Chapter 14 Radiofrequency Rhizotomy for Facet Syndrome
 At the cervical level, the facet joint appears to be an important source of pain with degenerative neck symptoms. More than 50% of patients presenting to a pain clinic with neck pain may have facet joint–related pain.
At the cervical level, the facet joint appears to be an important source of pain with degenerative neck symptoms. More than 50% of patients presenting to a pain clinic with neck pain may have facet joint–related pain. The most common symptom is unilateral pain without radiation to the arm. The history should exclude risk factors for serious underlying pathology (red flags).
The most common symptom is unilateral pain without radiation to the arm. The history should exclude risk factors for serious underlying pathology (red flags). Conservative treatment options for cervical facet pain, such as physiotherapy, manipulation, mobilization, and pharmacological management, although supported by little evidence, are frequently applied before considering interventional treatments.
Conservative treatment options for cervical facet pain, such as physiotherapy, manipulation, mobilization, and pharmacological management, although supported by little evidence, are frequently applied before considering interventional treatments. Interventional pain management techniques, including medial branch blocks and RF treatment, may be considered.
Interventional pain management techniques, including medial branch blocks and RF treatment, may be considered. The zygapophyseal (facet) joints account for between 5% and 15% of cases of chronic axial low back pain.
The zygapophyseal (facet) joints account for between 5% and 15% of cases of chronic axial low back pain. The most frequent complaint is axial low back pain with referred pain perceived in the flank, hip, and thigh.
The most frequent complaint is axial low back pain with referred pain perceived in the flank, hip, and thigh. The strongest indicator for lumbar facet pain is pain reduction after anesthetic blocks of the rami mediales (medial branches) of the rami dorsales that innervate the facet joints.
The strongest indicator for lumbar facet pain is pain reduction after anesthetic blocks of the rami mediales (medial branches) of the rami dorsales that innervate the facet joints. In patients with injection-confirmed facet joint pain, procedural interventions can be undertaken in the context of a multidisciplinary, multimodal treatment regimen that includes pharmacotherapy; physical therapy; regular exercise; and if indicated, psychotherapy.
In patients with injection-confirmed facet joint pain, procedural interventions can be undertaken in the context of a multidisciplinary, multimodal treatment regimen that includes pharmacotherapy; physical therapy; regular exercise; and if indicated, psychotherapy. In the group of patients attending a pain clinic for neck pain, facet joints are probably involved in more than 50% of cases.
In the group of patients attending a pain clinic for neck pain, facet joints are probably involved in more than 50% of cases. Medial branch block of the ramus dorsalis of the segmental nerve is primarily considered as a diagnostic aid; however, (repetitive) infiltration of local anesthetic was shown to provide therapeutic effect. Infiltrations need to be repeated every 14 to 16 weeks, which makes it burdensome for the patient.
Medial branch block of the ramus dorsalis of the segmental nerve is primarily considered as a diagnostic aid; however, (repetitive) infiltration of local anesthetic was shown to provide therapeutic effect. Infiltrations need to be repeated every 14 to 16 weeks, which makes it burdensome for the patient. The evidence of RF treatment of the ramus medialis (medial branch) of the ramus dorsalis for chronic cervical pain originating from the zygapophyseal (facet) joints is derived from observational studies. One RCT in patients with whiplash-associated disorders showed a positive effect for RF treatment compared to sham intervention.
The evidence of RF treatment of the ramus medialis (medial branch) of the ramus dorsalis for chronic cervical pain originating from the zygapophyseal (facet) joints is derived from observational studies. One RCT in patients with whiplash-associated disorders showed a positive effect for RF treatment compared to sham intervention. Lumbar paravertebral tenderness is suggestive of pain originating from the zygapophyseal (facet) joints.
Lumbar paravertebral tenderness is suggestive of pain originating from the zygapophyseal (facet) joints. Confirmation of pain originating from the zygapophyseal (facet) joints is obtained by a positive anesthetic block of the ramus medialis (medial branch) of the ramus dorsalis.
Confirmation of pain originating from the zygapophyseal (facet) joints is obtained by a positive anesthetic block of the ramus medialis (medial branch) of the ramus dorsalis. For patients suffering from chronic lumbar pain originating from the zygapophyseal (facet) joints, refractory to conservative treatment, RF treatment has the highest evidence with a low complication rate.
For patients suffering from chronic lumbar pain originating from the zygapophyseal (facet) joints, refractory to conservative treatment, RF treatment has the highest evidence with a low complication rate.Cervical Facet Pain
Establishing the Diagnosis
Background
Neck pain is defined as pain in the area between the base of the skull and the first thoracic vertebra. Pain extending into adjacent regions is defined as radiating neck pain. Pain may radiate into the head (cervicogenic headache), shoulder, or upper arm (radicular or nonradicular pain).1 A distinction is made between trauma-related neck pain (whiplash-associated disorders) and degenerative neck problems. Because the causes of neck pain often are unclear, a distinction is made between the cause and source.2 The following innervated structures in the neck may be sources of pain: vertebrae, discs, uncovertebral (Luschka) joints, ligaments, muscles, and facet (zygapophyseal) joints.
Cervical facet syndrome is defined as a combination of symptoms:
There is a great deal of research into degenerative signs of the cervical vertebral column. In the discus intervertebralis (1) annular tears, (2) discus intervertebralis prolapse, and (3) endplate damage and internal discus intervertebralis disruption have been identified as potential structural discus intervertebralis pathologies.3 Other structures in the neck, such as the facet joints and uncovertebral joints, may also show degenerative signs. The hypothesis that discus intervertebralis degeneration and discus intervertebralis narrowing increase facet joint loading and consequently facet osteoarthritis seems plausible but has yet to be proven. Some researchers claim that the discus intervertebralis and the facet joints can be seen as independent pain generators.4
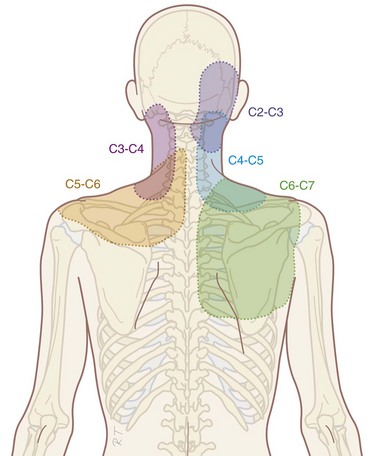
Compared with research on lumbar facet pain, research on cervical facet dysfunction is relatively recent. In 1988, Bogduk and Marsland5 described the positive effect of injection of local anesthetics close to the facet joints in patients with neck pain. Dwyer et al6 showed that injection of irritating substances into the facet joints results in a specific radiation pattern (Fig. 14-1). The same radiation pattern is seen with mechanical and electrical stimulation. However, because it was later demonstrated that stimulation of the discus intervertebralis produces the same radiation pattern as stimulation of the facet joints, this is probably a segmental phenomenon.
Physical Examination
Flexion and extension: passive and active
Lateroflexion: passive and active
Local pressure pain over the facet joints can indicate problems arising from the facet joints. Recent research showed that local pressure, defined as pain with pressure of at least 4 kg, is a predictor of success of RF treatment (see Radiofrequency Treament sections).7 When the neck pain is accompanied by radiation to the shoulder region, shoulder pathology should be excluded.
There is no evidence for the relation between the results of clinical examination and the history with the presence of pain originating from the cervical facet joints.8 In daily clinical practice, history and physical examination are useful to exclude serious pathology and to obtain a working diagnosis. An indication of the segmental level (high, mid, or low cervical) involved can be obtained.
Diagnostic Blocks
The working diagnosis of facet pain based on history and clinical examination may be confirmed by performing a diagnostic block. Local anesthetic can be injected intraarticularly or adjacent to the ramus medialis (medial branch) of the ramus dorsalis of the segmental nerve.2,9 Diagnostic medial branch block procedures are performed under fluoroscopy. There is no consensus about the definition of a successful diagnostic block. Some authors claim that 100% pain relief should be achieved.10 But Cohen et al7 showed that there is no difference in outcome of the RF treatment of patients reporting 80% and those reporting more than 50% pain reduction after a diagnostic block. In daily clinical practice, we consider a diagnostic block successful if more than 50% pain reduction is reported.
To minimize the number of false-positive results, a number of researchers have suggested that a second block should be carried out using a local anesthetic with different duration of action (e.g., lidocaine and bupivacaine; comparative double blocks). Only if the patient responds concordantly (longer or shorter pain reduction depending on the duration of action of the local anesthetic) is this indicative of facet joint pain. This is not an etiological but a pharmacological criterion. These researchers suggest that double blocks are the gold standard for the diagnosis of facet pain. A gold standard, however, should be generally accepted and used. The concept of double blocks has theoretical and practical shortcomings. A best evidence synthesis on the assessment of neck pain concluded that diagnostic facet injections have not been validated to identify facet joint pain.11
In summary, on the basis of history and physical examination, a working diagnosis of cervical facet pain is defined. One diagnostic block can be recommended for confirming the clinical working diagnosis of facet pain. A block is considered positive when the patient experiences 50% pain reduction.7
Differential Diagnosis
Chronic pain diagnoses such as segmental dysfunction, instability, and muscle strain are not sufficiently documented to be included in the differential diagnosis.2 A summary of the differential diagnosis is represented in Table 14-1.
Table 14-1 Differential Diagnosis in Axial Neck Pain without Irradiation
| Tumors | |
| Infections | Discitis, septic arthritis, osteomyelitis, meningitis, epidural abscess |
| Trauma | Fractures, whiplash-associated disorders |
| Rheumatoid arthritis | |
| Crystal arthropathies | e.g., Gout |
| Vascular disorders | Aneurysm |
| Neurological causes* | Neuromas |
| Degenerative disorders | Spondylosis, osteoarthrosis |
* Neurological causes are almost always accompanied with loss of neurological function.
Anatomy
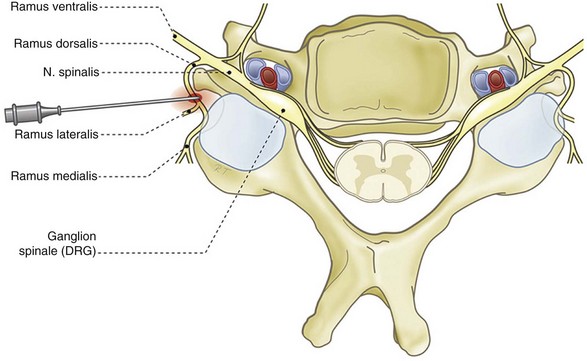
The cervical facet joint capsules are longer and looser than the facet joint capsules in the thoracic and lumbar regions.12 The more or less flat facet joint surfaces from C2 to C7 form an angle of approximately 45 degrees with the longitudinal axis through the cervical spinal column with a large range of intra- and interindividual variability. Compared with the lumbar facet joints, the cervical facet joints have a high density of mechanoreceptors. McLain took twenty-one cervical facet joint capsules and surrounding tissue from three human subjects by block-excision and processed them in a modified gold chloride technique.13 In twenty-five micron serial histological sections he identified, according to the classification of Freeman and Wyke, encapsulated Type I, II, and III mechanoreceptors and non-encapsulated Type IV mechanoreceptors. The Type II receptors were found most frequently, mainly localized in the dens, fibrous joint capsule. Fewer Type I receptors were identified in both the capsule and the areolar, loose connective tissue. Only a handful of Type III receptors were found, at the junction between the capsule and the loose connective, sub-synovial tissue. Unencapsulated, nociceptive Type IV free nerve endings were present throughout the capsule, synovium, and areolar tissue.14
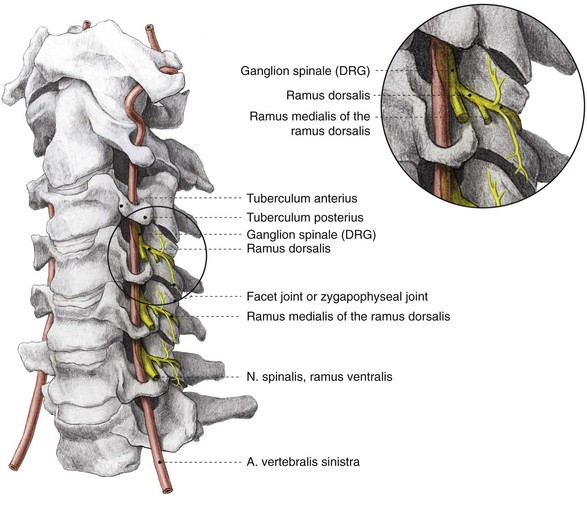
The facet joints from C3 to C7 are innervated by the ramus medialis (medial branch) of the ramus dorsalis of the segmental nerve (Fig. 14-2). Each facet joint is innervated by nerve branches from the upper and lower segments (Fig. 14-3).15
Imaging
In specific cases, plain radiography of the cervical spinal column (two or three directions) can be indicated to exclude tumor or fracture. Plain radiography does not, however, provide information for establishing the diagnosis of facet problems. This examination can help in estimating the degree of degeneration. The anterior spinal column is inspected for narrowing of the discus intervertebralis and anterior and posterior osteophyte formation. The posterior spinal column is inspected for facet osteoarthritis (facet sclerosis and osteophyte formation). In 1963, Kellgren et al16 stated that when degenerative changes are seen on plain radiography, anatomical degeneration has already reached an advanced stage.
With progressing age, degenerative changes are more frequently seen: 25% at the age of 50 years up to 75% at the age of 70 years.17 An age-related prevalence study concerning facet joint involvement in chronic neck pain indicates a comparable prevalence among all age groups.18
Degenerative changes of the cervical spinal column are present in asymptomatic patients, indicating that degenerative changes do not always cause pain. However, the conclusion that there is no relation between degeneration and pain cannot be drawn. There are studies indicating a relationship between degenerative changes and pain symptoms.17,19
Guidelines
Recently, the following classification for neck pain and associated symptoms has been proposed:20
 Grade I neck pain: No symptoms indicating serious pathology and minimal influence on daily activities
Grade I neck pain: No symptoms indicating serious pathology and minimal influence on daily activities Grade III neck pain: No symptoms indicating serious pathology, presence of neurological disorders such as decreased reflexes, muscle weakness, or decreased sensory function
Grade III neck pain: No symptoms indicating serious pathology, presence of neurological disorders such as decreased reflexes, muscle weakness, or decreased sensory function Grade IV neck pain: Indications of serious underlying pathology such as fracture, myelopathy, or neoplasm
Grade IV neck pain: Indications of serious underlying pathology such as fracture, myelopathy, or neoplasmThe following interventions are published options for the treatment of cervical joint pain:
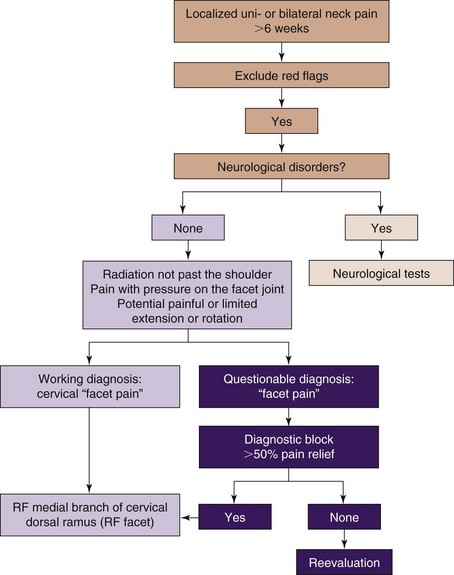
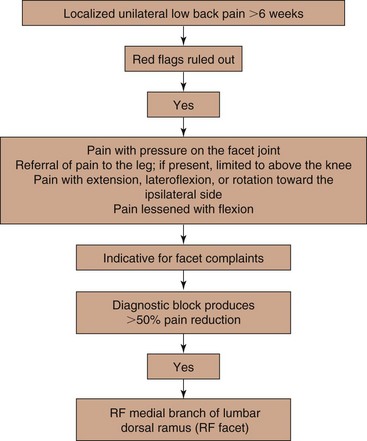
A practice algorithm for the management of facet pain is illustrated in Fig. 14-4.
Indications and Contraindications
Chronic neck pain can be caused by the facet joints. Percentages between 25% and 65% are described, depending on the patient group and selection method. In the group of patients attending a pain clinic for neck pain, it is likely to be more than 50%.21,22 Conservative treatment options for cervical facet pain such as physiotherapy, manipulation, mobilization, and pharmacological treatment are frequently applied before considering interventional treatments.
Corticosteroid injections are performed after a careful risk-to-benefit analysis in patients with metabolic diseases such as diabetes. Psychological factors such as avoidance behavior and catastrophizing are less commonly related to neck symptoms in contrast to patients with low back problems.2
Technique
Percutaneous Facet Denervation
The (postero-)lateral approach in the supine position is described below (Fig. 14-5). The advantage of this technique is that it is possible to maintain eye contact with the patient. Sedation is rarely required.
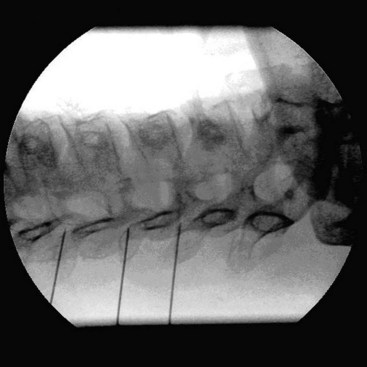
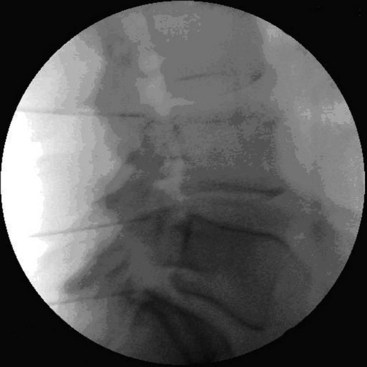
The patient is placed in the supine position with the head slightly extended on a small cushion. The C-arm is placed in an oblique position (±30 degrees). In this position, the beam runs parallel with the exiting nerve root that runs somewhat caudofrontal. Also in this position, the pedicles from the contralateral side are projected on the anterior half of the vertebral body. In the frontal plane (anteroposterior [AP] direction), the C-arm is positioned in a small angle with respect to the transverse plane. In this position, the discus intervertebralis space and neuroforamen are visible (Fig. 14-6). The ramus medialis (medial branch) of the ramus dorsalis runs over the base of the processus articularis superior. The injection point is marked on the skin, slightly posterior and caudal to the end point of the needle that is dorsal to the posterior boundary of the facet column. The first needle is introduced in a horizontal plane, slightly cranially so the tip of the needle points in the direction of the end point. It is important to understand that this is not a “tunnel-view” technique. The needle is slowly advanced anteriorly and cranially until bony contact with the facet column occurs. The farther the needle is advanced, the more difficult it becomes to change the direction. Therefore, the position of the needle needs to be checked frequently. If the needle points too much in the direction of the neuroforamen without contacting bone, the direction needs to be corrected to be more posterior. If there is no bone contact in the posterior direction, there is a risk that the needle will enter the spinal canal between the laminae. To prevent this, the needle position can be checked in the AP direction. The final position of the needle in the AP direction is in the concave “waist” of the facet column. After placement of the first needle, the other needles are introduced in the same way. The first needle acts as a guide to direction and depth.
The same technique is used for the facet joints of C3-C4 to C6-C7. For the facet joint of C2-C3, a different end point for the needle is used, just beneath the C2-C3 joint.23
Outcomes Evidence
The recently published Evidence-Based Practice Guidelines based on clinical diagnoses based the recommendations on the “grading strength of recommendations and quality of evidence in clinical guidelines” described by Guyatt et al24 and adapted by van Kleef et al25 in an editorial in Pain Practice (Table 14-2).
Table 14-2 Summary of Evidence Scores and Implications for Recommendation
| Score | Description | Implication |
|---|---|---|
| 1A+ | Effectiveness demonstrated in various RCTs of good quality. The benefits clearly outweigh the risks and burdens. | } Positive recommendation |
| 1B+ | One RCT or more RCTs with methodological weaknesses demonstrate effectiveness. The benefits clearly outweigh the risks and burdens. | |
| 2B+ | One or more RCTs with methodological weaknesses demonstrate effectiveness. The benefits are closely balanced with the risks and burdens. | |
| 2B± | Multiple RCTs, with methodological weaknesses, yield contradictory results better or worse than the control treatment. The benefits are closely balanced with the risks and burdens or there is uncertainty in the estimates of benefits, risks, and burdens. | } Considered, preferably study related |
| 2C+ | Effectiveness is only demonstrated in observational studies. Given that there is no conclusive evidence of the effect, benefits are closely balanced with the risks and burdens. | |
| 0 | There is no literature or there are case reports available, but these are insufficient to prove effectiveness or safety. These treatments should only be applied in relation to studies. | Only study related |
| 2C− | Observational studies indicate no or too short-lived effectiveness. Given that there is no positive clinical effect, the risks and burdens outweigh the benefits. | } Negative recommendation |
| 2B− | One or more RCTs with methodological weaknesses or large observational studies that do not indicate any superiority to the control treatment. Given that there is no positive clinical effect, the risks and burdens outweigh the benefits. | |
| 2A− | RCT of a good quality that does not exhibit any clinical effect. Given that there is no positive clinical effect, the risks and burdens outweigh the benefits. |
RCT, randomized controlled trial.
Intraarticular Steroid Injections
No reports on quality studies regarding the effect of intraarticular steroid injections are known until now.26 There are also no comparative studies between intraarticular steroid injections and RF therapy.
Local Infiltration of the Ramus Medialis of the Ramus Dorsalis
Medial branch block of the ramus dorsalis is primarily considered as a diagnostic aid; however, (repetitive) infiltration of local anesthetic was shown to provide therapeutic effect.27,28
In a randomized controlled trial (RCT) comparing the effect of medial branch blocks with bupivacaine alone versus blocks with the same local anesthetic plus steroid a comparable pain reduction was observed in both groups for mean duration of 14 and 16 weeks, respectively. During the follow-up period of 1 year, the mean numbers of procedures were 3.5 and 3.4, respectively. Patients were selected for participation in this study by controlled blocks providing ≥80% pain relief.28 These findings suggest that the addition of corticosteroid to local anesthetic does not provide better outcome. Moreover, as described above, the diagnostic procedure used in the RCT is burdensome for the patient, and repeat infiltrations are needed every 14 to 16 weeks. Therefore, this therapy cannot be recommended as an initial option.
Radiofrequency Treatment of the Ramus Medialis of the Ramus Dorsalis
Percutaneous RF treatment of cervical pain has been intensively studied. The data from original articles were summarized in seven systematic reviews.9,26,29–32 However, only one RCT studied RF treatment of the ramus medialis of the ramus dorsalis in patients with whiplash-associated disorder.10 The effectiveness of RF treatment for degenerative neck pathology was shown in observational studies.7,33,34
A retrospective chart analysis on the effect of repeat RF facet denervations illustrated that the mean duration of effect of the first intervention was 12.5 months. Patients who responded positively to the first intervention received a second up to a seventh intervention. Percentages of success were over 90% after each repeat intervention, and the duration of effect was between 8 and 12 months.35
The summary of the evidence for interventional management of cervical facet pain is given in Table 14-3.
Table 14-3 Evidence for Interventional Management of Cervical Facet Pain
| Technique | Score |
|---|---|
| Intraarticular injections | 0 |
| Therapeutic (repetitive) medial branch block (local anesthetic with or without corticosteroids) | 2B+ |
| RF treatment of the ramus medialis of the cervical ramus dorsalis (for degenerative facet pain) | 2C+ |
| RF treatment of the ramus medialis of the cervical ramus dorsalis (for whiplash-associated disorders) | 2B+ |
RF, radiofrequency.
Risk and Complication Avoidance
Complications are rare. Nevertheless, one should be aware that the arteria vertebralis may be punctured if the needle is pushed too far anterior into the neuroforamen. Verification of the needle point position should be made under AP fluoroscopy to prevent intrathecal injection of the local anesthetic. In an observational study,36 the incidence of inadvertent intravascular penetration for medial branch blocks at spinal level was reported to be 3.9%, comparable to the incidence at lumbar lever (3.7%). Some patients experienced short-term vasovagal reactions. The intravascular uptake of local anesthetic and contrast solution was thought to be responsible for false-negative diagnostic blocks. No systemic effects were reported.36 A report on transient tetraplegia after cervical facet joint injection, done without imaging, illustrates the vulnerability of the cervical arteries.37
A recent report on septic arthritis of the facet joints included two cases of cervical facet joints. In these cases, the port of entry could not be identified, but in one lumbar case, percutaneous injection was directly linked to this severe complication.38 Other potential complications of facet joint interventions are related to needle placement and drug administration; they include dural puncture, spinal cord trauma, spinal anesthesia, chemical meningitis, neural trauma, pneumothorax, radiation exposure, facet capsule rupture, hematoma formation, and side effects of corticosteroids.39
After RF treatment, postoperative burning pain is regularly reported. This pain disappears after 1 to 3 weeks.40 Smith et al41 found contrast enhancement on MRI typical for paraspinal abscess, even without apparent infection, which was attributed to a noninfectious post inflammatory process.
There are no incidence data on side effects and complications after cervical RF facet denervation. At the lumbar level, the incidence of complications was lower than 1%.42
Pain Originating from the Lumbar Facet Joints
Establishing a Diagnosis
Background
Pain emanating from the lumbar facet joints is a common cause of low back pain in the adult population. Golthwaite was the first to describe the syndrome in 1911, and Ghormley is generally credited with coining the term “facet syndrome” in 1933. Facet pain is defined as pain that arises from any structure that is part of the facet joints, including the fibrous capsule, synovial membrane, hyaline cartilage, and bone.43–45 The reported prevalence rate varies widely in different studies from less than 5% to as high as 90%, being heavily dependent on diagnostic criteria and selection methods.46–53 Based on information from studies that were done on well-selected patient populations, we estimate the prevalence to range between 5% and 15% of the population with axial low back pain.21,54–56 Because arthritis is a prominent cause of facetogenic pain, the prevalence rate increases with age.18,57 Although some experts have expressed doubts about the validity of “facet syndrome,” studies conducted in patients and volunteers have confirmed its existence.58–63 In rare cases, facet joint pain can result from a specific traumatic event (i.e., high-energy trauma associated with a combination of hyperflexion, extension, and distraction).64 More commonly, it is the result of repetitive stress or cumulative low-level trauma. This leads to inflammation, which can cause the facet joint to fill with fluid and swell, which in turn results in stretching of the joint capsule and subsequent pain generation.65 Inflammatory changes around the facet joint can also irritate the spinal nerve via neuroforaminal narrowing, resulting in sciatica. In addition, Igarashi et al66 found that inflammatory cytokines released through the ventral joint capsule in patients with facet joint degeneration may be partially responsible for the neuropathic symptoms in individuals with spinal stenosis. Predisposing factors for facet joint pain include spondylolisthesis or lysis, degenerative discus intervertebralis disease, and advanced age.45 The treatment of facet pain is the subject of great controversy. In 1963, Hirsch et al59 were the first group to describe the technique of facet joint injections, and in the mid-1970s, Shealy67,68 published the first reports of RF treatment of the facet joints under radiographic guidance. Because each facet joint receives dual innervation from adjacent levels, and most individuals have multilevel pathology, several levels usually need to be treated.69–71
The possible causes of lumbar pain are listed in Table 14-4.
| Lumbar causes | Intervertebral disc | Annular tears |
| Internal disc disruption | ||
| Discitis | ||
| Vertebral body | Fractures | |
| Malignant tumors | ||
| Osteomyelitis | ||
| Endplate damage | ||
| Facet joint | Osteoarthrosis | |
| Synovitis | ||
| Inflammatory arthritides | ||
| Fractures | ||
| Sacroiliac joint | Synovitis | |
| Inflammatory arthritides | ||
| Fractures | ||
| Osteoarthrosis | ||
| Ligamentous injury | ||
| Enthesopathy | ||
| Musculoligamentous | Strain | |
| Infection, abscess | ||
| Abdominal causes | Vascular | Aortic aneurysm |
| Renal | Pyelonephritis | |
| Kidney stones | ||
| Malignancy | ||
| Intestines | Malignancy |
History
A number of researchers have attempted to elucidate the clinical entity “facetogenic pain,” mostly through provocation of pain in volunteers.61,72–77 The most frequent complaint is axial low back pain. Although bilateral symptoms are more common than for sacroiliac joint pain, centralization of pain is less predictive of response to analgesic blocks than it is for discogenic pain.78,79 Sometimes pain may be referred into the groin or thigh.61 Whereas pain originating from the upper facet joints often extends into the flank, hip, and lateral thigh regions, pain from the lower facet joints typically radiates into the posterior thigh. Pain distal to the knee is rarely associated with facet pathology. Other causes of predominantly axial low back pain that must be considered in the differential diagnosis include discogenic pain, sacroiliac joint pathology, ligamentous injury, and myofascial pain. Within the context of facet pathology, inflammatory arthritides, such as rheumatoid arthritis, ankylosing spondylitis, gout, psoriatic arthritis, reactive arthritis, and other spondyloarthropathies, as well as osteoarthrosis and synovitis, must also be considered.
Physical Examination
There are no physical examination findings that are pathognomonic for diagnosis. Because facet pain originates from the mobile elements of the back, examination of motion seems relevant. In a series of cadaveric studies, Ianuzzi et al80 determined that the largest strain on the lower lumbar facet joints occurred during flexion and lateral bending, with extension also stressing L5/S1. It is therefore possible that pain worsened by flexion and extension is suggestive of pathology originating from the lowest lumbar segment(s). Revel et al54,77 were the first to correlate symptoms and physical examination signs with the response to placebo-controlled blocks.
The Revel criteria for lumbar facet joint pain are as follows:
However, previous and subsequent studies have failed to corroborate these findings.81–83 It is widely acknowledged that lumbar paravertebral tenderness is indicative of facetogenic pain, which is a claim supported by clinical trials.84 Recently, indicators of facet pain have been described based on a survey of an expert panel. They specified a panel of 12 indicators that creates the framework for a diagnosis of facet pain.85 These indicators are not in line with previous studies.77,84,86
Anatomy
The general joint features already described are also applicable to the lumbar spine.
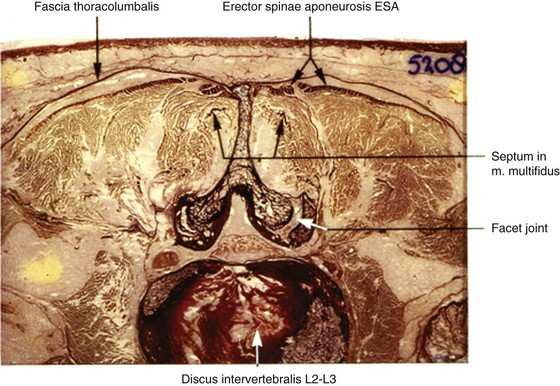
The processus articularis superior of the facet joint bears slightly concave vertical facets that face medially and posteriorly. The processus articularis inferior has slightly convex vertical facets directed laterally and anteriorly (Fig. 14-7). In maximal retroflexion (lordosis), the curved joint surfaces of these “pins and claws” enable flexion and extension of the lumbar spine. Axial rotation and lateroflexion are only possible when the segment comes out of this maximal retroflexion.
Xu et al87 correlated axial MRI, CT, and cryomicrotome sections of 66 lumbar facet joints in nine cadavers (three female, six male; ages 21 to 95 years). Although one should be skeptical of interpretations of in vivo tissue continuities using cadaveric specimens, the authors, by intraarticular injection of a contrast-fast green dye medium, in the MRI, but to a lesser extent in the CT images, could recognize extensions of the synovium and joint space along the processus articularis superior and inferior, under and into the ligamentum flavum. On the anterior aspect of the lumbar facet joint, the images showed the joint space extending into the ligamentum flavum or between the ligamentum flavum and the lamina. On the posterior aspect, the images showed a prominence of the fibrous joint capsule where the joint space extended under it along the processus articularis inferior or superior. The results were correlated to cryomicrotome sections of the scanned specimens in planes and levels corresponding to those of the MRI and CT.
As in all spinal segments, the facet joints from L1 to L5 are innervated by the ramus medialis (medial branch) of the ramus dorsalis of the segmental nerve. Each facet joint is innervated by nerve branches from the upper and lower segment (Fig. 14-8).69–71
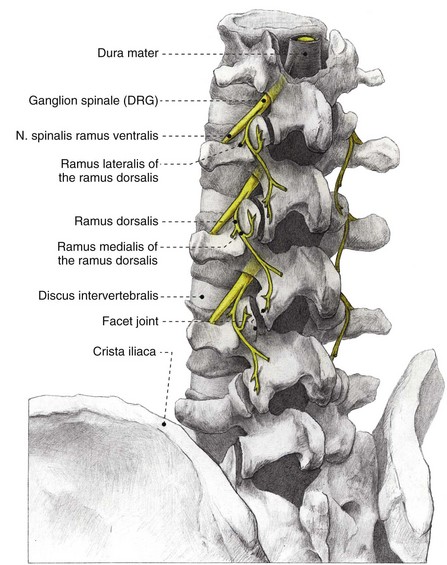
Fig. 14-8 Anatomy of the lumbar spinal column. DRG, dorsal root ganglion.
(Illustrated by Rogier Trompert Medical Art. http://www.medical-art.nl.)
For the rami dorsales L1-L4, besides the medial branch, Bogduk et al88 described a lateral and an intermediate branch. Medial, lateral, and intermediate branches distribute, respectively, to the musculus multifidus, musculus iliocostalis, and musculus longissimus. The ramus dorsalis L5 forms only a medial and intermediate branch, which is not surprising because the musculus iliocostalis does not attach to L5.
Imaging
The prevalence rate of pathological changes in the facet joints on radiological examination depends on the mean age of the subjects, the radiological technique used, and the definition of “abnormality.” Degenerative facet joints can be best visualized via CT examination.89 CT studies conducted in patients with low back pain show a prevalence rate of facet joint degeneration ranging between 40% and 80%.52,90 MRI scans may be somewhat less sensitive in the detection of facet pathology.47,89 Interestingly, the number of studies demonstrating a positive correlation between radiological abnormalities and the response to diagnostic blocks is roughly equivalent to the number showing no correlation.47,51–54,57,70,74,81,90–92 Supplementary radiological examination may also be necessary to rule out so-called “red flags” such as malignancy, compression fracture, or spinal infection.93
Guidelines
Conservative Management
The treatment of patients with facet pain should ideally occur in a multidisciplinary fashion and include conservative (pharmacological treatment; cognitive behavioral therapy; manual medicine; exercise therapy and rehabilitation; and if necessary, a more detailed psychological evaluation) as well as interventional pain management techniques. Because there have been no clinical studies evaluating pharmacological or noninterventional treatments for patients with injection-confirmed facet joint pain, one must extrapolate from studies that have been conducted on patients with chronic nonspecific low back complaints. Although nonsteroidal antiinflammatory drugs are often used, scientific evidence supporting their long-term use for low back complaints is scant.93 Antidepressants appear to be effective, but the treatment effect is small.94 Manipulation can also be effective,95,96 although one study showed no difference with “sham” therapy.97
Interventional Management
Currently, the gold standard for treating facetogenic pain is RF treatment. The major advantage of temperature-controlled RF treatment compared with voltage-controlled and other “neurolytic” techniques is that it produces controlled and reproducible lesion dimensions.98 RF facet treatment can also be repeated without a loss of efficacy, which is important because the duration of benefit is limited by the inexorable rate of nerve regeneration.99 There are currently no randomized studies comparing RF facet treatment with intraarticular injections.45
Intraarticular Corticosteroid Injections
The use of intraarticular corticosteroid injections in the facet joints is controversial. Uncontrolled studies have mostly demonstrated transient beneficial effects, but the results of controlled studies have been mostly disappointing. Lilius et al100 performed the largest randomized study, involving 109 patients. They found no difference between large-volume (8 mL) intraarticular saline injections, intraarticular corticosteroid and local anesthetic, and the same mixture injected around two facet joints. In a randomized, controlled study, Carette et al101 found only a small difference between the injection of saline (10% good effect) and depot corticosteroid (22% good effect) up to 6 months after treatment. One caveat with placebo-controlled trials that is not commonly recognized is that the intraarticular injection of saline may itself provide therapeutic benefit.102 Observational studies involving intraarticular local anesthetic and corticosteroid typically show symptom palliation lasting for up to 3 months.91,103 Based on the literature, one can conclude that intraarticular corticosteroid injections are of very limited value in the treatment of unscreened patients with suspected facetogenic pain. However, subgroup analyses have revealed that patients with positive single-photon emission computed tomography scans may be more likely to respond than patients without an acute inflammatory process.103,104
Radiofrequency Treatment
RF treatment is frequently performed for various forms of spinal pain, although the scientific evidence for this intervention remains controversial. The first controlled study was published by Gallagher et al105 in 1994. The authors selected 41 patients with chronic low back complaints who responded with some pain relief to diagnostic intraarticular injections and randomized them to receive either “sham” or true RF treatment of the rami mediales (medial branches). The two study groups were then subdivided into patients who obtained “good” and “equivocal” relief after the diagnostic block. After 6 months, a significant difference was found only between treatment and control subjects who had experienced good relief from the test blocks. In a well-designed placebo-controlled study, Van Kleef et al106 demonstrated good results after RF treatment lasting up to 12 months after treatment. Leclaire et al107 did not establish a therapeutic effect for RF treatment in a placebo-controlled trial, but this study has been criticized because the criterion for a positive “diagnostic” block was 24 hours or more of pain relief after lidocaine infiltration, which is inconsistent with the drug’s pharmacokinetics. In addition, 94% of the screened patients with back pain were selected for participation, which is much greater than the presumed prevalence for lumbar facetogenic pain (17% 30%) in this cohort. For these reasons, this study is judged to have major methodological flaws.
Van Wijk et al108 also found no difference between the treatment and control groups with regard to visual analog scale pain score, medication usage, and function. However, the RF group in this study did report 50% or more reduction in complaints significantly more often (62% vs. 39%) than those who received a sham procedure. However, the evaluation method was subject to discussion.
Finally, in the most recent randomized placebo-controlled trial, which was undertaken in 40 patients who obtained significant pain relief after three diagnostic blocks, a significantly greater improvement in pain symptoms, global perception of improvement, and quality of life was observed after 6 months in subjects allocated to RF treatment.56 In two randomized studies comparing pulsed and conventional RF treatment for facetogenic pain, both showed conventional RF to be superior.109,110
From these seven controlled studies, one can conclude that RF treatment of the facet joints can provide intermediate-term benefit in carefully selected patients. However, in a recent review, the value of this intervention was questioned.111 In a letter to the editor, the methodology was questioned, and a meta-analysis was performed. When including the six RCTs, RF was significantly better than placebo. Even when only the two trials without shortcomings were included, the difference in favor of RF treatment remained significant.112
There is presently no evidence to support the use of operative interventions for injection-confirmed facetogenic pain.45 Although several devices have been used and advocated for percutaneous facet joint fusion, none has been evaluated in rigorous trials.
Indications and Contraindications
 Eliciting or worsening of the pain by unilateral pressure on the facet joint or the processus transversus
Eliciting or worsening of the pain by unilateral pressure on the facet joint or the processus transversusTechnique and Equipment
Diagnostic Blocks
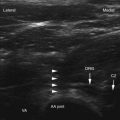
Diagnostic blocks are most frequently performed under radiographic guidance but can also be done under ultrasonography.94,113 Although intraarticular injection and medial branch (facet joint nerve) blocks are often described as “equivalent,” this has yet to be demonstrated in a comparative, crossover study design.45 Neither of these approaches has been shown to be superior.60 Both medial branch and intraarticular blocks are associated with significant false-positive and false-negative rates. For both techniques, the rate of false-positive results is most often cited as ranging between 15% and 40%.45 Regarding the false-negative rate, Kaplan et al114 found that 11% of volunteers retained the ability to perceive capsular distention after appropriately performed medial branch blocks, which was attributed to aberrant innervation.
Other causes of false-negative blocks include inappropriate needle placement, failure to detect vascular uptake, and inability of the patient to discern baseline from procedure-related pain.115 False-positive results can be ascribed to several phenomena, including placebo response; use of sedation; or the excessive use of superficial local anesthesia, which can obscure myofascial pain.116,117 In addition, the local anesthetic can spread to surrounding pain-generating structures. More than 70 years ago, Kellegren118 noted that an intramuscular injection of 0.5 mL of fluid spreads over an area encompassing 6 cm2 of tissue; this was later confirmed by Cohen and Raja.45,118 Dreyfuss et al115 found that either epidural or neuroforaminal spread occurred in 16% of blocks using the traditional target point at the superior junction of the processus transversus and the processus articularis superior. Given the close proximity of the ramus lateralis and intermedius to the ramus medialis (medial branch) of the primary ramus dorsalis, it is not possible to selectively block one without the others. During intraarticular facet blocks, the capsule can rupture after the injection of 1 to 2 mL of fluid with the resultant spread of the local anesthetic to other potential pain-generating structures. Perhaps because of their safety, simplicity, and prognostic value, diagnostic medial branch blocks are done more frequently than intraarticular injections.
Dreyfuss et al115 researched the ideal needle position for diagnostic medial branch blocks. They compared two different target sites—one with the needle tip positioned on the upper edge of the processus transversus and the other with the needle tip located halfway between the upper edge of the processus transversus and the mamillo-accessory ligament. The authors found that the lower (i.e., latter) target position was associated with a lower incidence of inadvertent injectate spread to the segmental nerves and epidural space when a volume of 0.5 mL was used. It is thus recommended to use the lower target site when performing diagnostic medial branch blocks. After the procedure, the patient is given a pain diary with instructions to discount procedure-related discomfort and engage in normal activities to permit adequate assessment of effectiveness. Failure to properly discriminate between baseline pain and that related to the procedure is a common cause of false-negative blocks. In general, a definitive treatment is carried out if a patient experiences 50% or greater pain reduction lasting for the duration of action of the local anesthetic (e.g., >30 minutes with lidocaine and 3 hours with bupivacaine). Because double, comparative blocks are associated with a significant false-negative rate and have not been shown to be cost-effective, the “double-block” paradigm is not advisable at this time.119–121
Radiofrequency Treatment of the Lumbar Facet Joints
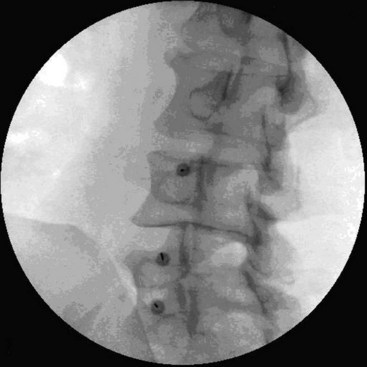
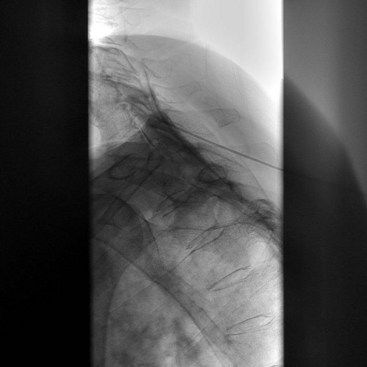
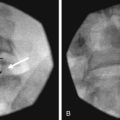
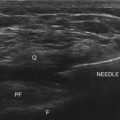
There are several ways to perform lumbar facet RF treatment, and comparative studies among different techniques are lacking. This section describes just one technique. RF treatment is a procedure that requires continuous feedback from the patient during the procedure. Therefore, if sedation is used, it should be light enough to enable conversation. The patient is placed in a prone position on an examination table. A cushion is placed under the abdomen to straighten the physiological lumbar lordosis. First, the anatomical structures are identified with an AP examination. Next, the C-arm is rotated axially to align the x-ray beam parallel with the L4-L5 discus intervertebralis to remove parallax of the endplates. The C-arm is then rotated approximately 15 degrees obliquely to the ipsilateral side so the junction between the processus articularis superior and the processus transversus, the traditional target point, is more easily accessible. Several preclinical studies have demonstrated that placing the active tip parallel to the course of the nerve maximizes lesion size.122,123 Hence, if the practitioner desires to orient the electrode parallel to the targeted nerve in a coaxial view to facilitate placement, the image intensifier can be further angled in the caudad direction. The injection point is then marked on the skin. The traditional target is the cephalad junction between the processus articularis superior and the processus transversus. However, one cadaveric study and literature review determined the optimal needle position to be with the electrode tip lying across the lateral neck of the processus articularis superior.122 When inserting the electrode, one should first make contact with the processus transversus as close as possible to the processus articularis superior. After contacting bone, the needle is advanced slightly in a cranial direction so the tip slides over the processus transversus (Fig. 14-9). In the lateral fluoroscopic view, the electrode tip should now lie at the base of the processus articularis superior in the plane formed by the so-called facet column at the lower aspect of the neuroforamen, approximately 1 mm dorsal to its posterior border (Fig. 14-10). When proper needle position is confirmed in multiple views, the impedance is checked, and a sensory stimulus current of 50 Hz is applied. The electrode position is generally deemed adequate if concordant stimulation is obtained at 0.5 V or less. Motor stimulation at 2 Hz serves to confirm correct needle placement via contraction of the musculus multifidus and to ensure the absence of distal muscle contractions in the leg, which indicates improper placement. Local muscle contractions in the back can generally be observed and palpated by the practitioner, but these are not always detectable. If leg movement is observed or the patient feels contractions in the leg, the needle must be repositioned. When the practitioner is confident the needle is properly positioned, 0.5 mL of local anesthetic is injected. After a brief interval in which the local anesthetic takes effect, a ≥67-degree lesion is applied for at least 1 minute. The nerve location and technique are the same for the ramus medialis (medial branch) of the nerves L1-L4. For L5, it is the ramus dorsalis itself that is amenable to lesioning because it courses along the junction between the ala ossis sacri and the processus articularis ossis sacri. At this level, 2 Hz of stimulation does not always produce prominent contraction of the musculus multifidus, yet motor stimulation should be performed to prevent inadvertent lesioning too close to the segmental nerve.
Outcomes Evidence
In the Evidence-Based Practice Guideline on the management of lumbar facet pain, the grade of recommendations defined as described above are listed in Table 14-5. The practice algorithm derived from the evidence assessment is illustrated in Fig. 14-11.
Table 14-5 Evidence for Interventional Pain Management of Lumbar Facet Pain
| Technique | Assessment |
|---|---|
| Intraarticular injections | 2B± |
| RF treatment of the ramus medialis (medial branch) of the L1-L4 primary rami dorsales and of the L5 primary ramus dorsalis | 1B+ |
RF, radiofrequency.
Risk and Complication Avoidance
Complications of Radiofrequency Treatment
The complications and side effects of RF treatment have been previously described in a small retrospective study by Kornick et al.42 Of 116 procedures, the two most commonly occurring complications were transient, localized burning pain and self-limiting back pain lasting longer than 2 weeks, each occurring with a frequency of 2.5% per procedure. In this study, no infections or motor or new sensory deficits were identified. Unlike diagnostic blocks, which in rare instances have been complicated by spinal infection(s), RF treatment has never been associated with infectious complications.124 This may be because heat lesioning serves a protective function. In rare instances, local burns and motor weakness have been reported.45,125
1 Guzman J, Hurwitz EL, Carroll LJ, et al. A new conceptual model of neck pain: linking onset, course, and care: the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl):S14-S23.
2 Bogduk N, McGuirk B. Management of acute and chronic neck pain. In: Pain research and clinical management. St. Louis: Elsevier; 2006.
3 Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151-2161.
4 Bogduk N, Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213-217.
5 Bogduk N, Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610-617.
6 Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns. I: a study in normal volunteers. Spine. 1990;15:453-457.
7 Cohen SP, Bajwa ZH, Kraemer JJ, et al. Factors predicting success and failure for cervical facet radiofrequency denervation: a multi-center analysis. Reg Anesth Pain Med. 2007;32:495-503.
8 Kirpalani D, Mitra R. Cervical facet joint dysfunction: a review. Arch Phys Med Rehabil. 2008;89:770-774.
9 Manchikanti L, Boswell MV, Singh V, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699-802.
10 Lord SM, Barnsley L, Wallis BJ, et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721-1726.
11 Nordin M, Carragee EJ, Hogg-Johnson S, et al. Assessment of neck pain and its associated disorders: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. J Manipulative Physiol Ther. 2009;32(suppl):S117-S140.
12 Moore K. Clinically oriented anatomy, ed 3. Baltimore: Williams & Wilkins; 1992.
13 De Avila GA, O’Connor BL, Visco DM, Sisk TD. The mechanoreceptor innervation of the human fibular collateral ligament. J Anat. 1989;162:1-6.
14 McLain RF. Mechanoreceptor endings in human cervical facet joints. Iowa Orthop J. 1993;13:149-154.
15 Groen GJ, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282-296.
16 Kellgren J, Jeffrey M, Ball J. The epidemiology of chronic rheumatism. Oxford: Blackwell; 1963.
17 Friedenberg ZB, Miller WT. Degenerative disc disease of the cervical spine. J Bone Joint Surg Am. 1963;45:1171-1178.
18 Manchikanti L, Manchikanti KN, Cash KA, et al. Age-related prevalence of facet-joint involvement in chronic neck and low back pain. Pain Physician. 2008;11:67-75.
19 van der Donk J, Schouten JS, Passchier J, et al. The associations of neck pain with radiological abnormalities of the cervical spine and personality traits in a general population. J Rheumatol. 1991;18:1884-1889.
20 Haldeman S, Carroll L, Cassidy JD, et al. The Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders: executive summary. Spine. 2008;33(suppl):S5-S7.
21 Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15.
22 Yin W, Bogduk N. The nature of neck pain in a private pain clinic in the United States. Pain Med. 2008;9:196-203.
23 Govind J, King W, Bailey B, Bogduk N. Radiofrequency neurotomy for the treatment of third occipital headache. J Neurol Neurosurg Psychiatry. 2003;74:88-93.
24 Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129:174-181.
25 van Kleef M, Mekhail N, van Zundert J. Evidence-based guidelines for interventional pain medicine according to clinical diagnoses. Pain Pract. 2009;9:247-251.
26 Falco FJ, Erhart S, Wargo BW, et al. Systematic review of diagnostic utility and therapeutic effectiveness of cervical facet joint interventions. Pain Physician. 2009;12:323-344.
27 Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55:99-106.
28 Manchikanti L, Singh V, Falco FJ, et al. Cervical medial branch blocks for chronic cervical facet joint pain: a randomized, double-blind, controlled trial with one-year follow-up. Spine. 2008;33:1813-1820.
29 Geurts JW, van Wijk RM, Stolker RJ, Groen GJ. Efficacy of radiofrequency procedures for the treatment of spinal pain: a systematic review of randomized clinical trials. Reg Anesth Pain Med. 2001;26:394-400.
30 Niemisto L, Kalso E, Malmivaara A, et al. Radiofrequency denervation for neck and back pain: a systematic review within the framework of the Cochrane Collaboration back review group. Spine. 2003;28:1877-1888.
31 Manchikanti L, Singh V, Vilims BD, et al. Medial branch neurotomy in management of chronic spinal pain: systematic review of the evidence. Pain Physician. 2002;5:405-418.
32 Boswell MV, Trescot AM, Datta S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
33 McDonald GJ, Lord SM, Bogduk N. Long-term follow-up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery. 1999;45:61-67. discussion 67-68
34 Barnsley L. Percutaneous radiofrequency neurotomy for chronic neck pain: outcomes in a series of consecutive patients. Pain Med. 2005;6:282-286.
35 Husted DS, Orton D, Schofferman J, Kine G. Effectiveness of repeated radiofrequency neurotomy for cervical facet joint pain. J Spinal Disord Tech. 2008;21:406-408.
36 Verrills P, Mitchell B, Vivian D, et al. The incidence of intravascular penetration in medial branch blocks: cervical, thoracic, and lumbar spines. Spine. 2008;33:E174-E177.
37 Heckmann JG, Maihofner C, Lanz S, et al. Transient tetraplegia after cervical facet joint injection for chronic neck pain administered without imaging guidance. Clin Neurol Neurosurg. 2006;108:709-711.
38 Michel-Batot C, Dintinger H, Blum A, et al. A particular form of septic arthritis: septic arthritis of facet joint. Joint Bone Spine. 2008;75:78-83.
39 Boswell MV, Colson JD, Sehgal N, et al. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10:229-253.
40 Haspeslagh SR, Van Suijlekom HA, Lame IE, et al. Randomised controlled trial of cervical radiofrequency lesions as a treatment for cervicogenic headache [ISRCTN07444684]. BMC Anesthesiol. 2006;16:1.
41 Smith M, Ferretti G, Mortazavi S. Radiographic changes induced after cervical facet radiofrequency denervation. Spine J. 2005;5:668-671.
42 Kornick C, Kramarich SS, Lamer TJ, Todd Sitzman B. Complications of lumbar facet radiofrequency denervation. Spine. 2004;29:1352-1354.
43 Goldthwaite J. The lumbosacral articulation: an explanation of many cases of lumbago, sciatica, and paraplegia. Boston Med Surg J. 1911:365-372.
44 Ghormley R. Low back pain with special reference to the articular facts, with presentation of an operative procedure. JAMA. 1933:1773-1777.
45 Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591-614.
46 Long DM, BenDebba M, Torgerson WS, et al. Persistent back pain and sciatica in the United States: patient characteristics. J Spinal Disord. 1996;9:40-58.
47 Murtagh FR. Computed tomography and fluoroscopy guided anesthesia and steroid injection in facet syndrome. Spine. 1988;13:686-689.
48 Destouet JM, Gilula LA, Murphy WA, Monsees B. Lumbar facet joint injection: indication, technique, clinical correlation, and preliminary results. Radiology. 1982;145:321-325.
49 Lau LS, Littlejohn GO, Miller MH. Clinical evaluation of intra-articular injections for lumbar facet joint pain. Med J Aust. 1985;143:563-565.
50 Moran R, O’Connell D, Walsh MG. The diagnostic value of facet joint injections. Spine. 1988;13:1407-1410.
51 Raymond J, Dumas JM. Intraarticular facet block: diagnostic test or therapeutic procedure? Radiology. 1984;151:333-336.
52 Carrera GF. Lumbar facet joint injection in low back pain and sciatica: description of technique. Radiology. 1980;137:661-664.
53 Lewinnek GE, Warfield CA. Facet joint degeneration as a cause of low back pain. Clin Orthop Relat Res. 1986:216-222.
54 Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain: identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73:824-828.
55 Dreyfuss P, Halbrook B, Pauza K, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine. 2000;25:1270-1277.
56 Nath S, Nath CA, Pettersson K. Percutaneous lumbar zygapophysial (Facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain: a randomized double-blind trial. Spine. 2008;33:1291-1297. discussion 1298
57 Hicks GE, Morone N, Weiner DK. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine (Phila Pa 1976). 2009;34:1301-1306.
58 Cavanaugh JM, Ozaktay AC, Yamashita HT, King AI. Lumbar facet pain: biomechanics, neuroanatomy and neurophysiology. J Biomech. 1996;29:1117-1129.
59 Hirsch C, Ingelmark BE, Miller M. The anatomical basis for low back pain. Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta Orthop Scand. 1963;33:1-17.
60 Marks RC, Houston T, Thulbourne T. Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain. 1992;49:325-328.
61 McCall IW, Park WM, O’Brien JP. Induced pain referral from posterior lumbar elements in normal subjects. Spine. 1979;4:441-446.
62 Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181-187.
63 Mooney V, Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976:149-156.
64 Song KJ, Lee KB. Bilateral facet dislocation on L4-L5 without neurologic deficit. J Spinal Disord Tech. 2005;18:462-464.
65 Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9:557-565.
66 Igarashi A, Kikuchi S, Konno S. Correlation between inflammatory cytokines released from the lumbar facet joint tissue and symptoms in degenerative lumbar spinal disorders. J Orthop Sci. 2007;12:154-160.
67 Shealy CN. Facet denervation in the management of back and sciatic pain. Clin Orthop Relat Res. 1976:157-164.
68 Shealy CN. Percutaneous radiofrequency denervation of spinal facets. J Neurosurg. 1975;43:448-451.
69 Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg. 1979;51:172-177.
70 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
71 Schwarzer AC, Wang SC, Bogduk N, et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100-106.
72 Marks R. Distribution of pain provoked from lumbar facet joints and related structures during diagnostic spinal infiltration. Pain. 1989;39:37-40.
73 Fukui S, Ohseto K, Shiotani M, et al. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain. 1997;13:303-307.
74 Fairbank JC, Park WM, McCall IW, O’Brien JP. Apophyseal injection of local anesthetic as a diagnostic aid in primary low-back pain syndromes. Spine. 1981;6:598-605.
75 Helbig T, Lee CK. The lumbar facet syndrome. Spine. 1988;13:61-64.
76 Schwarzer AC, Aprill CN, Derby R, et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine. 1994;19:801-806.
77 Revel M, Poiraudeau S, Auleley GR, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine. 1998;23:1972-1976. discussion 1977
78 Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101:1440-1453.
79 Laslett M, McDonald B, Aprill CN, et al. Clinical predictors of screening lumbar zygapophyseal joint blocks: development of clinical prediction rules. Spine J. 2006;6:370-379.
80 Ianuzzi A, Little JS, Chiu JB, et al. Human lumbar facet joint capsule strains: I. During physiological motions. Spine J. 2004;4:141-152.
81 Jackson RP, Jacobs RR, Montesano PX. 1988 Volvo award in clinical sciences. Facet joint injection in low-back pain. A prospective statistical study. Spine. 1988;13:966-971.
82 Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
83 Laslett M, Oberg B, Aprill CN, McDonald B. Zygapophysial joint blocks in chronic low back pain: a test of Revel’s model as a screening test. BMC Musculoskelet Disord. 2004;5:43.
84 Cohen SP, Hurley RW, Christo PJ, et al. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23:45-52.
85 Wilde VE, Ford JJ, McMeeken JM. Indicators of lumbar zygapophyseal joint pain: survey of an expert panel with the Delphi technique. Phys Ther. 2007;87:1348-1361.
86 Cohen SP, Argoff CE, Carragee EJ. Management of low back pain. BMJ. 2008;337:a2718.
87 Xu GL, Haughton VM, Carrera GF. Lumbar facet joint capsule: appearance at MR imaging and CT. Radiology. 1990;177:415-420.
88 Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383-397.
89 Weishaupt D, Zanetti M, Boos N, Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28:215-219.
90 Carrera GF, Williams AL. Current concepts in evaluation of the lumbar facet joints. Crit Rev Diagn Imaging. 1984;21:85-104.
91 Dolan AL, Ryan PJ, Arden NK, et al. The value of SPECT scans in identifying back pain likely to benefit from facet joint injection. Br J Rheumatol. 1996;35:1269-1273.
92 Schwarzer AC, Wang SC, O’Driscoll D, et al. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine. 1995;20:907-912.
93 Airaksinen O, Brox JI, Cedraschi C, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(suppl 2):S192-S300.
94 Greher M, Kirchmair L, Enna B, et al. Ultrasound-guided lumbar facet nerve block: accuracy of a new technique confirmed by computed tomography. Anesthesiology. 2004;101:1195-1200.
95 Andersson GB, Lucente T, Davis AM, et al. A comparison of osteopathic spinal manipulation with standard care for patients with low back pain. N Engl J Med. 1999;341:1426-1431.
96 Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502. discussion 1502-1493
97 Licciardone JC, Stoll ST, Fulda KG, et al. Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine. 2003;28:1355-1362.
98 Silvers HR. Lumbar percutaneous facet rhizotomy. Spine (Phila Pa 1976). 1990;15:36-40.
99 Schofferman J, Kine G. Effectiveness of repeated radiofrequency neurotomy for lumbar facet pain. Spine. 2004;29:2471-2473.
100 Lilius G, Laasonen EM, Myllynen P, et al. [Lumbar facet joint syndrome. Significance of non-organic signs. A randomized placebo-controlled clinical study]. Rev Chir Orthop Reparatrice Appar Mot. 1989;75:493-500.
101 Carette S, Marcoux S, Truchon R, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325:1002-1007.
102 Egsmose C, Lund B, Bach Andersen R. Hip joint distension in osteoarthrosis. A triple-blind controlled study comparing the effect of intra-articular indoprofen with placebo. Scand J Rheumatol. 1984;13:238-242.
103 Pneumaticos SG, Chatziioannou SN, Hipp JA, et al. Low back pain: prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology. 2006;238:693-698.
104 Holder LE, Machin JL, Asdourian PL, et al. Planar and high-resolution SPECT bone imaging in the diagnosis of facet syndrome. J Nucl Med. 1995;36:37-44.
105 Gallagher J, Vadi PLP, Wesley JR. Radiofrequency facet joint denervation in the treatment of low back pain-a prospective controlled double-blind study in assess to efficacy. Pain Clin. 1994;7:193-198.
106 van Kleef M, Barendse GA, Kessels F, et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine. 1999;24:1937-1942.
107 Leclaire R, Fortin L, Lambert R, et al. Radiofrequency facet joint denervation in the treatment of low back pain: a placebo-controlled clinical trial to assess efficacy. Spine. 2001;26:1411-1416. discussion 1417
108 van Wijk RM, Geurts JW, Wynne HJ, et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain: a randomized, double-blind, sham lesion-controlled trial. Clin J Pain. 2005;21:335-344.
109 Tekin I, Mirzai H, Ok G, et al. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clin J Pain. 2007;23:524-529.
110 Kroll HR, Kim D, Danic MJ, et al. A randomized, double-blind, prospective study comparing the efficacy of continuous versus pulsed radiofrequency in the treatment of lumbar facet syndrome. J Clin Anesth. 2008;20:534-537.
111 Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34:1078-1093.
112 Van Zundert J, Vanelderen P, Kessels AG, et al. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34:1078-1093. Spine (Phila Pa 1976) 35:841; author reply 841-842, 2010
113 Shim JK, Moon JC, Yoon KB, et al. Ultrasound-guided lumbar medial-branch block: a clinical study with fluoroscopy control. Reg Anesth Pain Med. 2006;31:451-454.
114 Kaplan M, Dreyfuss P, Halbrook B, Bogduk N. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine (Phila Pa 1976). 1998;23:1847-1852.
115 Dreyfuss P, Schwarzer AC, Lau P, Bogduk N. Specificity of lumbar medial branch and L5 dorsal ramus blocks. A computed tomography study. Spine. 1997;22:895-902.
116 Ackerman WE, Munir MA, Zhang JM, Ghaleb A. Are diagnostic lumbar facet injections influenced by pain of muscular origin? Pain Pract. 2004;4:286-291.
117 Cohen SP, Larkin TM, Chang AS, Stojanovic MP. The causes of false-positive medial branch (facet joint) blocks in soldiers and retirees. Mil Med. 2004;169:781-786.
118 Kellegren J. On the distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clin Sci. 1939;4:35-46.
119 Lord SM, Barnsley L, Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11:208-213.
120 O’Neill C, Owens DK. Lumbar facet joint pain: time to hit the reset button. Spine J. 2009;9:619-622.
121 Bogduk N, Holmes S. Controlled zygapophysial joint blocks: the travesty of cost-effectiveness. Pain Med. 2000;1:24-34.
122 Lau P, Mercer S, Govind J, Bogduk N. The surgical anatomy of lumbar medial branch neurotomy (facet denervation). Pain Med. 2004;5:289-298.
123 Bogduk N, Macintosh J, Marsland A. Technical limitations to the efficacy of radiofrequency neurotomy for spinal pain. Neurosurgery. 1987;20:529-535.
124 Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
125 Ogsbury JS3rd, Simon RH, Lehman RA. Facet “denervation” in the treatment of low back syndrome. Pain. 1977;3:257-263.