Chapter 66 Pyogenic liver abscess
Overview
Pyogenic liver abscess (PLA) may be defined as solitary or multiple collections of pus within the liver, the result of bacterial infection. In 1938, Ochsner and coworkers reported the first series of patients with hepatic abscesses in the modern surgical era treated by surgical drainage. This study included 47 patients and reported an overall survival of 67%. The advent of antibiotic therapy marked the basis of the contemporary treatment of liver abscesses, becoming a major part of the therapy, combined with surgical drainage. The first landmark report in minimally invasive treatment of liver abscess was that of McFadzean and colleagues in 1953. They presented a group of 14 patients who underwent percutaneous drainage for PLAs with no deaths within the group. Although potentially effective for dealing with the liver problem, such approaches present the disadvantage of overlooking the underlying abdominal pathology because of the lack of surgical exploration.
The development of clinical ultrasound (US) in the 1960s and the introduction of computed tomography (CT) in the 1970s represent the two major advances in the diagnosis and treatment of PLAs. Surgical exploration as a diagnostic tool was replaced by abdominal imaging, thus allowing minimally invasive techniques to become the first choice of treatment. Currently, percutaneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) have become standard methods for both single and multiple PLAs (see Chapter 28). Surgical debridement, done either in an open or laparoscopic fashion, has a limited therapeutic role for patients in whom nonoperative treatment fails or in those requiring surgical treatment for the underlying cause of the abscess. In addition, surgical exploration may be indicated as the initial procedure when coexistence of peritonitis is suspected as result of abscess rupture into the peritoneal cavity.
Etiology
During the nineteenth century, PLAs were well known as a complication of acute appendicitis. Since then, etiology and presentation have dramatically changed. Inflammatory abdominal diseases are no longer the most common underlying conditions for PLAs, being replaced in later decades by a higher incidence of biliary causes, including malignancies, immunocompromised status, and advanced age (Branum et al, 1990). In 1996, Huang and colleagues (1996) presented a review that spanned more than 40 years in the treatment of PLAs. They analyzed and compared patterns of clinical presentation in 80 patients treated between 1952 and 1972 with a second group of 153 patients treated from 1973 to 1993. The authors concluded that the increased incidence of biliary malignancies as a cause of PLAs was due to a more aggressive approach in the treatment of this pathology, which includes more frequent instrumentation of the biliary tree (see Chapters 28 and 50D). Hilar cholangiocarcinoma was the most frequent single condition found during the second period reviewed, with the use of biliary stents and broad-spectrum antibiotics leading to the emergence of mixed bacterial and fungal infections. Biliary malignancy was an important risk factor for hospital mortality (Lok et al, 2008).
Elucidating the subjacent condition that caused a liver abscess is as important therapeutically as the correct treatment of a PLA. In a simplified schema, infection may get to the liver by five different avenues: 1) portal vein, 2) hepatic artery, 3) biliary tree, 4) adjacent organ infection, and 5) direct trauma to the liver. The term cryptogenic PLA applies when no underlying pathology is identified (Box 66.1).
Box 66.1 Classification of PLA According to the Underlying Mechanism of Dissemination
Portal pyemia is often a consequence of intraabdominal infection, such as acute appendicitis or diverticulitis. The incidence of this mechanism as a cause of PLA has markedly decreased in the past years. However, portal vein patency must be evaluated with Doppler US or dynamic CT scan in patients with PLAs. Gastrointestinal malignancies, such as colorectal adenocarcinoma or even gastric carcinoma, may also lead to a disruption of the mucosal barrier, leading to pyemia and liver abscess in the absence of liver metastasis (Tzur et al, 2003). This phenomenon should be particularly considered in the evaluation of patients with a cryptogenic PLA (Lim & Lim, 2004). Adequate investigation of etiology after PLA treatment may lead to a diagnosis of an underlying disease previously unknown for the patient (Mohsen et al, 2002).
Hematogenous spread of infection through the hepatic artery may also cause PLA. Frequent examples are bacterial endocarditis and intravenous drug abuse, but certain immunosuppressive conditions may also be associated with this mechanism. Liver cirrhosis is often associated with immunodeficiency, and the incidence of liver abscesses in cirrhotic patients compared with the general population is increased (see Chapter 9; Molle et al, 2001). Loss of hepatic filter function, impaired immunity, and frequent abdominal infections may also be responsible factors. Immunosuppressive drug use, alcoholism, chronic pancreatitis, pyelonephritis, and acquired immune deficiency syndrome are also associated with PLA (Huang et al, 1996).
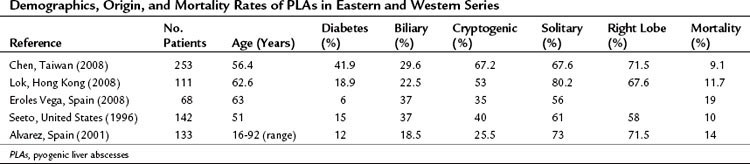
The incidence of diabetes mellitus varies among different series, but its presence is associated with a 10-fold increased risk for PLA compared with that of the general population. Diabetes mellitus was the most common comorbidity in a report of PLA from Taiwan and was present in 41 (16%) of 253 patients with PLA (Chen et al, 2008a). Biliary pathology has become the most identifiable cause of PLA in recent decades (Alvarez et al, 2001a). In this group of patients, infection may have different etiologies. Bile duct strictures, common bile duct stones, and hepatolithiasis are benign conditions that may cause PLA (see Chapters 43 and 44). Benign diseases are more commonly reported in Asia (Lok et al, 2008); in Western countries, however, biliary malignancy is a more prevalent condition. Instrumentation of bile duct obstruction by endoscopic stenting or percutaneous drainage is a frequent cause of cholangitis and PLA. Malignancy is a predictor of higher recurrence and mortality rates. The mortality rate of PLA caused by underlying malignant disease is almost twice as high as that of nonmalignant disease (Yeh et al, 1998).
Direct liver contamination by adjacent organ infection may produce a PLA, with acute cholecystitis being the most common example (see Chapter 31). However, gastric and duodenal perforation directly into the liver may also give rise to a PLA. Perforation secondary to foreign bodies, mainly from fish bones, have been also reported. In addition, liver parenchyma may become infected after direct damage. Intrahepatic biloma, hematoma, and necrotic parenchyma are favorable conditions for development of a PLA. This is a well-known consequence of blunt or penetrating liver trauma and liver resections.
In recent decades, local treatments for liver tumors have become a widespread alternative for many patients. These local techniques include ethanol injection, transarterial chemoembolization (TACE), selective internal radiation therapy, and radiofrequency ablation (RFA) (see Chapter 83, Chapter 84A, Chapter 84B, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ), which induce tumor necrosis either by chemical or thermal tissue destruction. In all these patients, PLA is a potentially life-threatening complication that may occur up to 5 months after a successful procedure. Transarterial liver embolization may be helpful in patients with hepatocellular carcinoma or liver metastases of gastrointestinal sarcomas and neuroendocrine tumors. The incidence of liver abscess after this procedure is low. Ong and colleagues (2004) reported PLAs in 0.26% of 3878 embolizations; the mortality rate in these patients was 33%. Patients with bilioenteric anastomosis are at particular risk for this complication. The odds ratio (OR) for developing PLA in this population was 894 in a series from Philadelphia (Kim et al, 2001). Subjacent pathology is not a predisposing factor for this complication (Song et al, 2001), but RFA of liver tumors may also cause a PLA. In a multicenter study from Italy, Livraghi and coworkers (2003) reported 2320 patients with 3554 lesions, with a 0.3% incidence of PLA. This incidence was slightly higher (1.7%) in another group of patients with hepatocellular carcinoma treated by RFA (Choi et al, 2005). De Baere and colleagues (2003) reported a 100% incidence of liver abscess in patients with prior bilioenteric anastomosis who underwent RFA. A further prospective analysis showed an incidence of PLA of 50% in patients with biliary diversions, but the risk was not increased if this diversion was performed synchronously with the ablation procedure (Elias et al, 2006).
In some patients with a PLA, no underlying cause is identified; they are considered to have a cryptogenic PLA. These cases account for up to 67% in some published data (Chen et al, 2008a). Etiology and presentation are summarized in Table 66.1.
Incidence
PLA is a rare disease, and actual incidence rates are poorly described in the literature. However, recent investigations demonstrated a marked increase of PLA incidence in the general population in the last few years. Huang and colleagues (1996) showed that PLA accounted for 13 per 100,000 hospital admissions in 1973, which increased to up to 20 per 100,000 only 20 years later. In a recent study from a teaching hospital in the United Kingdom, PLA had an incidence of 18.5 per 100,000 hospital admissions, with an estimated crude incidence of 2.3 cases per 100,000 the general population (Mohsen et al, 2002).
The second, a national series from Denmark (Jepsen et al, 2005), analyzed 1448 cases in a 25-year period; 54% were male. The incidence of PLA in Danish men was 6 per million in 1977 and 18 per million in 2002. In women, the incidence rose from 8 per million to 12 per million in the same period. Mortality rates in 1977 were 40% and 50% for men and women, respectively, and decreased to a global 10% in 2002.
Clinical Presentation
Liver abscesses may have a broad array of symptoms and physical findings, which may vary according to the patient’s underlying condition. Cryptogenic lesions are often found after several days of nonspecific symptoms. The most frequent clinical features include fever of more than 38° C and chills. Other symptoms that may be present are general malaise and anorexia. Patients may also have abdominal pain, nausea and vomiting, diarrhea, and weight loss. Although more often found with abscesses of biliary origin, jaundice may be an indicator of systemic sepsis response, and septic shock may be a dramatic form of the presentation. There are no significant differences in clinical presentation between single and multiple lesions, although single PLAs are more frequent in the right lobe of the liver. These symptoms may be present from several days up to several weeks before hospital admission (Chou et al, 1997; Seeto & Rockey, 1996). In another group of patients, PLA is a consequence of an underlying pathologic process that may lead to a faster diagnosis. Benign or malignant biliary disease, intraabdominal infections, and abdominal surgery are frequent causes of abscesses, and imaging studies should be systematically carried out to exclude the presence of a PLA in a septic patient.
Finally, some patients may be seen with systemic complications as a result of metastatic septic lesions that originate from a PLA. Although rare, cases of endophtalmitis; meningitis; cellulitis; lung abscess; prostate, kidney, and joint infections; pulmonary embolisms; and even cardiac tamponade due to pericardic effusion have been reported in the literature (Cahill et al, 2000; Vong et al, 2007). Infection by Klebsiella pneumoniae genotype K1 and an underlying condition of immunosuppression resulting from diabetes mellitus and alcoholism may be predisposing factors for these complications (Chen et al, 2006; Fang et al, 2007).
Diagnosis
The most common laboratory findings are nonspecific alterations as a result of infection. In a large series from Hong Kong, low albumin levels were found in 92.8% of patients presenting with PLAs (Lok et al, 2008). Leukocytosis (74.8%), increased alkaline phosphatase (72.1%), and elevated alanine aminotransferase levels (ALT; 58.6%) were frequent laboratory abnormalities also present in these patients. Jaundice may be seen in up to 50% of patients, and alkaline phosphatase, gamma-glutamyl transferase, erythrocyte sedimentation rate, and glutamic oxaloacetic transaminase levels are usually elevated (Cosme et al, 2010). Alvarez and colleagues (2001) analyzed laboratory data obtained from patients younger than and older than 60 years. The only significant difference between these two groups was that older patients tended to present with higher blood urea nitrogen and serum creatinine levels. Hemoglobin level, serum C-reactive protein, blood urea nitrogen, creatinine levels, prothrombin time, and total bilirubin levels must be part of systematic laboratory tests in patients with PLA.
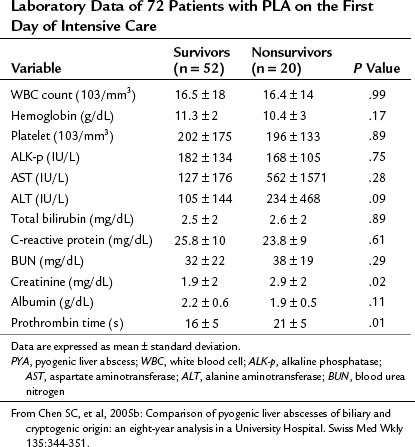
Some findings at presentation may be prognostic and associated with increased mortality rate. Chen and colleagues (2008b) presented a review of 72 patients admitted to the intensive care unit. Low levels of serum albumin, increased serum creatinine, and prolonged prothrombin time were significant risk factors for death. These authors’ findings are shown in Table 66.2.
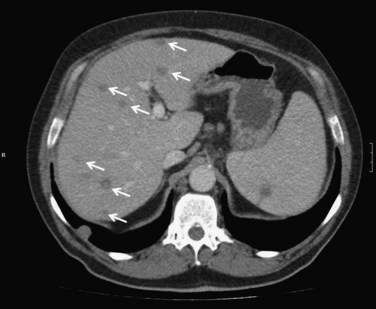
CT has a sensitivity of more than 97% in detecting PLA and is considered the most important imaging modality. Examination should be routinely performed with intravenous contrast enhancement. During the arterial phase, parenchyma surrounding the abscess may show segmental enhancement as a result of altered portal microcirculation in the infected tissue. The typical CT image description of PLA is that of a target-like sign (see Chapter 16) that appears as a single or multiloculated mass with a central hypodense region and peripheral contrast enhancement during the portal phase of examination.
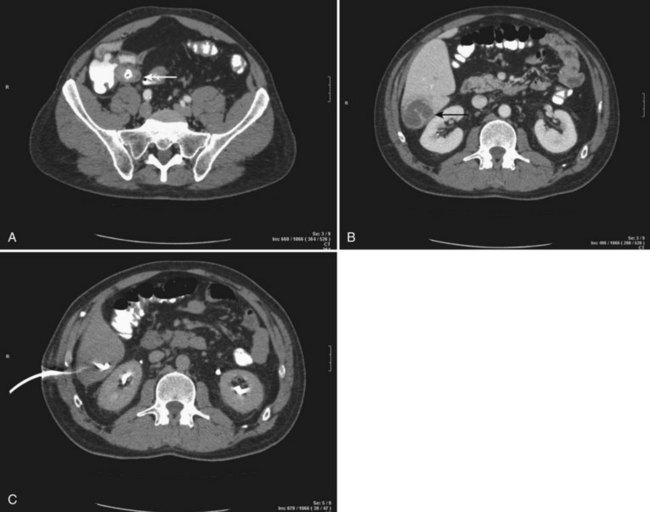
CT scan offers the advantage of detecting other intraabdominal pathology that may be the cause of the abscess, such as acute diverticulitis or appendicitis. It may also provide information about portal vein patency and may demonstrate local complications that may include pleural effusion, vascular complications, and spontaneous rupture into the peritoneal cavity, retroperitoneum, and even the pericardium (Yang et al, 2004b). Finally, CT scan is generally necessary to guide percutaneous drainage of the abscess (Fig. 66.1).
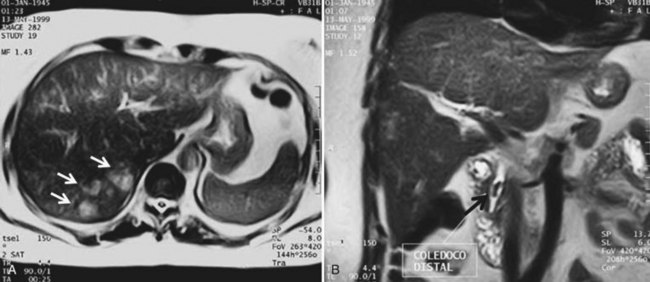
Magnetic resonance imaging (MRI) is an alternative to CT for the diagnosis of PLA. In MRI sequences, PLA appears much the same as other fluid-containing lesions: hypointense in T1-weighted and hyperintense in T2-weighted images. After contrast administration, most PLAs show a hyperintense enhancement rim, which may persist in later phases (Balci et al, 1999). Infected tumors or metastases may show the same pattern, and differentiation by MRI may be difficult, although diffusion techniques may play a role in distinguishing between benign and neoplasic lesions. MRI is not widely accepted as a first-line diagnostic approach for several reasons: examinations take more time, patient cooperation is needed, and MRI cannot be used as guidance for percutaneous treatment. Nevertheless, it has the advantage of providing data regarding the biliary tract in patients with underlying biliary pathology, findings that could change the therapeutic approach (Fig. 66.2).
Nuclear medicine has a limited role in PLA diagnosis, but in case of doubt, it may be helpful for detecting intraabdominal abscesses. This may be important, for example, in a patient with polycystic liver disease and suspected infection of a single lesion. Indium-111 leukocyte scintigraphy readily detects most hepatic abscess. Gallium-67 may also be used, but it is normally taken up by the liver, making interpretation of the results more difficult. In both cases, prior technetium-99m sulfur colloid scan provides more diagnostic accuracy (Youssef et al, 2005).
Conflicting results have been reported regarding PLA characteristics according to different origins (Chen et al, 2005). Single lesions tend to be cryptogenic, whereas multiple abscesses are more likely to have a biliary origin (Alvarez et al, 2001). Regardless of etiology, patients are most commonly seen initially with a single lesion (Chan et al, 2005). In both single and multiple forms, abscesses are more frequently located in the right hepatic lobe, followed by a left and bilateral distribution (Chen et al, 2008a; Seeto & Rockey, 1996).
A PLA less than 2 cm in diameter is described as a microabscess. Multiple microabscesses may present as widely scattered lesions or with a cluster pattern that tends to aggregate focally (Ralls, 2002). The diffuse miliary pattern of pyogenic microabscesses is usually staphylococcal in origin, the result of hematogenous spread of an endovascular infection. The cluster pattern is often associated with coliform and enteric organisms and may occur in cholangitic abscesses secondary to biliary obstruction. It is probable that the clustering of pyogenic microabscesses is an early stage of an evolving pyogenic abscess cavity, and the tendency of coliform microabscesses to coalesce into a larger abscess with intercommunicating cavities explains the success of single-catheter drainage of multiseptate PLAs.
Microbiology
Although PLAs may originate from a broad spectrum of microorganisms, the underlying etiology and geographic location of PLAs are often related to specific pathogens. Identification of pathogens may be achieved by direct puncture of the abscess or by blood cultures. The positive abscess culture rates are higher than those of blood culture samples, and only up to 50% of patients with PLA have both cultures positive (Chen et al, 2005). Moreover, negative abscess cultures may be found in 20% of patients (Lok et al, 2008). Poor culture technique may be the reason in some cases, but negative cultures can also be caused by the use of broad-spectrum antibiotics before cultures are obtained. Patients with cryptogenic PLAs are more likely to have negative cultures from the blood, whereas patients with PLAs secondary to biliary tract disorders are more likely to have positive cultures from blood and aspirated pus (Seeto & Rockey, 1996).
In Asian populations, Klebsiella pneumoniae is the most frequent pathogen associated with cryptogenic PLAs. The incidence of infection with K. pneumoniae appears to have markedly increased in this population, ranging from 50% to 88% of all cases occurring in Taiwan in the last few decades to become an endemic disease. Diabetes mellitus is suggested to be an important risk factor, but the pathogenesis is still unclear. No mutations or clonal spread strains have been found. Environmental or host factors may be responsible for the different incidence rates in Asia compared with Western series (Cheng et al, 2002). Although PLAs secondary to K. pneumoniae infections are associated with an increased risk of septic metastases, overall mortality, even in diabetic populations, is decreased in this group of patients (Tsai et al, 2008; Yang et al, 2004a).
Gas-forming PLAs are uncommon and often associated with compromised immunity in diabetic patients, and they present higher mortality rates than non–gas-forming PLAs. Escherichia coli, Enterobacteriaceae, and K. pneumoniae are well-known gas-forming microorganisms. The mechanisms of gas formation involves mixed acid fermentation of glucose, increased production of gas, impaired transportation of gas, and equilibrium between the gas in local tissues and that in abscesses (Lee et al, 2004).
E. coli is the most common pathogen in Western countries, in both monomicrobial and polymicrobial isolates, followed by Streptococcus milleri. Anaerobes may also be cultured from PLAs, and Bacteroides spp. are the most common isolated organism (Alvarez et al, 2001b; Mohsen et al, 2002).
An abscess secondary to biliary tract disease or originating from a gastrointestinal source is more likely to be polymicrobial with aerobic gram-negative organisms and anaerobes. PLAs that result from hematogenous spread, on the other hand, are more likely to be monomicrobial, and staphylococci and streptococci are the most frequent bacteria isolated in these patients. A summarized spectrum of possible pathogens is listed in Box 66.2.
Treatment
PLAs must be treated with a prolonged course of antibiotics. Initially, antibiotics should be administrated parenterally. This therapy must be maintained until stabilization of the patient’s condition is achieved and for at least 2 weeks thereafter. Criteria for clinical stability include subjective and objective evidence of improvement in the inflammatory response produced by the infection. The patient should be afebrile, with a white cell count trend toward normal and adequate oral intake and gastrointestinal absorption. The patient may then be switched to oral antibiotic therapy, perhaps even continued on an outpatient basis for at least 2 more weeks. This sequential modality has proven effective in the treatment of PLAs compared with continuous intravenous administration, reducing both the cost of therapy and the length of hospital stay (Ng et al, 2002).
Drainage of all lesions may not be possible in patients with multiple small abscesses. In such cases, percutaneous puncture of any of the abscess may be indicated for isolation of the causative bacteria for a rational antibiotic therapy (Fig. 66.3). If a biliary origin of these lesions is suspected, biliary decompression is mandatory, either by endoscopic, percutaneous, or surgical means (see Chapters 27 through 29). The mortality rates reported in patients with multiple liver abscesses are high, ranging from 22% to 44%. However, Giorgio and colleagues (2006) reported 0% mortality in 39 patients treated with an aggressive approach consisting of percutaneous needle aspiration of all visible lesions under sonographic guidance and antibiotic therapy.
Percutaneous Treatment
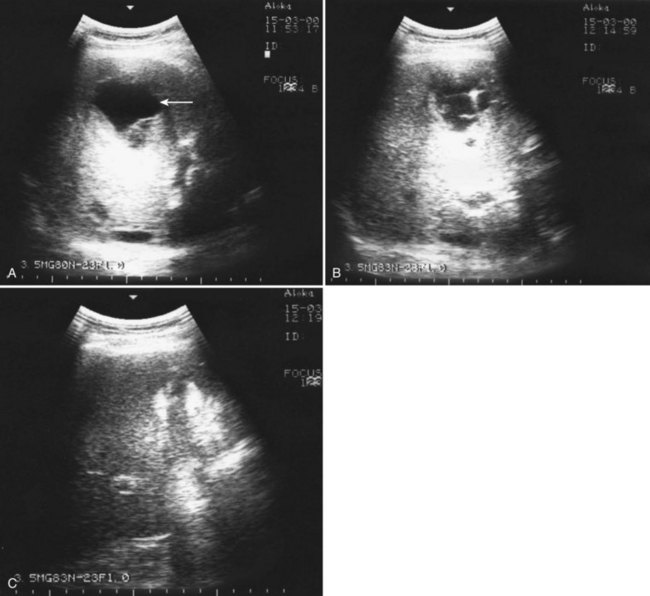
Percutaneous drainage is a safe and effective treatment for almost all PLAs (Pearce et al, 2003; see Chapter 28). This procedure can be performed under sonographic or CT guidance. The US exam provides real-time guidance of the needle, and it is fast and reliable (Fig. 66.4). Fluoroscopy control and operating theaters under aseptic conditions are recommended. The freehand technique allows dynamic changes in the needle pattern and does not have the limitations of attached needle guides. CT guidance is recommended for multiple abscess drainage, when multiple catheters may be placed or in cases of hazardous anatomic locations. Local anesthesia and minimal sedation is used for most of these procedures (Giorgio et al, 2006).
After aspiration of a PLA to obtain samples for culture and to confirm the diagnosis, the treatment options are complete aspiration of pus (PNA) or catheter drain insertion (PCD). Controversy persists regarding the optimal procedure, particularly for small abscesses. The first randomized trial conducted by Rajak and colleagues in 1998 showed 100% resolution of PLAs in the PCD group with a 60% success rate in the FNA group. Although FNA patients were given only a second chance of aspiration before placing a PCD, the thickly viscous nature of the pus was considered a major cause of failure in this group. In a subsequent randomized study conducted by Yu and colleagues in 2004 using intermittent needle aspiration, no significant differences were found between PCD and FNA, suggesting that FNA may be as effective as catheter drainage. However, a tendency toward a shorter hospitalization and lower mortality rate was found in the FNA group. Finally, a third trial randomized 60 patients to FNA or PCD (Zerem & Hadzic, 2007). The authors succeeded in all 30 patients undergoing one or two PCDs. In the FNA group, a 33% failure rate was reported after three aspiration procedures. None of the patients with multiloculated lesions was effectively treated by FNA. The authors recommended PCD as first-line treatment option but considered FNA a valid alternative in simple abscesses smaller than 50 mm in diameter. Several nonrandomized studies report experiences with both therapeutic approaches with good results. The clinical experience of the treating physicians is important in the evaluation of each patient. FNA could be considered as an alternative to routine PCD in patients with small uniloculated lesions, no viscous content, and with no communication with the biliary tree.
Placement of a PCD may cause bacteremia in spite of antibiotic administration. The most important preventive measure is to avoid excessive manipulation of infected pus. Overdistension of the abscess with contrast agents during the procedure and saline injections to wash the cavity are associated with an increased risk of bacteremia and are therefore not recommended (Thomas et al, 2006). Flushing the catheter may be useful for maintaining its patency, especially in cases of viscous pus. Furthermore, in such cases, larger catheters may be needed to drain the cavity efficiently. In some cases, after apparent successful drainage, imaging may show incomplete evacuation of the abscess cavity, and repositioning of the catheter or even placement of additional catheters may be necessary. An intercostal approach may cause pneumothorax, pleural effusion, or contamination of the pleural space, and a chest tube should be placed without removal of the abscess drain.
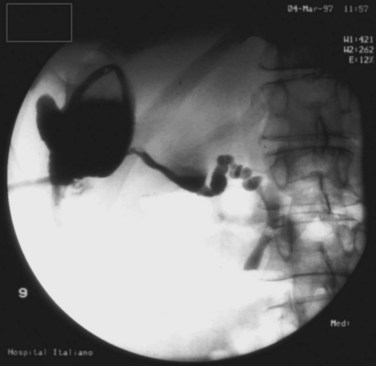
Failure of PCD may occur in up to 10% of patients, and the chronicity of a PLA may result in a thick, fibrous rind that will not collapse. In other cases, a cluster of abscesses may be misinterpreted as a multiloculated PLA on imaging, and the absence of communication with the main cavity results in incomplete drainage. Persistent drainage of bile through the catheter after several days or weeks is commonly seen when the abscess communicates with the biliary tree (Fig. 66.5). Endoscopic retrograde cholangiopancreatography with endoscopic sphincterotomy and transpapillary stent may help resolve this problem. Sugiyama and Atomi (2002) found that in all cases of biliary communication, complete cure of abscesses after PCD required additional treatment to relieve the obstruction.
Surgical Treatment
Surgical treatment of PLAs as a first therapeutic approach is not usually indicated. Percutaneous procedures have been increasingly performed, leaving surgical drainage to salvage cases where percutaneous treatment fails (Tan et al, 2005). However, surgical treatment of PLAs may be indicated in certain specific clinical situations.
Primary surgical therapy is indicated in patients with intraperitoneal rupture, in which complete exploration and lavage of the abdomen are indicated. Laparoscopic approach combined with intraoperative US examination appears to be a minimally invasive therapeutic option for this rare condition (Siu et al, 1997). In some other patients, surgery may be indicated for the treatment of complications of the percutaneous drainage, such as bleeding or intraperitoneal leakage of pus.
Large, multiloculated abscesses containing thick pus are more prone to not respond to percutaneous treatment. In these patients, complete evacuation and removal of necrotic tissue and debris may be more easily achieved surgically (Hope et al, 2008). Percutaneous drainage may, on the other hand, delay effective treatment, which would increase the number of treatment failures, secondary procedures, and the length of hospital stay (Tan et al, 2005). Failure to institute appropriate surgical treatment in a timely fashion could be harmful in critically ill patients who require urgent resolution of the septic illness (Hsieh et al, 2008) and in whom salvage surgery may be associated with a mortality rate of 46% (Ng et al, 2008). However, a multidisciplinary team of surgeons and interventional radiologists is mandatory for treating patients in these complex situations. Laparoscopic drainage may play an important role in diminishing wound-related complications. With a laparoscopic approach, intraoperative US plays an important role to assess location and extension of the lesion (Yeh et al, 2007); however, it should be emphasized that an open approach should be used if there is any question regarding the adequacy of drainage achievable laparoscopically. No prospective studies compare open versus laparoscopic approach in the treatment of PLAs. Wang and colleagues (2004) presented a retrospective comparison of five patients treated with open drainage with 18 patients who underwent laparoscopic approach. Although mortality rate in the series was 0%, the laparoscopic group had shorter operating times and earlier oral intake in the postoperative period with less intraoperative blood loss and shorter hospitalization. Given the bias associated with patient selection, these results cannot be taken as evidence of the superiority of the laparoscopic approach but only as evidence that laparoscopic treatment is feasible in selected patients.
Liver resection for the treatment of PLAs is rare because almost all PLAs can be cured by either percutaneous or operative drainage, but in some cases, PLAs with different physical and etiologic characteristics may need a different surgical approach. Liver atrophy and multiple abscesses may be the consequences of chronic obstruction of a segmentary bile duct branch as a result of hepatholitiasis or intrahepatic strictures (see Chapter 39, Chapter 42A, Chapter 42B, Chapter 44 ). Resection of the affected liver segments is the recommended treatment for these patients. Although more frequent in the left lobe, this condition may also be present in the right lobe of the liver. This aggressive approach is also indicated for multiple pyogenic abscesses with severe destruction of surrounding liver parenchyma (Chou et al, 1997). In an impressive series, Strong and colleagues (2003) reported 49 patients who underwent liver resections for PLAs over a 15-year period. Indications for surgery were failure of medical treatment or underlying hepatobiliary pathology. Two deaths (4%) occurred after intraperitoneal rupture of the PLA.
Treatment Summary
Imaging and clinical response are good indicators of treatment success. On occasion, a residual cavity may persist despite drainage. In this situation, if the patient remains asymptomatic and the lesion imaging is stable, no further procedure may be needed (Johannsen et al, 2000). After clinical stabilization, antibiotics may be switched to oral administration. A complete course of 4 weeks of antibiotics is recommended, although some authors suggest 2 weeks of treatment (Bowers et al, 1990).
Outcome and Prognosis
After successful treatment, recurrence of a PLA is rare; however, it is important to differentiate PLA recurrence from relapse as a result of incomplete treatment. Some patients with underlying risk factors may be at risk for recurrence. Cheng and colleagues (2008) reported 601 patients with PLAs with a median follow-up of 72 months. Recurrence rates were low in the cryptogenic (2%) and diabetes (4.4%) groups compared with those whose disease was of a biliary origin (23.8%). Except for patients with biliary pathology, recurrences tended to be caused by the same organisms that generated the first PLA. However, the authors found no difference in recurrence rates comparing E. coli and K. pneumoniae organisms as causative agents.
PLA is a potentially life-threatening disease. Since the first report by Oschner in 1938, improvement in antibiotic therapy, new imaging techniques, and minimally invasive procedures have significantly decreased mortality. In series published in the last decades, mortality rates ranged between 6% and 20% (Chung, 2008). Although different prognostic factors have been identified in patients with PLAs, others remain controversial. Abscesses caused by K. pneumoniae have a higher risk of metastatic infection; however, mortality rates in these patients are lower compared with those whose PLA is caused by other organisms, even if compared with E. coli infections (Yang et al, 2004a). Diabetes is a well-known risk factor for PLA, but mortality rates in patients with diabetes are not increased compared with the general population in both Eastern and Western series (Alvarez et al, 2001; Tsai et al, 2008). Liver cirrhosis is a strong risk factor for developing PLA and is associated with poor prognosis and high short-term mortality rates (Molle et al, 2001). In general, the prognosis of patients with malignancy is poor, but in the absence of comorbidities, age is not associated with worse prognosis (Alvarez et al, 2001a).
Systemic response to septic injury is a major determinant of outcome. This may be indicated by the presence of septic shock, clinical jaundice, coagulopathy, leukocytosis, and hypoalbuminemia. An Acute Physiologic, Age, and Chronic Health Evaluation (APACHE II) score at admission of 15 or higher, multidrug-resistant isolates, gas-forming abscess, anaerobic infections, and a blood urea nitrogen level of 7.86 mmol/L or greater have been identified as high mortality–associated risk factors (Chen et al, 2008a).
Alvarez J, et al. Pyogenic liver abscesses: a comparison of older and younger patients. HPB (Oxford). 2001;3:201-206.
Alvarez JA, et al. Single and multiple pyogenic liver abscesses: etiology, clinical course, and outcome. Dig Surg. 2001;18:283-288.
Balci NC, et al. Pyogenic hepatic abscesses: MRI findings on T1- and T2-weighted and serial gadolinium-enhanced gradient-echo images. J Magn Reson Imaging. 1999;9:285-290.
Bowers ED, Robinson DJ, Doberneck RC. Pyogenic liver abscess. World J Surg. 1990;14:128-132.
Branum GD, et al. Hepatic abscess: changes in etiology, diagnosis and management. Ann Surg. 1990;212:655-662.
Cahill M, Chang B, Murray A. Bilateral endogenous bacterial endophthalmitis associated with pyogenic hepatic abscess. Br J Ophthalmol. 2000;84:1436.
Chan KS, et al. Pyogenic liver abscess: a retrospective analysis of 107 patients during a 3-year period. Jpn J Infect Dis. 2005;58:366-368.
Chen SC, et al. Comparison of pyogenic liver abscesses of biliary and cryptogenic origin: an eight-year analysis in a University Hospital. Swiss Med Wkly. 2005;135:344-351.
Chen SC, et al. Risk factors for developing metastatic infection from pyogenic liver abscesses. Swiss Med Wkly. 2006;136:119-126.
Chen SC, et al. Predictors of mortality in patients with pyogenic liver abscess. Neth J Med. 2008;66:196-203.
Chen W, et al. Clinical outcome and prognostic factors of patients with pyogenic liver abscess requiring intensive care. Crit Care Med. 2008;36:1184-1188.
Cheng HC, et al. Long-term outcome of pyogenic liver abscess: factors related with abscess recurrence. J Clin Gastroenterol. 2008;42:1110-1115.
Cheng HP, et al. Klebsiella pneumoniae liver abscess in Taiwan is not caused by a clonal spread strain. J Microbiol Immunol Infect. 2002;35:85-88.
Choi D, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860-1867.
Chou FF, et al. Single and multiple pyogenic liver abscesses: clinical course, etiology, and results of treatment. World J Surg. 1997;21:384-388. discussion 388-389
Chung YF. Pyogenic liver abscess: predicting failure to improve outcome. Neth J Med. 2008;66:183-184.
Cosme A, et al. Pyogenic versus amoebic liver abscesses: a comparative clinical study in a series of 58 patients. Rev Esp Enferm Dig. 2010;102:90-99.
de Baere T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695-700.
Elias D, et al. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30:823-827.
Eroles Vega G, et al. Liver abscess: retrospective review of 68 cases. An Med Interna. 2008;25:335-341.
Fang CT, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284-293.
Giorgio A, et al. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. AJR Am J Roentgenol. 2006;187:1585-1590.
Hope WW, et al. Optimal treatment of hepatic abscess. Am Surg. 2008;74:178-182.
Hsieh HF, et al. Aggressive hepatic resection for patients with pyogenic liver abscess and APACHE II score ≥ 15. Am J Surg. 2008;196:346-350.
Huang CJ, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg. 1996;223:600-607.
Jepsen P, et al. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977-2002. Aliment Pharmacol Ther. 2005;21:1185-1188.
Johannsen EC, Sifri CD, Madoff LC. Pyogenic liver absecesses. Infect Dis Clin North Am. 2000;14:547-563.
Kim W, et al. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:965-968.
Lee HL, et al. Clinical significance and mechanism of gas formation of pyogenic liver abscess due to Klebsiella pneumoniae. J Clin Microbiol. 2004;42:2783-2785.
Lim WC, Lim CC. Silent colorectal carcinoma and pyogenic liver abscess. J Gastroenterol Hepatol. 2004;19:945-946.
Livraghi T, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451.
Lok KH, et al. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41:483-490.
McFadzean AJ, Chang KP, Wong CC. Solitary pyogenic abscess of the liver treated by closed aspiration and antibiotics; a report of 14 consecutive cases with recovery. Br J Surg. 1953;41:141-152.
Mohsen AH, et al. Liver abscess in adults: ten years experience in a UK centre. QJM. 2002;95:797-802.
Molle I, et al. Increased risk and case fatality rate of pyogenic liver abscess in patients with liver cirrhosis: a nationwide study in Denmark. Gut. 2001;48:260-263.
Ng FH, et al. Sequential intravenous/oral antibiotic vs. continuous intravenous antibiotic in the treatment of pyogenic liver abscess. Aliment Pharmacol Ther. 2002;16:1083-1090.
Ng SS, Lee JF, Lai PB. Role and outcome of conventional surgery in the treatment of pyogenic liver abscess in the modern era of minimally invasive therapy. World J Gastroenterol. 2008;14:747-751.
Ochsner A, DeBakey M, Murray S. Pyogenic abscess of the liver: an analysis of forty-seven cases with review of the literature. Am J Surg. 1938;40:292-319.
Ong GY, et al. Liver abscess complicating transcatheter arterial embolization: a rare but serious complication—a retrospective study after 3878 procedures. Eur J Gastroenterol Hepatol. 2004;16:737-742.
Pearce N, et al. Non-operative management of pyogenic liver abscess. HPB (Oxford). 2003;5:91-95.
Rajak CL, et al. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR Am J Roentgenol. 1998;170:1035-1039.
Ralls PW. Inflammatory disease of the liver. Clin Liver Dis. 2002;6:203-225.
Seeto RK, Rockey DC. Pyogenic liver abscess: changes in etiology, management, and outcome. Medicine (Baltimore). 1996;75:99-113.
Siu WT, et al. Laparoscopic management of ruptured pyogenic liver abscess. Surg Laparosc Endosc. 1997;7:426-428.
Song SY, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol. 2001;12:313-320.
Strong R, et al. Hepatectomy for pyogenic liver abscess. HPB (Oxford). 2003;5:86-90.
Sugiyama M, Atomi Y. Pyogenic hepatic abscess with biliary communication. Am J Surg. 2002;183:205-208.
Tan YM, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg. 2005;241:485-490.
Thomas J, et al. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? AJR Am J Roentgenol. 2006;186:1419-1422.
Tsai FC, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14:1592-1600.
Tzur T, et al. Liver abscesses caused by Streptococcus milleri: an uncommon presenting sign of silent colonic cancer. Isr Med Assoc J. 2003;5:206-207.
Vong SC, et al. Cardiac tamponade secondary to pyogenic liver abscess. J Clin Gastroenterol. 2007;41:635-636.
Wang W, et al. Laparoscopic drainage of pyogenic liver abscesses. Surg Today. 2004;34:323-325.
Yang CC, et al. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37:176-184.
Yang DM, et al. Complications of pyogenic hepatic abscess: computed tomography and clinical features. J Comput Assist Tomogr. 2004;28:311-317.
Yeh TS, et al. Pyogenic liver abscesses in patients with malignant disease: a report of 52 cases treated at a single institution. Arch Surg. 1998;133:242-245.
Yeh TS, et al. Efficacy of color sonography and Harmonic Scalpel in laparoscopic management of multiple/lobulated liver cysts and abscesses. Hepatogastroenterology. 2007;54:485-488.
Youssef IM, et al. Importance of Tc-99m sulfur colloid liver-spleen scans performed before indium-111 labeled leukocyte imaging for localization of abdominal infection. Clin Nucl Med. 2005;30:87-90.
Yu SC, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932-938.
Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. AJR Am J Roentgenol. 2007;189:W138-W142.