Chapter 16 Pulsed Radiofrequency
 During PRF application, an attempt is made to maximize the delivery of RF currents while the thermal tissue injury is minimized by maintaining the tissue temperature below the neurodestructive range.
During PRF application, an attempt is made to maximize the delivery of RF currents while the thermal tissue injury is minimized by maintaining the tissue temperature below the neurodestructive range. The RF currents are applied in a pulsatile manner so the heat can dissipate in between the RF pulses.
The RF currents are applied in a pulsatile manner so the heat can dissipate in between the RF pulses. PRF is applied by placing an electrode in the vicinity of the target nociceptive structure; however, because the electrical currents extend radially from the electrode tip, juxtapositioning of the electrode tip parallel to the target nerve is considered unnecessary.
PRF is applied by placing an electrode in the vicinity of the target nociceptive structure; however, because the electrical currents extend radially from the electrode tip, juxtapositioning of the electrode tip parallel to the target nerve is considered unnecessary. It is assumed that sustained high-density electrical fields generated during PRF application stress the biomolecules and cause cellular dysfunction and death of the target neural structure.
It is assumed that sustained high-density electrical fields generated during PRF application stress the biomolecules and cause cellular dysfunction and death of the target neural structure.Introduction and History
Radiofrequency (RF) currents have been used clinically to create predictable and quantifiable thermal lesions since the 1950s and have been used in the treatment of pain since the early 1970s.1 During conventional RF (CRF) application for the treatment of pain, the RF currents are passed through an electrode that is placed in the vicinity of a nociceptive pathway. Consequently, the electrical energy imparted to the tissues immediately surrounding the active electrode tip creates a thermal lesion, likely interrupting the nociceptive impulses.2 Because tissue temperatures above 45° C are known to be neurodestructive,3 tissue temperatures are characteristically raised to well above the neurodestructive range but below the point of gas formation—80° C to 90° C. Although selective destruction of unmyelinated C and A-δ fibers by CRF thermal lesioning has been suggested,4 further studies showed indiscriminate destruction of all nerve fiber types during thermal CRF lesioning.5 Because of the possible risk of injury to the motor nerve fibers, local neuritis, loss of sensation, and deafferentation pain, the clinical use of CRF has generally been limited to facet denervation.6 Observation that low temperature non–tissue-destructive CRF application had results similar to high temperature tissue-destructive CRF generated immense interest. It was theorized that electrical currents rather than temperature determined the outcomes of CRF application.7 During pulsed RF (PRF), an attempt is made to maximize the delivery of electrical currents to the tissues by using higher voltage RF currents, and the risk of thermal tissue injury is minimized by maintaining the tissue temperatures below the neurodestructive range. These contradictory goals are achieved by applying the RF currents in a pulsatile manner to allow the heat to dissipate in between the RF pulses.8
Mechanism of Effect
Sluijter et al8 in their first description of PRF use described its possible mechanism of action. These authors assumed that by maintaining the electrode temperatures below the thermal destructive range that thermal tissue injury was obviated and the sustained high-density electrical fields that were generated stressed the biomolecules and caused cellular dysfunction and death. However, later investigators observed the slow response time of the temperature-measuring devices used during PRF application and concluded that the generation of brief high-temperature spikes could not be excluded, suggesting a combined role of electrical and thermal tissue injury.9,10 In laboratory studies, evidence of neuronal activation,11,12 cellular stress,13 and damage to cellular substructure9 have been demonstrated after PRF application. Conversely, other experimental studies showed that the observed PRF effects are predominantly a function of the set temperature and undermined the role of electrical currents.14,15 Among other postulated mechanisms, neuromodulation as a possible mechanism of PRF effect has also been suggested.16 The exact mechanism of the clinical effects of PRF is therefore unclear, and no evidence currently exists of nociceptive pathway interruption in response to PRF application.
Technique
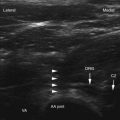
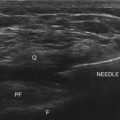
PRF is applied via an electrode placed in the vicinity of the target nociceptive structure. However, as the electrical currents extend radially from the electrode tip, unlike the CRF technique, juxtapositioning of the electrode tip parallel to the target nerve is deemed unnecessary. During a typical PRF application, the RF currents are applied for 20 msec, at 2 Hz, for a total duration of 120 seconds; for the majority of the duration of the lesion—480 of 500 msec. Therefore, no RF current is applied. The electrical currents are applied at higher voltage, but the maximum electrode temperature is maintained below 42° C.8 Variations from this standard PRF protocol have been infrequent with the exception of longer lesion duration, and PRF was been applied for 4, 8, and 20 minutes in clinical studies.
Clinical Uses
The clinical use of PRF is relatively widespread, and it has been used for both painful and some nonpainful conditions.17 PRF has been applied to the dorsal root ganglia (DRG) at all spinal levels; in the treatment of multiple pain syndromes, including radicular pain, postherpetic neuralgia, herniated intervertebral disc, postamputation stump pain, and inguinal herniorrhaphy pain.17 It has also been applied to a wide variety of peripheral nerves for the following pain syndromes: medial branch nerve for facet syndrome; suprascapular nerve for shoulder pain; intercostal nerves for postsurgical thoracic pain; lateral femoral cutaneous nerve for meralgia paresthetica; pudendal nerve for pudendal neuralgia; dorsal penile nerves for premature ejaculation; splanchnic nerves for chronic benign pancreatic pain; sciatic nerve for phantom limb pain; obturator and femoral nerves for hip pain; glossopharyngeal nerve for glossopharyngeal neuralgia; occipital nerve for occipital neuralgia; and genitofemoral, ilioinguinal, and iliohypogastric nerves for groin pain and orchialgia.17 PRF was applied to various central nervous system and autonomic ganglia, including the gasserian ganglion for trigeminal neuralgia; the sphenopalatine ganglion for head, neck, and facial pain; and the lumbar sympathetic chain in the treatment of complex regional pain syndrome.17 In some reports, the target neural structure for PRF application was unclear, such as sacroiliac joint for sacroiliac joint dysfunction, intradiscally for discogenic pain, myofascial trigger points for myofascial pain, scar neuromas for postsurgical scar pain, spermatic cord for testicular pain, and intraarticularly for arthrogenic pain.17
Clinical Efficacy
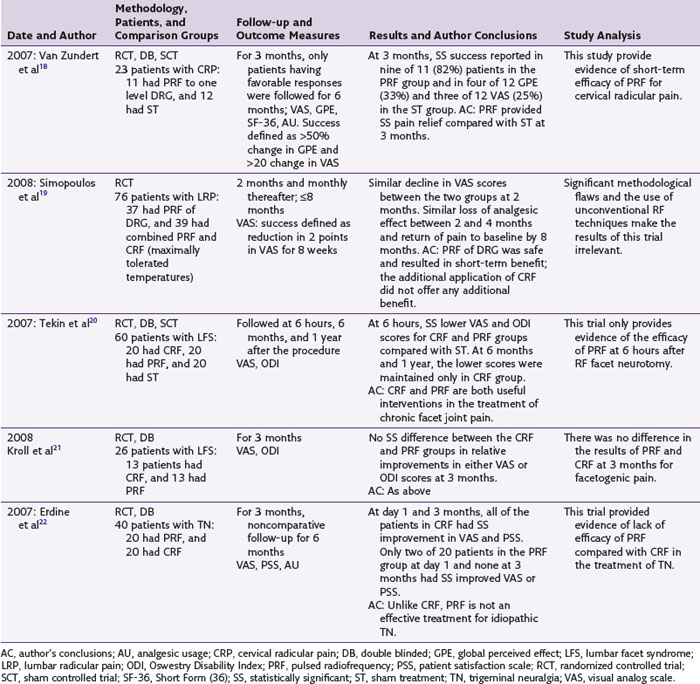
PRF has been used most frequently for the treatment of lumbar and cervical radicular pains. Seven of the nine studies reporting this PRF use are observational and reported its successful use.17 Of the two randomized controlled trials (RCT) available on this topic, one is an RCT of 23 patients with chronic cervical radicular pain that compared PRF applied with the DRG in 11 patients to similarly performed sham intervention in 12 patients (Table 16-1).18 The results of this trial showed statistically significant improvement in pain and patient satisfaction scores at 3 months in the PRF group. However, in addition to its small size, this trial reported only short-term results at 3 months. The second RCT include 76 patients with lumbar radicular pain and compared PRF with combined PRF and CRF application to the involved DRG.19 In both study groups, the patients experienced significant pain relief at 2 months, but there was significant loss of analgesic effect by 4 months and return of pain by the eighth month. Although the study results concluded that the PRF of the DRG resulted in short-term benefits and no additional benefit was gained by CRF application, this trial compared PRF with a combined PRF and CRF technique not used clinically and with variable lesion temperatures and durations (i.e., CRF applied until the patient felt radicular pain).
The second most commonly reported PRF application is in the treatment of facet syndrome, (FS) and two RCTs and three observational studies are available on this topic.17 In one RCT of 60 patients with chronic lumbar FS, the effects of CRF, PRF, and sham treatment were compared.20 The three study groups were evaluated immediately and at 6 and 12 months after the procedure. The patients in both the CRF and the PRF groups had lower pain and disability scores immediately after the procedure compared with the sham group. However, the pain relief and functional improvement were maintained in the CRF group only at 6 and 12 months. The significance of these lowered pain scores in the postprocedural period in terms of long-term pain relief is unclear. The second RCT included 50 patients with lumbar FS of more than 1 month’s duration.21 Only 26 patients, of whom 13 received CRF and 13 PRF, completed their follow-up evaluations. No significant difference in the pain and disability scores was found at 3 months between the two groups. This trial reported a dropout rate of 48%, and only short-term results at 3 months were reported. The available controlled trials of PRF for the treatment of FS therefore provide inconclusive evidence of its long-term efficacy for this condition. The three available observational studies of PRF application for FS all reported its efficacy.17
In an RCT of 40 patients with idiopathic trigeminal neuralgia, the effects of PRF application to gasserian ganglion were compared with CRF.22 At 3 months, patients in the PRF group reported no significant pain relief or improved satisfaction compared with the CRF group. The results of this study concluded that compared with CRF, PRF was not an effective method of treatment for idiopathic trigeminal neuralgia. One case series reported the efficacy of PRF in the treatment of trigeminal neuralgia.17
Successful application of PRF to suprascapular nerve for shoulder pain has been reported in four case reports or case series.17 A case report and a prospective case series reported successful application of PRF to sphenopalatine ganglion for head, neck, and facial pain.17 The use of PRF for the remaining clinical conditions described earlier is based on single case report or a case series, almost all of which described the successful use of PRF for that condition.17
Side Effects and Complications
Although bleeding, infection, and nerve damage from needle placement and burns from incorrect placement of the grounding pad have been reported,23 no noticeable side effects or complications have been directly attributed to PRF use.
1 Hunsperger RW, Wyss O. Production of localized lesions in nervous tissue by coagulation with high frequency current. Helvetica Physiologica Pharmacologica Acta. 1953;11:283-304.
2 Shealy CN. Percutaneous radiofrequency denervation of spinal facets: treatment for chronic back pain and sciatica. J Neurosurg. 1975;43:448-451.
3 Brodkey J, Miyazaki Y, Ervin FR, et al. Reversible heat lesions, a method of stereotactic localization. J Neurosurg. 1964;21:49-53.
4 Letcher FS, Goldring S. The effect of radiofrequency current and heat on peripheral nerve action potential in the cat. J Neurosurg. 1968;29:42-47.
5 Smith HP, McWorther JM, Challa VR. Radiofrequency neurolysis in a clinical model. J Neurosurg. 1981;55:246-253.
6 Uematsu S, Udvarhelyi GB, Benson DW, et al. Percutaneous radiofrequency rhizotomy. Surg Neurol. 1974;2:319-325.
7 Slappendel R, Crul BJ, Braak GJ, et al. The efficacy of radiofrequency lesioning of the cervical spinal dorsal root ganglion in a double-blinded, randomized study: no difference between 40 degrees C and 67 degrees C treatments. Pain. 1997;73:159-163.
8 Sluijter ME, Cosman ER, Rittman WB, Van Kleef M. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion—a preliminary report. Pain Clin. 1998;11:109-117.
9 Erdine S, Yucel A, Cimen A, et al. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9:251-256.
10 Cosman ER, Cosman ERSr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6:405-424.
11 Higuchi Y, Nashold BS, Sluijter M, et al. Exposure of dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery. 2002;50:850-855.
12 Van Zundert J, de Louw AJ, Joosten EA, et al. Pulsed and continuous radiofrequency current adjacent to the cervical dorsal root ganglion of the rat induces late cellular activity in the dorsal horn. Anesthesiology. 2005;102:125-131.
13 Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;10:171-176.
14 Podhajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continues radiofrequency lesions at 42 degrees C to rat dorsal root ganglion and sciatic nerve. Spine. 2005;30:1008-1013.
15 Heavner JE, Boswell MV, Racz GB. A comparison of pulsed radiofrequency and continuous radiofrequency on thermocoagulation of egg white in vitro. Pain Physician. 2006;9:135-137.
16 Cahana A, Vutskits L, Muller D. Acute differential modulation of synaptic transmission and cell survival during exposure to pulsed and continuous radiofrequency energy. J Pain. 2003;4:197-202.
17 Malik K, Benzon HT. Pulsed radiofrequency: a critical review of its efficacy. Anaesth Intensive Care. 2007;35:863-873.
18 Van Zundert J, Patijn J, Kessels A, et al. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain. 2007;127:173-182.
19 Simopoulos TT, Kraemer J, Nagda JV, et al. Response to pulsed and continuous radiofrequency lesioning of the dorsal root ganglion and segmental nerves in patients with chronic lumbar radicular pain. Pain Physician. 2008;11:137-144.
20 Tekin I, Mirzai H, Ok G, et al. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clin J Pain. 2007;23:524-529.
21 Kroll HR, Kim D, Danic MJ, et al. A randomized, double-blind, prospective study comparing the efficacy of continuous versus pulsed radiofrequency in the treatment of lumbar facet syndrome. J Clin Anesth. 2008;20:534-537.
22 Erdine S, Ozyalcin NS, Cimen A, Celik M, et al. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11:309-313.
23 Cohen SP, Foster A. Pulsed radiofrequency as a treatment for groin pain and orchialgia. Urology. 2003;61:645.