Chapter 58 Radiation Retinopathy
Introduction
Radiation retinopathy (RR) is a slowly progressive, delayed-onset occlusive microangiopathy of the retinal vasculature that occurs with variable latency after exposure of the retina to ionizing radiation. First described by Stallard in 1933,1 the term encompasses all retinal vascular changes, including ischemic and proliferative RR (PRR) and radiation maculopathy. It is a potentially devastating sequela of exposure of the eye to any source of radiation, including local plaque radiation treatment (brachytherapy), external-beam radiation treatment (ERBT), proton beam radiation, helium ion radiotherapy and gamma knife radiotherapy of the eye, ocular adnexa, orbit, and head and neck structures.2–6 Radiotherapy offers an alternative to enucleation for patients with retinoblastoma, choroidal melanoma, and ocular metastases,7–9 as well as life-saving treatment of orbital, sinus, and intracranial tumors. Since the Collaborative Ocular Melanoma Study showed similar survival rates after radiotherapy and enucleation, the shift towards globe-salvaging therapeutic strategies has increased the use of radiation and consequently increased its complications, with reports of the incidence of RR ranging from 3% to over 20%.10,11 The current interest in use of radiation in the treatment of neovascular age-related macular degeneration (AMD) might further increase the risk of this complication.12
The risk of RR is related to characteristics of the radiation treatment itself, the presence of systemic disease, and exposure to radiation sensitizers such as chemotherapy. Fundoscopic findings can be highly variable, ranging from scattered retinal hemorrhages, microaneurysms, and cotton-wool spots to macular edema, large-vessel occlusion, extensive ischemic retinopathy and maculopathy and consequent retinal and ocular neovascularization. Within the retina, the posterior pole is particularly sensitive to this pathology,13 with grave implications for visual prognosis. Further, RR has a long latency and may not be clinically detectable for 8 years or more.14
Etiology, pathogenesis, and histopathology
RR can be described as a progressive obliterative arteritis that initiates a characteristic pattern of degenerative and proliferative vascular changes and microvascular dysfunction. Radiotherapy is the most frequent cause. Radiotherapy generates its effective tumoricidal effects both directly, via injury to the DNA of rapidly dividing cells, and indirectly, via the production of free radicals. However, these processes damage vascular and interstitial support structures of both pathological and healthy tissue. Histopathologically, ionizing radiation of the retina induces an acute transudative as well as a slowly progressive occlusive vasculopathy.15 The initial pathologic change, and the fundamental abnormality, is retinal vascular endothelial cell injury and loss15–18 and associated inflammation,19 which occurs primarily in capillaries, followed by capillary closure, clearly visible with fluorescein angiography (FA),14,20–22 retinal ischemia, nerve tissue necrosis, and fibrovascular proliferation.13,18,21,23,24
The loss of capillary cellularity leads to the development of microaneurysms, and hemodynamic alterations produce fenestrated telangiectatic retinal vessels. Larger retinal vessels become involved later in the course of the retinopathy, with diameter reduction of up to 75% in major retinal arteries and veins demonstrated in animal models.19 Closure of blood vessels is the single most characteristic finding on FA. Ghost vessels are later visible ophthalmoscopically. Central retinal artery occlusion has also been described as a consequence of high-dose irradiation,25 as has interruption of the choroidal circulation.26,27 The choriocapillaris also suffers, and hypoperfusion is detectable several months after treatment,27 leading to choroidopathy and chorioretinal atrophy. The widespread vascular occlusion induces the production of vascular endothelial growth factor (VEGF),18 leading to neovascularization and an increase in vascular permeability, both of which result in vascular leakage and tissue edema.28 Uveal effusion can also be present.
The disappearance of choriocapillaris, retinal pigment epithelium (RPE), photoreceptors and retinal nerve fibers, along with leukocyte invasion, has been demonstrated histologically.29 Areas devoid of photoreceptor cells correspond with areas of pigment dispersion with reduced numbers of melanocytes. There is a thickening of arteriolar and capillary walls and endothelial cell loss. In contrast to diabetic retinopathy, in which pericytes are initially affected, RR exhibits an early loss of endothelial cells. However, pericytes can be affected in severely damaged capillaries.
On optical coherence tomography (OCT) images, significant thinning of the inner plexiform, inner nuclear, and outer plexiform layers is visible, suggesting that radiation-induced damage is confined to the inner layers of the retina.30 However, secondary functional changes may occur in the outer retina.31
Natural history and clinical features
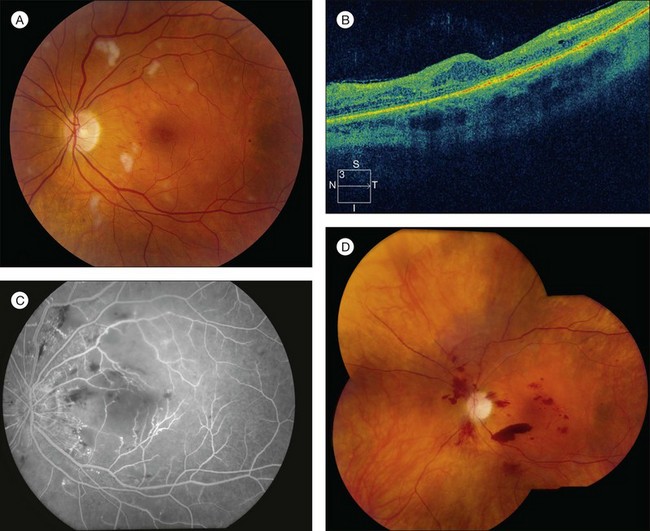
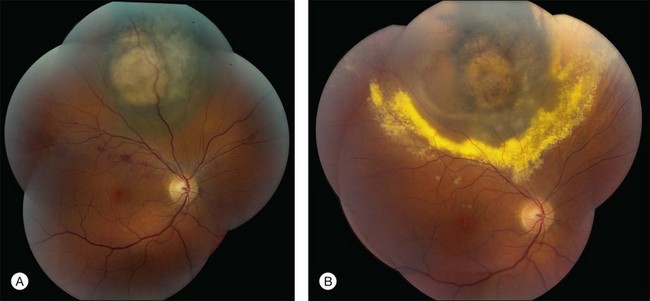
RR displays clinical and angiographic features that are virtually identical to those seen in diabetic retinopathy.32 This is to be expected, since both radiation and diabetes primarily damage the retinal capillaries. Ophthalmoscopically, microaneurysms are the first to appear and are near-universally present,33 closely followed by intraretinal hemorrhages, macular capillary dilation and nonperfusion, and nerve fiber layer infarcts (cotton-wool spots; Fig. 58.1).14 Retinal edema, hard exudates, telangiectasia, and perivascular sheathing may follow in variable sequence and latency. Hard exudates have been found to be more prevalent after brachytherapy (Fig. 58.2), whereas retinal hemorrhages and microaneurysms are more commonly found after EBRT.13 The telangiectatic-like vessels are a feature of established retinopathy and are likely to represent collateral vasculature at the edges of capillary occlusion.14 Confluent areas of capillary closure lead to the development of large areas of retinal capillary nonperfusion.
The fluorescein angiographic hallmarks of the condition are the presence of severe retinal capillary nonperfusion, capillary dilation, and microaneurysms, frequently in combination with macular edema or ischemia.13 Central macular chorioretinal anastomosis has also been described.34 Neovascularization may develop later (Fig. 58.1), and does so in approximately 32% of eyes with RR.35 This development is referred to as PRR, and its presence is ominous. Analogous to proliferative diabetic retinopathy, it suggests profound ischemia and carries a worse prognosis for long-term visual acuity. If left untreated, it can lead to neovascular glaucoma, vitreous hemorrhage, and tractional retinal detachment caused by fibrovascular proliferation similar to that seen in diabetic retinopathy. However, in contrast to diabetic retinopathy and other retinal vasculopathies, neovascularization does not typically extend through the internal limiting membrane into the vitreous.18 Nevertheless, vitreous hemorrhage can occur and its presence is associated with a poor prognosis for both vision and globe salvage, as well as for ghost cell or neovascular glaucoma. It also impedes the clinician’s ability to monitor the retinopathy and to employ laser photocoagulation to treat progression (Table 58.1).
| Complication | Incidence |
|---|---|
| Nonproliferative radiation retinopathy | 68% |
| Proliferative radiation retinopathy | 32% |
| Clinically significant macular edema | 76% |
| Macular ischemia | 76% |
| Radiation optic neuropathy | 55% |
| Cataract | 52% |
| Vitreous hemorrhage | 24% |
| Glaucoma | 12% |
| Radiation keratopathy | 10% |
| Tractional retinal detachment | 5% |
(Adapted from Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc 2008;106:325–35.)
Classification
There is currently no standard method of classification for RR, although several methods have been developed. These can be categorized as clinical or ophthalmoscopic, fluorescein angiographic and OCT. Finger and Kurli proposed a four-stage, prognosis-related classification that uses ophthalmoscopic and fluorescein angiographic findings to classify macular and extramacular changes.36 In this system, stage 1 includes extramacular ischemic changes, stage 2 includes macular ischemic changes, stage 3 includes additional macular edema and retinal neovascularization, and stage 4 encompasses vitreous hemorrhage and at least 5 disc areas of retinal ischemia, whether macular or extramacular. The Early Treatment Diabetic Retinopathy Study (ETDRS) definition of clinically significant macular edema has been applied to macular edema associated with radiation damage. In these cases, it is referred to as clinically significant radiation macular edema, which follows the ETDRS definitions: retinal thickening within 500 mm of the fovea; edema-associated hard exudates within 500 mm of the fovea; and one or more zones of retinal thickening ≥1 disc area, any part of which is within 1 disc diameter of the fovea.37 FA allows the clinician to determine the condition of the macula, allowing maculopathy to be subdivided into ischemic and nonischemic variants, which has a significant impact on the visual outcome. FA also allows for the classification of macular edema, which is useful if grid or focal laser photocoagulation is to be administered. OCT is increasingly used to guide diagnosis and treatment decisions for macular edema in various retinal disorders.38 Horgan et al. proposed a five-point OCT-based grading scale based on standard reference OCT images: grade 1, extrafoveolar, noncystoid edema; grade 2, extrafoveolar cystoid edema; grade 3, foveolar noncystoid edema; grade 4, mild-to-moderate foveolar cystoid edema; and grade 5, severe foveolar cystoid edema.39 This study demonstrated OCT evidence of macular edema as early as 4 months after plaque radiotherapy for uveal melanoma, with a peak incidence at 12–18 months, and that it is associated with significant vision loss. The use of OCT is important, because macular edema is visible on OCT approximately 5 months earlier than clinically detectable radiation maculopathy.39 The classification of RR into PRR and nonproliferative (NPRR) subtypes is also of great prognostic importance. A recent retrospective study reported an incidence of PRR of 5.8% at 5 years and 7% at 10 and 15 years in 3841 eyes treated with plaque radiotherapy for uveal melanoma.40
Risk factors
Risk factors for the development of RR can be divided into internal (inherent/patient) and external (iatrogenic) factors. The primary patient factor is concomitant vascular disease such as diabetes mellitus.41 Since both diabetes and radiation primarily damage the retinal capillaries, this synergistic effect is to be expected, as the capillaries experience an early loss of pericytes due to diabetes and endothelial cell loss due to radiation. Destruction of these two cell types leaves little cellular structural support for capillaries and thus results in capillary closure, aneurysms, vessel leakage, and hemorrhage. Indeed, it has been suggested that concomitant diabetes mellitus is also a poor prognostic indicator for visual acuity, increasing the risk of visual loss by nearly 300%.42 This is in part due to the fact that diabetes mellitus is associated with an increased incidence and severity of neovascular complications, including neovascular glaucoma43 and radiation papillopathy44 following radiotherapy.45 Other vascular disorders predisposing to RR are arterial hypertension and coronary artery disease.46 Tumor characteristics likely to lead to a worse prognosis include large tumors and close proximity to the macula and optic disc.47
Chemotherapy, whether or not concurrent/concomitant with the radiation therapy, also increases the risk,13,48 as may pregnancy.49 Chemotherapy increases the vulnerability of the retinal vasculature to radiation damage, possibly via an increase in oxygen-derived free radicals.23 It also increases the risk of proliferative (or neovascular) RR, which carries a worse prognosis than the nonproliferative type.50 The concomitant administration of chemotherapy increases the risk of visual complications,13,23,48,51–53 potentiates the development of RR at lower radiation doses,13 and may shorten the latent period between exposure and retinopathy.48,52,54
Incidence and dosimetry
The most important external or iatrogenic risk factors relate to the radiation itself. These are radiation type, treatment modality (external beam versus brachytherapy or plaque), total radiation dose and the fractionation schedule of that dosage, the total elapsed time in the course of irradiation treatment, and errors in treatment technique and/or dosage calculations.53,55
Radiation type
If a large enough dose is applied, any type of radiation can result in retinopathy and its associated complications. RR is found in 87% of patients after plaque radiotherapy for juxtapapillary melanomas, and radiation maculopathy in 89% after proton beam irradiation. However, neither of these radiation types is as highly associated with severe sight-limiting complications (such as rubeosis and neovascular glaucoma) as gamma knife treatment, which may lead to complete loss of vision in nearly 50%.5
Total radiation dose
The association between dose and RR is well established.25 However, a precise threshold has been difficult to determine. The condition does not usually occur at total doses <45 Gy unless an additional risk factor such as diabetes is present.55,56 In a study of 68 eyes that underwent EBRT for primary extracranial head and neck tumors, the dose–response curve for retinopathy was characterized by a dramatic increase in incidence between 45 and 55 Gy.56 Nearly all patients receiving higher doses developed retinopathy. A more recent retrospective review suggests that the incidence can be significantly reduced by hyperfractionated (twice-daily doses of 1.1–1.2 Gy) EBRT.55 However, above 70 Gy, the overall incidence of retinopathy approaches 40%.
Fractionation schedule
During EBRT, increased fraction size correlates with an increased incidence of retinal complications.13,23,53,57,58 A dose per fraction below 1.9 Gy/fraction has been shown to decrease the incidence of retinopathy.56 This is referred to as hyperfractionation, which theoretically allows the retina enough time to repair single-strand DNA breaks before a second nearby insult can occur.55 This reduces late toxicity to the retina. However, for brachytherapy, which is primarily used for the treatment of choroidal melanoma, fractionation is not possible, since the radiation is delivered continuously at a predetermined dose for a certain period of time.
Differential diagnosis and diagnostic evaluation
RR can occur many years after radiation therapy. Because of this potential delay, RR may not be recognized as the cause of visual loss. Further, due to the ophthalmoscopic and angiographic similarities, RR may be misdiagnosed as diabetic or hypertensive retinopathy. However, a dilated ophthalmic examination combined with a careful history, including questioning of the patient and a thorough review of the treatment records to determine whether the eyes were included in the field of radiation, will usually lead to the diagnosis. The diagnosis should be considered following cephalic radiation for any reason, including treatment for orbital inflammatory disease such as thyroid disease60 and orbital pseudotumor,61 sinus malignancies and periocular cutaneous lesions. A feature of RR that distinguishes it from diabetic retinopathy is the atrophy of the RPE sometimes seen after radiation treatment.
Bone marrow transplant retinopathy can mimic RR, and it can be very difficult to distinguish the two unless affected patients have a history of exposure to one (e.g., radiation) and not the other (e.g., bone marrow transplantation).62 Other potential causes of the observed retinal abnormalities include multiple branch retinal artery occlusions, multiple retinal venous occlusive episodes or retinal telangiectasia from other causes. Severe anemia, leukemia, and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) must be excluded before the diagnosis of RR can be definitively made.
Although the diagnosis can usually be made clinically, further evaluation should be considered. Fluorescein and indocyanine green angiography can be useful to define the extent of retinal ischemia and vascular anomalies.63,64 If macular pathology is suspected, OCT should be carried out to determine the degree of macular edema, since macular thickness on OCT correlates with visual acuity for up to 2 years after plaque treatment.39
Prevention and treatment
There is no widely accepted treatment protocol for RR. Treatment has until now been based on its clinical, histopathologic, and angiographic similarities with diabetic retinopathy, a disease for which large randomized controlled studies have already been successfully conducted. Indeed, several groups have applied treatment guidelines of the ETDRS to eyes with RR, with favorable results.21,65 Further, the Branch Retinal Vein Occlusion study provided strong evidence that laser photocoagulation can decrease the probability of vision loss due to macular edema and vitreous hemorrhage in patients with regional retinal ischemia.66 Although the proven, currently available therapeutic options are limited both in number and in success rate, and its management remains challenging, once RR has developed, all efforts should be made to minimize its impact on the patient’s visual acuity. Nevertheless, once macular ischemia has developed, the likelihood of visual improvement is very low.
Retinal laser photocoagulation remains the gold standard in the treatment of most forms of ischemic retinopathies. It has shown definite promise in the treatment of RR since 1981.67 Since then, various studies have demonstrated the utility of this modality in inducing regression of plaque-associated RR,68,69 although no large randomized, controlled studies have yet been completed. The rationale behind laser photocoagulation is that the destruction of oxygen-consuming photoreceptor cells and RPE decreases the intraocular VEGF concentrations, thereby inhibiting active retinal neovascularization.70 Treatment has been shown to be anatomically beneficial by decreasing macular edema, neovascularization, and vitreous hemorrhages. However, the visual acuity results of treatment have been disappointing. This may be due to differences between RR and diabetic retinopathy. First, it seems that there is more widespread capillary nonperfusion in RR than in diabetic retinopathy. Second, RR follows acute injury, whereas diabetic retinopathy is due to a long-term metabolic insult. Third, the optic nerve is affected more frequently and severely in RR than in diabetic retinopathy, likely contributing to more vision loss. Fourth, the damage to the RPE and choroid is more severe in radiation retinopathy than in diabetic retinopathy.17,71–73 Attempts to improve visual acuity results might include development of a protocol to identify affected patients as early as possible so that treatment can be instituted before vision loss due to macular edema or proliferative retinopathy has occurred. Indeed, even prophylactic treatment may be of benefit. Finger et al. reported the use of laser photocoagulation to obliterate the ischemic zone caused by ophthalmic plaque brachytherapy.36 They observed regression of early-stage RR in 64% of treated eyes and found that only 18.75% of the “high-risk” patients who were treated prophylactically with sector scatter laser later developed RR. Typical laser settings include a 200 µm spot size, 0.1–0.2 ms duration, and 100–300 mW.74 Laser photocoagulation has also been used for the treatment of macular edema secondary to RR. Although macular ischemia is currently not treatable, the frequently associated macular edema may respond to photocoagulation. Hykin et al. reported a visual acuity improvement of at least one line on the Snellen chart in 42% of affected eyes at 6 months, although the difference between treated patients and controls was not significant at 12 and 24 months.74
Corticosteroids have both angiostatic and vascular antipermeability properties that have been harnessed in the treatment of neovascular AMD, diabetic retinopathy, and macular edema due to various conditions.75 This treatment modality has recently been tested for RR. Intravitreal triamcinolone has been reported to be of both visual and anatomic benefit in the treatment of both radiation maculopathy76,77 and optic neuropathy.78 However, when compared with intravitreal injection, periocular triamcinolone is associated with lower rates of steroid-induced glaucoma,79 cataracts, retinal detachment, and endophthalmitis.80,81 In a prospective, randomized, controlled trial with 163 patients newly diagnosed with uveal melanoma undergoing iodine-125 plaque radiotherapy, Horgan et al. found prophylactic periocular injection of triamcinolone acetonide (40 mg in 1 mL), administered at the time of plaque radiotherapy and 4 and 8 months later, to be both safe and beneficial in reducing the risk of macular edema, moderate vision loss, and poor visual acuity.82
During the past several years, the focus has shifted to anti-VEGF agents for the treatment of intraocular neovascularization and vascular permeability. The discovery of VEGF-A’s role in the pathogenesis of neovascular ocular disease provided a strong rationale for the use of anti-VEGF agents.83 These agents have been proven in large trials to be effective for neovascular AMD84 and diabetic retinopathy.85 Finger et al. first reported the efficacy of intravitreal bevacizumab therapy for the treatment of radiation maculopathy and optic neuropathy.86 This led to the regression of retinal edema, hemorrhages, exudates, and neovascularization after ophthalmic plaque radiation therapy. Since this early report, many reports have been published, suggesting the crucial role of intravitreal injection of both bevacizumab87 and ranibizumab83,88 in the treatment of radiation maculopathy89 and optic neuropathy86 due to plaque brachytherapy90 and EBRT.91 Anti-VEGF agents have also been used to treat the secondary complications of RR, namely neovascular glaucoma and exudative retinal detachment.83,92 Systemic administration of bevacizumab might be beneficial in the treatment of macular edema after EBRT.93 However, systemic bevacizumab has been reported to carry a risk of systemic thromboembolic events.94 In contrast, intravitreal administration, which requires a much smaller dose, minimizes systemic risks but increases ocular risks.95 Whether it is most effective to administer anti-VEGF agents prophylactically or only upon development of pathology has not yet been conclusively determined. However, a small study by Gupta et al. suggested that patients with shorter duration of macular edema benefited more than those with long-standing retinal disease.89 Further, the optimal administration schedule has not yet been determined. Pharmaceutical companies have begun sponsoring studies to study this issue in depth; a recent phase I, open-label, Genentech-sponsored study of 5 patients with RR-related macular edema showed visual acuity improvement and decreased foveal thickness after monthly injections of ranibizumab for at least 4 months.88 In sum, most of the published studies suggest that anti-VEGF agents may have a role in the treatment of RR and its secondary complications. Further studies, such as the Treatment of Radiation Retinopathy (TORR) trial, are necessary to delineate the best administration schedules. The TORR trial will determine whether treatment with intravitreal ranibizumab (0.5 mg) or intravitreal triamcinolone acetonide (4.0 mg) is associated with improved visual acuity at 1 year, as compared to natural history. Results of this trial are expected in late 2012.96
Prognosis
The visual prognosis is largely dependent on the extent and location of capillary nonperfusion97 as well as the presence of neovascularization. If the perifoveal capillary net is involved, the prognosis for central vision is grave, due to either retinal atrophy or persistent macular edema, as well as from neovascularization, which occurs at the interface of perfused and nonperfused retina as well as on the optic nerve head. PRR, present in approximately one-third of eyes with RR, is likely to carry an inferior long-term visual prognosis (mean visual acuity 20/440) than the nonproliferative type (mean visual acuity 20/100), although this retrospective study had a very different follow-up duration for PRR (109 months) and NPRR (27 months).98 The same study reported macular edema to be more common in PRR (85.7%) than in NPRR (71.2%). Shields et al. found predictors of poor visual acuity at long-term follow-up following plaque radiotherapy included patient age ≥60 years, tumor base ≥10 mm, tumor thickness >8 mm, radiation dose to the tumor base of ≥33 300 cGy, and increasing radiation dose to the optic disc.99 Tumor location is also of great importance, with tumors located posterior to the equator at increased risk for radiation maculopathy and loss of central vision.100
1 Stallard HB. Radiant energy as (a) a pathogenic and (b) a therapeutic agent in ophthalmic disorders. Br J Ophthalmol Suppl. 1933;6:1–126.
2 Finger PT, Chin KJ, Duvall G, Palladium-103 for Choroidal Melanoma Study Group. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology. 2009;116:790–796.
3 Krema H, Somani S, Sahgal A, et al. Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol. 2009;93:1172–1176.
4 Gragoudas ES, Seddon JM, Egan K, et al. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology. 1987;94:349–353.
5 Haas A, Pinter O, Papaefthymiou G, et al. Incidence of radiation retinopathy after high-dosage single-fraction gamma knife radiosurgery for choroidal melanoma. Ophthalmology. 2002;109:909–913.
6 Levy RP, Fabrikant JI, Frankel KA, et al. Heavy-charged-particle radiosurgery of the pituitary gland: Clinical results of 840 patients. Stereotact Funct Neurosurg. 1991;57:22–35.
7 Finger PT, Chin KJ, Duvall G. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology. 2009;116:790–796.
8 Finger PT. Radiation therapy for orbital tumors: Concepts, current use, and ophthalmic radiation side effects. Surv Ophthalmol. 2009;54:545–568.
9 Finger PT, Pro MJ, Schneider S, et al. Visual recovery after radiation therapy for bilateral subfoveal acute myelogenous leukemia (AML). Am J Ophthalmol. 2004;138:659–662.
10 Shields CL, Naseripour M, Cater J, et al. Plaque radiotherapy for large posterior uveal melanomas (> or = 8 mm thick) in 354 consecutive patients. Ophthalmology. 2002;109:1838–1849.
11 Gunduz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1999;117:609–614.
12 Evans JR, Sivagnanavel V, Chong V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 5, 2010. CD004004
13 Brown GC, Shields JA, Sanborn G, et al. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501.
14 Amoaku WM, Archer DB. Cephalic radiation and retinal vasculopathy. Eye. 1990;4:195–203.
15 Archer DB, Amoaku WMK, Gardinar TA. Radiation retinopathy: Clinical, histopathological, ultrastructural and experimental correlations. Eye. 1991;5:239–251.
16 Irvine AR, Alvarado JA, Wara WM, et al. Radiation retinopathy: An experimental model for the ischemic-proliferative retinopathies. Trans Am Ophthalmol Soc. 1981;79:103–122.
17 Egbert PR, Fajarado LF, Donaldson SS, et al. Posterior ocular abnormalities after irradiation for retinoblastoma: A histopathological study. Br J Ophthalmol. 1980;64:660–665.
18 Irvine RA, Wood IS. Radiation retinopathy as an experimental model for ischemic proliferative retinopathy and rubeosis iridis. Am J Ophthalmol. 1987;103:790–797.
19 Hiroshiba N, Ogura Y, Sasai K, et al. Radiation-induced leukocyte entrapment in the rat retinal microcirculation. Invest Ophthalmol Vis Sci. 1999;40:1217–1222.
20 Hayreh SS. Post-radiation retinopathy: A fluorescence fundus angiographic study. Br J Ophthalmol. 1970;54:705–714.
21 Kinyoun JL, Chittum ME, Wells CG. Photocoagulation treatment of radiation retinopathy. Am J Ophthalmol. 1988;105:470–478.
22 Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy – clinical, histopathological, ultrastructural and experimental correlations. Eye. 1991;5:239–251.
23 Wara WM, Irvine AR, Neger RE, et al. Radiation retinopathy. Int J Radiat Oncol Biol Phys. 1979;5:81–83.
24 Irvine AR, Alvarado JA, Wara WM, et al. Radiation retinopathy: An experimental model for the ischemic-proliferative retinopathies. Trans Am Ophthalmol Soc. 1981;79:103–122.
25 Takeda A, Shigematsu N, Suzuki S, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated-dose area and severity. Int J Radiat Oncol Biol Phys. 1999;44:599–605.
26 Archer DB, Gardiner TA. Ionizing radiation and the retina. Curr Opin Ophthalmol. 1994;5:59–65.
27 Midena E, Segato T, Valenti M, et al. The effect of external eye irradiation on choroidal circulation. Ophthalmology. 1996;103:1651–1660.
28 Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611.
29 Krebs IP, Krebs W, Merriam JC, et al. Radiation retinopathy: electron microscopy of retina and optic nerve. Histol Histopathol. 1992;7:101–110.
30 Raman R, Pal SS, Krishnan T, et al. High-resolution optical coherence tomography correlates in ischemic radiation retinopathy. Cutan Ocul Toxicol. 2010;29:57–61.
31 Levitz LM. The use of optical coherence tomography to determine the severity of radiation retinopathy. Ophthalmic Surg Lasers Imaging. 2005;36:410–411.
32 Gass J. Stereoscopic atlas of macular diseases, diagnosis and treatment, 3rd ed. St Louis: Mosby; 1987. 404–5
33 Li M, Qiu G, Luo W, et al. Clinical investigation of radiation retinopathy fundus and fluorescein angiographic features. Yan Ke Xue Bao. 1999;15:183–186.
34 Berker N, Aslan O, Batman C, et al. Choroidal neovascular membrane in radiation retinopathy. Clin Experiment Ophthalmol. 2006;34:625–626.
35 Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:325–335.
36 Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2005;89:730–738.
37 Early Treatment of Diabetic Retinopathy Study Group. Photocoagulation for macular edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch Ophthalmol. 1985;103:1796–1806.
38 Catier A, Tadayoni R, Paques M, et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol. 2005;140:200–206.
39 Horgan N, Shields CL, Mashayekhi A, et al. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina. 2008;28:263–273.
40 Bianciotto C, Shields CL, Pirondini C, et al. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117:1005–1012.
41 Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: case report and review. B J Ophthalmol. 1991;75:629–632.
42 Packer S, Rotman M. Radiotherapy of choroidal melanoma with iodine 125. Int Ophthalmol Clin. 1980;20:135–142.
43 Conway R, Poothullil A, Daftari I, et al. Estimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapy. Arch Ophthalmol. 2006;124:839–843.
44 Rudoler SB, Corn BW, Shields CL, et al. External beam irradiation for choroid metastases: identification of factors predisposing to long-term sequelae. Int J Radiat Oncol Biol Phys. 1997;38:251–256.
45 Gunduz K, Shields CL, Shields JA, et al. Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol. 1999;117:170–177.
46 Wakelkamp IM, Tan H, Saeed P, et al. Orbital irradiation for Graves’ ophthalmopathy: Is it safe? A long-term follow-up study. Ophthalmology. 111, 2004. 1557e62
47 Stack R, Elder M, Abdelaal A, et al. New Zealand experience of I125 brachytherapy for choroidal melanoma. Clin Experiment Ophthalmol. 2005;33:490–494.
48 Chan RC, Shukovsky LJ. Effects of irradiation on the eye. Radiology. 1976;120:673–675.
49 Kumar B, Palimar P. Accelerated radiation retinopathy in diabetes and pregnancy. Eye. 2004;14:107–108.
50 Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol. 1996;114:1097–1100.
51 Bagan SM, Hollenhorst RW. Radiation retinopathy after irradiation of intracranial lesions. Am J Ophthalmol. 1979;88:694–697.
52 Lopez PF, Steinberg P, Dabbs CK, et al. Bone marrow transplant retinopathy. Am J Ophthalmol. 1991;112:635–646.
53 Chacko DC. Considerations in the diagnosis of radiation injury. JAMA. 1981;245:1255–1258.
54 Bagan SM, Hollenhorst RW. Radiation retinopathy after irradiation of intracranial lesions. Am J Ophthalmol. 1979;88:694–697.
55 Monroe AT, Bhandare N, Morris CG, et al. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys. 2005;61:856–864.
56 Parsons JT, Bova FJ, Fitzgerald CR, et al. Radiation retinopathy after external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:765–773.
57 Nikoskelainen E, Joensuu H. Retinopathy after irradiation for Graves’ ophthalmopathy (letter). Lancet. 1989;2:690–691.
58 Miller M, Goldberg S, Bullock J. Radiation retinopathy after standard radiotherapy for thyroid-related ophthalmopathy. Am J Ophthalmol. 1991;112:600–601.
59 Durkin SR, Roos D, Higgs B, et al. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand. 2007;85:240–250.
60 Bradley EA, Gower EW, Bradley DJ, et al. Orbital radiation for graves ophthalmopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:398–409.
61 Eng TY, Boersma MK, Fuller CD, et al. The role of radiation therapy in benign diseases. Hematol Oncol Clin North Am. 2006;20:523–557.
62 Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: case report and review. Br J Ophthalmol. 1991;75:629–632.
63 Spaide RF, Borodoker N, Shah V. Atypical choroidal neovascularization in radiation retinopathy. Am J Ophthalmol. 2002;133:709–711.
64 Spaide RF, Leys A, Hermann-Delamazure B, et al. Radiation-associated choroidal neovasculopathy. Ophthalmology. 1999;106:2254–2260.
65 Kinyoun JL, Zamber RW, Lawrence BS, et al. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol. 1995;70:144–149.
66 Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Arch Ophthalmol. 1986;104:34–41.
67 Chaudhuri PR, Austin DJ, Rosenthal AR. Treatment of radiation retinopathy. Br J Ophthalmol. 1981;65:623–625.
68 Augsburger JJ, Roth SE, Magargal LE, et al. Panretinal photocoagulation for radiation-induced ocular ischemia. Ophthalmic Surg. 1987;18:589–593.
69 Finger PT. Radiation therapy for choroidal melanoma. Surv Ophthalmol. 1997;42:215–232.
70 Barnstable CJ, Tombran-Tink J. Neuroprotective and anti-angiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Ret Eye Res. 2004;23:561–577.
71 Hidayat AA, Fine BS. Diabetic choroidopathy: light and electron microscopic observations of seven cases. Ophthalmology. 1985;92:512–522.
72 Takahashi K, Kishi S, Muraoka K, et al. Radiation choroidopathy with remodeling of the choroidal venous system. Am J Ophthalmol. 1998;125:367–373.
73 Flyczkowski AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol. 1989;13:269–279.
74 Hykin PG, Shields CL, Shields JA, et al. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105:1425–1429.
75 Ciulla TA, Walker JD, Fong DS, et al. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Curr Opin Ophthalmol. 2004;15:211–220.
76 Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation-induced macular edema. Arch Ophthalmol. 2003;121:1491–1493.
77 Shields CL, Demirci H, Dai V, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25:868–874.
78 Shields CL, Demirci H, Marr BP, et al. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina. 2006;26:537–544.
79 Iwao K, Inatani M, Kawaji T, et al. Frequency and risk factors for intraocular pressure elevation after posterior sub-Tenon capsule triamcinolone acetonide injection. J Glaucoma. 2007;16:251–256.
80 Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: Two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–1538.
81 Conti SM, Kertes PJ. The use of intravitreal corticosteroids, evidence-based and otherwise. Curr Opin Ophthalmol. 2006;17:235–244.
82 Horgan N, Shields CL, Mashayekhi A, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: a randomized controlled trial. Ophthalmology. 2009;116:1383–1390.
83 Dunavoelgyi R, Zehetmayer M, Simader C, et al. Rapid improvement of radiation-induced neovascular glaucoma and exudative retinal detachment after a single intravitreal ranibizumab injection. Clin Exp Ophthalmol. 2008;35:878–880.
84 CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908.
85 Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615.
86 Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (Avastin) for radiation retinopathy. Arch Ophthalmol. 2007;125:751–756.
87 Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys. 2008;70:974–977.
88 Finger PT, Chin KJ. Intravitreous ranibizumab (lucentis) for radiation maculopathy. Arch Ophthalmol. 2010;128:249–252.
89 Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina. 2008;28:964–968.
90 Wen JC, McCannel TA. Treatment of radiation retinopathy following plaque brachytherapy for choroidal melanoma. Curr Opin Ophthalmol. 2009;20:200–204.
91 Finger PT, Mukkamala SK. Intravitreal anti-VEGF bevacizumab (Avastin) for external beam related radiation retinopathy. Eur J Ophthalmol. 2011;21:446–451.
92 Vasquez LM, Somani S, Altomare F, et al. Intracameral bevacizumab in the treatment of neovascular glaucoma and exudative retinal detachment after brachytherapy in choroidal melanoma. Can J Ophthalmol. 2009;44:106–107.
93 Solano JM, Bakri SJ, Pulido JS. Regression of radiation-induced macular edema after systemic bevacizumab. Can J Ophthalmol. 2007;42:748–749.
94 Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–1047.
95 Rosenfeld PJ, Schwartz SD, Blumenkranz MS, et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology. 2005;112:1048–1053.
96 http://clinicaltrials.gov/ct2/show/NCT00811200.
97 Noble KG, Kupersmith MJ. Retinal vascular remodelling in radiation retinopathy. Br J Ophthalmol. 1984;68:475–478.
98 Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:325–335.
99 Shields CL, Shields JA, Cater J, et al. Plaque radiotherapy for uveal melanoma: long-term visual outcome in 1106 consecutive patients. Arch Ophthalmol. 2000;118:1219–1228.
100 Finger PT. Tumour location affects the incidence of cataract and retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2000;84:1068–1070.