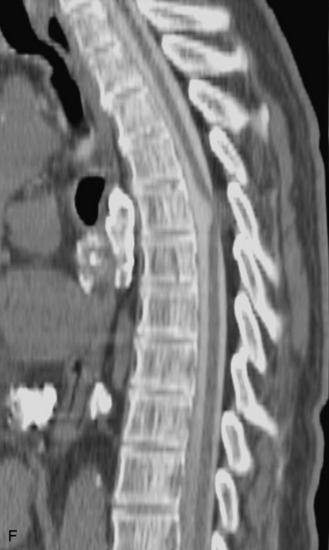
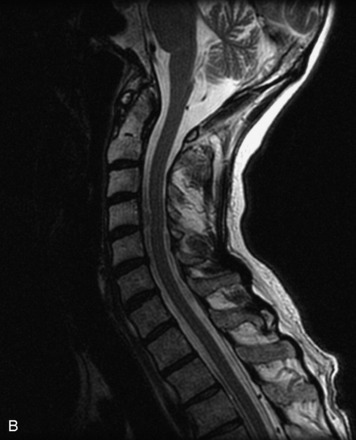
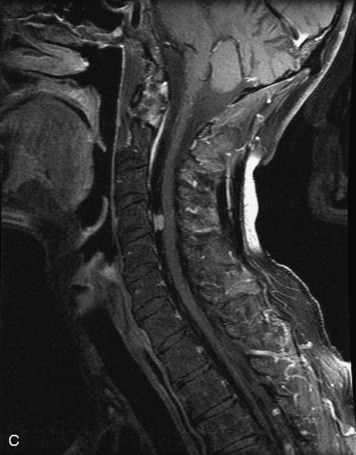
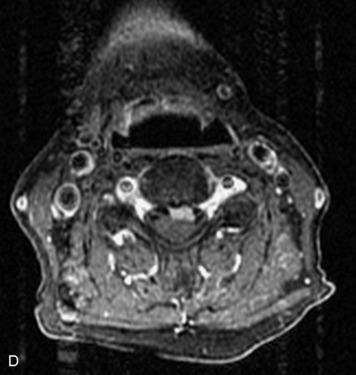
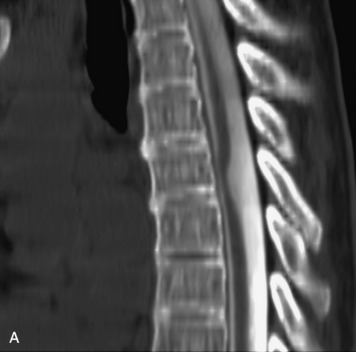
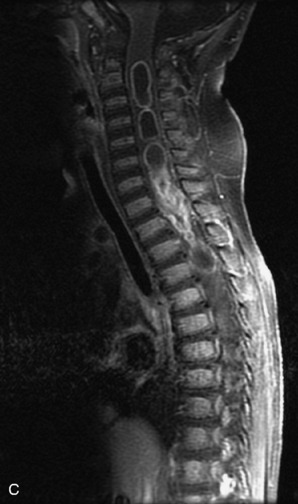
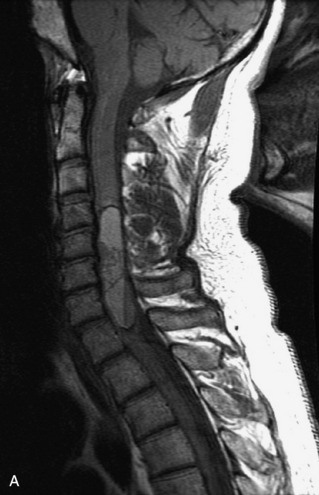
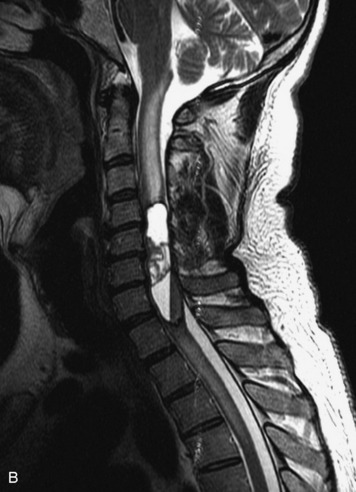
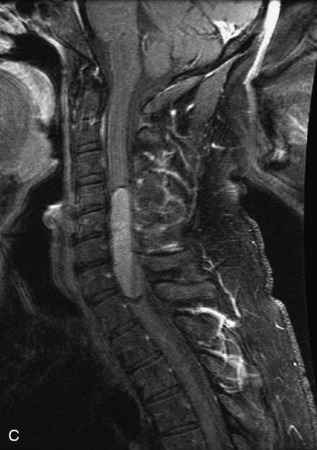
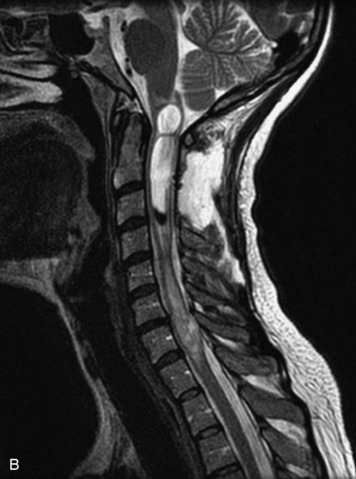
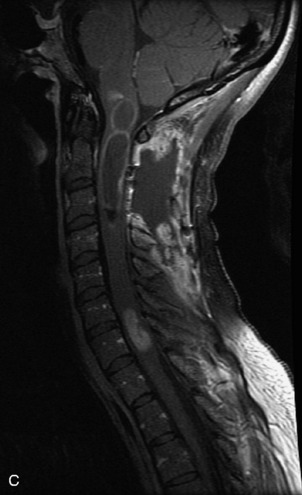
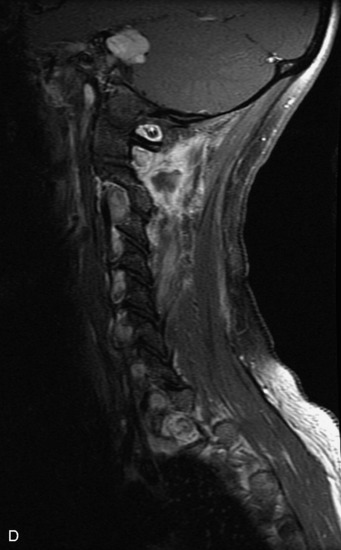
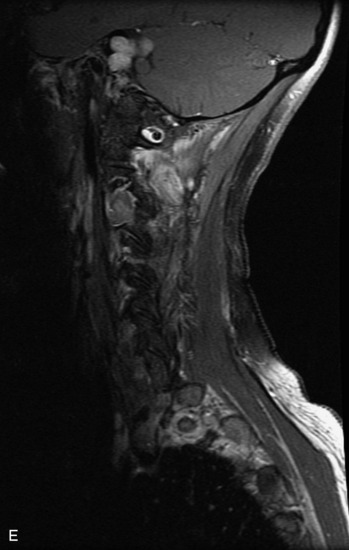
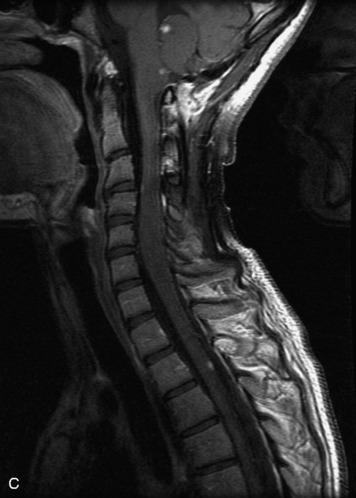
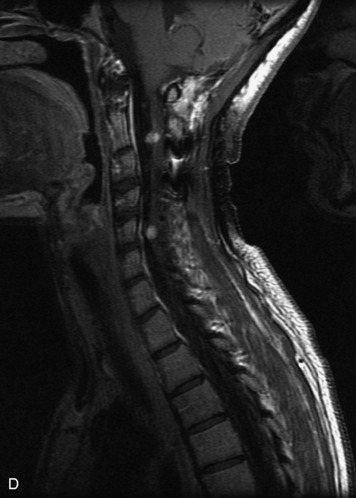
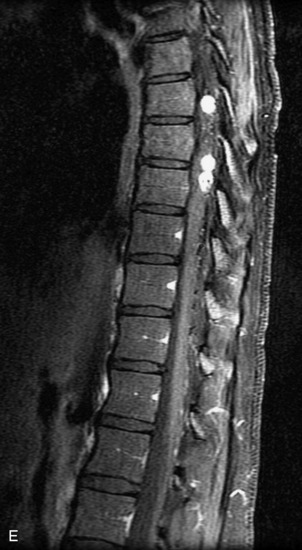
Epidural spinal infection with abscess formation usually results from hematogenous spread of disease primarily to the intervertebral disc, typically from infection of the skin, urinary tract, or respiratory system. Direct spread from paraspinal infection also may occur but is less common. Predisposing factors include diabetes mellitus, trauma, alcoholism, and intravenous drug abuse (Box 10-5). Epidural anesthesia and analgesia also are associated with increased risk. Pyogenic organisms (most often Staphylococcus aureus) are the most common pathogens.31,32 MRI is the imaging tool of choice and should include the entire spinal axis to exclude multiple sites of involvement.32 The posterior epidural space may be more commonly involved than the anterior, and the abscess can extend over multiple segments.33 Abscesses are classically isointense to hypointense to the cord on T1WI, hyperintense on T2WI and STIR images, and demonstrate rim enhancement (Fig. 10-6). Homogeneously or heterogeneously enhancing phlegmon may often be seen in some infections without frank abscess formation (Figs. 10-7 and 10-8).34 Associated findings of spondylodiscitis, including vertebral body endplate erosion and disc space narrowing, are helpful in some cases in making the diagnosis. High signal on DWI with reduced ADC has been demonstrated in spinal epidural abscesses and also may be helpful to confirm the diagnosis.35
Box 10-5 Epidural Abscess
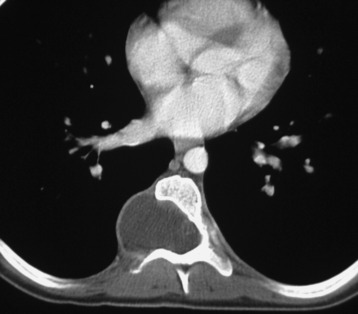
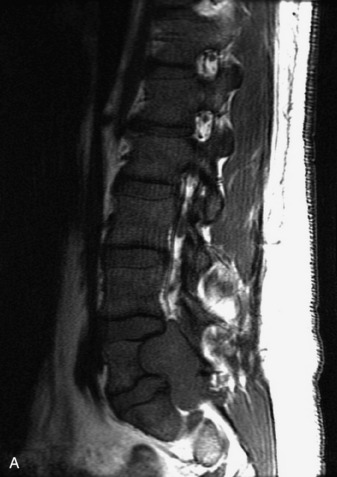
SYNOVIAL CYST
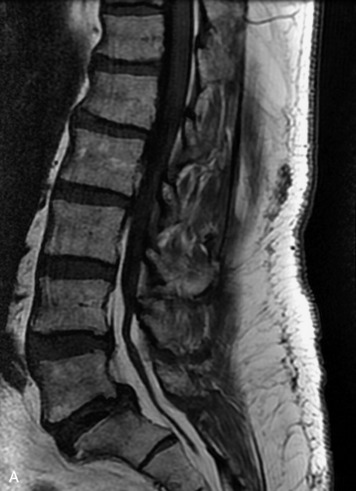
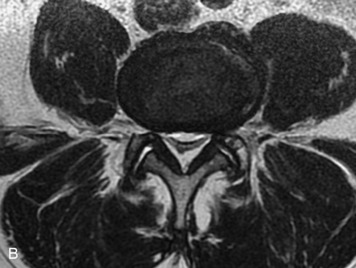
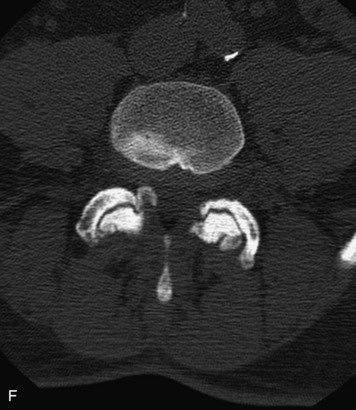
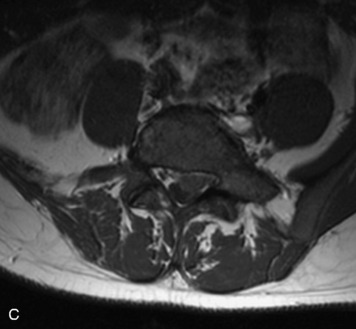
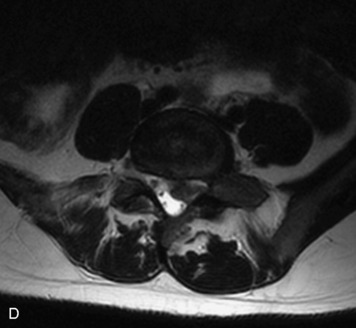
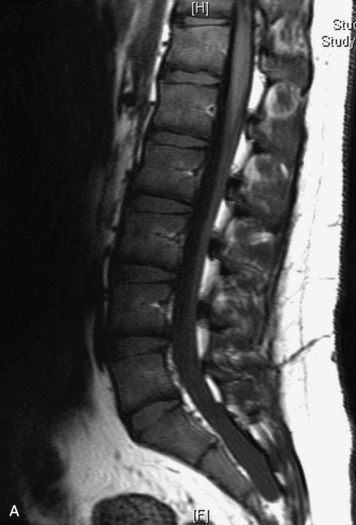
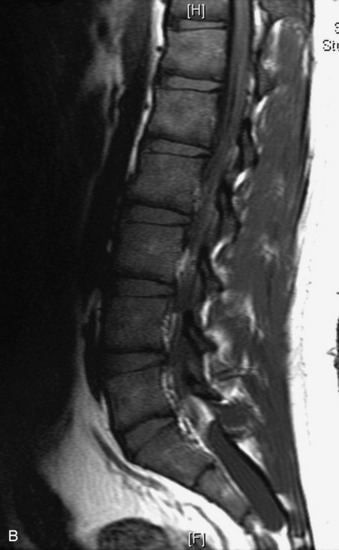
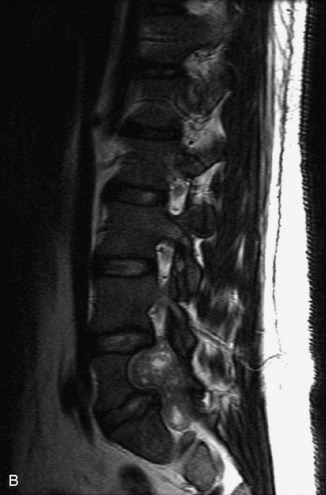
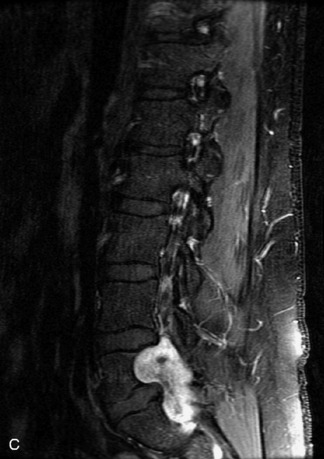
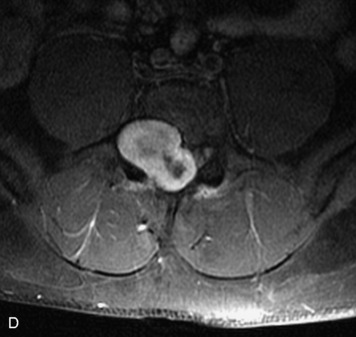
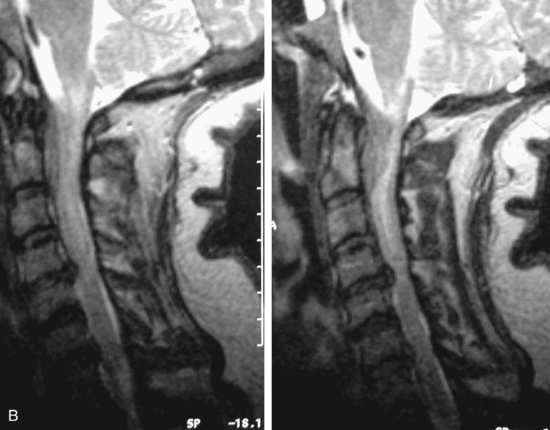
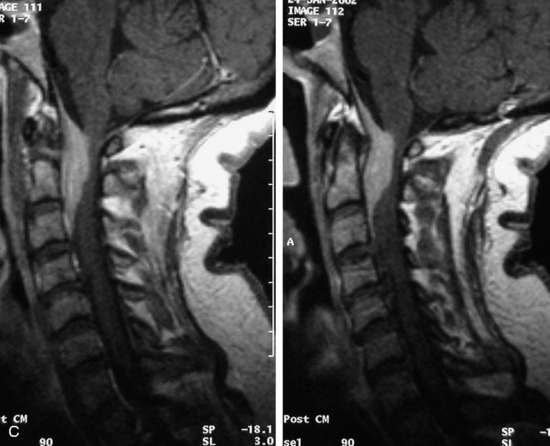
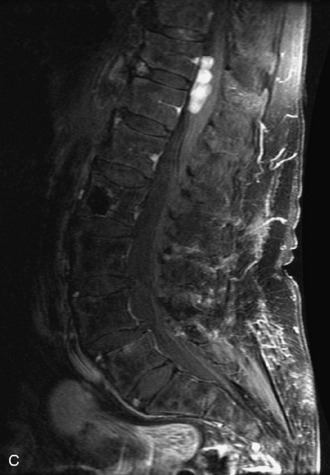
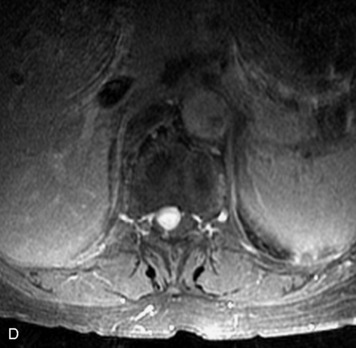
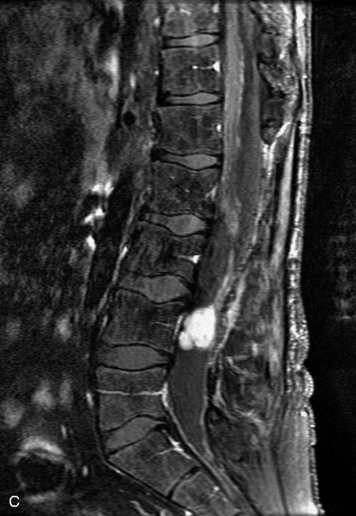
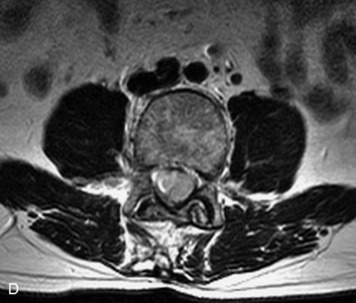
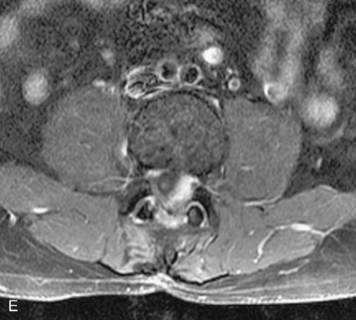
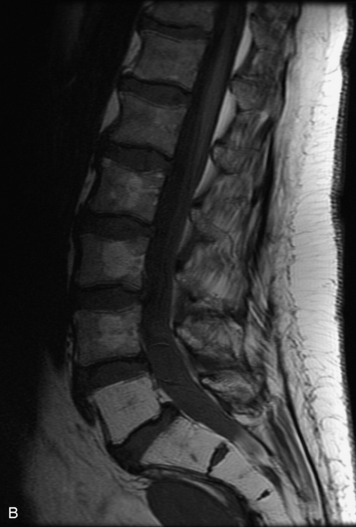
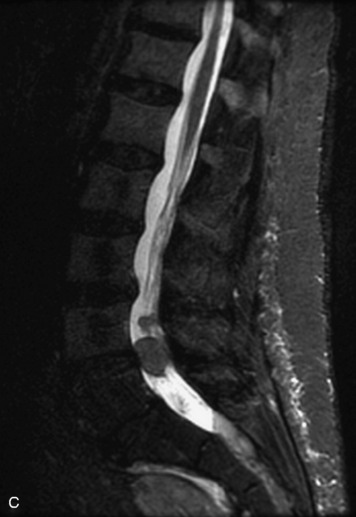
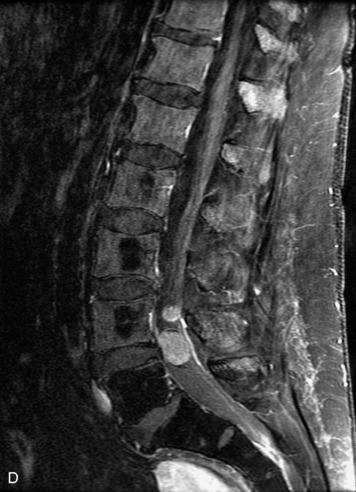
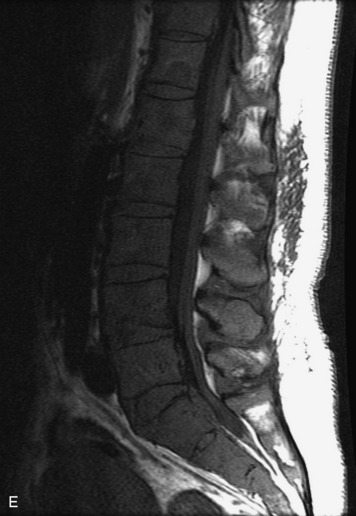
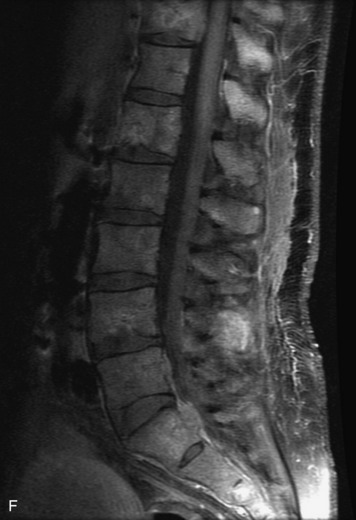
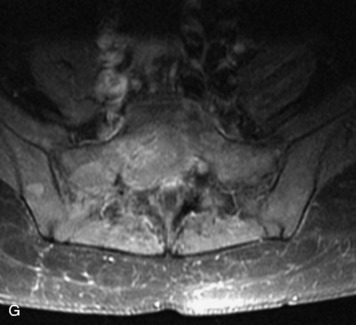
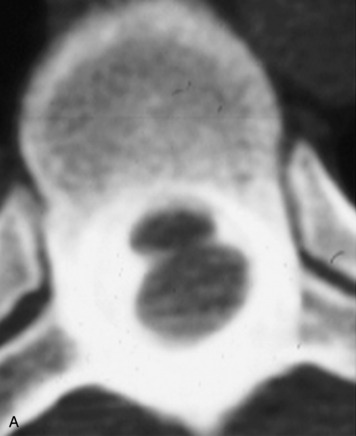
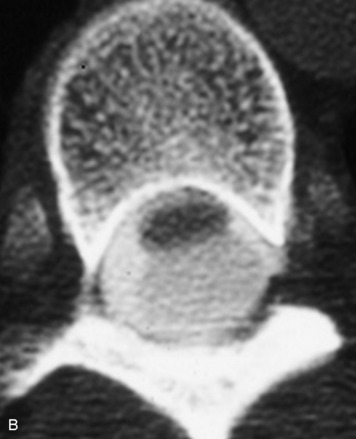
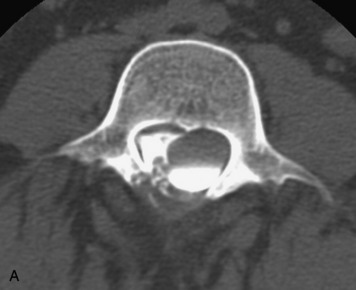
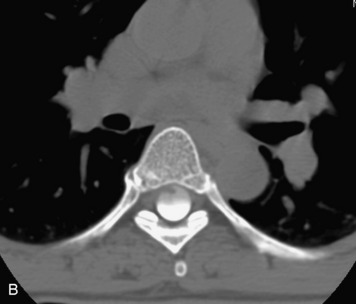
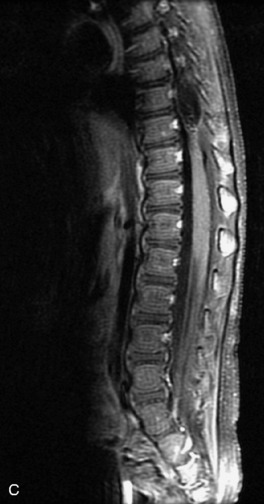
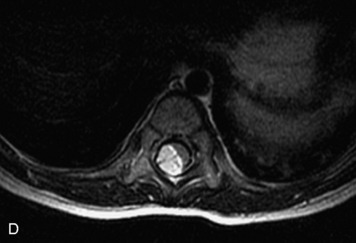
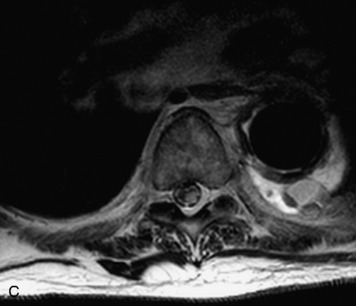
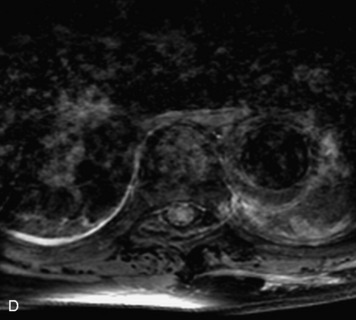
Synovial cysts are almost always associated with degenerated facet joints and usually do not present a diagnostic dilemma. The appearance of a round, sharply marginated, posterolateral, extradural cystic mass communicating with the facet joint is characteristic (Fig. 10-9). MRI signal intensity of these lesions varies depending on the protein content and the presence of calcification or hemorrhage (Fig. 10-10). Mild rim enhancement may be seen (Fig. 10-11). These lesions may be difficult to detect on CT unless calcification, intracystic gas, or hemorrhage is present (Box 10-6).36,37
SEQUESTERED DISC FRAGMENT
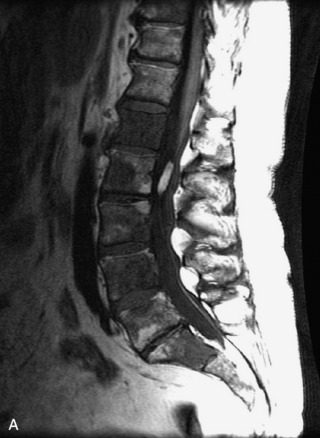
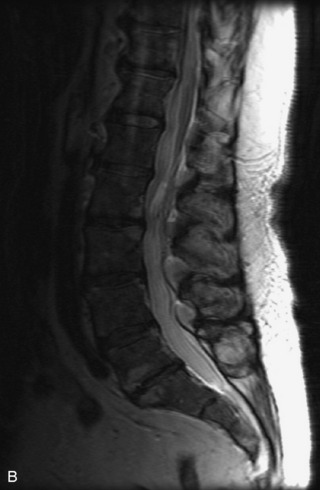
Protruded disc material may detach and become displaced from the parent disc. The extruded, sequestered disc fragment usually is found in the anterior epidural space but may occasionally migrate posteriorly or through the neural foramen.38 Although peripheral enhancement may be seen, the disc material itself usually does not enhance.17,38,39 Additional findings of degenerative disc disease are helpful in making the diagnosis (Fig. 10-12).
EXTRADURAL ARACHNOID CYST
An extradural arachnoid cyst (EAC) is a congenital or acquired CSF-filled outpouching of arachnoid that protrudes through a dural defect (Box 10-7). Most EACs are found in the lower thoracic spine, posterior or posterolateral to the cord.5 MRI demonstrates a long-segment extradural mass that follows CSF signal characteristics on all sequences. CT myelography may show communication with the CSF space.17,40
Box 10-7 Extradural Arachnoid Cyst
INTRADURAL EXTRAMEDULLARY LESIONS
Intradural extramedullary lesions arise inside the dura but outside of the spinal cord or cauda equina. These lesions tend to displace the spinal cord and enlarge the ipsilateral subarachnoid space with a sharp interface between the undersurface of the mass and the CSF space (Box 10-8). Nerve sheath tumors (schwannomas and neurofibromas) and meningiomas account for the vast majority of intradural lesions. Paragangliomas and CSF-disseminated metastases are relatively rare neoplasms of the intradural space. Tumor-like cysts, including arachnoid cysts, epidermoids, and neurenteric cysts, are non-neoplastic lesions that may be seen in the intradural space and may cause symptoms such as spinal cord or nerve root compression. Lipomas are considered congenital developmental lesions and are associated with a tethered cord but may be an incidental finding and may not present until adulthood.
SCHWANNOMA
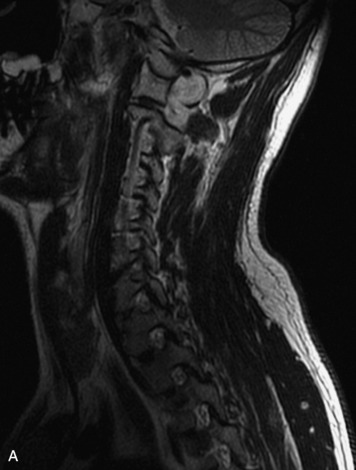
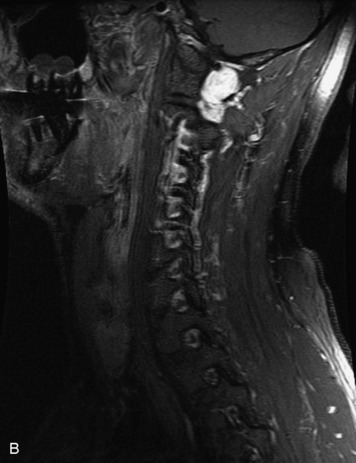
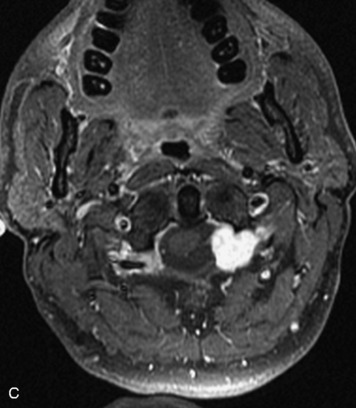
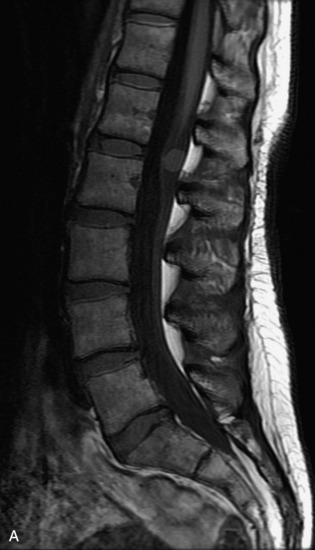
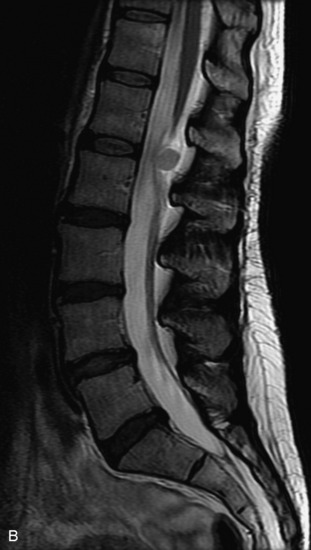
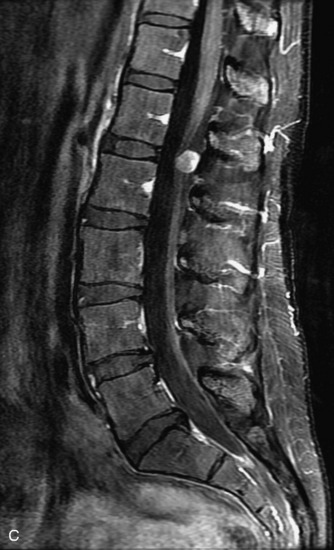
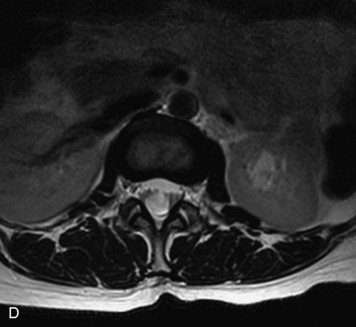
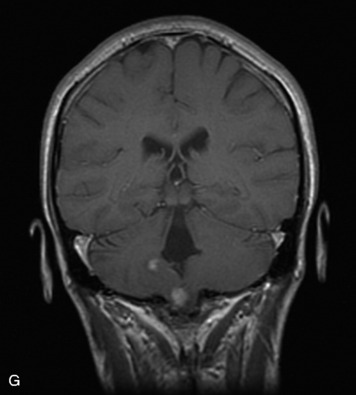
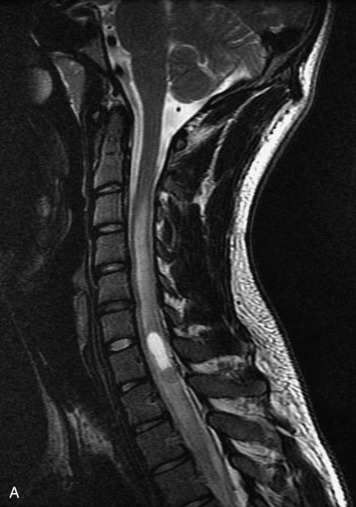
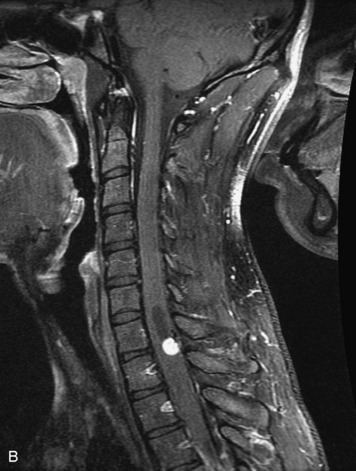
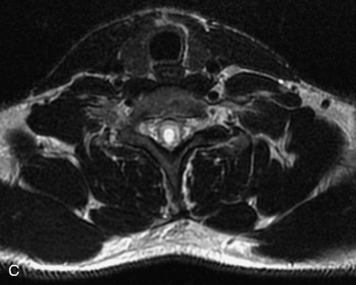
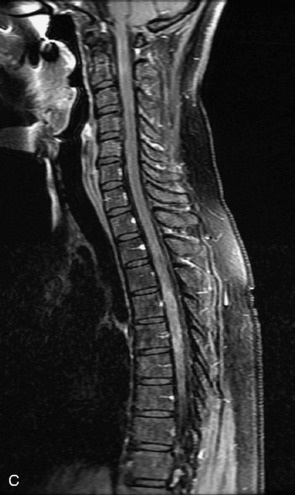
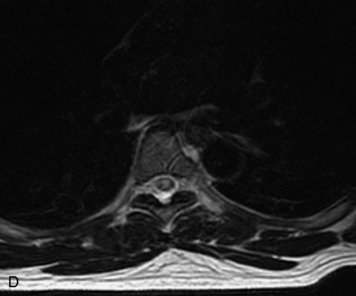
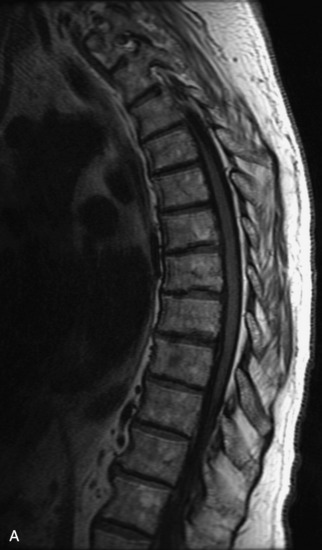
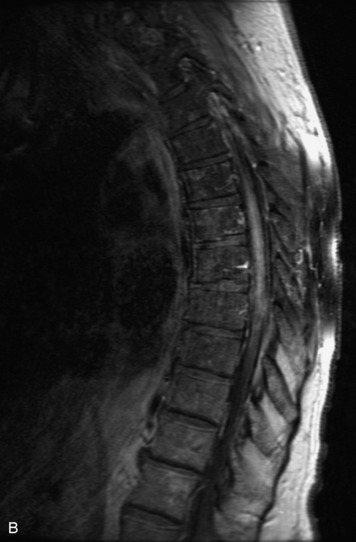
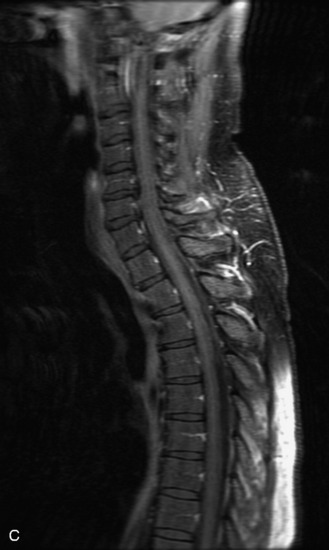
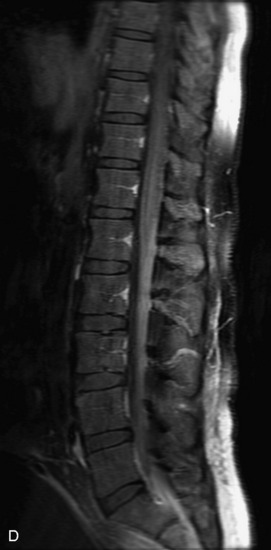
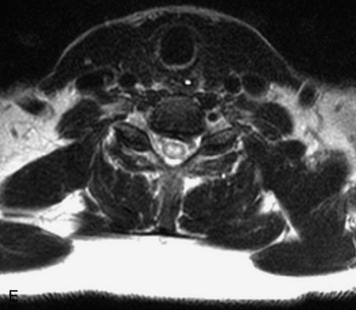
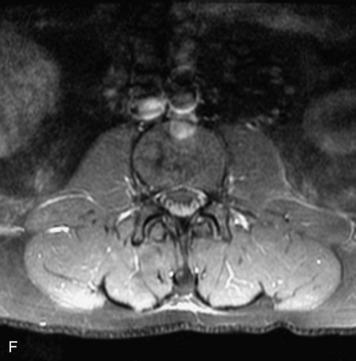
Schwannomas are grossly encapsulated neoplasms of the nerve sheath in the peripheral nervous system that typically arise from the dorsal sensory root. They account for approximately 30% of all spinal tumors and 70% of intraspinal tumors and occur with fairly uniform distribution throughout the spine.18 Most lesions are sporadic with peak incidence between 30–60 years of age (Box 10-9). Lesions are usually solitary unless associated with an underlying genetic condition such as neurofibromatosis type 2 (NF-2), schwannomatosis, or Carney syndrome.20 Lesions are generally benign, although malignant degeneration may occur.41 Classically, schwannomas are well-circumscribed, lobulated or dumbbell shaped, enhancing spinal masses that often show cystic degeneration and/or hemorrhage. Approximately 70–75% of lesions are intradural, 15% are completely extradural, and 15% are a combination of intradural and extradural.17 Intradmedullary schwannomas have been reported but are rare (<1%).42,43 On CT, lesions are usually isodense to the spinal cord with lower density cystic changes, adjacent bony remodeling (enlarged neural foramen and expanded canal), and either peripheral or solid enhancement.20,44 Calcification is rare. On MRI, lesions are typically isointense to the cord on T1WI, with the exception of rare melanotic schwannomas, which are hyperintense.45,46 Most lesions are bright on T2WI, with some lesions demonstrating a “target” appearance with a bright rim and dark center. Enhancement is usually intense and may be homogeneous, heterogeneous, or peripheral (Figs. 10-17 to 10-19).41,44,45,47 Melanotic schwannomas are a rare variant of schwannomas composed of melanin-producing cells with features of Schwann cells. These lesions have an increased incidence of local recurrence after resection as well as an increased likelihood of metastasis compared with typical schwannomas and require careful long-term follow-up. Melanotic schwannomas also may be challenging to differentiate from malignant melanoma, which has a much worse prognosis.48
Box 10-9 Schwannoma
NEUROFIBROMA
Neurofibromas are unencapsulated neoplasms of the nerve sheath. They are less common than schwannomas and have a peak incidence between 20–30 years of age. Most lesions are sporadic, solitary tumors (Box 10-10). However, multiple neurofibromas (especially those of the plexiform type) are typically seen in NF-1. Lesions may be localized, diffuse, or plexiform in morphology.20 Imaging characteristics may be indistinguishable from schwannomas, especially for the localized types. However, hemorrhage and cystic degeneration are less common. A target appearance of peripheral hyperintensity on T2WI with central low to intermediate signal intensity has been described as more common in neurofibromas than in schwannomas.49,50
Box 10-10 Neurofibroma
MENINGIOMA
Meningiomas are slow-growing benign tumors that arise from arachnoid cap cell rests and are based on the dura. They are the second most common type of intradural lesions, accounting for 25% of spinal tumors, and occur most commonly in the fifth and sixth decades of life, with a female predilection (Box 10-11). Most lesions are solitary and sporadic, but multiple lesions can be seen in the setting of multiple meningiomatosis or NF-2, and some meningiomas may occur as the sequelae of prior radiation.17,18,41,51 The vast majority of meningiomas (90%) are intradural. The remaining 10% are either extradural or dumbbell-shaped (intradural and extradural), with the rare occurrence of intramedullary or paraspinal meningiomas reported.52–55 Meningiomas are most commonly found in the thoracic region in a posterolateral location. Cervical meningiomas are more commonly anterior to the cord.56 The classic appearance is of a round, well-circumscribed, uniformly enhancing, broad-based mass against the dura with a dural tail. On CT, most meningiomas are isointense to slightly hyperdense to the spinal cord; demonstrate strong, homogeneous enhancement; and have an enhancing dural tail. Variable amounts of calcification may be present within meningiomas, and some are so heavily calcified that enhancement may not be apparent. Cystic change may occur but is rare. Hyperostosis of the adjacent bone is a feature that, if present, is helpful to make the diagnosis. Similar features are demonstrated on MRI. Most lesions are isointense to the spinal cord on T1- and T2-weighted sequences and enhance uniformly, with variation of signal intensity and enhancement dependent on the degree of calcification of the lesion (Figs. 10-22 to 10-25).17,18,41,47,57 DWI may demonstrate reduced ADC in some meningiomas and may be helpful in making the diagnosis and determining the likelihood of recurrence.58,59 Highly vascular meningiomas may demonstrate internal flow voids on MRI.60 On angiography, meningiomas demonstrate early capillary blush and remain opacified well into the venous phase—arriving early and staying late—thus inspiring the “mother-in-law” sign.
Box 10-11 Meningioma
CEREBROSPINAL FLUID DISSEMINATED METASTASES
Spread of a malignant tumor through the subarachnoid space, also termed leptomeningeal spread of disease, is much less common than vertebral body/extradural involvement. CSF metastases may result from hematogenous spread of primary neoplasms, such as lung, breast, or melanoma, or may be caused by drop metastases of primary CNS tumors, such as anaplastic astrocytoma, glioblastoma (GBM), and ependymoma in adults and primitive neuroectodermal tumor (PNET)/medulloblastoma, germinoma, ependymoma, and choroid plexus papilloma/carcinoma in children.18 Any point along the CSF pathway may be involved, including the central canal of the spinal cord (Box 10-12). CSF metastases may present as a solitary mass at the bottom of the thecal sac or along the cord surface; as multifocal discrete nodules along the cord, nerve roots, or central canal; or as a diffuse sheet-like coating of the cord and nerve roots, termed leptomeningeal carcinomatosis. Disease may be subtle or impossible to detect on CT.61 MRI findings reflect the aforementioned patterns of disease. On T1WI, diffuse disease may cause apparent mild increased signal of the CSF space and blurring of the cord and nerve roots. On T2WI, thickening of the nerve roots, small nodules, or a dominant mass may be seen (Figs. 10-27 to 10-29). These are typically isointense to the cord and nerve roots and stand out against the background of bright CSF. However, both T1- and T2-weighted standard sequences may fail to detect disease; however, gadolinium markedly improves sensitivity of MRI, making it the preferred modality (Fig. 10-30).62–64 Post-contrast images generally demonstrate avid enhancement of lesions and, in diffuse disease, may give a “sugar-coating” appearance to the spinal cord and nerve roots.62 One must be careful, however, because diffuse enhancement of the cord surface and nerve roots is nonspecific and can be seen with postoperative reactive changes, inflammatory disorders such as sarcoidosis, and leptomeningeal carcinomatosis.62
Box 10-12 Cerebrospinal Fluid Disseminated Metastases
PARAGANGLIOMA
Paragangliomas are tumors composed of chromaffin cells, a group of cells associated with the autonomic nervous system. They most commonly arise from the adrenal glands (where they are referred to as pheochromocytomas) and along the sympathetic chain (where they are called glomus tumors). The spine is a rare extra-adrenal site of paragangliomas (Box 10-13).17,65 Spinal paragangliomas are usually intradural and located at the conus medullaris, cauda equina, or filum terminale, but have been described within the thoracic spine and within extradural locations.41,66,67 They are well-defined, lobular, vascular masses that often have associated hemorrhage. MRI demonstrates heterogeneous signal on T1WI, high signal on T2WI, and strong enhancement (Fig. 10-31).17,41,68–70 Flow voids are commonly seen within lesions.17,41
INTRADURAL ARACHNOID CYST
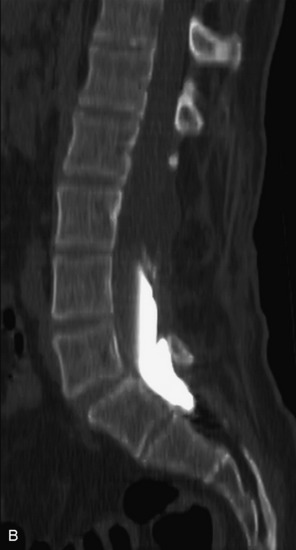
Intradural arachnoid cysts are loculated intradural CSF collections that may be congenital or acquired. Primary arachnoid cysts most commonly occur in the dorsal mid-thoracic spine.71 As one might expect, they are well-circumscribed, nonenhancing lesions that follow CSF characteristics on all imaging modalities and displace the cord and spinal nerve roots (Box 10-14). One may see immediate, delayed, or no filling of an arachnoid cyst with myelographic contrast relative to the CSF space with CT myelography (Figs. 10-32 and 10-33).72,73 Idiopathic spinal cord herniation, a syndrome in which the thoracic cord is prolapsed through an anterior dural defect, can have similar MR appearance to an intradural arachnoid cyst and is important to recognize because it is a cause of progressive myelopathy and can be treated effectively with surgery. MRI demonstrates focal ventral displacement of the mid-thoracic spinal cord and focal cord atrophy with or without an associated dorsal intradural arachnoid cyst present. CT myelography with thin slices may be helpful to demonstrate the focal dural defect (Fig. 10-34).74
Box 10-14 Intradural Arachnoid Cyst
EPIDERMOID
Epidermoids are benign spinal masses composed of cells that embryologically comprise the skin and dermal appendages (hair follicles, sweat glands, and sebaceous glands). These lesions can be congenital or may be acquired from iatrogenically implanted epithelial cells (e.g., as a result of lumbar puncture).75 The majority of epidermoids are extramedullary and intradural, but intramedullary lesions have been reported.76 On MRI, the signal characteristics of epidermoids are similar but not identical to those of CSF (Box 10-15). They are isointense to mildly hyperintense to CSF on T1WI and T2WI.76–79 FLAIR and DWI may be helpful to distinguish these lesions from arachnoid cysts, because epidermoids remain hyperintense on FLAIR and demonstrate reduced ADC, unlike arachnoid cysts, which behave identically to CSF.20,79,80 Epidermoids demonstrate minimal or no enhancement, unless infected (Figs. 10-35 to 10-37).17,20,76–79
Box 10-15 Epidermoid
LIPOMA
Spinal lipomas arise from premature separation (dysjunction) of the cutaneous ectoderm from the underlying neuroectoderm during neurulation and therefore are considered developmental rather than neoplastic lesions (Box 10-16). Imaging demonstrates a lobulated mass with characteristics of fat, including low attenuation on CT, high signal on T1-weighted MR images, low signal on fat suppressed images, and lack of enhancement (Figs. 10-38 to 10-40). Lesions may grow during infancy. Associated findings of tethered cord and spinal dysraphism may be seen.17,20
Box 10-16 Lipoma
POLYNEUROPATHIES
A number of conditions may affect multiple spinal nerve roots including trauma, infection, radiation, neurofibromatosis, lymphoma, and hereditary hypertrophic polyneuropathies. An in-depth discussion of these entities is beyond the scope of this chapter. The hereditary hypertrophic polyneuropathies encompass several hereditary disorders characterized by focal or diffuse peripheral nerve enlargement with distal extremity atrophy. Imaging shows the enlarged nerves, which may or may not demonstrate abnormal enhancement and should not be confused with a neoplastic process, such as lymphoma, in the appropriate clinical setting (Fig. 10-41). In the acute stage, high signal may be seen within denervated muscles on T2WI.20
INTRAMEDULLARY LESIONS
Intramedullary masses are by definition lesions within the spinal cord (Box 10-17). The majority of these lesions are malignant primary neoplasms, most of which are astrocytomas or ependymomas.41 Gangliogliomas are relatively rare neoplasms in adults but are the second most common intramedullary tumor in children, despite being previously underdiagnosed because of limited or uncertain methods of studying pathological specimens.81–84 Hemangioblastomas are considered the third most common intramedullary tumor in adults, accounting for approximately 3% of spinal cord tumors and are often associated with von Hippel–Lindau (VHL) disease.8,18,41 Intramedullary metastases are rare but should be considered in the setting of a known primary tumor. Tumor-like masses, including benign syrinx, multiple sclerosis (MS) plaques, transverse myelitis, and cord infarcts, are important to recognize and to not mistake for neoplasms.
ASTROCYTOMA
Astrocytomas are primary neoplasms of astrocytic origin that comprise the second most common type of spinal cord neoplasm in adults and are the most common type in children. Peak incidence is in the third to fourth decades, and lesions most commonly occur in the cervical and upper thoracic spine.17,18,24 Seventy-five percent of spinal cord astrocytomas are low-grade World Health Organization (WHO) grade I or II lesions (Box 10-18). An even higher proportion of astrocytomas are low-grade lesions in children than in adults.17 The diffuse fibrillary type of astrocytoma is more common in the spine than pilocytic astrocytomas.84 Spinal astrocytomas are typically enhancing, infiltrating masses that expand the cord and secondarily expand the spinal canal. Lesions are often eccentric within the cord or may even be exophytic. Tumor margins may be ill defined but are better characterized and differentiated from associated cysts, syrinx, and edema on post-gadolinium images. Astrocytomas are typically hypointense on T1, hyperintense on T2, and nearly always show a mild to moderate degree of heterogeneous enhancement (Figs. 10-42 to 10-47). Astrocytomas commonly have cystic components, and calcification is rare.8,17,18,20,41,85–88
Box 10-18 Astrocytoma
EPENDYMOMA
Ependymomas are neoplasms of the ependyma lining the central canal of the spinal cord. They comprise the most common type of primary spinal cord tumor in adults. Peak incidence is in the fourth to fifth decades. Histologically, spinal cord ependymomas are almost always of either the cellular or myxopapillary type.41,84 The cellular type is most common and typically presents as an intramedullary, well-circumscribed mass within the cervical or thoracic cord that is hypointense on T1WI and hyperintense on T2WI and causes symmetric cord expansion (Figs. 10-48 to 10-50).41,89–91 Myxopapillary ependymomas constitute approximately 30% of spinal cord ependymomas and are almost exclusively found in the conus and filum terminale (Box 10-19). These lesions tend to be more heterogeneous than the cellular type, and can demonstrate areas of T1 hyperintensity (Figs. 10-51 and 10-52).20,41,92 Cysts and hemorrhage are common in all types of ependymomas. Calcification is uncommon. Ependymomas typically enhance intensely but may demonstrate heterogeneous or rim enhancement.17,18,20,41,89–93 There is significant overlap in the appearances of spinal cord ependymomas and astrocytomas, and imaging is often unable to accurately identify these intramedullary tumors correctly. Certain helpful imaging features are listed in Table 10-4.
Box 10-19 Ependymoma
Table 10-4 Imaging Features of Spinal Cord Astrocytoma vs. Ependymoma
| Astrocytoma | Ependymoma | |
|---|---|---|
| Age | Children | Adults |
| Location | 75% cervicothoracic Eccentric |
Conus, cauda equina Central cord |
| Characteristics | Enhancing, infiltrating mass expanding the cord Hemorrhage less common 30% have cystic components |
Well-defined Hemosiderin common (may present with subarachnoid hemorrhage) Intense enhancement with sharp borders Cyst formation common |
HEMANGIOBLASTOMA
Hemangioblastomas are low-grade vascular neoplasms that may be seen sporadically or in one-third of cases are associated with VHL disease. VHL disease is an autosomal dominant condition with increased incidence of cerebellar and/or spinal hemangioblastomas; retinal angiomatosis; renal cell carcinoma; pheochromocytomas; and cysts in the kidneys, liver, and pancreas. The majority of spinal hemangioblastomas are intramedullary lesions within the posterior aspect of the cord, but extramedullary lesions (both intradural and extradural) are not uncommonly seen.20,41 Small hemangioblastomas tend to be homogeneously hypointense on T1WI, hyperintense on T2WI, and enhance brightly and homogeneously (Box 10-20). Larger lesions classically demonstrate a tumor cyst with a bright enhancing mural nodule, but appearances are variable and these tumors may be heterogeneous or solid. Associated edema is often seen within the cord surrounding hemangioblastomas (Fig. 10-54).94 Because these tumors are so highly vascular, adjacent flow voids caused by feeding arteries or, more often, by draining veins can be seen surrounding the tumor (Fig. 10-55). On angiography, intense tumor stain and prominent venous drainage are seen.8,20
Box 10-20 Hemangioblastomas
GANGLIOGLIOMA
Gangliogliomas have traditionally been considered rare tumors of the spinal cord, but it is likely that they are more common than originally reported, because many gangliogliomas were misclassified as astrocytomas in the original literature.81 Gangliogliomas are likely the second most common spinal cord tumor in children.83 Their appearance may be identical to that of spinal cord astrocytomas, but tumor cysts and heterogeneous enhancement may be more common in gangliogliomas (Fig. 10-58).82 The upper cord is the most common location (Box 10-21). Associated scoliosis and bony erosion may be seen in up to 50% of cases.20,82
METASTASES
Intramedullary metastases are less common than intradural or extradural metastases but are now more often diagnosed as cancer patients survive longer. The context of a known primary malignancy is extremely important for diagnosis. Similar to intradural metastatic spread of disease, metastases may either originate as drop metastases from primary CNS neoplasms or may spread hematogenously from non-neurogenic primary neoplasms (most commonly lung, breast, melanoma, colon, renal, lymphoma, and leukemia). Intramedullary metastatic lesions are typically small at presentation (Box 10-22). The most common appearance is that of a small, avidly enhancing mass with surrounding cord edema out of proportion to the lesion’s size (Fig. 10-59). Spinal cord metastases are rarely associated with cysts.8
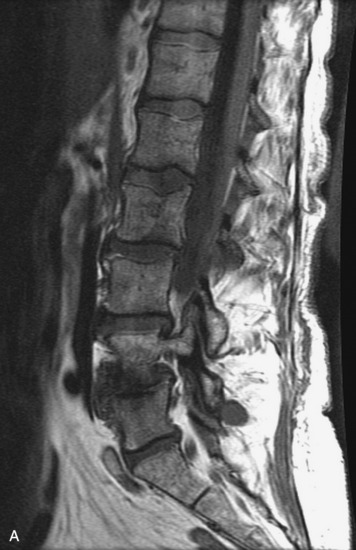
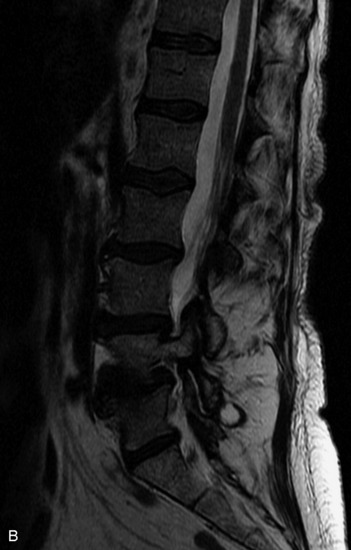
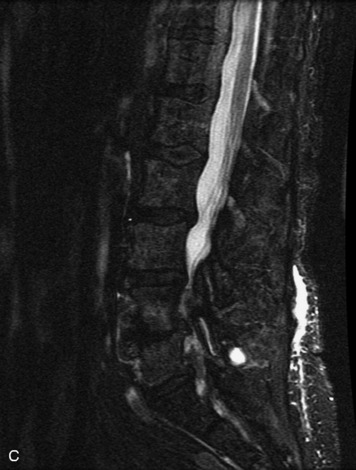
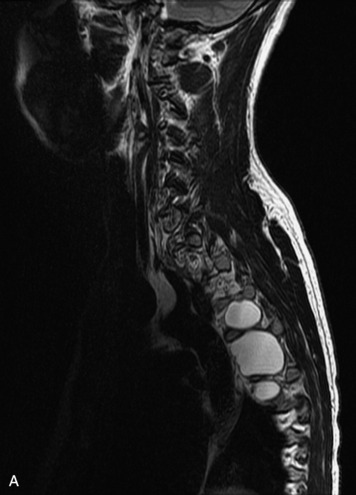
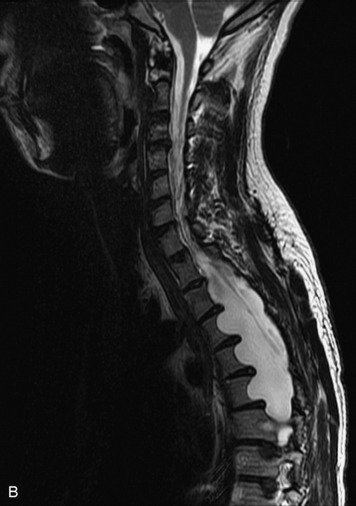
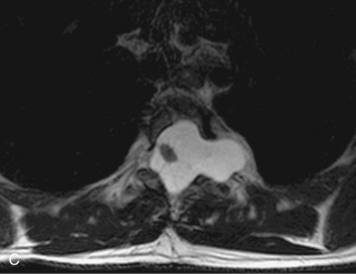
HYDROSYRINGOMYELIA
Hydromyelia refers to expansion of the central cord that is lined by ependyma. Syringomyelia refers to an eccentric cystic space that is lined by gliotic tissue. Because hydromyelia and syringomyelia cannot be distinguished by imaging, the term hydrosyringomyelia is often used to encompass the two conditions. Patients typically develop hydrosyringomyelia in the setting of abnormal CSF flow, such as in Chiari malformation, arachnoiditis, cord injury, or chronic cord compression caused by an intramedullary or extramedullary mass (Box 10-23).20,95 In some patients, no predisposing factor is found. The favored hypothesis for syrinx formation is that abnormal CSF flow around the cord forces fluid into the cord through abnormal perivascular and extracellular spaces, forming intramedullary microcysts that then expand to become cystic cavities. This hypothesis is supported by the so-called “pre-syrinx state” of intramedullary high T2 signal that may be observed before frank cyst formation.96 Because hydrosyringomyelia may be caused by an underlying neoplasm, contrast should be administered when the syrinx is first observed (Fig. 10-60).
Box 10-23 Hydrosyringomyelia
MULTIPLE SCLEROSIS
Multiple sclerosis (MS) is a primary demyelinating disease of the central nervous system characterized by multiple lesions disseminated in time and space. Only 10-20% of patients with MS have isolated spinal cord disease; therefore, imaging the brain for concomitant intracranial lesions is often helpful in making the diagnosis.20,97 MS lesions within the spinal cord generally present as well-circumscribed foci of increased T2 signal peripherally within the cord that extend over two or fewer vertebral segments (Box 10-24). Little if any cord expansion is seen, and cord atrophy may be present, especially in the secondary progressive form of the disease.98 Enhancement is variable and is only seen during the acute to subacute phase of a lesion, disappearing within 1 to 2 months (Fig. 10-61).20,99
SPINAL CORD INFARCT
Spinal cord infarction is rare in those younger than 50 years of age and is associated with atherosclerotic risk factors, such as diabetes, cigarette smoking, and hypertension. Abrupt onset of symptoms, including weakness or loss of sensation, is helpful to make the diagnosis.20 Focal hyperintensity is seen within the cord on T2WI, usually extending over more than one vertebral body segment and causing mild cord expansion. The signal abnormality may involve only gray matter or may extend to involve the adjacent white matter or even the entire cross-sectional area of the cord.100,101 Patchy, ill-defined enhancement can be seen in the subacute phase (Fig. 10-62). Associated vertebral body infarct, although uncommon, may help confirm the diagnosis (Box 10-25), presenting as marrow T2 hyperintensity in the anterior vertebral body or in the deep medullary portion near the endplate.102 Similarly, reduced diffusion demonstrated on DWI supports the diagnosis of spinal cord infarct.103
TRANSVERSE MYELITIS
Transverse myelitis refers to acute inflammation of the spinal cord that may be idiopathic or caused by direct viral infection or post-viral immunological attack. MRI demonstrates diffuse T2 hyperintensity as a result of cord edema with associated fusiform cord enlargement over multiple contiguous segments. On T1WI, central low signal may be seen within the cord similar to a syrinx but with a slightly higher signal than CSF. Variable patchy enhancement of the involved cord can be seen (Fig. 10-63).20,104,105 Symptoms often peak at 1 week and typically regress over a 4- to 6-week period, although complete recovery is uncommon.20